Introduction
Coronary heart disease (CHD) is the most common
cause of mortality in Euramerican countries (1). Coronary vessels in patients with CHD
undergo damage and stenosis, the abnormal narrowing of blood
vessels, due to atherosclerosis (AS) (2). Coronary restenosis, additional vessel
narrowing, may occur following percutaneous coronary intervention,
the primary treatment for CHD, which may lead to severe
complications. Dysregulation of immune responses has been shown to
be an important initiating factor for the inflammatory reactions
that characterize AS (3). Clinical
and animal studies have suggested that the humoral and cellular
immune responses of patients with CHD are in a hyperfunctional
state, which may have an important role in the formation and
development of atheromatous plaques in coronary arteries (3,4).
Cellular immunity is mediated by T cells and involves the
synergistic effects of numerous inflammatory cells and inflammatory
factors. The abnormal activation of T cells has been shown to have
a role during each stage in the occurrence and development of AS
(5,6).
Costimulatory molecules, including cluster of
differentiation (CD)40 and CD134, bind to their ligands (CD40L and
CD134L) to mediate interactions between T lymphocytes and dendritic
cells. These molecules have an important role in the innate and
adaptive immune responses, and have been implicated in the
pathogenesis of AS (7).
Costimulatory molecules may also activate atheroma-associated cells
to produce intercellular adhesion molecules (ICAM), cytokines,
matrix metalloproteinases (MMP) and tissue factors that are
involved in AS (8,9).
Cyclosporin A (CSA), which is a type of
immunosuppressant that exerts its effects predominantly via the
inhibition of T cell activation processes, is widely used during
organ transplantation and to treat autoimmune diseases (10,11).
Therefore, the present study aimed to investigate the roles of the
costimulatory molecules CD40 and CD134, and inflammatory factors,
in the formation and development of CHD and RS in a rabbit model,
and to determine whether CSA intervention was able to attenuate the
development of AS.
Materials and methods
Animals
A total of 48 healthy male New Zealand White rabbits
(age, 3–5 months; weight, 2.3–3.0 kg) were randomly divided into
six groups (8 rabbits/group), as follows: Normal control (N) group;
N + CSA group; CHD model group; CHD + CSA group; RS model group;
and RS + CSA group. The present study was approved by the ethics
committee of Tianjin Chest Hospital. The N and N + CSA groups
received a normal diet of vegetables and grains. The CHD and CHD +
CSA groups received a 1.5% cholesterol diet and underwent iliac
artery balloon injury at the end of week 4. The RS and RS + CSA
groups also received a 1.5% cholesterol diet, but underwent iliac
artery balloon injury at the ends of weeks 4 and 8. In addition,
the N + CSA, CHD + CSA and RS + CSA groups were administered 10
mg/kg/day CSA (Novartis International AG, Basel, Switzerland) for 4
weeks by gavage. Rabbits were maintained in individual cages at
temperatures of 19–25°C and 42–68% humidity, under a natural
day/night cycle. Rabbits had unlimited access to water, and were
sacrificed at the end of week 12 for harvesting of the iliac
arteries. This was performed by inhalation of anesthesia; rabbits
were administered 10 mg/ml ketamine hydrochloride at a volume of 1
ml/kg of body weight (Jiuan Wuhan Pharmaceutical Co., Ltd., Wuhan,
China)
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Sections of the iliac arteries were used to measure
the mRNA expression levels of CD40/CD40L, CD134/CD134L and the
inflammatory factors MMP-1, MMP-9, vascular cell adhesion protein
(VCAM)-1, interleukin (IL)-6 and tumor necrosis factor (TNF)-α by
RT-qPCR. Briefly, total RNA was extracted from the iliac arteries
and homogenized in liquid nitrogen, following which 1 ml TRIzol
(Promega Corporation, Madison, WI, USA) was added. The purity and
quantity of total RNA was determined using an ultraviolet
spectrophotometer, and was used at a concentration of 1.08–1.752
µg/µl. Reverse transcription was conducted in a two-step reaction,
as follows: i) 0.4 µl random primers, 7.6 µl double-distilled
H2O and 2.0 µl RNA, incubated at 70°C for 5 min; and ii)
2.5 µl double-distilled H2O, 2.0 µl 10 µM
deoxynucleotide solution, 4.0 µl 5X buffer, 0.5 µl RNase, 1.0 µl
Moloney Murine Leukemia Virus Reverse Transcriptase (all Promega
Corporation) at 37°C for 60 min, followed by incubating the
reaction mix at 95°C for 5 min. qPCR was performed on the Applied
Biosystems 7500 Real-Time PCR System (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) using 10.0 µl SYBR Green PCR Premix (Takara
Bio, Inc., Otsu, Japan) with a reaction system consisting of 0.4 µl
ROX Reference Dye II, 0.5 µl each of the forward and reverse
primers, 1.0 µl cDNA (diluted 10 times), and 7.6 µl
double-distilled H2O. PCR cycling conditions were 95°C
for 30 sec, followed by 45 cycles of 95°C for 5 sec and 62°C for 34
sec. Primer sequences were as follows: CD40 forward,
5′-AATGCGTAGACGGCACCAAC-3′ and reverse, 5′-CAGGCATCGCTGATGCAATG-3′;
CD40L forward, 5′-AACGAGAAGGGTCCTTATCC-3′ and reverse,
5′-CCAAAGTGTTGCTCATGGTG-3′; CD134 forward,
5′-CGAGGCTGTCAACTACCAAG-3′ and reverse, 5′-GTCCGCTTCCCAGCTAAGG-3′;
CD134L forward, 5′-TTCTGTGCCTCACCTACGTC-3′ and reverse,
5′-CACACTGCAGGATGACGACTGAG-3′; and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) forward, 5′-GTGATGCTGGTGCCGAGTAC-3′ and
reverse, 5′-GGTGGCAGTGATGGCGTGC-3′. These were provided by Beijing
Liuhe Huada Gene Technology Company, Beijing, China. RT-qPCR was
performed in 3 repeats. Relative mRNA expression levels normalized
to GAPDH were determined using the 2−ΔΔCq method
(12).
Histological analysis
Another part of the iliac arteries was used for
histological analyses. Briefly, the rabbit iliac arteries were
embedded in paraffin and cut into 5-µm sections. The sections were
dried at 45°C for ~4 h. Following dewaxing with dimethylbenzene,
the sections were treated with 3,3′-diaminobenzidine and
hematoxylin and eosin. Subsequently, the sections were mounted
using neutral gum and observed under a light microscope.
Immunostaining
For immunostaining, the sections were incubated with
rabbit anti-CD40 monoclonal antibody (cat. no. ab58391; 1:400;
Abcam, Cambridge, UK), rabbit anti-CD40L monoclonal antibody (cat.
no. ab52750; 1:400; Abcam), rabbit anti-CD134 polyclonal antibody
(cat. no. ab76000; 1:400; Abcam) and rabbit anti-CD134L polyclonal
antibody (cat. no. ab76130; 1:400; Abcam) for 1 h at 37°C. These
were then incubated with MaxVision HRP-Polymer anti-mouse/rabbit
IHC Kit (Fuzhou Maixin Biotechnology Co. Ltd., Fuzhou, Fujian) for
20 min at 37°C and visualized using IHC Antibody Diluent (Fuzhou
Maixin Biotechnology Co. Ltd.). The sections were observed under a
microscope, and the levels of expression were analyzed using a
HMIAS-2000 analysis system. The positive rate was defined as the
percentage of cells with yellow or brown membranes or plasma in a
high-power field. For every section, images were captured of three
high-power fields to obtain a mean positive rate.
Statistical analysis
SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analyses. Continuous variables are
presented as the mean ± standard deviation. One-way analysis of
variance was performed to compare differences among groups, while
Fisher's least significant difference test was used to compare two
groups. Categorical variables are expressed as percentages or
frequencies. To compare count data, the χ2 test was
applied, although Fisher's exact probability method was used when
any form of the theory frequency was <5. Furthermore, a linear
correlation analysis was performed. P<0.05 was considered to
indicate a statistically significant difference.
Results
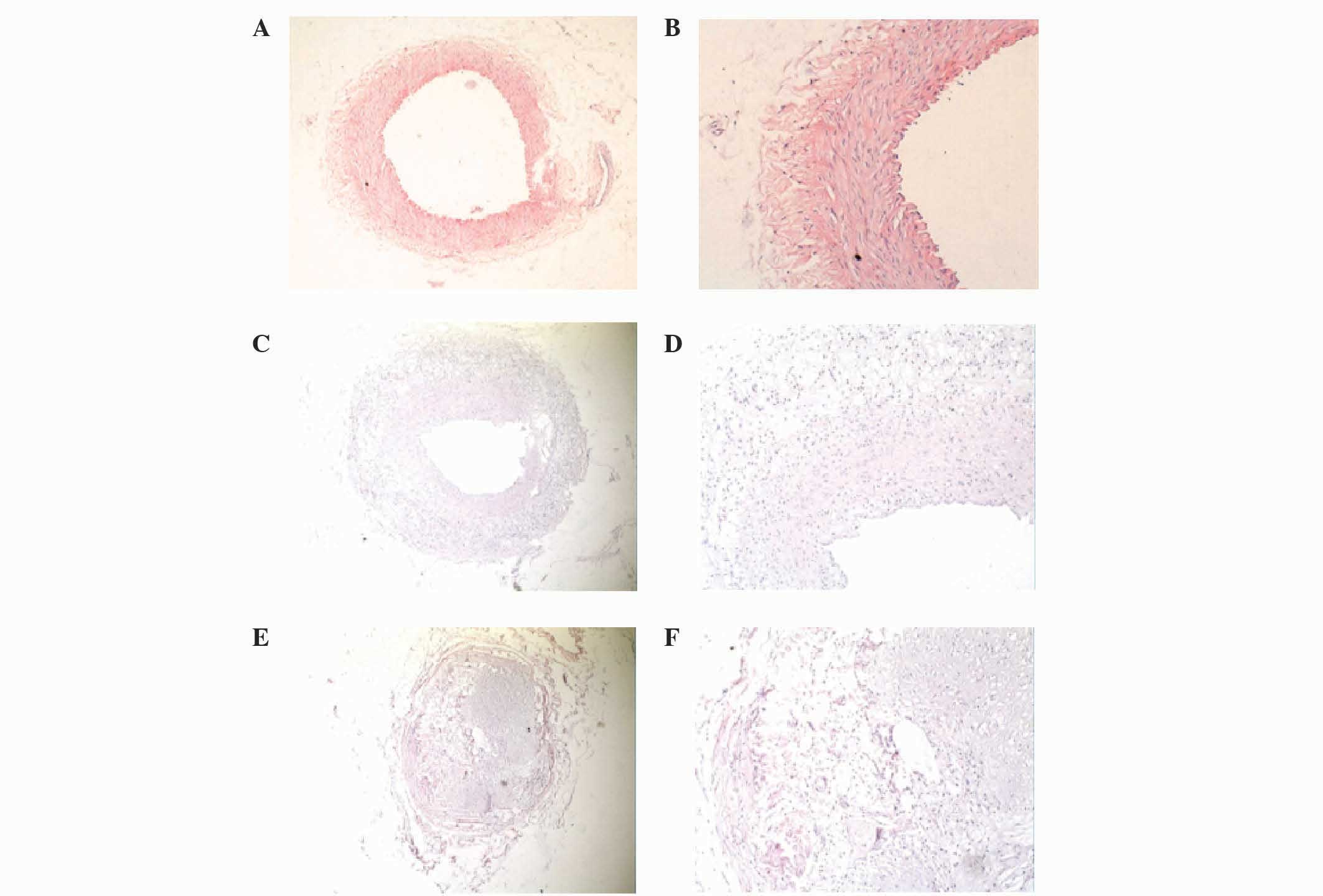
Morphology of iliac arteries
In the N and N + CSA groups, the lumens of the iliac
arteries were regular, the thickness of the vessel wall was
uniform, the vessel walls were thin and the inner and outer elastic
plates were clear and complete. In addition, the endothelial cell
nuclei were stained blue and were aligned, and the intima was shown
to be predominantly composed of monolayer endothelial cells and
inner elastic plate. In the N group, smooth muscle cells (SMCs)
were aligned, the nuclei were fusiform and SMCs had not migrated to
the endothelium (Fig. 1A and B).
The blood vessel walls of the CHD group were thicker
compared with the N group, and the lumen was narrower with
eccentric stenosis. Furthermore, the intima of the vessel wall was
significantly hyperplastic, the inner elastic plate had a
discontinuous fracture and SMCs were arranged randomly. A large
number of SMCs had migrated to the endothelium and hyperplasia
after breaking through the inner elastic, and had formed foam cells
and fibrous connective tissue in the CHD group. The pathological
appearance of the CHD + CSA group was similar to the CHD group
(Fig. 1C and D).
In the RS and RS + CSA groups, the vascular lumen
showed severe stenosis, with some of the lumens appearing almost
entirely occluded. In addition, the intimal hyperplasia was more
severe compared with the CHD group, and the hyperplastic cells were
disordered in their arrangement. A large number of SMCs, phagocytes
and foam cells were observed, and fibrous connective tissue and
small blood vessel regeneration was apparent. The damage to the
inner elastic plate was severe, while there was no clear boundary
between the endangium and tunica media in the RS group (Fig. 1E and F).
mRNA expression levels of inflammatory
factors
mRNA expression levels of MMP-1 were decreased in
the experimental groups, as compared with the N group, although the
difference was not significant (P>0.05). The mRNA expression
levels of IL-6 were significantly increased in the CHD group, as
compared with the N group (P<0.05); a decrease in mRNA
expression levels of IL-6 in CHD + CSA and RS + CSA groups compared
with the CHD and RS groups, respectively, but this was not
statistically significantly different (P>0.05). The mRNA
expression levels of MMP-9, VCAM-1, and TNF-α were markedly
elevated in the CHD and RS groups, as compared with the N group,
and the mRNA expression levels of TNF-α were significantly
increased in the RS group, as compared with the N and N + CSA
groups (P<0.05). Furthermore, there were significant differences
in the mRNA expression levels of MMP-9, VCAM-1 and TNF-α between
the CHD and CHD + CSA groups, and the RS and RS + CSA groups
(P<0.05). However, as compared with the CHD group, there was no
significant difference in the mRNA expression levels of any of
these inflammatory factors in the RS group (Table I).
 | Table I.Relative mRNA expression levels of
MMP-1, MMP-9, VCAM-1, IL-6 and TNF-α in rabbit ilial arteries
(n=8/group). |
Table I.
Relative mRNA expression levels of
MMP-1, MMP-9, VCAM-1, IL-6 and TNF-α in rabbit ilial arteries
(n=8/group).
| Group | MMP-1 | MMP-9 | VCAM-1 | IL-6 | TNF-α |
|---|
| N | 1.04±0.41 | 1.00±0.03 | 1.03±0.33 | 1.01±0.17 | 1.03±0.24 |
| N + CSA | 0.74±0.07 | 1.08±0.12 | 1.37±0.43 | 1.01±0.10 | 1.06±0.48 |
| CHD | 0.69±0.18 |
2.25±0.62a |
3.20±2.59b |
1.09±0.33b |
2.24±0.62a |
| CHD + CSA | 0.64±0.19 |
1.81±0.51c |
0.99±1.05c | 0.95±0.30 |
2.08±0.97c |
| RS |
0.60±0.21a,b |
1.60±0.81b |
3.03±1.19b | 0.88±0.37 |
3.34±1.72a,c |
| RS + CSA | 0.77±0.26 |
0.75±0.46d |
2.81±0.86d | 0.85±0.06 |
2.24±2.10d |
mRNA expression levels of CD40/CD40L
and CD134/CD134L
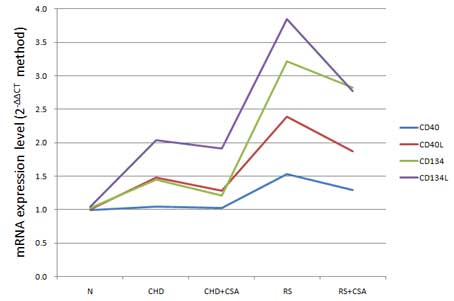
As is shown in Fig.
2, the mRNA expression levels of CD40/CD40L and CD134/CD134L
exhibited an increasing trend in the N, CHD and RS groups, with a
significant difference among the groups (P<0.05; Table II). Notably, the relative mRNA
expression levels of CD40/CD40L and CD134/CD134L were significantly
decreased in the CHD + CSA group, as compared with the CHD group
(P<0.05), and the same was observed for the RS + CSA group, as
compared with the RS group (P<0.05; Table II). Linear correlation analysis
showed that the mRNA expression levels of CD40/CD40L were
positively correlated with those of CD134/CD134L (P<0.01).
Specific correlation coefficients are shown in Table III.
 | Table II.Relative mRNA expression levels of
CD40/CD40L and CD134/CD134L in the rabbit iliac arteries
(n=8/group). |
Table II.
Relative mRNA expression levels of
CD40/CD40L and CD134/CD134L in the rabbit iliac arteries
(n=8/group).
| Group | CD40 | CD40L | CD134 | CD134L |
|---|
| N | 0.99±0.28 | 1.01±0.15 | 1.03±0.00 | 1.05±0.00 |
| N+CSA | 1.00±0.20 | 1.03±0.32 | 1.18±0.23 | 1.03±0.39 |
| CHD | 1.05±0.34 | 1.48±0.01 | 1.45±1.16 | 2.04±0.21 |
| CHD + CSA | 1.03±0.29 |
1.28±0.00a |
1.21±0.76b |
1.91±0.10a |
| RS | 1.53±0.13 | 2.39±0.34 |
3.22±0.38 | 3.85±0.35 |
| RS + CSA |
1.29±0.70c |
1.87±0.17c |
2.82±0.47c |
2.77±0.29c |
| P-value | 0.052 | 0.031 | 0.024 | 0.012 |
 | Table III.Correlation of the mRNA expression
levels of CD40/CD40L and CD134/CD134L. |
Table III.
Correlation of the mRNA expression
levels of CD40/CD40L and CD134/CD134L.
| Group | CD134 | CD134L |
|---|
| CD40 | 0.970a | 0.961a |
| CD40L | 0.962a | 0.992a |
Protein expression levels of
CD40/CD40L and CD134/CD134L
The rates of CD40/CD40L- and CD134/CD134L-positive
cells in the vessel walls were significantly increased in the CHD
and RS groups compared with the N group (P<0.05). In addition,
there were significant differences in the rates of CD40/CD40L- and
CD134/CD134L-positive cells between the CHD + CSA and CHD groups,
and the RS + CHD and RS groups (P<0.05; Table IV).
 | Table IV.Rates of CD40/CD40L and CD134/CD134L
positive cells in rabbit ilial arteries (n=8/group). |
Table IV.
Rates of CD40/CD40L and CD134/CD134L
positive cells in rabbit ilial arteries (n=8/group).
| Group | CD40 (%) | CD40L (%) | CD134 (%) | CD134L (%) |
|---|
| N | 10.42±8.32 | 4.09±5.37 | 3.01±2.05 | 6.97±3.11 |
| N + CSA | 11.23±6.37 | 9.01±6.79 | 2.57±1.97 | 5.52±2.35 |
| CHD |
63.34±8.52a |
50.53±17.98a |
26.05±12.32a |
41.73±11.77a |
| CHD + CSA |
58.37±9.81b |
45.09±11.37b |
25.91±10.33b |
37.03±14.82b |
| RS |
64.86±9.34a |
53.00±18.53a,b |
27.74±10.73a |
43.06±17.61a |
| RS + CSA |
62.32±8.35c |
49.31±17.35c |
24.41±14.38c |
39.15±16.84c |
Correlation analysis
Correlation analysis demonstrated that the mRNA
expression levels of CD40/CD40L and CD134/CD134L were positively
correlated with the intimal thickness, intimal area, internal
elastic lamin and external elastic lamina of the rabbit iliac
arteries (P<0.05; Table V). In
addition, the rates of CD40/CD40L- and CD134/CD134L- positive cells
were positively correlated with the intima hyperplasia index
(P<0.05), but negatively correlated with the lumen area
(P<0.05; Table VI).
 | Table V.Correlations between the mRNA
expression levels of CD40/CD40L and CD134/CD134L and neointimal
proliferation and vascular remodeling. |
Table V.
Correlations between the mRNA
expression levels of CD40/CD40L and CD134/CD134L and neointimal
proliferation and vascular remodeling.
| Marker | IT | IA | IA/MA | IHI | LA | IEL | EEL |
|---|
| CD40 | 0.894a | 0.953a | 0.851 | 0.778 | −0.536 | 0.939a | 0.945a |
| CD40L | 0.953a | 0.936a | 0.911a | 0.880a | −0.685 | 0.923a | 0.929a |
| CD134 | 0.939a | 0.995b | 0.883a | 0.833 | −0.599 | 0.991b | 0.991b |
| CD134L | 0.959b | 0.916a | 0.941a | 0.904a | −0.737 | 0.902a | 0.911a |
 | Table VI.Correlations between the rates of
CD40/CD40L and CD134/CD134L positive cells and neointimal
proliferation and vascular remodeling. |
Table VI.
Correlations between the rates of
CD40/CD40L and CD134/CD134L positive cells and neointimal
proliferation and vascular remodeling.
| Marker | IT | IA | IM | IHI | LA | IEL | EEL |
|---|
| CD40 | 0.911a | 0.721 | 0.897a | 0.972b | −0.955a | 0.725 | 0.727 |
| CD40L | 0.811 | 0.546 | 0.845 | 0.925a | −0.994b | 0.545 | 0.555 |
| CD134 | 0.785 | 0.512 | 0.842 | 0.903a | −0.985b | 0.507 | 0.522 |
| CD134L | 0.819 | 0.556 | 0.859 | 0.928a | −0.990b | 0.552 | 0.564 |
Discussion
CSA is a type of immune inhibitor with a high
efficiency and low toxicity that is widely used in the treatment of
various autoimmune diseases, aplastic anemia and other
hematological diseases (13). To
date, few studies have investigated the inhibitory effect of CSA on
the local inflammatory responses and factors associated with the
formation of AS (14,15). Therefore, the present study aimed to
evaluate the effect of CSA intervention on the expression levels of
local inflammatory factors in the iliac arteries of rabbit models
of CHD and RS, in order to determine whether CSA exerts a
protective effect against AS.
In a previous study, MMPs expressed by macrophages
were shown to degrade extracellular matrix, thin the fibrous cap of
plaque and promote plaque rapture (16). The expression of MMPs is regulated by
numerous cytokines, although it is predominantly regulated via the
induction of CD40/CD40L signaling (17,18).
MMP-9 is more prevalent and has a higher activity in plaque than
other subtypes of MMPs (19).
Consistent with this, the present study did not demonstrate a
significant difference in MMP-1 expression among the groups, except
between the RS and N groups. Conversely, the expression of MMP-9
showed an increasing trend in the RS and CHD groups, as compared
with the normal feeding group, thus suggesting that MMP-9
expression is upregulated during the process of AS. Notably, the
expression levels of MMP-9 were significantly reduced following CSA
intervention in both the CHD + CSA and RS + CSA groups.
CSA has been shown to inhibit inflammatory processes
by mediating T lymphocyte inactivation and inhibiting various
signaling pathways in the cytokine network. In the experimental
groups, the expression levels of VCAM-1 and TNF-α were
significantly decreased by CSA intervention. Cyclophilin A (CyPA)
is a receptor of CSA that also participates in cellular cholesterol
transport (20,21). Under the action of
oxidized-low-density lipoprotein (LDL), CyPA activation may occur,
which promotes cholesterol and LDL accumulation and leads to the
surface expression of VCAM-1 on endothelial cells, thereby
exacerbating inflammation (15). In
addition, in a previous study, CyPA induced endothelial cell
dysfunction and apoptosis via TNF (4). Therefore, the binding of CSA to CyPA
may inhibit AS. Previous studies of organ transplantation in rats
have suggested that CSA is able to downregulate the expression of
TNF-α (22–24). In the present study, the relative
expression levels of IL-6 were slightly reduced in the CSA
intervention groups as compared with the groups that did not
receive the intervention, although the difference was not
significant. Conversely, a previous study reported that CSA did not
reduce the serum levels of IL-6 in a rabbit model of CHD (25). The differences in these findings may
be due to the animal species, animal specificity of the antibody
and the total sample size used.
Costimulatory molecules, CD40/CD40L and
CD134/CD134L, have an important role in the signal transduction
pathway of inflammation (26). The
interaction of CD40 with its ligand can induce T lymphocytes to
produce cytokines, including IL-1, IL-6 and TNF-α, and to
upregulate the expression of adhesion and costimulatory molecules
(VCAM-1, ICAM-1, CD80 and CD86), resulting in the induction of
monocytes, SMCs and endothelial cells (9,27). A
combination of these inflammatory factors and CD40/CD40L
interaction initiates immune responses, leading to the
proliferation of endothelial cells, migration of SMCs, release of
cytokines from fat cells, and the subsequent acceleration of the
formation and progression of AS (27). Zirlik et al (28) demonstrated that CD40L interacts with
the integrin Mac-1 on the surface of macrophages to promote the
adhesion and migration of inflammatory cells. However, there is a
limited number of previous studies that have investigated the
association between CD134/CD134L and CHD. CD134/CD134L was shown to
regulate the differentiation of T lymphocytes to promote T cell
responses (29), and to induce the
proliferation and differentiation of B lymphocytes to secrete
immunoglobulin (30). It was
suggested that the CD134L gene was an AS susceptibility gene in
rats, and various genotypes of CD134L were associated with the
incidence of human myocardial infarction (31). Together, these findings suggested
that the interaction of CD40/CD40L and CD134/CD134L axes underlie
the pathophysiological process of CHD and RS in animal models,
which is consistent with the results of the present study.
CSA has been shown to inhibit protein kinase C and
calcineurin, and block the expression of the CD40L gene and
functioning of CD40-dependent T lymphocytes (32). Furthermore, a previous study
demonstrated that CSA downregulated the expression of CD40L by
inhibiting transcription factors of the nuclear factor of activated
T cell family, which are substrates of calcineurin and are required
for the expression of CD40L (32).
Research has shown that the mitochondrial permeability transition
pore (mPTP), which is closed during myocardial ischemia and open
during reperfusion, may cause reperfusion injury in the open state
(33). Therefore, as an mPTP
blocker, CSA may attenuate reperfusion injury and improve the
prognosis of patients with CHD (34). In addition, the results of the
present study suggested that CSA may influence the occurrence and
development of CHD via inhibition of the CD40/CD40L and
CD134/CD134L axes.
The present study had some limitations. The protocol
used only observed the expression of five inflammatory cytokines of
local tissue and alterations in their expression levels following
CSA intervention; thus, the study failed to further clarify the
role of other inflammatory factors in the pathogenesis of AS and
RS, and the intervention effect of CSA. Although CSA may attenuate
AS by inhibiting immune responses, the underlying mechanisms remain
unclear.
In conclusion, the present study demonstrated that
CSA significantly decreases expression of inflammatory factors
(MMP-9, VCAM-1 and TNF-α) and costimulatory molecules (CD40, CD40L,
CD134 and CD134L) in humoral and cellular immune responses, which
are closely associated with the occurrence of AS and CHD. It
suggested that CSA intervention exerted beneficial effects on CHD
and RS. These findings provide a basis for future studies, which
will provide novel ideas and strategies for the prevention and
treatment of CHD and RS.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant no. 51171058), the Tianjin
Science and Technology Major Program (grant no. 12ZCZDSY03200), the
Tianjin Municipal Health Bureau Key Program in Science and
Technology (grant no. 10KG122), and the Tianjin Municipal Health
Bureau Science and Technology Foundation (grant no. 2011KZ64).
References
|
1
|
Grundy SM, Benjamin IJ, Burke GL, Chait A,
Eckel RH, Howard BV, Mitch W, Smith SC Jr and Sowers JR: Diabetes
and cardiovascular disease: A statement for healthcare
professionals from the American heart association. Circulation.
100:1134–1146. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeFeudis FV: Coronary atherosclerosis:
Current therapeutic approaches and future trends. Life Sci.
49:689–705. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hansson GK and Libby P: The immune
response in atherosclerosis: A double-edged sword. Nat Rev Immunol.
6:508–519. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lamb DJ, Eales LJ and Ferns GA:
Immunization with bacillus Calmette-Guerin vaccine increases aortic
atherosclerosis in the cholesterol-fed rabbit. Atherosclerosis.
143:105–113. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salonen JT, Ylä-Herttuala S, Yamamoto R,
Butler S, Korpela H, Salonen R, Nyyssönen K, Palinski W and Witztum
JL: Autoantibody against oxidised LDL and progression of carotid
atherosclerosis. Lancet. 339:883–887. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hansson GK: Immune mechanisms in
atherosclerosis. Arterioscler Thromb Vasc Biol. 21:1876–1890. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lievens D, Zernecke A, Seijkens T,
Soehnlein O, Beckers L, Munnix IC, Wijnands E, Goossens P, van
Kruchten R, Thevissen L, Boon L, et al: Platelet CD40L mediates
thrombotic and inflammatory processes in atherosclerosis. Blood.
116:4317–4327. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schönbeck U, Mach F, Sukhova GK, Atkinson
E, Levesque E, Herman M, Graber P, Basset P and Libby P: Expression
of stromelysin-3 in atheorsclerotic lesions: Regulation via
CD40-CD40Ligand signaling in vitro and in vivo. J Exp Med.
189:843–853. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schönbeck U, Mach F, Sukhova GK, Herman M,
Graber P, Kehry MR and Libby P: CD40Ligation induces tissue factor
expression in human vascular smooth muscle cells. Am J Pathol.
156:7–14. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lallemand F, Felt-Baeyens O, Besseghir K,
Behar-Cohen F and Gurny R: Cyclosporine A delivery to the eye: A
pharmaceutical challenge. Eur J Pharm Biopharm. 56:307–318. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noble S and Markham A: Cyclosporin: A
review of the pharmacokinetic properties, clinical efficacy and
tolerability of a microemulsion-based formulation. Drugs.
50:924–941. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Muehlschlegel JD: Closing the pore on
reperfusion injury: Myocardial protection with cyclosporine.
Anesthesiology. 121:212–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sazliyana S, Shahrir MS Mohd, Kong CT, Tan
HJ, Hamidon BB and Azmi MT: Implications of immunosuppressive
agents in cardiovascular risks and carotid intima media thickness
among lupus nephritis patients. Lupus. 20:1260–1266. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nigro P, Satoh K, O'Dell MR, Soe NN, Cui
Z, Mohan A, Abe J, Alexis JD, Sparks JD and Berk BC: Cyclophilin A
is an inflammatory mediator that promotes atherosclerosis in
apolipoprotein E-deficient mice. J Exp Med. 208:53–66. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Poston RN, Haskard DO, Coucher JR, Gall NP
and Johnson-Tidey RR: Expression of intercellular adhesion
molecule-1 in atherosclerotic Plaques. Am J Pathol. 140:665–673.
1992.PubMed/NCBI
|
|
17
|
Zeitler H, Ko Y, Zimmermann C, Nickenig G,
Glänzer K, Walger P, Sachinidis A and Vetter H: Elevated serum
concentrations of soluble adhesion molecules in coronary artery
disease and acute myocardial infarction. Eur J Med Res. 2:389–394.
1997.PubMed/NCBI
|
|
18
|
Bou Khzam L, Boulahya R, Abou-Saleh H,
Hachem A, Zaid Y and Merhi Y: Soluble CD40 ligand stimulates the
pro-angiogenic function of peripheral blood angiogenic outgrowth
cells via increased release of matrix metalloproteinase-9. PLoS
One. 8:e842892013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ll H, Cybulsdy MI, Gimbrone MA Jr and
Libby P: Inducible expression of vascular cell adhesion molecule-1
by vascular smooth muscle cells in vitro and within rabbit
atheroma. Am J Pathol. 143:1551–1559. 1993.PubMed/NCBI
|
|
20
|
Smart EJ, Ying Y, Donzell WC and Anderson
RG: A role for caveolin in transport of cholesterol from
endoplasmic reticulum to plasma membrane. J Biol Chem.
271:29427–29435. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu J, Zhang Z, Shen WJ and Azhar S:
Cellular cholesterol delivery, intracellular processing and
utilization for biosynthesis of steroid hormones. Nutr Metab
(Lond). 7:472010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamada H, Suzuki H, Abe J, Suzuki Y,
Suenaga T, Takeuchi T, Yoshikawa N, Shibuta S, Miyawaki M, Oishi K,
Yamaga H, et al: Inflammatory profiles during Cyclosporin treatment
for immunoglobulin-resistant Kawasaki disease. Cytokine.
60:681–685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Joo YH, Chang DY, Kim JH, Jung MH, Lee J,
Cho HJ, Jeon SY, Kim SJ and Kim SW: Anti-inflammatory effects of
intranasal cyclosporine for allergic rhinitis in a mouse model. Int
Forum Allergy Rhinol. 2016. View Article : Google Scholar
|
|
24
|
Massuda TY, Nagashima LA, Leonello PC,
Kaminami MS, Mantovani MS, Sano A, Uno J, Venancio EJ, Camargo ZP
and Itano EN: Cyclosporin A treatment and decreased fungal
load/antigenemia in experimental murine paracoccidioidomycosis.
Mycopathologia. 171:161–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Houssen ME, Haron MM, Metwally SS and
Ibrahim TM: Effects of immunomodulatory drugs on plasma
inflammatory markers in a rabbit model of atherosclerosis. J
Physiol Biochem. 67:115–120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Watts TH: TNF/TNFR family members in
costimulation of T cell responses. Annu Rev Immunol. 23:23–68.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Antoniades C, Bakogiannis C, Tousoulis D,
Antonopoulos AS and Stefanadis C: The CD40/CD40 ligand system:
Linking inflammation with atherothrombosis. J Am Coll Cardiol.
54:669–677. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zirlik A, Maier C, Gerdes N, MacFarlane L,
Soosairajah J, Bavendiek U, Ahrens I, Ernst S, Bassler N, Missiou
A, et al: CD40Ligand mediates inflammation independently of CD40 by
interaction with Mac-1. Circulation. 115:1571–1580. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hauer AD, Uyttenhove C, de Vos P,
Stroobant V, Renauld JC, van Berkel TJ, van Snick J and Kuiper J:
Blockade of interleukin-12 function by protein vaccination
attenuates atherosclerosis. Circulation. 112:1054–1062. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee TS, Yen HC, Pan CC and Chau LY: The
role of interleukin 12 in the development of atherosclerosis in
ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 19:734–742.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Ria M, Kelmenson PM, Eriksson P,
Higgins DC, Samnegård A, Petros C, Rollins J, Bennet AM, Wiman B,
et al: Positional identification of TNFSF4, encoding OX40 ligand,
as a gene that influences atherosclerosis susceptibility. Nat
Genet. 37:365–372. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gupta AK, Giaglis S, Hasler P and Hahn S:
Efficient neutrophil extracellular trap induction requires
mobilization of both intracellular and extracellular calcium pools
and is modulated by cyclosporine A. PLoS One. 9:e970882014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mewton N, Croisille P, Gahide G, Rioufol
G, Bonnefoy E, Sanchez I, Cung TT, Sportouch C, Angoulvant D, Finet
G, et al: Effect of cyclosporine on left ventricular remodeling
after reperfused myocardial infarction. J Am Coll Cardiol.
55:1200–1205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dąbrowski MJ: Is further improvement of
the treatment of acute coronary syndromes still possible. Postepy
Kardiol Interwencyjnej. 9:41–44. 2013.PubMed/NCBI
|