Introduction
Insulin autoimmune syndrome (IAS) is an uncommon
cause of spontaneous hypoglycemia, and it was first reported by
Hirata et al in 1970 (1). In
patients with IAS, insulin levels were found to be significantly
elevated, usually higher than 100 mIU/ml, along with positive test
results for insulin autoantibodies. Insulin autoimmune syndrome is
usually diagnosed in patients with a negative history of exposure
to exogenous insulin. The occurrence of insulin-induced IAS is
rare. Herein, we report a case study of IAS induced by exogenous
insulin.
Case report
A 56-year-old Han Chinese man presented to Ningbo
First Hospital (Ningbo, China) on March 24, 2014 with a complaint
of repeated episodes of hypoglycemia. He had been diagnosed with
type 2 diabetes mellitus in 2004 and was subsequently treated with
oral antidiabetic agents, including metformin 500 mg BID
(Bristol-Myers Squibb, New York, NY, USA) and gliclazide 60 mg QD
(Servier, Beijing, China). He had been prescribed Novo Mix 30R
therapy (insulin aspart 30, 12 U before breakfast and 10 U before
dinner) for the past 5 years. In 2012, he was admitted in a local
hospital in a comatose state after a short prodrome of syncope,
diaphoresis, hunger sensation and palpitation. At that time, his
blood glucose level decreased to 2.3 mmol/l (normal range, 3.9–6.1
mmol/l). His hypoglycemic symptoms were relieved quickly with
intravenous glucose injection and discontinuation of insulin
therapy. Thereafter, he experienced three more episodes of
symptomatic hypoglycemia even after the treatment. The patient was
admitted to Ningbo First Hospital for further evaluation and
treatment on March 24, 2014. On admission, his random blood glucose
level was 2.2 mmol/l. He had a history of poliomyelitis and was not
aware of any medical history of hypoglycemic episodes in his family
members.
His physical vital signs were as follows: Height,
163 cm; weight, 50.5 kg; body mass index, 19 kg/m2; and
blood pressure, 122/78 mm Hg. Hyperinsulinemia was initially
suspected, and then confirmed by 75 g oral glucose tolerance test
(Table I). Laboratory investigations
showed positive insulin autoantibodies (Table II). Abdominal contrast-enhanced
computed tomography (CT) revealed a normal pancreas and
gastrointestinal tract, with no suspicious masses resembling
insulinomas. Thus, IAS was considered as the probable cause of
repeated hypoglycemia.
 | Table I.75 g oral glucose tolerance test. |
Table I.
75 g oral glucose tolerance test.
| Indicators | 0 min | 120 min | Normal range |
|---|
| One day after
admission |
|
|
|
| Blood
glucose (mmol/l) | 3.41 | 16.64 | 3.9–6.1 |
| Insulin
(mIU/l) | >1,000 | >1,000 |
1.9–23.0 |
| C-peptide
(ng/ml) | 5.69 | 6.39 | 1.1–5.0 |
| One week after
admission |
|
|
|
| Blood
glucose (mmol/l) | 3.3 | 15.3 | 3.9–6.1 |
| Insulin
(mIU/l) | >1,000 | >1,000 |
1.9–23.0 |
| C-peptide
(ng/ml) | 6.51 | 6.62 | 1.1–5.0 |
| 15 days after
discharge |
|
|
|
| Blood
glucose (mmol/l) | 6.72 | 10.2 | 3.9–6.1 |
| Insulin
(mIU/l) | 642.1 | 650.2 |
1.9–23.0 |
| C-peptide
(ng/ml) | 4 | 7.26 | 1.1–5.0 |
| Six months after
discharge |
|
|
|
| Blood
glucose (mmol/l) | 6.67 | 11.3 | 3.9–6.1 |
| Insulin
(mIU/l) | 16.5 | 53.8 |
1.9–23.0 |
| C-peptide
(ng/ml) | 4.3 | 6.89 | 1.1–5.0 |
 | Table II.Laboratory investigations of the
patient. |
Table II.
Laboratory investigations of the
patient.
| Parameter | Value | Normal range |
|---|
| Hemoglobin
A1c (%) | 7.6 |
4.0–6.0 |
| Total cholesterol
(mmol/l) | 4.62 |
2.8–5.67 |
| High density
lipoprotein-cholesterol (mmol/l) | 1.33 |
0.8–1.92 |
| Low density
lipoprotein-cholesterol (mmol/l) | 2.45 | 2.1–3.3 |
| Triglycerides
(mmol/l) | 0.63 |
0.1–1.8 |
| Homocysteine
(µmol/l) | 6 | 0–10 |
| Creatinine
(µmol/l) | 57.3 |
40–104 |
| Blood urea nitrogen
(mmol/l) | 8.96 | 1.79–7.14 |
| Alanine
aminotransferase (IU/l) | 45 | 9–50 |
| Aspartate
aminotransferase (IU/l) | 35 | 15–40 |
| Total thyroxin
(µg/dl) | 7.6 |
6.09–12.23 |
| Free thyroxin
(ng/dl) | 1.01 | 0.61–1.12 |
| Total
triiodothyronine (ng/ml) | 0.97 | 0.87–1.76 |
| Free triiodothyronine
(pg/ml) | 3.09 | 2.5–3.9 |
| Thyroid-stimulating
hormone (mIU/l) | 0.85 | 0.34–5.6 |
| Anti-tiroperoxidasa
(IU/ml) | 1.1 | 0–9 |
| Follicle-stimulating
hormone (IU/l) |
28.74 |
1.27–19.26 |
| Luteinizing hormone
(IU/l) | 9.73 | 1.24–8.62 |
| Prolactin (µg/l) | 7.83 |
4–14.4 |
| Testosterone
(ng/ml) | 6.06 | 1.75–7.81 |
| Growth hormone
(ng/ml) | 0.06 | 0.01–1 |
| Cortisol, 8 AM
(µg/dl) | 9.54 |
8.7–22.4 |
| Cortisol, 4 PM
(µg/dl) | 4.89 | 0–10 |
| Adrenocorticotropic
hormone, 8 AM (pg/ml) |
20.05 |
9.89–79.14 |
| Adrenocorticotropic
hormone, 4 PM (pg/ml) |
13.92 |
4.95–39.57 |
| Insulin
autoantibodies |
Positive |
Negative |
| Islet cell
antibodies |
Negative |
Negative |
| Glutamic acid
decarboxylase antibodies |
Negative |
Negative |
| Tumor markers |
|
|
|
Alpha-fetoprotein | 2.98 |
0–9.00 |
|
Carcinoembryonic antigen | 0.86 |
0–5.00 |
|
Carbohydrate antigen 19–9 | 4.4 |
0–25.0 |
| Cancer
antigen 125 | 8.7 |
0–35.0 |
| Cancer
antigen 15–3 | 9.9 |
0–14.0 |
|
Neuron-specific enolase | 9.04 |
0–15.20 |
|
CYF211 | 1.07 |
0–3.30 |
The patient was prescribed oral antidiabetic agents
only, including repaglinide (1 mg at 6:00 a.m., 1 mg at 10:00 a.m.,
and 2 mg at 4:00 a.m.; Novo Nordisk, Bagsværd, Denmark), acarbose
(50 mg TID; Huadong Medicine Group Co., Ltd., Hangzhou, China) and
pioglitazone (15 mg QD; Huadong Medicine Group Co., Ltd.) for 10
days. However, no signs of remission of hypoglycemia were detected.
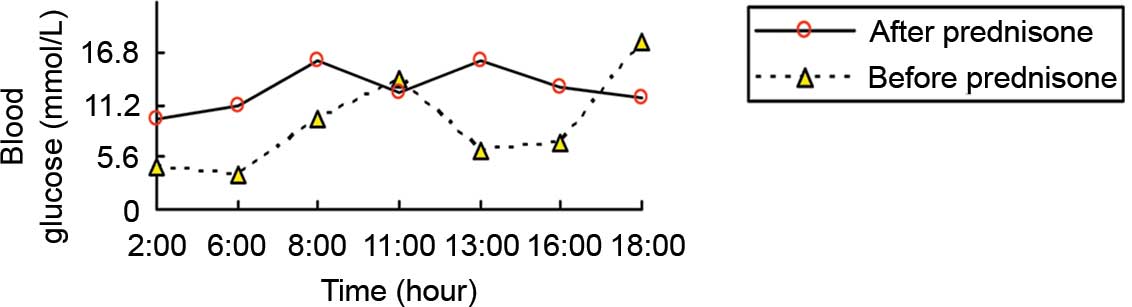
His fasting blood glucose level was normal, but postprandial blood
glucose level was elevated (Fig. 1).
One week after admission, prednisone (10 mg PO QD) was added to
control his hypoglycemia. His symptoms disappeared and there was no
recurrence of hypoglycemia (Fig. 1).
Then, his prednisone dose was maintained at 5 mg PO QD after 4
weeks. At day 15 after discharge, he had elevated blood glucose and
insulin levels (Table I) and
positive insulin autoantibodies, but had no symptomatic
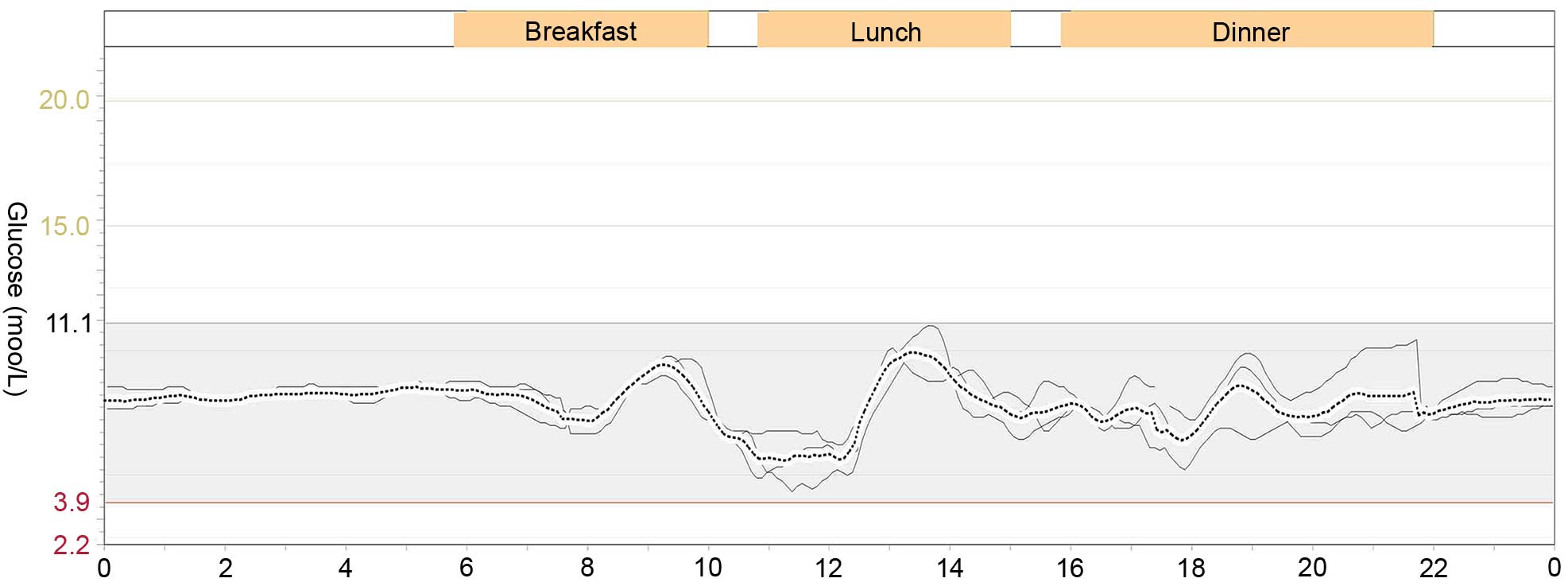
hypoglycemia. Six months after discharge, the glucose excursion was
evaluated using a continuous glucose monitoring system (MRT;
Medtronic, Ltd., Dublin, Ireland) without prednisone on three
consecutive days (2). He had a
stable blood glucose level without any episode of hypoglycemia or
hyperglycemia (Fig. 2). The
parameters of blood glucose variability were calculated as follows:
Mean absolute glucose excursion was 2.4 mmol/l, mean of daily
difference was 0.69 mmol/l. The mean blood glucose on three
consecutive days was 7.1±1.5 mmol/l, and his fasting insulin level
was 16.5 mIU/l. The initially positive result for insulin
autoantibodies turned to negative.
Discussion
Insulin autoimmune syndrome, following insulinoma
and extrapancreatic neoplasms, is the third leading cause of
spontaneous hypoglycemia in Japan (3). The incidence of IAS is similar in men
and women, and it is more frequently observed in patients aged
>40 years (3). In the present
study, we excluded the possibility of insulinoma or extrapancreatic
neoplasms by abdominal contrast-enhanced CT. The classical IAS is
characterized by postprandial hypoglycemic episodes, elevated
insulin levels, and positive insulin autoantibodies. In this case,
the patient did not have postprandial hypoglycemic episodes,
suggesting an atypical presentation of IAS. Considering that the
patient was given insulin therapy, the source of insulin
autoantibodies should be distinguished. Usually, autoantibodies
against exogenous insulin are relatively weak and disappear
spontaneously (4). However, in the
present patient insulin autoantibodies tested positive for a
relatively long period after discontinuing insulin therapy; it
indicates that these insulin autoantibodies may have been
endogenous.
Insulin receptor autoimmune diseases may cause
insulin resistance and paradoxical hypoglycemia, which are also
considered in the differential diagnosis (5). In this condition, hypoglycemia usually
occurs when the stomach is empty and disappears after taking food
(5). These patients may have other
autoimmune diseases, such as acanthosis nigricans and
hyperandrogenism. Importantly, insulin receptor antibodies can be
positive while insulin autoantibodies are negative in these cases,
which are helpful in the differential diagnosis; however, these
were unavailable in our hospital. Considering a high level of
insulin and positive insulin autoantibodies, in this case, IAS
seems to be the more likely diagnosis.
At present, the exact mechanism underlying IAS is
unknown. The commonly accepted hypothesis is use of medications
containing a sulfhydryl group might induce insulin autoantibodies
and influence the binding and release of insulin by autoantibodies
(6). Alpha-lipoic acid, a
nutritional supplement for treating diabetic neuropathy, has also
been described to cause IAS (7).
Insulin secretes when glucose concentration rises in the blood.
However, autoantibodies can prevent normal action of insulin by
binding with it (6). When the
glucose concentration decreases, the autoantibodies gradually
dissociate from insulin, resulting in a surplus of insulin, which
may contribute to hypoglycemia (7).
Certain diseases such as Graves' disease, systemic lupus
erythematosus and rheumatoid arthritis, may also trigger the
production of autoantibodies (8).
Insulin aspart, insulin glulisine and endogenous insulin may induce
IAS, but the association among them remains unclear (9,10). In
the present case, the patient did not have a history of immune
diseases or taking any of the oral drugs mentioned above. However,
he received long-term insulin treatment, and continued to
experience hypoglycemic attacks even after discontinuation of his
insulin treatment.
As the majority of patients with IAS can achieve a
remission soon after drug withdrawal, surgery is not the first
choice (11). Small, frequent meals
are recommended to reduce or avoid hypoglycemic episodes (12). In addition, glucocorticoids and
immunosuppressants may be useful as adjuvant therapies for IAS
control (13). A Japanese study
showed that corticosteroid therapy reduced the amount of insulin
receptor binding sites and avoided hypoglycemic attacks, suggesting
the usefulness of corticosteroid therapy in the treatment of IAS
(14). In the present case, a small
dose of prednisone was used, with an initial dosage of 10 mg QD and
maintaining at 5 mg QD. The patient achieved a remission and did
not experience symptomatic hypoglycemia again. His insulin
autoantibodies turned negative after prednisone treatment for 12
weeks. Acarbose, diazoxide, octreotide, pancreatectomy and
plasmapheresis have also been the treatment of choice for the
successful management of IAS (15).
Insulin autoimmune syndrome should be considered in
any patient who suffers hyperinsulinemic hypoglycemia, particularly
in a patient in which a neoplasm was not detected inside or outside
of the pancreas. Insulin-induced IAS rarely occurs. The causal
association between insulin and IAS as well as the underlying
mechanisms require further investigation.
References
|
1
|
Hirata Y, Ishizu H and Ouchi N: Insulin
autoimmunity in a case with spontaneous hypoglycaemia. J Japan Diab
Soc. 13:312–320. 1970.
|
|
2
|
Philippon M, Sejil S, Mugnier M, Rocher L,
Guibergia C, Vialettes B and Delenne B: Use of the continuous
glucose monitoring system to treat insulin autoimmune syndrome:
Quantification of glucose excursions and evaluation of treatment
efficacy. Diabet Med. 31:e20–e24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Savas-Erdeve S, Agladioglu S Yilmaz, Onder
A, Kendirci HN Peltek, Bas VN, Sagsak E, Cetinkaya S and Aycan Z:
An uncommon cause of hypoglycemia: Insulin autoimmune syndrome.
Horm Res Paediatr. 82:278–282. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lamy PJ, Sault C and Renard E: High
fasting serum insulin level due to autoantibody interference in
insulin immunoassay discloses autoimmune insulin syndrome: A case
report. Ann Biol Clin. 74:490–494. 2016.
|
|
5
|
Wang YL, Yao PW, Zhang XT, Luo ZZ, Wu PQ
and Xiao F: Insulin autoimmune syndrome: 73 Cases of clinical
analysis. Chin Med J. 128:2408–2409. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ismail AA: The insulin autoimmune syndrome
(IAS) as a cause of hypoglycaemia: An update on the
pathophysiology, biochemical investigations and diagnosis. Clin
Chem Lab Med. Apr 12–2016.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wong SL, Priestman A and Holmes DT:
Recurrent hypoglycemia from insulin autoimmune syndrome. J Gen
Intern Med. 29:250–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eisenbarth GS: Immunoendocrinology:
Scientific and Clinical Aspects. Humana Press; New York, NY:
2011
|
|
9
|
Kawasaki M, Oikawa Y, Katsuki T, Kabeya Y,
Tomita M, Okisugi M and Shimada A: Insulin glulisine may cause a
disease resembling insulin autoimmune syndrome: Case report.
Diabetes Care. 36:e195–e196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki K, Hirayama S and Ito S: A case of
a non-insulin dependent diabetic patient with regular spontaneous
hypoglycemic attacks, which were due to insulin-binding antibodies
induced by human insulin therapy. Tohoku J Exp Med. 182:163–173.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kandaswamy L, Raghavan R and Pappachan JM:
Spontaneous hypogly hypoglycemia: diagnostic evalution and
management. Endocrine. 53:47–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lanas A, Paredes A, Espinosa C, Caamaño E,
Pérez-Bravo F, Pinto R, Iñiguez G, Martínez D and Soto N: Insulin
autoimmune syndrome: Report of two cases. Rev Med Chil.
143:938–942. 2015.(in Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saxon DR, McDermott MT and Michels AW:
Novel management of insulin autoimmune sydrome with rituximab and
continuous glucose monitoring. J Clin Endocrinol Metab.
101:1931–1934. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohtsuka Y, Kondo T, Shimada M, Murakami K,
Ide H and Kawakami Y: Erythrocyte insulin receptor in insulin
autoimmune syndrome: Effects of corticosteroid therapy. Tohoku J
Exp Med. 151:181–190. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lupsa BC, Chong AY, Cochran EK, Soos MA,
Semple RK and Gorden P: Autoimmune forms of hypoglycemia. Medicine
(Baltimore). 88:141–153. 2009. View Article : Google Scholar : PubMed/NCBI
|