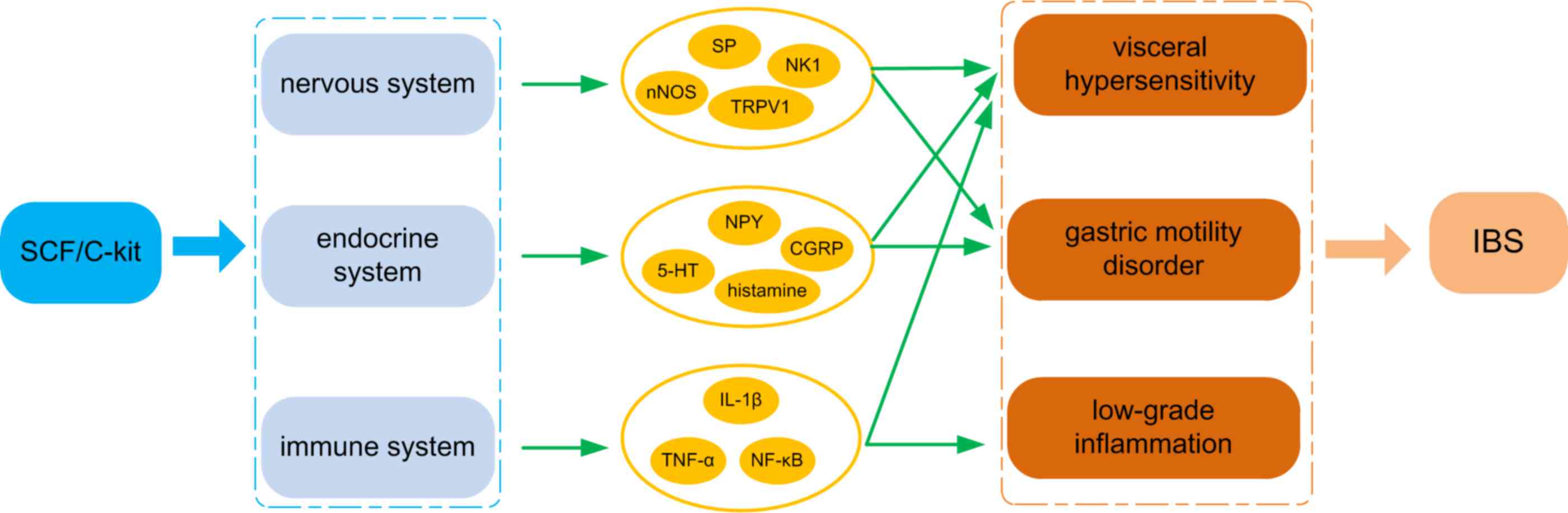
Introduction
Irritable bowel syndrome (IBS) is one of the most
prevalent chronic and functional bowel diseases, resulting in
considerable misery across the globe (1–3). The
disease is characterized by substantial abdominal pain and
discomfort; however, it lacks anatomical or histological
aberrations or consistent changes in clinical chemistry (4,5). The
current incidence of IBS is between 7–21% worldwide, a phenomenon
attributed to an increased pace of life and alterations in diet
(6). Identified risk factors
requiring further investigation include psychological stress,
changes in social environment, noxious gut stimuli and specific
dietary factors. Experimental and emerging treatments, including
fecal transplantation, have also been linked to IBS pathogenesis
(7), further demonstrating the need
for more detailed study into the IBS risk factors. Greater
understanding of factors associated with the disease is potentially
critical for future improvements in disease prevention and
therapy.
The obscurity of IBS pathogenesis is a hindrance to
such progress, though it is generally accepted that high visceral
sensitivity and disturbed gut motility, in combination with
low-grade inflammation, cause IBS via neuroendocrine and immune
dysfunction (8). However,
identifying the factors that mediate this dysfunction is still a
major challenge in current IBS research. Clues into the etiology of
the disease may be provided by study into the neurotransmitter
dynamics of the brain-gut axis and associated endocrinological
factors (9,10), as well as the intestinal flora,
though a single factor alone cannot explain the complexity of IBS
pathogenesis. Nevertheless, research should provide important
insights into pathomechanism of IBS that potentially lead to novel
drug development for the treatment of IBS.
The available evidence suggests that stem cell
factor (SCF) expression is increased in clinical IBS (11). The system composed of SCF and its
cognate receptor, c-Kit, is a principal regulator of survival and
functionality for a multitude of neural crest-derived cell types,
in particular for those involved in visceral perception, smooth
muscle contraction and inflammation (12–14).
Disorders of the neuro-endocrine-immunological network resulting
from alterations in the SCF/c-Kit system provide an explanation for
the high visceral sensitivity, abnormal bowel contraction strength
and low-grade inflammation experienced in IBS, suggesting that this
system may be an important target for intervention (15,16). In
the present review, the current trends in IBS are investigated,
highlighting the regulation of SCF/c-Kit.
Biological functions of the SCF/c-Kit
system
The c-Kit receptor is the product of the
c-kit proto-oncogene and belongs to the receptor tyrosine
kinase (RTK) superfamily, with the members of this family being the
cardinal regulators of cellular fate in the mammalian body
(17). As an important member of the
type III RTK family, it has a highly specific and restricted
expression pattern, with prominent c-Kit levels expressed on the
surface of hematopoietic cells, mast cells (MCs) and interstitial
cells of Cajal (ICC) (18,19). The cognate ligand of c-Kit is SCF,
alternatively known as dry factor or MC growth factor, which is
synthesized in abundance by the gastrointestinal (GI) tract smooth
muscle cells (SMCs) (20). The
expression patterns of SCF and c-Kit are thus consistent with their
potential involvement in IBS.
In the SCF/c-Kit mechanism, as presented in Fig. 1, extracellular SCF binds specifically
with c-Kit, with few alternate receptors proposed. Following SCF
binding, c-Kit homodimers are formed via activation of the
enzymatic kinase domain within the receptor (21). This provokes autophosphorylation of
tyrosine residues within the receptor's cytoplasmic C-termini. The
phosphotyrosine residues serve as docking sites for receptor
adaptor proteins, in turn provoking activation of a range of signal
transduction pathways (22). These
can involve the following signaling molecules: Phosphatidylinositol
3-kinase (PI3K), single-subunit small GTPases/extracellular
regulated protein kinases (Ras/Erk), janus kinase/signal
transducers and activators of transcription (JAK/STAT),
phospholipase C (PLC)-γ and the tyrosine-protein kinase Src
(16,22). Specific expression of genes typically
results and a range of biological signals are initiated to regulate
the survival, proliferation, differentiation, apoptosis, motility
and migration of c-Kit bearing cells (22).
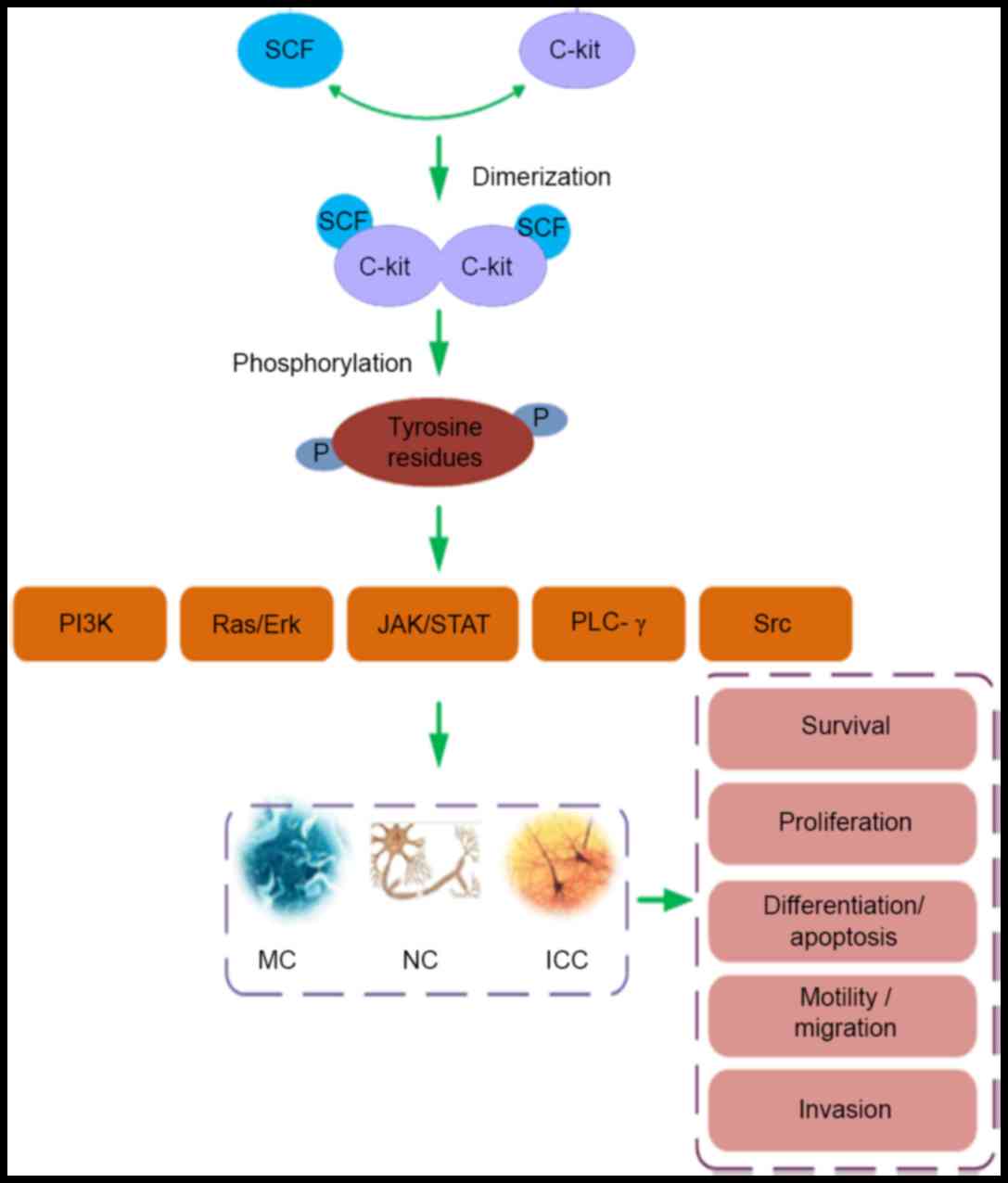 | Figure 1.The SCF/c-Kit ligand/receptor system
affects the biological function of MCs, NCs and ICC. Dimerization
of c-Kit after engagement of SCF with the receptor induces
phosphorylation of tyrosine residues in the receptor's cytoplasmic
tail. The phosphotyrosines serve as docking sites for adaptor
proteins which mediate further signal transduction, leading to
activation of the following pathways: PI3K, Ras/Erk, JAK/STAT,
PLC-γ and Src. In turn these pathways regulate the survival,
proliferation, differentiation, apoptosis, motility, migration and
invasion of MCs, NCs and the ICC. Stem cell factor (SCF)/c-Kit,
ligand/receptor tyrosine kinase signaling system; MC, mast cell;
NC, nerve cell, ICC, intestinal cells of Cajal; SCF, stem cell
factor; PI3K, phosphatidylinositol 3-kinase; Ras, a
guanosine-nucleotide-binding protein (G protein); Erk,
extracellular regulated protein kinases, JAK, janus kinase; STAT,
signal transducer and activator of transcription; PLC-γ,
phospholipase C-γ; Src, a tyrosine-protein kinase. |
Regulation of the SCF/c-Kit system affects
the function of a variety of cells
Cell lines regulated by the SCF/c-Kit system
prominently include ICC, other enteric nerve cells (NCs) and MCs.
In general, SCF is a trophic factor for neural crest derivatives,
with similar effects on ICC in terms of differentiation and
proliferation (23). SCF
specifically increases expression of the key gap junction protein
connexin 43 (Cx43), resulting in improved network function by
promoting intercellular conduction of electric stimuli (24). Furthermore, SCF promotes MC
hyperplasia and enhances the release of MC-derived pro-inflammatory
mediators (25–27). The main mediators released are
histamine, serotonin and arachidonic acid-derived compounds
(leukotrienes, prostaglandins (28),
interleukin), which in turn reduce the integrity of the local
microcirculation and elicit an inflammatory response of body
(29–31). Additionally, SCF increases the
intrinsic pacemaker rhythm of ICC that regulates GI smooth muscle
contraction, via phosphorylation of substance P (SP), neurokinin-1
(NK1), transient receptor potential vanilloid-1 (TRPV1) receptors
and by promoting the conduction of pain signals towards the central
nervous system (CNS) (32). When
considering the consequences of alterations in these biological
signals, a potential role of the SCF/c-Kit axis in IBS
pathophysiology emerges. It can be argued that this axis
contributes to the high visceral sensitivity, exaggerated
contraction and inflammation observed in IBS, thus explaining how
stress and psychological factors are related to abdominal pain and
discomfort in IBS.
In this context, it is noteworthy that structural
loss in the ICC is a potential factor in IBS pathogenesis (15,33).
ICCs constitute an elaborate network of contraction-controlling
cells, present in all muscle layers of the GI tract and other
internal structures, including the stomach, small intestine,
pancreas, colon and bladder, where they regulate a range of
biological functions. Based on their morphology, distribution and
anatomical relationship with nerve plexus and smooth muscle, ICC
are categorized into four subtypes (34): i) Myenteric ICC (IC-MY), located
between circular and longitudinal muscle layers of the stomach,
small intestine, colon and other muscles (35); ii) submucosal ICC (IC-SM),
distributed along the submucosal layer of the surficial colon
circular beam (36); iii) deep
muscular ICC, (IC-DMP), located between the inner thin layer and
outer thick layer of the intramuscular ring, particularly in the
small intestine (37); and iv)
intramuscular ICC, (IC-IM), located in all areas of the muscle
layers above (35). MY and IC-SM
serve mainly as GI pacemakers. IC-DMP and IC-IM are typically
associated with the transmission of enteric nerve signals. The ICC
types constitute an intricate network, fundamental to GI
electrophysiological activity (22).
Importantly, cross-talk with the CNS exists and the ICC stimulate
the sensory nerve of the GI tract through direct synaptic contact
(38–40). The ICC are key to gastroenterological
function and, conversely, the SCF/c-Kit axis is important for the
development and phenotypic differentiation of the ICC, as well as
ICC membrane polarization and pacemaker activity. Thus, a defining
role of the axis in transcriptional regulation effecting intestinal
bowel movement and intestinal rhythm is possible (41). Indeed, in experimental rodents,
neutralizing antibody targeting c-Kit resulted in major loss of ICC
from the jejunum, whereas high-dose SCF reversed this effect
(23). Together with the cardinal
functionality of ICC in visceral motility (including pacemaker
activity, regulation of peristaltic bowel movement and other
aspects of smooth muscle cell functionality), these results
indicate that key aspects of GI physiology require functional
SCF/c-Kit signaling. Though it is noteworthy that the SCF/c-Kit
axis controls mainly long-term changes in ICC physiology, where it
is directly controlled by receptors for NK1, NO,
5-hydroxytryptamine (5-HT) and SP, expressed on the ICC
surface.
Other cell types controlled by the SCF/c-Kit axis
include MCs, a specific type of immune cell required for barrier
protection in the intestinal mucosa. An important event in IBS
pathogenesis is MC degranulation (42), whereby MC release 5-HT, histamine,
TNF-α and various interleukins, all of which are known to aggravate
intestinal inflammation and affect visceral perception (43). The development and migration of MCs
depend on SCF. For instance, SCF promotes the adhesion and
proliferation of MCs by regulating expression of intercellular
adhesion molecule-1 (44). As a
chemotactic factor of MCs, SCF may promote the regeneration of MC
from CD34+ progenitor cells, MC survival and adhesion to
the extracellular matrix (25).
Importantly, histamine levels decline substantially when the
SCF/c-Kit system is repressed and the SCF/c-Kit axis has been
repeatedly identified as one of the most promising targets for
controlling MC inflammation (26,45).
Indeed, evidence suggests that SCF inhibition potentially lowers
visceral sensitivity via modulation of the MC compartment (46).
Additionally, control of neuronal electrical
activity is key to reducing excessive intestinal motility in IBS.
The signaling pathway provoked by SCF/c-Kit activity maintains
survival, proliferation and nutrition of the neural crest cells, as
well as inducing their differentiation and migration (47). c-Kit expression is a typical
characteristic of post-mitotic nerve cells in the early stages of
lineage differentiation from neurons to glial cells. SCF stimulates
nerve regeneration both in experimental rodents and in vitro
(48). Although indirect, these
associations point to a central role of the SCF/c-Kit axis in
organizing neural networks and represent targets for IBS
treatment.
ICC, MC and NC are not independent structures and
undergo interactions when IBS occurs throughout the nervous system
(NS), digestive system, immune system and other regions (49–52).
They constitute a large and intricate network system, potentially
of critical importance in IBS due to its control of a range of
intestinal functions, including visceral sensitivity and
inflammatory responses. However, it is also important to note that
the ICC, MCs and NCs are not the sole targets of SCF/c-Kit
activity, with other cell targets of SCF/c-Kit having potential
involvement in IBS.
Relationship between the SCF/c-Kit axis and
neuroendocrine-immune regulation in IBS
SCF/c-Kit and neurological
disorders
Neurological disorders, including those of the
enteric nervous system (ENS), the autonomic nervous system (ANS)
and the CNS, contribute to visceral hypersensitivity and GI
motility disorder in IBS (53). Upon
stimulation by intestinal irritation and/or psychological or
emotional factors, the three strands of the NS integrate the
stimulus information to generate a GI effector response and cause
pain sensation. Appropriate execution of this process depends on
the structural integrity and electrophysiological properties of the
intestinal neurons involved, which are in turn controlled by the
SCF/c-Kit axis: SCF is constitutively expressed in various regions
of the NS including the CNS, ENS and ANS, but specific expression
levels are influenced by external stimuli (54). SCF directly affects neurotransmission
by binding its receptor c-Kit and this influences the efficacy of
the NS response to external stimuli (12). Indeed, experimental studies in a
depression mouse model revealed a correlation between decreased
c-Kit expression in the hippocampus and impaired neuronal
differentiation and migration (55).
It is well established that severe depression is a key factor
predisposing to IBS development (56,57).
Therefore, a relationship between altered SCF/c-Kit signals with
the emotional, psychological and physical stimulated state of IBS
patients is possible (58).
Potential effector mechanisms may depend on SCF/c-Kit-mediated
effects on the phosphorylation status of receptor systems involved
in sensing neurotransmitter levels, including the neuronal nitric
oxide synthase (nNOS), SP, NK1 and TRPV1 systems (59,60).
This has been shown to result in overstimulation of the
nerve-ICC-smooth muscle signal transfer system, promoting
development of IBS-like symptoms (61). Similarly, in experimental IBS,
changes in the regulation of SCF/c-Kit may stimulate strong nerve
reflexes and enhance the rhythm of smooth muscle contraction, while
simultaneously stimulating pain perception (14,62).
However, the contributions of such activity to clinical IBS remains
to be established.
SCF/c-Kit axis and abnormalities of
the endocrine system
It is well established that malfunction of the
brain-gut axis is a key mechanism in explaining IBS pathogenesis
(63). In addition to direct
innervation between the brain and the gut, it is often assumed that
the endocrine system is an important connection between the two
systems. Indeed, there is an established link between disturbed
serum hormone levels and IBS (60,64), due
to hormone secretion typically being related to the psychological
state of the patient. This may explain IBS-related stress and other
psychological disorders. Relevant mediators include 5-HT, nerve
peptide Y, calcitonin gene-related peptide and histamine (65,66). The
secretion of these is altered by the patient's psychological state,
with the secreted factors targeting the intestinal ICC network. The
SCF/c-Kit axis may facilitate the perception of such
endocrinological signals, as it has been found that stimulation of
c-Kit provokes substantial leukotriene C4 release via the
activation of cytosolic phospholipase A2 (67). This is then associated with increased
affinity of histamine and 5HT receptors for their ligands (68,69). As
such, the SCF/c-Kit axis promotes sensitivity to GI hormones and
thus may have a detrimental influence on pain perception,
inflammation and bowel movement.
SCF/c-Kit and the immune system
Low-grade intestinal inflammation is established as
a key characteristic of IBS (70,71) and
is related to MC activation, as well as altered permeability of the
intestinal mucosa to antigens (71,72). The
general principal genomic regulator of the inflammatory response is
nuclear factor-κB (NF-κB). Inactive NF-κB is sequestered in the
cytoplasm, while activated NF-κB enters the nucleus and stimulates
transcription of a range of proinflammatory factors, including
tumor necrosis factor-α (TNF-α) and interleukin-1β. C-kit may
activate NF-κB and promote the release of TNF-α when it is
sensitized, which may be one way of SCF/C-kit initiating the immune
inflammatory response (51,73). In addition, the trophic action of
SCF/c-Kit on the MC compartment is directly associated with
diarrhea-predominant IBS. Furthermore, c-Kit-mediated cysteinyl
production results in sensitization of MC receptors involved in
secretion of proinflammatory mediators, in turn enhancing the
inflammatory response. These findings demonstrate a mild
inflammatory response is stimulated through SCF/c-Kit signaling,
which potentially links to IBS pathogenesis.
SCF/c-Kit and visceral hypersensitivity in
IBS
Zhang et al developed an IBS rat model via
infection with Trichinella spiralis (74). Rat models are generally not
appropriate targets for genetic intervention, however, imatinib
mesylate (STI-571), a moderately specific blocker of c-Kit
(75), is amenable to experimental
investigation in this model. A previous study revealed that the
change of intestinal ICC activator rectus muscle electricity and
dorsal commissural nucleus (DCN) were higher in the IBS rat,
compared with the other rat model, whereas they decreased
significantly following STI-571 exposure. It was indicated that
visceral hypersensitivity in IBS rats may be suppressed when
blocked the SCF/C-kit signal (74).
These results indicate the importance of the SCF/c-Kit axis in IBS
and the pharmacological implications of this axis, with imatinib
offering a potential therapeutic option for targeting visceral
hypersensitivity.
Visceral hypersensitivity is a key characteristic of
IBS. The strong dependence of IBS-related pain on psychological and
environmental conditions indicates that the pain is under CNS
control, however, activity and sensitivity of the spinal sensory
nerve fibers may additionally be dependent on endocrinological
control (9,10). In this instance, the neurotrophic
action of SCF may directly affect and stimulate neurotransmitter
responses via the SCF-c-Kit axis (12). In the ENS, periodic slow wave
potentials between the ICC and SMCs are generated and perceived by
neurotransmitter receptors, including those for SP, vasoactive
intestinal peptide, histamine, serotonin and acetylcholine,
expressed on the ICC membranes. In turn, these receptors mediate
contractile and relaxant effects in GI smooth muscle and pain
perception (76). As stated above,
MCs may further amplify the pain signals (77), though the pain threshold itself
appears largely dependent on the activation state of the SCF/c-Kit
axis. Small intestinal resection and transmission microscopy
observations indicate that modifying the c-Kit pathway or
activating the SCF/c-Kit pathway affects the depolarization and
pacemaker functions of the ICC and alters intestinal rhythm
(78). However, whether clinical
trials employing imatinib provide benefit for these effects is
unclear and warrants further study.
Conclusion
IBS is a functional disease associated with multiple
systems in the body, though it is particularly associated with
brain-gut cross-talk via modulation of the endocrine system. The
results of this include aberrant GI motility and visceral
hypersensitivity, in turn leading to abdominal discomfort and
abnormal defecation patterns. The SCF/c-Kit signaling system is
critical in controlling many of the elements involved (Fig. 2), including ICC MC and nerve cells,
and thus shows potential as a pharmacological, intervention aimed
at combatting visceral sensitivity and GI disorder in IBS patients.
The ICC serve as the pacemakers for GI smooth muscle contraction
and integrate neuroendocrine physiology (72). ICCs depend on SCF/c-Kit interaction
for growth and development and respond to its signaling by
upregulating neurotransmission. Pain perception in ICCs is
increased through the effects of SCF/c-Kit on MCs. Fortunately,
pharmacological antagonists for SFC/c-Kit signaling are clinically
available. For instance, inhibitors including imatinib, sorafenib,
lapatinib and sunitinib are capable at a minimum of partially
targeting this signaling system (73–75). In
addition, imatinib is reasonably c-Kit-specific, however the
physiological importance of SCF/c-Kit signal transduction in nearly
all cell types derived from the neuronal crest lineage may prevent
the use of inhibitory strategies. As a factor involved in GI
motility, visceral sensitivity and inflammatory signaling, blockade
of SCF/c-Kit may trigger collateral damage, which would likely
preclude its use in a non-lethal disease such as IBS (76,77).
Therefore, further studies with animal models are required to
develop acceptable interventions. In addition, further insight is
required into the mechanisms mediating SCF/c-Kit signaling. Design
of new drugs specifically inhibiting SCF/c-Kit signal transduction,
that preferentially act locally in the intestine, may be critical
for successful outcomes. Such targeting of SCF/c-Kit inhibition is
a novel strategy in IBS therapy.
Acknowledgements
This study was supported by the Guangdong province
Nature Science Foundation of China (grant no. 2014A030313404), the
Natural Science Foundation of China (grant no. 81673842) and the
‘Excellent Doctoral Dissertation Incubation Grant’ from the First
Clinical School of Guangzhou University of Chinese Medicine (grant
no. YB201402).
Glossary
Abbreviations
Abbreviations:
|
IBS
|
irritable bowel syndrome
|
|
SCF
|
stem cell factor
|
|
ICC
|
intestinal cells of Cajal
|
|
RTK
|
receptor tyrosine kinase
|
|
GI
|
gastrointestinal
|
|
SMC
|
smooth muscle cell
|
|
Src
|
a tyrosine-protein kinase
|
|
SP
|
substance P
|
|
NK1
|
neurokinin-1
|
|
TRPV
|
transient receptor potential
vanilloid
|
|
5-HT
|
5-hydroxytryptamine
|
|
MC
|
mast cell
|
|
NC
|
nerve cell
|
|
NS
|
nervous system
|
|
ENS
|
enteric nervous system
|
|
ANS
|
autonomic nervous system
|
|
CNS
|
central nervous system
|
|
NF-κB
|
nuclear factor-κB
|
|
TNF-α
|
tumor necrosis factor-α
|
References
|
1
|
Chassany O, Bonaz B, Bruley DES, Varannes
S, Bueno L, Cargill G, Coffin B, Ducrotté P and Grangé V: Acute
exacerbation of pain in irritable bowel syndrome: Efficacy of
phloroglucinol/trimethylphloroglucinol. A randomized, double-blind,
placebo-controlled study. Aliment Pharmacol Ther. 25:1115–1123.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Horwitz BJ and Fisher RS: The irritable
bowel syndrome. N Engl J Med. 344:1846–1850. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moghimi-Dehkordi B, Vahedi M,
Pourhoseingholi MA, Mansoori B Khoshkrood, Safaee A, Habibi M,
Pourhoseingholi A and Zali MR: Economic burden attributable to
functional bowel disorders in Iran: A cross-sectional
population-based study. J Dig Dis. 12:384–392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schonrich S, Brockow T, Franke T, Dembski
R, Resch KL and Cieza A: Analyzing the content of outcome measures
in clinical trials on irritable bowel syndrome using the
international classification of functioning, disability and health
as a reference. Rehabilitation (Stuttg). 45:172–180. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spinelli A: Irritable bowel syndrome. Clin
Drug Investig. 27:15–33. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chey WD, Kurlander J and Eswaran S:
Irritable bowel syndrome: A clinical review. JAMA. 313:949–958.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Konstantinov SR and Peppelenbosch MP:
Fecal microbiota transfer may increase irritable bowel syndrome and
inflammatory bowel diseases-associated bacteria. Gastroenterology.
144:e19–e20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saha L: Irritable bowel syndrome:
Pathogenesis, diagnosis, treatment, and evidence-based medicine.
World J Gastroenterol. 20:6759–6773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bajwa SJ and Haldar R: Endocrinological
disorders affecting neurosurgical patients: An intensivists
perspective. Indian J Endocrinol Metab. 18:778–783. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baldinger P, Kranz G, Höflich A, Savli M,
Stein P, Lanzenberger R and Kasper S: The effects of hormone
replacement therapy on mind and brain. Nervenarzt. 84:14–19.
2013.(in German). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang J, Shi YQ and Zhao XY: Expression and
significance of SCF and 5-HT in the intestinal mucosa of patients
with diarrhea predominant irritable bowel syndrome. Jilin Medicine.
4:646–647. 2015.
|
|
12
|
Sun YG, Gracias NG, Drobish JK, Vasko MR,
Gereau RW and Chen ZF: The c-kit signaling pathway is involved in
the development of persistent pain. Pain. 144:178–186. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin QH, Shen HX, Wang H, Shou QY and Liu
Q: Curcumin improves expression of SCF/c-kit through attenuating
oxidative stress and NF-κB activation in gastric tissues of
diabetic gastroparesis rats. Diabetol Metab Syndr. 5:122013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu G, Chen ZY, Ma L, Lou X, Li SJ and
Wang YL: Intracranial hemangiopericytoma: MR imaging findings and
diagnostic usefulness of minimum ADC values. J Magn Reson Imaging.
38:1146–1151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eshraghian A and Eshraghian H:
Interstitial cells of Cajal: A novel hypothesis for the
pathophysiology of irritable bowel syndrome. Can J Gastroenterol.
25:277–279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou YL, Zhang W, Gao EL, Dai XX, Yang H,
Zhang XH and Wang OC: Preoperative BRAF mutation is predictive of
occult contralateral carcinoma in patients with unilateral
papillary thyroid microcarcinoma. Asian Pac J Cancer Prev.
13:1267–1272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng ZC, Riopel M, Popell A and Wang R: A
survival Kit for pancreatic beta cells: Stem cell factor and c-Kit
receptor tyrosine kinase. Diabetologia. 58:654–665. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edling CE and Hallberg B: c-Kit-a
hematopoietic cell essential receptor tyrosine kinase. Int J
Biochem Cell Biol. 39:1995–1998. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tamada H and Kiyama H: Existence of c-Kit
negative cells with ultrastructural features of interstitial cells
of Cajal in the subserosal layer of the W/Wv mutant mouse colon. J
Smooth Muscle Res. 51:1–9. 2015. View Article : Google Scholar
|
|
20
|
Morimoto M: Intestinal smooth muscle cells
locally enhance stem cell factor (SCF) production against
gastrointestinal nematode infections. J Vet Med Sci. 73:805–807.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ali S and Ali S: Role of c-kit/SCF in
cause and treatment of gastrointestinal stromal tumors (GIST).
Gene. 401:38–45. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang J, Wu YL, Chen BJ, Zhang W, Tanaka Y
and Sugiyama H: The C-kit receptor-mediated signal transduction and
tumor-related diseases. Int J Biol Sci. 9:435–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tong W, Jia H, Zhang L, Li C, Ridolfi TJ
and Liu B: Exogenous stem cell factor improves interstitial cells
of Cajal restoration after blockade of c-kit signaling pathway.
Scand J Gastroenterol. 45:844–851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan YY, Ji ZL, Zhao G, Jiang JR, Wang D
and Wang JM: Decreased SCF/c-kit signaling pathway contributes to
loss of interstitial cells of Cajal in gallstone disease. Int J
Clin Exp Med. 7:4099–4106. 2014.PubMed/NCBI
|
|
25
|
Das Roy L, Curry JM, Sahraei M, Besmer DM,
Kidiyoor A, Gruber HE and Mukherjee P: Arthritis augments breast
cancer metastasis: Role of mast cells and SCF/c-Kit signaling.
Breast Cancer Res. 15:R322013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reber L, Da Silva CA and Frossard N: Stem
cell factor and its receptor c-Kit as targets for inflammatory
diseases. Eur J Pharmacol. 533:327–340. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okayama Y and Kawakami T: Development,
migration, and survival of mast cells. Immunol Res. 34:97–115.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bos CL, Richel DJ, Ritsema T,
Peppelenbosch MP and Versteeg HH: Prostanoids and prostanoid
receptors in signal transduction. Int J Biochem Cell Biol.
36:1187–1205. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Movat HZ: The role of histamine and other
mediators in microvascular changes in acute inflammation. Can J
Physiol Pharmacol. 65:451–457. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Magierowski M, Jasnos K, Kwiecien S,
Drozdowicz D, Surmiak M, Strzalka M, Ptak-Belowska A, Wallace JL
and Brzozowski T: Endogenous prostaglandins and afferent sensory
nerves in gastroprotective effect of hydrogen sulfide against
stress-induced gastric lesions. PLoS One. 10:e01189722015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Di Gennaro A and Haeggström JZ: The
leukotrienes: Immune-modulating lipid mediators of disease. Adv
Immunol. 116:51–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huizinga JD, Robinson TL and Thomsen L:
The search for the origin of rhythmicity in intestinal contraction;
from tissue to single cells. Neurogastroenterol Motil. 12:3–9.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jee SR, Morales W, Low K, Chang C, Zhu A,
Pokkunuri V, Chatterjee S, Soffer E, Conklin JL and Pimentel M: ICC
density predicts bacterial overgrowth in a rat model of
post-infectious IBS. World J Gastroenterol. 16:3680–3686. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Burns AJ, Herbert TM, Ward SM and Sanders
KM: Interstitial cells of Cajal in the guinea-pig gastrointestinal
tract as revealed by c-Kit immunohistochemistry. Cell Tissue Res.
290:11–20. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Blair PJ, Bayguinov Y, Sanders KM and Ward
SM: Interstitial cells in the primate gastrointestinal tract. Cell
Tissue Res. 350:199–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mitsui R and Komuro T: Distribution and
ultrastructure of interstitial cells of Cajal in the gastric antrum
of wild-type and Ws/Ws rats. Anat Embryol (Berl). 206:453–460.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miyamoto-Kikuta S, Ezaki T and Komuro T:
Distribution and morphological characteristics of the interstitial
cells of Cajal in the ileocaecal junction of the guinea-pig. Cell
Tissue Res. 338:29–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gao J, Du P, O'Grady G, Archer R, Gibbons
SJ, Farrugia G and Cheng LK: Cellular automaton model for
simulating tissue-specific intestinal electrophysiological
activity. Conf Proc IEEE Eng Med Biol Soc. 2013:5537–5540.
2013.PubMed/NCBI
|
|
39
|
Bassotti G and Villanacci V: Colonic
diverticular disease: Abnormalities of neuromuscular function. Dig
Dis. 30:24–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang X and Xu WX: The pacemaker functions
of visceral interstitial cells of Cajal. Sheng Li Xue Bao.
62:387–397. 2010.PubMed/NCBI
|
|
41
|
Shin DH, Lee MJ, Jiao HY, Choi S, Kim MW,
Park CG, Na J, Kim SW, Park IK, So I and Jun JY: Regulatory roles
of endogenous mitogen-activated protein kinases and tyrosine
kinases in the pacemaker activity of colonic interstitial cells of
cajal. Pharmacology. 96:16–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sohn W, Lee OY, Lee SP, Lee KN, Jun DW,
Lee HL, Yoon BC, Choi HS, Sim J and Jang KS: Mast cell number,
substance P and vasoactive intestinal peptide in irritable bowel
syndrome with diarrhea. Scand J Gastroenterol. 49:43–51. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Braak B, Klooker TK, Wouters MM, Welting
O, van der Loos CM, Stanisor OI, van Diest S, van den Wijngaard RM
and Boeckxstaens GE: Mucosal immune cell numbers and visceral
sensitivity in patients with irritable bowel syndrome: Is there any
relationship? Am J Gastroenterol. 107:715–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tsang CM, Wong CK, Ip WK and Lam CW:
Synergistic effect of SCF and TNF-alpha on the up-regulation of
cell-surface expression of ICAM-1 on human leukemic mast cell line
(HMC)-1 cells. J Leukoc Biol. 78:239–247. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Draber P, Halova I, Polakovicova I and
Kawakami T: Signal transduction and chemotaxis in mast cells. Eur J
Pharmacol. 778:11–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Collmann E, Bohnacker T, Marone R, Dawson
J, Rehberg M, Stringer R, Krombach F, Burkhart C, Hirsch E,
Hollingworth GJ, et al: Transient targeting of phosphoinositide
3-kinase acts as a roadblock in mast cells' route to allergy. J
Allergy Clin Immunol. 132:959–968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kojima T, Hirota Y, Ema M, Takahashi S,
Miyoshi I, Okano H and Sawamoto K: Subventricular zone-derived
neural progenitor cells migrate along a blood vessel scaffold
toward the post-stroke striatum. Stem Cells. 28:545–554.
2010.PubMed/NCBI
|
|
48
|
Jin K, Mao XO, Sun Y, Xie L and Greenberg
DA: Stem cell factor stimulates neurogenesis in vitroin vivo. J
Clin Invest. 110:311–319. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Drăghici IM, Drăghici L, Cojocaru M,
Gorgan CL and Vrabie CD: The immunoprofile of interstitial Cajal
cells within adenomyosis/endometriosis lesions. Rom J Morphol
Embryol. 56:133–138. 2015.PubMed/NCBI
|
|
50
|
Lu T, Luo Y, Sun H, Qin W and Li Y:
Electroacupuncture improves behavioral recovery and increases
SCF/c-kit expression in a rat model of focal cerebral
ischemia/reperfusion. Neurol Sci. 34:487–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Micheva-Viteva SN, Shou Y, Nowak-Lovato
KL, Rector KD and Hong-Geller E: c-KIT signaling is targeted by
pathogenic Yersinia to suppress the host immune response. BMC
Microbiol. 13:2492013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guo S, Tao X, Wang Y, Tang J, Shen L and
Song C: SCF/c-Kit signaling promotes invasion of T24 cells via PI3K
pathway. Nan Fang Yi Ke Da Xue Xue Bao. 34:507–510. 2014.(In
Chinese). PubMed/NCBI
|
|
53
|
Zheng Z and Tang H: Decreased
neuroplasticity may play a role in irritable bowel syndrome:
Implication from the comorbidity of depression and irritable bowel
syndrome. J Neurogastroenterol Motil. 21:298–299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Da Silva CA, Reber L and Frossard N: Stem
cell factor expression, mast cells and inflammation in asthma.
Fundam Clin Pharmacol. 20:21–39. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xiao Y: SCF/c-Kit signaling acts as a new
etiologic factor of depression by regulation adult neurogenesis.
PhD dissertationShanghai Jiaotong University China: 2010
|
|
56
|
Keightley P, Pavli P, Platten J and Looi
JC: Gut feelings 1. Mind, mood and gut in irritable bowel syndrome:
Approaches to psychiatric care. Australas Psychiatry. 23:403–406.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Muscatello MR, Bruno A, Scimeca G,
Pandolfo G and Zoccali RA: Role of negative affects in
pathophysiology and clinical expression of irritable bowel
syndrome. World J Gastroenterol. 20:7570–7586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Guo XZ: SCF/c-Kit signaling acts as a new
etiological factor of depression by regulating adult hippocampal
neurogenesis. In: Proceedings of the Chinese society of genetic
model organisms and human health conference. 2010
|
|
59
|
Zhao X, Suo HY, Qian Y, Li GJ, Liu ZH and
Li J: Therapeutic effects of Lactobacillus casei Qian treatment in
activated carbon-induced constipated mice. Mol Med Rep.
12:3191–3199. 2015.PubMed/NCBI
|
|
60
|
Matsumoto K, Hosoya T, Tashima K, Namiki
T, Murayama T and Horie S: Distribution of transient receptor
potential vanilloid 1 channel-expressing nerve fibers in mouse
rectal and colonic enteric nervous system: Relationship to
peptidergic and nitrergic neurons. Neuroscience. 172:518–534. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
DiNitto JP, Deshmukh GD, Zhang Y, Jacques
SL, Coli R, Worrall JW, Diehl W, English JM and Wu JC: Function of
activation loop tyrosine phosphorylation in the mechanism of c-Kit
auto-activation and its implication in sunitinib resistance. J
Biochem. 147:601–609. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yamamoto T, Watabe K, Nakahara M, Ogiyama
H, Kiyohara T, Tsutsui S, Tamura S, Shinomura Y and Hayashi N:
Disturbed gastrointestinal motility and decreased interstitial
cells of Cajal in diabetic db/db mice. J Gastroenterol Hepatol.
23:660–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Okumura T: Brain-gut interaction in the
pathophysiology of IBS. Nihon Shokakibyo Gakkai Zasshi.
111:1334–1344. 2014.(In Japanese). PubMed/NCBI
|
|
64
|
Keszthelyi D, Troost FJ, Jonkers DM, van
Eijk HM, Dekker J, Buurman WA and Masclee AA: Visceral
hypersensitivity in irritable bowel syndrome: Evidence for
involvement of serotonin metabolism-a preliminary study.
Neurogastroenterol Motil. 27:1127–1137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sun J, Wu X, Meng Y, Cheng J, Ning H, Peng
Y, Pei L and Zhang W: Electro-acupuncture decreases 5-HT, CGRP and
increases NPY in the brain-gut axis in two rat models of
Diarrhea-predominant irritable bowel syndrome(D-IBS). BMC
Complement Altern Med. 15:3402015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Camilleri M, Oduyebo I and Halawi H:
Chemical and molecular factors in irritable bowel syndrome: Current
knowledge, challenges, and unanswered questions. Am J Physiol
Gastrointest Liver Physiol. 311:G777–G784. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Murakami M, Austen KF and Arm JP: The
immediate phase of c-kit ligand stimulation of mouse bone
marrow-derived mast cells elicits rapid leukotriene C4 generation
through posttranslational activation of cytosolic phospholipase A2
and 5-lipoxygenase. J Exp Med. 182:197–206. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Pynaert G, Grooten J, van Deventer SJ and
Peppelenbosch MP: Cysteinyl leukotrienes mediate histamine
hypersensitivity ex vivo by increasing histamine receptor numbers.
Mol Med. 5:685–692. 1999.PubMed/NCBI
|
|
69
|
Bloemers SM, Verheule S, Peppelenbosch MP,
Smit MJ, Tertoolen LG and de Laat S: Sensitization of the histamine
H1 receptor by increased ligand affinity. J Biol Chem.
273:2249–2255. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Martínez C, Lobo B, Pigrau M, Ramos L,
González-Castro AM, Alonso C, Guilarte M, Guilá M, de Torres I,
Azpiroz F, et al: Diarrhoea-predominant irritable bowel syndrome:
An organic disorder with structural abnormalities in the jejunal
epithelial barrier. GUT. 62:1160–1168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Vicario M, González-Castro AM, Martinez C,
Lobo B, Pigrau M, Guilarte M, de Torres I, Mosquera JL, Fortea M,
Sevillano-Aguilera C, et al: Increased humoral immunity in the
jejunum of diarrhoea-predominant irritable bowel syndrome
associated with clinical manifestations. GUT. 64:1379–1388. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Willot S, Gauthier C, Patey N and Faure C:
Nerve growth factor content is increased in the rectal mucosa of
children with diarrhea-predominant irritable bowel syndrome.
Neurogastroenterol Motil. 24:734–739, e347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Eby JM, Kang HK, Klarquist J, Chatterjee
S, Mosenson JA, Nishimura MI, Garrett-Mayer E, Longley BJ,
Engelhard VH, Mehrotra S and Le Poole IC: Immune responses in a
mouse model of vitiligo with spontaneous epidermal de- and
repigmentation. Pigment Cell Melanoma Res. 27:1075–1085. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang JY, Huang YX, Qin M and Wang JJ:
Effect of overactivation of stem cell factor/c-kit on hyperalgesia
in rats with irritable bowel syndrome. Journal of Shangxi Medical
University. 3:177–181. 2012.
|
|
75
|
Siehl J and Thiel E: C-kit, GIST, and
imatinib. Recent Results Cancer Res. 176:145–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
D'Antonio C, Wang B, McKay C and Huizinga
JD: Substance P activates a non-selective cation channel in murine
pacemaker ICC. Neurogastroenterol Motil. 21:e979–985. 2009.
|
|
77
|
Milenkovic N, Frahm C, Gassmann M, Griffel
C, Erdmann B, Birchmeier C, Lewin GR and Garratt AN: Nociceptive
tuning by stem cell factor/c-Kit signaling. Neuron. 56:893–906.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chen J, Du L, Xiao YT and Cai W:
Disruption of interstitial cells of Cajal networks after massive
small bowel resection. World J Gastroenterol. 19:3415–3422. 2013.
View Article : Google Scholar : PubMed/NCBI
|