Introduction
Diabetes has become one of the most important
diseases worldwide, with the proportion of the adult population
with diabetes expected to increase to 69% by 2030 (1). Diabetes mellitus is a metabolic disease
characterized by prolonged hyperglycemia, which can lead to the
development of microvascular and macrovascular disease (2). Hyperglycemia at the macrovascular level
causes coronary artery and cerebrovascular diseases, and at the
microvascular level injury mainly affects the eye, kidney and
liver. Diabetic hepatic damage is the most common cause of
end-stage liver disease, namely cirrhosis, and contributes to
disability and the high mortality rate in patients with diabetes.
The pathogenesis of diabetic hepatic damage is multifactorial, with
long-term hyperglycemia playing a crucial role (3,4). During
diabetes mellitus, the continuous hyperglycemic conditions are
associated with oxidative stress and the mitochondrial production
of free radicals, which lead to hepatic dysfunction (5). One of the main factors in the
initiation of the pathological response to oxidative stress is the
generation of reactive oxygen species (ROS). According to a
previous study, increased nicotinamide adenine dinucleotide
phosphate (NADPH) oxidase-4 (Nox-4)/p47phox is
associated with elevated ROS generation, implying the potential
importance of Nox-4-based NADPH oxidase in the oxidative damage
associated with diabetic hepatism (6). Moreover, increased ROS production is
associated with inflammation and indirectly damages cells by
activating a variety of stress-sensitive intracellular signaling
pathways. Also, ROS-mediated activation of nuclear factor (NF)-κB
and the c-Jun subunit of activator protein-1 (AP-1), two
redox-sensitive transcription factors, are evolutionarily conserved
and associated with a wide variety of pro-inflammatory mediators,
including cytokines, chemokines, and inducible effector enzymes
such as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2
(COX-2) (7).
Oenanthe javanica (water dropwort), which is
cultivated in Australia and East Asian countries, such as Korea,
China and Japan is a perennial herb. O. javanica, which
contains high levels of vitamins and minerals, is consumed as a
vegetable, and has also been used as a medicinal agent. It has a
long history of use for the treatment of inflammatory conditions,
including hepatitis (8,9). Hyperoside, persicarin and isorhamnetin
are the three major substances with pharmacological activities that
have been identified in O. javanica. These substances have
been shown to possess hepatoprotective, antithrombotic,
antiarrhythmic, antidiabetic, antihepatitis B virus,
neuroprotective and anticancer activities (9–16).
Persicarin has been reported to exhibit
neuroprotective and antioxidant activities against
glutamate-induced neurotoxicity in primary cultured rat cortical
cells (14). In addition, persicarin
has exhibited an anti-inflammatory effect against high mobility
group box 1-induced inflammatory responses in human endothelial
cells and in a cecal ligation and puncture model of septicemia in
mice (17). However, to the best of
our knowledge, no studies have yet been performed on the
antidiabetic activity of persicarin.
Therefore, in the present study, the effects of
persicarin on the oxidative stress-related factors involved in the
development of diabetic hepatic damage were investigated using
streptozotocin (STZ)-induced type 1 diabetic mice.
Materials and methods
Materials
Ethylenediaminetetraacetic acid (EDTA) was purchased
from Generay Biotech Co. Ltd. (Shanghai, China).
2′,7′-Dichlorofluorescein diacetate (DCFH-DA) was obtained from
Molecular Probes (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The protease inhibitor mixture was purchased from Wako Pure
Chemical Industries, Ltd. (Osaka, Japan). Dihydrorhodamine 123
(DHR123) and diethylene triamine penta-acetic acid (DTPA) were
purchased from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Isoflurane (1%) was purchased from Piramal Critical Care, Inc.,
(Bethlehem, PA, USA). The Bio-Rad protein assay kit and pure
nitrocellulose membrane were supplied by Bio-Rad Laboratories
(Seoul, Korea). Phenylmethylsulfonyl fluoride (PMSF) and sodium
dodecyl sulfate (SDS) were acquired from (Amresco, LLC, Solon, OH,
USA). PBS-Tween (P2006) was obtained from Biosesang, Inc.,
(Gyeonggi-do, Korea). Rabbit polyclonal antibodies against Nox-4
(cat. no. sc-30141), p47phox (cat. no. sc-14015) and
NF-κBp65 (cat. no. sc-372), and mouse monoclonal antibodies against
COX-2 (cat. no. sc-19999), iNOS (cat. no. sc-372), histone (cat.
no. sc-8030) and β-actin (cat. no. sc-4778), and goat polyclonal
antibodies against transforming growth factor (TGF)-β (cat. no.
sc-146) and tumor necrosis factor (TNF)-α (cat. no. sc-1351) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Rabbit polyclonal anti-AP-1 (cat. no. 2315S) was obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Goat anti-rabbit
(cat. no. sc-2774) and goat anti-mouse (cat. no. sc-2005)
immunoglobulin G (IgG) horseradish peroxidase (HRP)-conjugated
secondary antibodies were acquired from Santa Cruz Biotechnology,
Inc. ECL Western Blotting Detection Reagents were supplied by GE
Healthcare (Piscataway, NJ, USA).
Preparation of persicarin
extracts
The aerial parts of O. javanica were
collected from agricultural farms in Pyeongyang-ri, Cheongdo-gun,
Gyoungsanbuk-do, South Korea in April 2011, and identified by
Professor Tae Hoon Kim (Daegu University, Daegu, South Korea).
Freshly milled O. javanica plant material (1.4 kg) was
extracted with 70% ethanol (EtOH; 10 liters, thrice) at room
temperature. The solvent was then evaporated under vacuum, and the
combined crude EtOH extract (81.5 g) was suspended in 20% methanol
(MeOH; 3 liters), and partitioned sequentially against
n-hexane (3 liters, thrice), ethyl acetate (EtOAc; 3 liters,
thrice), and n-butanol (BuOH; 3 liters, thrice) to yield
dried n-hexane-(5.5 g), EtOAc-(1.8 g), n-BuOH (10.6
g) and H2O-soluble (53.6 g) residues. A portion (1.7 g)
of the EtOAc extract was chromatographed using a column containing
Toyopearl HW-40 [coarse grade; 3.0 cm internal diameter (i.d.)x48
cm] using water containing increasing amounts of MeOH in a stepwise
manner. The 100% H2O eluate obtained was subjected to
column chromatography using YMC GEL ODS AQ 120-50S (1.6 cm i.d.x37
cm) using aqueous MeOH, to yield pure compound 1 (75.9 mg;
retention time, 10.1 min). HPLC analysis was carried out using a
YMC-Pack ODS A-302 column (4.6 mm i.d.x150 mm; YMC Co., Kyoto,
Japan) with a linear gradient of 10% (v/v) acetonitrile (MeCN) in
0.1% formic acid/H2O (detection, UV 280 nm; flow rate,
1.0 ml/min; 40°C), which was increased to 90% MeCN over 30 min and
then to 100% MeCN over 5 min. Compound 1 was identified and
characterized as persicarin by 1H and 13C NMR
and by comparing peaks with literature values (18). The 1H NMR results were as
follows: (600 MHz, DMSO-d6) δ 8.06 (1H, d, J= 2.2 Hz,
H-2′), 7.61 (1H, d, J=8.8, 2.2 Hz, H-6′), 6.92 (1H, d, J=8.8 Hz,
H-5′), 6.43 (1H, d, J=2.2 Hz, H-8), 6.22 (1H, d, J=2.2 Hz, H-6),
3.83 (3H, s, MeO-3′). The 13C NMR results were as
follows: (150 MHz, DMSO-d6) δ 177.1 (C-4), 166.5 (C-7),
161.1 (C-5), 156.2 (C-2), 155.5 (C-9), 149.6 (C-3′), 147.1 (C-4′),
131.8 (C-5′), 121.2 (C-6′), 121.1 (C-1′), 115.2 (C-2′), 103.1
(C-10), 99.7 (C-6), 93.6 (C-8), 55.6 (MeO).
Experimental animals and
treatment
Animal experiments were performed according to the
Guidelines for Animal Experimentation and approved by Daegu Haany
University (approval no. DHU2015-011). A total of 24, 5-week-old
male ICR mice (23–28 g) were purchased from Orient Bio Inc.
(Gyeonggi, Korea). Mice were maintained under a 12-h light/dark
cycle, and housed at a controlled temperature (22±2°C) and humidity
(55±5%) with free access to food and water. After several days of
adaptation, the mice were randomly separated into normal control
(n=6) and diabetic groups. Mice in the diabetic group were injected
intraperitoneally with STZ (Sigma-Aldrich; 120 mg/kg body weight)
in 10 mM citrate buffer (pH 4.5). After 7 days of STZ injection,
the glucose levels of blood taken from the tail vein were measured,
and then the STZ-induced diabetic mice were divided into three
groups (each n=6). Treatment with persicarin was initiated after
confirming the induction of hyperglycemia in the diabetic mice by
weight (33.8±0.5 g) and serum glucose level (288.3±6.5 mg/dl). Mice
in the diabetic control group (Veh group) were given water orally,
while those in the other two diabetic groups were orally treated
with persicarin extracts daily for 10 days at a low or high dose
(2.5 and 5 mg/kg body weight, respectively). The diabetic groups
were compared with the normal (non-diabetic) control group. Body
weight, food intake and water intake were determined every day
during the experimental period. After administration for 10 days,
mice were anesthetized with 1% isoflurane and blood samples were
collected from the abdominal aorta of anesthetized mice. Serum was
separated immediately by centrifugation. Subsequently, each mouse
was perfused with ice-cold physiological saline, and then the liver
was harvested, snap-frozen in liquid nitrogen and stored at −80°C
until analyses were performed.
Analysis of serum and hepatic
functional parameters
The serum glucose level was measured using a
commercial kit (Glucose Test, cat. no. AM201; Asan Pharm. Co.,
Ltd., Hwaseong, South Korea). Hepatic functional parameters
[alanine aminotransferase (ALT) and aspartate aminotransferase
(AST)] were measured using a Transaminase CII-Test kit (cat. no.
431-30901; Wako Pure Chemical Industries, Ltd.).
Analysis of hepatic glucose
content
The hepatic glucose level was determined using the
method of Momose et al (19),
with minor modifications. Hepatic tissue was homogenized with
ice-cold 0.9% NaCl buffer, and then the homogenate was
deproteinized with 0.15 M Ba(OH)2 and 5%
ZnSO4. The supernatant was obtained by centrifugation at
1,670 × g for 15 min, and then the glucose level was determined
using the aforementioned glucose test kit.
Measurement of hepatic ROS generation
and thiobarbituric acid reactive substance (TBARS) levels
ROS generation was measured using the method of Ali
et al (20). Hepatic tissue
was homogenized on ice with 1 mM EDTA-50 mM sodium phosphate buffer
(pH 7.4), and then 25 mM DCFH-DA was added to the homogenates.
After incubation for 30 min, the changes in fluorescence values
were determined at an excitation wavelength of 486 nm and emission
wavelength of 530 nm. The TBA-reactive substance content was
determined using the method of Mihara and Uchiyama (21).
Measurement of peroxynitrite
(ONOO−) generation in the liver
ONOO− was measured by the method of Kooy
et al (22). Each sample was
mixed with rhodamine buffer (pH 7.4), 5 mM DTPA and 5 mM DHR123.
After incubation for 5 min at 37°C, the fluorescence intensity of
the oxidized DHR123 was measured with a microplate fluorescence
reader at excitation and emission wavelengths of 485 and 530 nm,
respectively.
Preparation of nuclear and
post-nuclear fractions
Nuclear protein extraction was performed using the
method reported by Komatsu (23).
Briefly, liver tissue was homogenized with ice-cold lysis buffer
containing 5 mM Tris-HCl (pH 7.5), 2 mM MgCl2, 15 mM
CaCl2 and 1.5 M sucrose, followed by the addition of a
0.1 M dithiothreitol (DTT) and a protease inhibitor mixture. After
centrifugation (10,500 × g for 20 min at 4°C), the pellet was
suspended with an extraction buffer containing 20 mM
2-[4-(2-hydroxyethyl)-1-piperazyl] ethanesulfonic acid (pH 7.9),
1.5 mM MgCl2, 0.42 M NaCl, 0.2 mM EDTA and 25% (v/v)
glycerol, followed by the addition of a 0.1 M DTT and protease
inhibitor mixture. The mixture was placed on ice for 30 min. The
nuclear fraction was prepared by centrifugation at 20,500 × g for 5
min at 4°C.
The post-nuclear fraction was extracted from the
kidneys of each mouse. Briefly, liver tissue was homogenized with
ice-cold lysis buffer (pH 7.4) containing 137 mM NaCl, 20 mM
Tris-HCl, 1% Tween 20, 10% glycerol, 1 mM PMSF, and a protease
inhibitor mixture. The homogenate was then centrifuged at 2,000 × g
for 10 min at 4°C, and the protein concentration in each fraction
was determined using a Bio-Rad protein assay kit.
Western blot analyses
To determine the expression of NF-κBp65, AP-1 and
histone, 10 µg protein from each nuclear fraction was separated by
8% SDS-PAGE. For Nox-4/p47phox, COX-2, iNOS, TGF-β and
β-actin , 10 µg protein of each post-nuclear fraction was separated
by 8–15% SDS-PAGE. The separated proteins were transferred to a
nitrocellulose membrane, blocked with a 5% (w/v) skimmed milk
solution for 1 h, and incubated separately with the primary
antibodies (NF-κBp65, AP-1, histone, Nox-4/p47phox,
COX-2, iNOS, TGF-β and β-actin) overnight at 4°C at a dilution of
1:1,000. Following washing with PBS-Tween, the blots were incubated
with the anti-rabbit or anti-mouse IgG HRP-conjugated secondary
antibody for 1 h at room temperature at a dilution of 1:3,000. Each
antigen-antibody complex was visualized using ECL Western Blotting
Detection Reagents and detected using SENSI-Q2000 (Lugen Sci. Co.,
Ltd., Gyeonggi, South Korea). The band densities were determined
using ATTO Densitograph software (CS Analyzer 2.0; ATTO
Corporation, Tokyo, Japan), and quantified as a ratio to β-actin.
The protein levels of the groups are expressed relative to those of
the normal mice (represented as 1).
Histological examination
The excised liver samples were immediately fixed in
10% neutral-buffered formalin and, after embedding in paraffin,
they were cut into 5-µm sections. After staining with hematoxylin
and eosin (H&E), the sections were examined with a light
microscope.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Statistical comparisons were performed using one-way
analysis of variance followed by a Dunnett's test using SPSS 11.5.1
for Windows, 2002 (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Body weight gain, liver weight, food
intake and water intake
The body weight gains, liver weight, food intake and
water intake during the experimental period are shown in Table I. The diabetic control mice showed a
significant reduction in body weight in comparison with
non-diabetic mice. However, the body weights of mice treated with
persicarin were notably higher than those diabetic control mice. In
addition, the administration of persicarin did not affect food and
water intake, but the liver weight was significantly increased by
~1.19- and 1.2-fold in the low and high dose groups,
respectively.
 | Table I.Body weight, liver weight, food
intake, and water intake. |
Table I.
Body weight, liver weight, food
intake, and water intake.
|
| Body weight |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Group | Initial (g) | Final (g) | Gain (g/10
days) | Liver weight (g/100
g body weight) | Food intake
(g/day) | Water intake
(g/day) |
|---|
| Non-diabetic
mice |
38.9±0.6a |
42.0±0.6a |
3.6±0.2a |
7.9±0.3a | 5.1±2.6 |
6.1±3.0 |
| Diabetic mice |
|
|
|
|
|
|
|
Veh | 33.9±0.8 | 33.2±0.8 | −1.3±0.2 | 6.1±0.1 | 8.4±0.5 | 28.7±4.9 |
|
Low | 33.8±1.1 | 33.9±1.5 |
−0.4±0.3b |
7.3±0.3b | 7.8±0.2 | 35.2±3.4 |
|
High | 33.8±1.1 | 33.3±1.2 | 0±0.2a |
7.3±0.1a | 8.4±0.4 | 38.6±4.0 |
Serum and hepatic functional
parameters
Table II shows that
the serum glucose level was significantly increased in diabetic
control mice; the increase was ~4.9-fold in comparison with that in
non-diabetic mice. Persicarin treatment led to a notable reduction
of glucose level in a dose-dependent manner. Hepatic functional
parameters, namely ALT and AST levels in the serum, of vehicle- and
persicarin-treated diabetic mice were investigated. The activities
of ALT and AST in the diabetic mice were significantly higher than
those of normal mice, while their activities in the persicarin
treatment groups were markedly reduced in a dose-dependent
manner.
 | Table II.Biochemical analyses. |
Table II.
Biochemical analyses.
|
|
| Diabetic mic |
|---|
|
|
|
|
|---|
| Variable | Non-diabetic
mice | Veh | Low | High |
|---|
| Serum glucose
(mg/dl) |
122.15±5.54a | 597.12±7.39 |
533.96±9.94a |
513.11±19.86b |
| Hepatic glucose
(mg/mg protein) |
31.53±1.50c | 36.73±0.46 | 34.47±1.81 |
33.56±0.35a |
| Serum ALT
(IU/l) |
15.43±0.73a | 31.27±0.30 | 27.32±1.69 |
25.64±0.51a |
| Serum AST
(IU/l) |
51.98±3.49a | 120.08±4.27 |
93.32±1.01a |
86.03±3.09a |
Hepatic glucose
As Table II
demonstrates, persicarin exhibited an effect on hepatic glucose.
Persicarin administration markedly reduced hepatic glucose levels
at a dose of 5 mg/kg.
Biomarkers associated with oxidative
stress in hepatic tissue
As shown in Fig. 1,
the biomarkers of oxidative stress, namely ROS, ONOO−
and TBARS in diabetic control mice were notably elevated compared
with those in normal mice. However, the elevated levels were
diminished by oral treatment with persicarin.
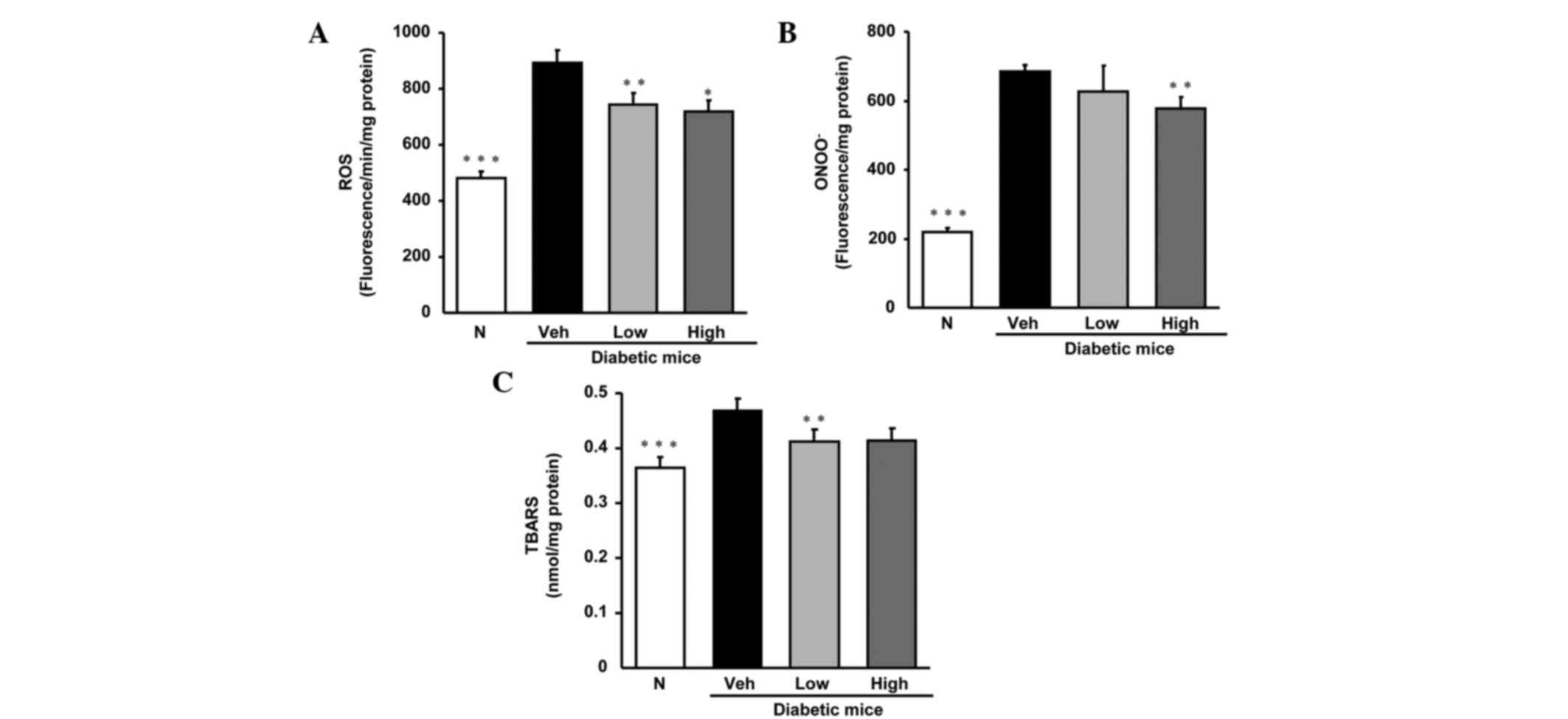 | Figure 1.Levels of (A) ROS, (B) ONOO- and (C)
TBARS in hepatic tissue. N, non-diabetic mice; veh, vehicle-treated
diabetic mice; low, persicarin 2.5 mg/kg body weight-treated
diabetic mice; high, persicarin 5 mg/kg body weight-treated
diabetic mice. Data are the means ± standard error of the mean
(n=6). *P<0.05, **P<0.01, ***P<0.001 vs. vehicle-treated
diabetic mice. ROS, reactive oxygen species; ONOO-, peroxynitrite;
TBARS, thiobarbituric acid-reactive substance. |
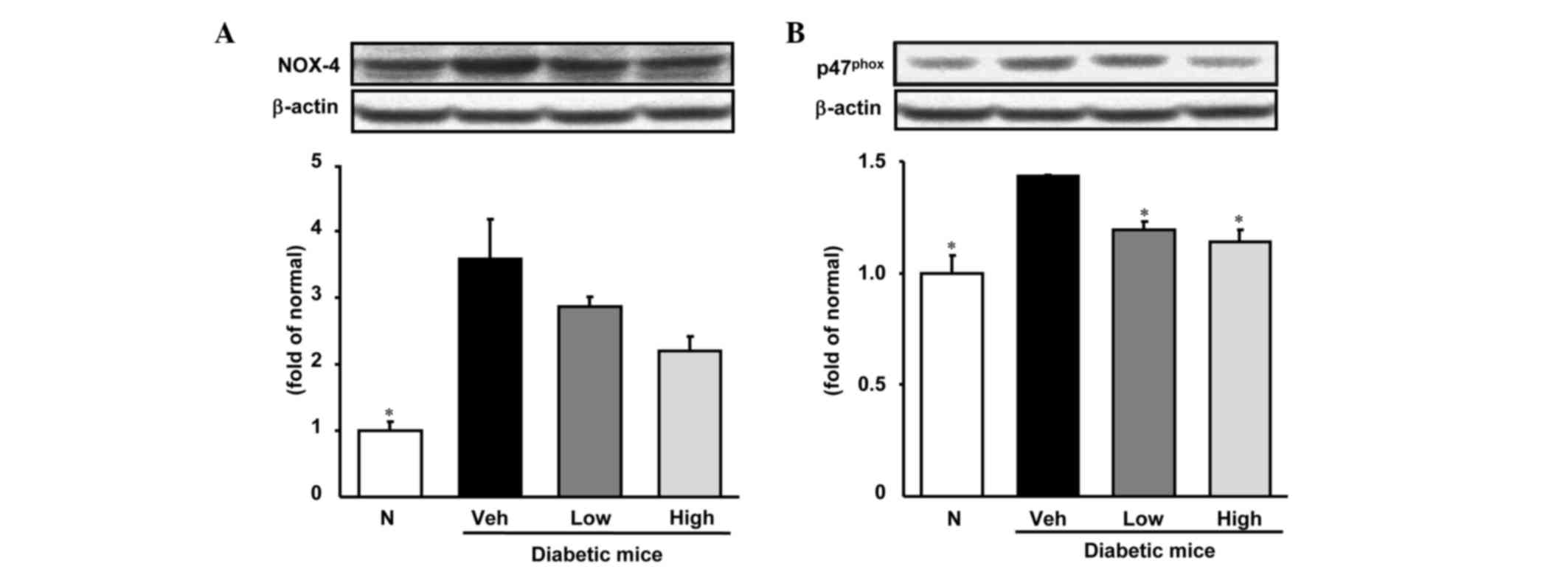
Expression of NADPH oxidase subunits
Nox-4 and p47phox in hepatic tissue
The results of western blot analysis of hepatic
Nox-4 and p47phox protein in the hepatic tissues of the
four groups are shown in Fig. 2.
Persicarin administration showed a tendency to decrease the Nox-4
level (although the reduction was not statistically significant),
whereas the p47phox protein expression level was
significantly decreased.
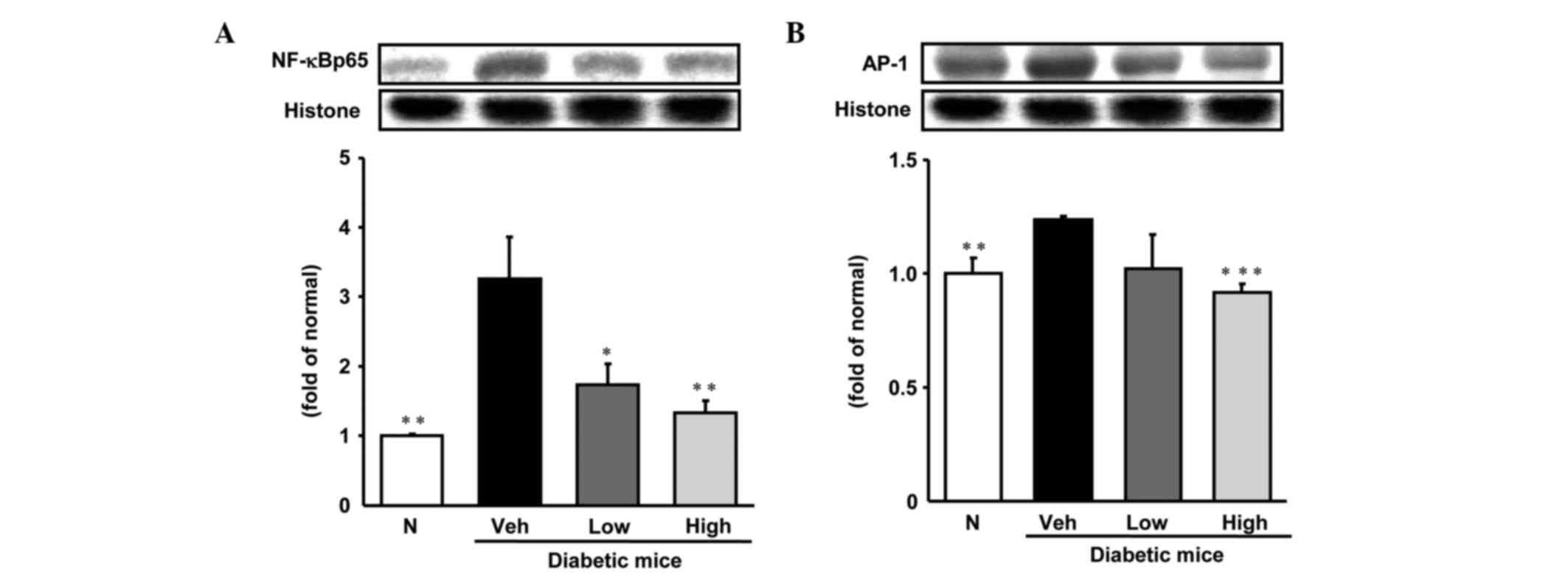
Inflammation-related protein
expression in hepatic tissue
As is shown in Figs.
3 and 4, the levels of
expression of various inflammation-related proteins, specifically,
NF-κBp65, AP-1, iNOS, COX-2 and TGF-β were significantly increased
in the livers of diabetic control mice compared with those in
non-diabetic mice. NF-κBp65 and AP-1 protein levels were reduced
significantly to become comparable with those of normal mice
following treatment with persicarin. In addition, TGF-β and COX-2
protein expression levels were notably decreased by the
administration of persicarin at a dose of 5 mg/kg. Furthermore,
iNOS protein expression was significantly decreased in a
dose-dependent manner by treatment with persicarin.
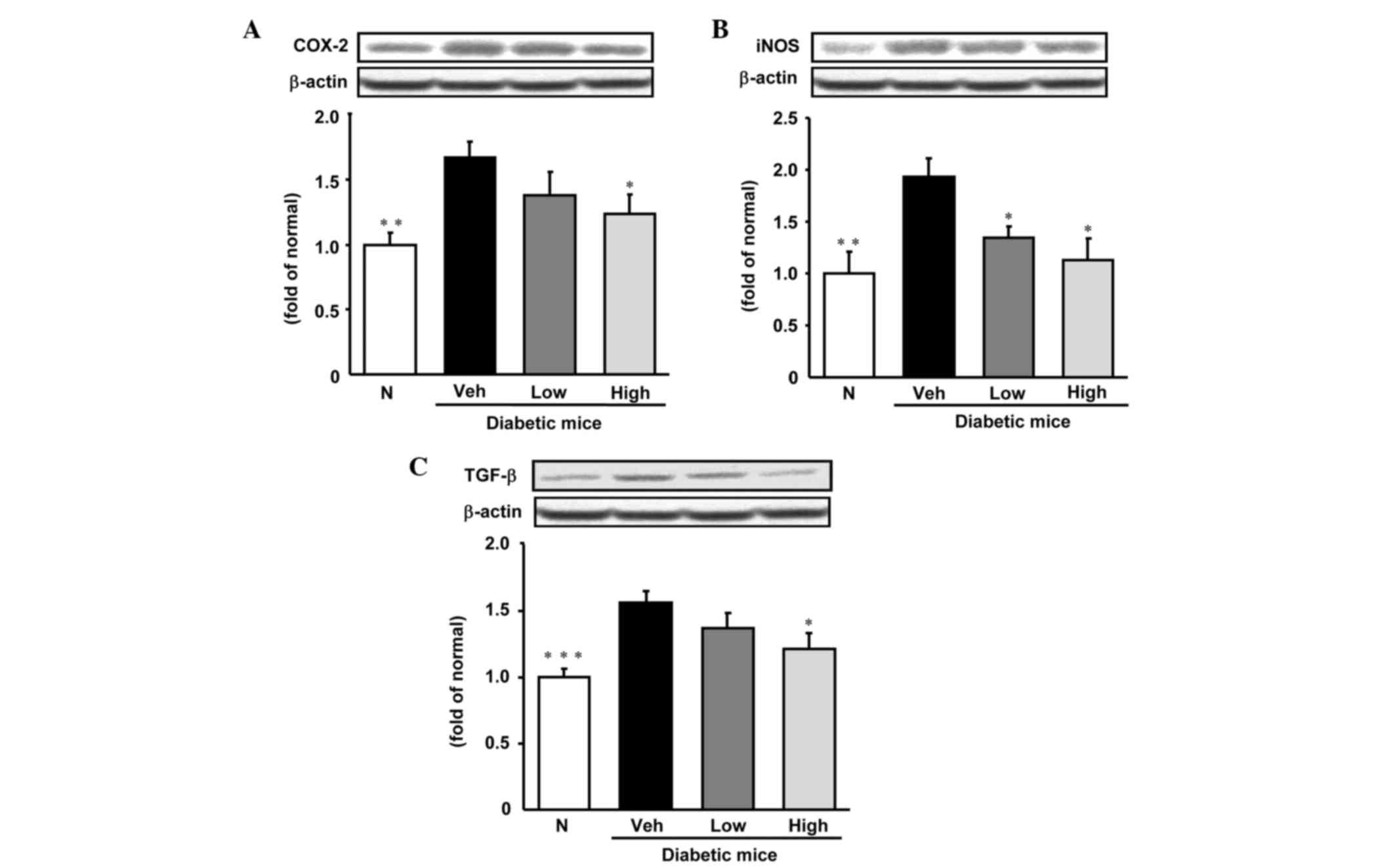 | Figure 4.Expression of (A) COX-2, (B) iNOS and
(C) TGF-β protein in hepatic tissue. N, non-diabetic mice; veh,
vehicle-treated diabetic mice; low, persicarin 2.5 mg/kg body
weight-treated diabetic mice; high, persicarin 5 mg/kg body
weight-treated diabetic mice; COX-2, cyclooxygenase-2; iNOS,
inducible nitric oxide synthase; TGF-β, transforming growth
factor-β. Data are the means ± standard error of the mean (n=6).
*P<0.05, **P<0.01, ***P<0.001 vs. vehicle-treated diabetic
mice. . |
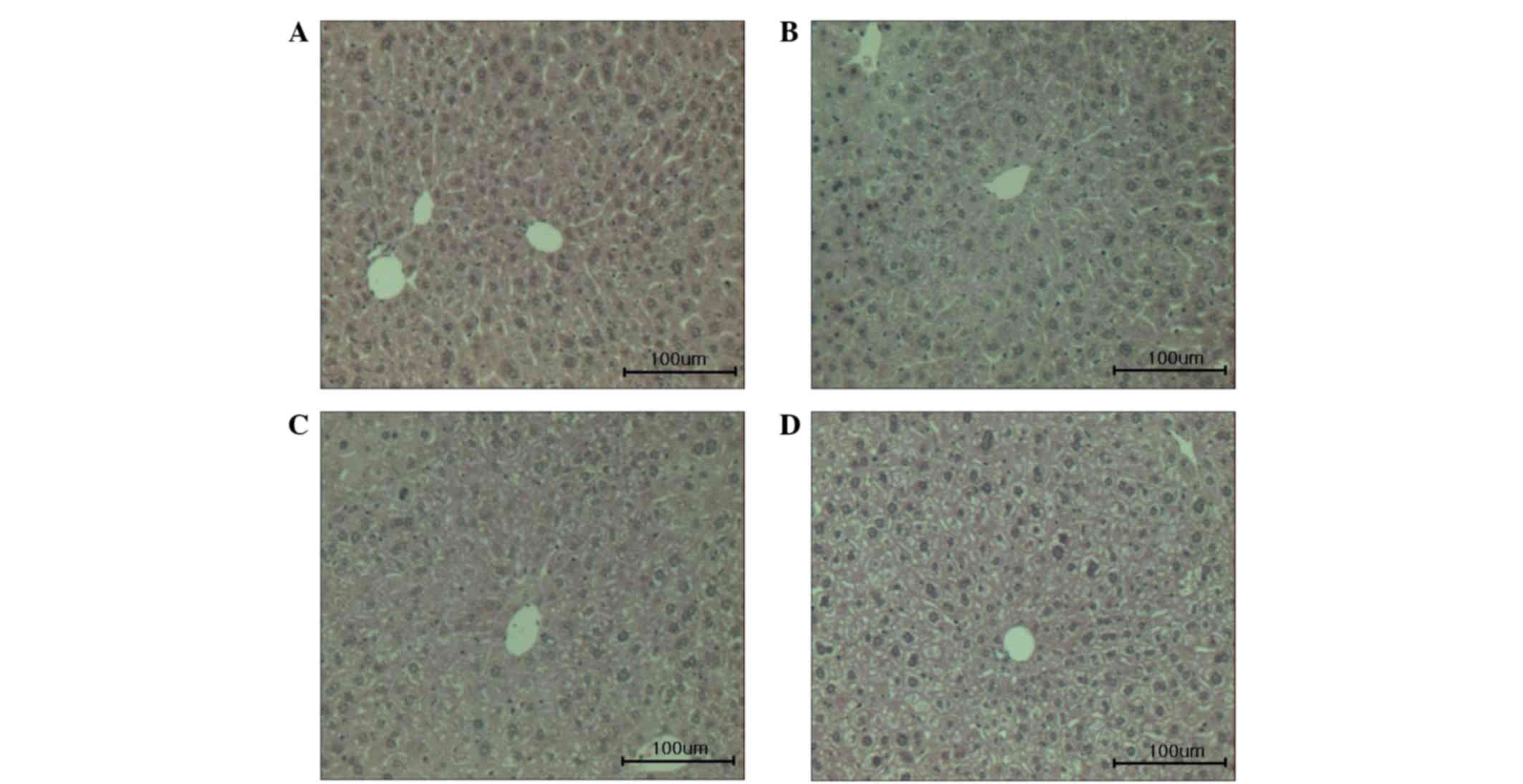
Hepatic histological examination
Histological evaluation of the hepatocellular damage
was conducted (Fig. 5). The level of
hepatocellular damage was higher in the vehicle-treated diabetic
mice compared with normal mice. However, the administration of
persicarin was observed to attenuate the hepatocellular damage in
STZ-treated diabetic mice.
Discussion
O. javanica has been used for many years for
the treatment of inflammatory conditions, including hepatic damage
(24). Previous studies have shown
that it possesses anti-hepatitis B virus (9), antithrombotic (10) and anticancer activities (15,16).
Moreover, persicarin, a major flavonoid component isolated from
O. javanica has been reported to have a hypoglycemic effect
in a type 1 diabetes model (12),
and to inhibit oxidative stress and inflammation in human
endothelial cells and sepsis-induced mice (17). In addition, persicarin has been
reported to exhibit hepatoprotective effects through the inhibition
of lipid peroxidation; however, its underlying beneficial effects
on diabetes-induced liver damage are unclear (8). In the present study, the protective
activities of persicarin against diabetes-induced liver damage were
evaluated.
Diabetes is characterized by polyuria, polydipsia
and polyphagia symptoms (25). STZ
also leads to abnormal metabolism, including increases in food
intake and water intake and decreases in body weight gain and liver
weight (26,27). In the present study, the
administration of persicarin for 10 days led to no change in food
or water intake, but body weight gain and liver weight were
significantly increased by both doses (Table I). These results indicate that oral
treatment with persicarin ameliorates some of the common symptoms
of diabetes. Diabetic mice exhibited serum and hepatic glucose
levels that were significantly increased compared with those in
normal mice. The serum and hepatic glucose levels of the
persicarin-treated diabetic mice were lower than those of the
vehicle-treated diabetic mice (Table
II).
The levels of glucose transporter 2 protein (GLUT2)
are upregulated in a dose-dependent manner according to glucose
concentration (28). In addition,
liver GLUT2 protein levels are increased in diabetic rats and
downregulated by insulin (29). On
this basis, it is suggested that the reduction of the glucose
content in the liver tissue involved an improvement of insulin
resistance through GLUT2 activity.
Hyperglycemia is a primary cause of increased
generation of ROS, leading to increased oxidative stress under
conditions where the antioxidant defense is damaged. Hyperglycemia
is a continuous cause of oxidative stress in diabetes (30–32).
Oxidative stress plays a important role in the progression of liver
damage (1). In addition, hepatic
injury has been found to be associated with an increase in
production of ROS (1,3). Mitochondria are one of the major
sources of ROS, and they increase the production of ROS from the
mitochondrial respiratory chain when they are functionally
disordered (33). In hyperglycemia,
NADPH oxidase is a main source of ROS production and the actions of
mitochondrial respiratory chain complex enzymes are damaged as a
result of continuous hyperglycemic conditions (6,34). It is
well recognized that in diabetic animal models, STZ results in the
overexpression of ROS and generates nitric oxide (NO). NO combines
with superoxide to form ONOO−, which causes lipid
peroxidation, DNA damage and cell death. Therefore, it has a direct
toxic effect on the liver leading to hepatic damage (35–37). In
the present study, the elevated levels of NADPH oxidase subunits
Nox-4 and p47phox protein in the hepatic tissues of
diabetic mice were notably reduced by the administration of
persicarin. Moreover, the hepatic functional parameters ALT and AST
of diabetic mice were markedly higher than those of normal mice;
however, ALT and AST levels in the diabetic mice were significantly
lowered by persicarin (Table II).
These results suggest that persicarin improved hepatic functional
parameters and exhibited hepatoprotective effects through
downregulated oxidative stress via the modulation of Nox-4 and
p47phox protein expression.
Oxidative stress mediated by hyperglycemia leads to
the overexpression of the redox responsive transcription factor
NF-κB and AP-1, which modulates the increased gene expression
required for the inflammatory response (38). Following an inflammatory response,
the activation of NF-κB induces downstream inflammatory mediators
such as TGF-β1, iNOS and COX-2 that have been reported to induce
toxic effects in the liver (39).
COX-2 is a key enzyme in prostaglandin biosynthesis from
arachidonic acid and is associated with inflammatory processes
(40). iNOS is another enzyme
involved in inflammation and it catalyzes the formation of NO. NO
reacts with superoxide to produce ONOO−, which is a
highly ROS and leads to increased destructive and nitrosative
stress (41). The overexpression of
AP-1 increases TGF-β transcription, and AP-1 elements are found in
the TGF-β promoter (42). In the
present study, the livers of vehicle-treated diabetic mice showed
significantly increased NF-κB and AP-1 levels in comparison with
those of normal mice. However, the increased liver levels of NF-κB
and AP-1 were notably reduced by persicarin. In addition, TGF-β,
COX-2 and iNOS expression levels in the livers of diabetic mice
were markedly increased compared with those of normal mice.
Administration of persicarin decreased the TGF-β and COX-2
expression levels; the reduction was significant at a dose of 5
mg/kg. In addition, the iNOS expression level was significantly
decreased by persicarin in a dose-dependent manner from a dose of
2.5 mg/kg through the NF-κB pathway. In the present study, the
elevated protein expression of transcription factors (NF-κB and
AP-1), pro-inflammatory enzymes (COX-2 and iNOS) and
pro-inflammatory cytokine (TGF-β1) in the livers of diabetic mice
were downregulated significantly by the administration of
persicarin, suggesting that persicarin attenuates the inflammatory
response by inhibiting the NF-κB and AP-1 pathway. Results also
indicate that persicarin suppressed the symptoms of type 1 diabetes
in STZ-induced mice. Persicarin ameliorated abnormal hepatic
metabolism by decreasing hyperglycemia and oxidative stress
(declining glucose and ROS production). Also, persicarin attenuated
the inflammation associated with AP-1, NF-κB, COX-2, TGF-β1, and
iNOS in the liver.
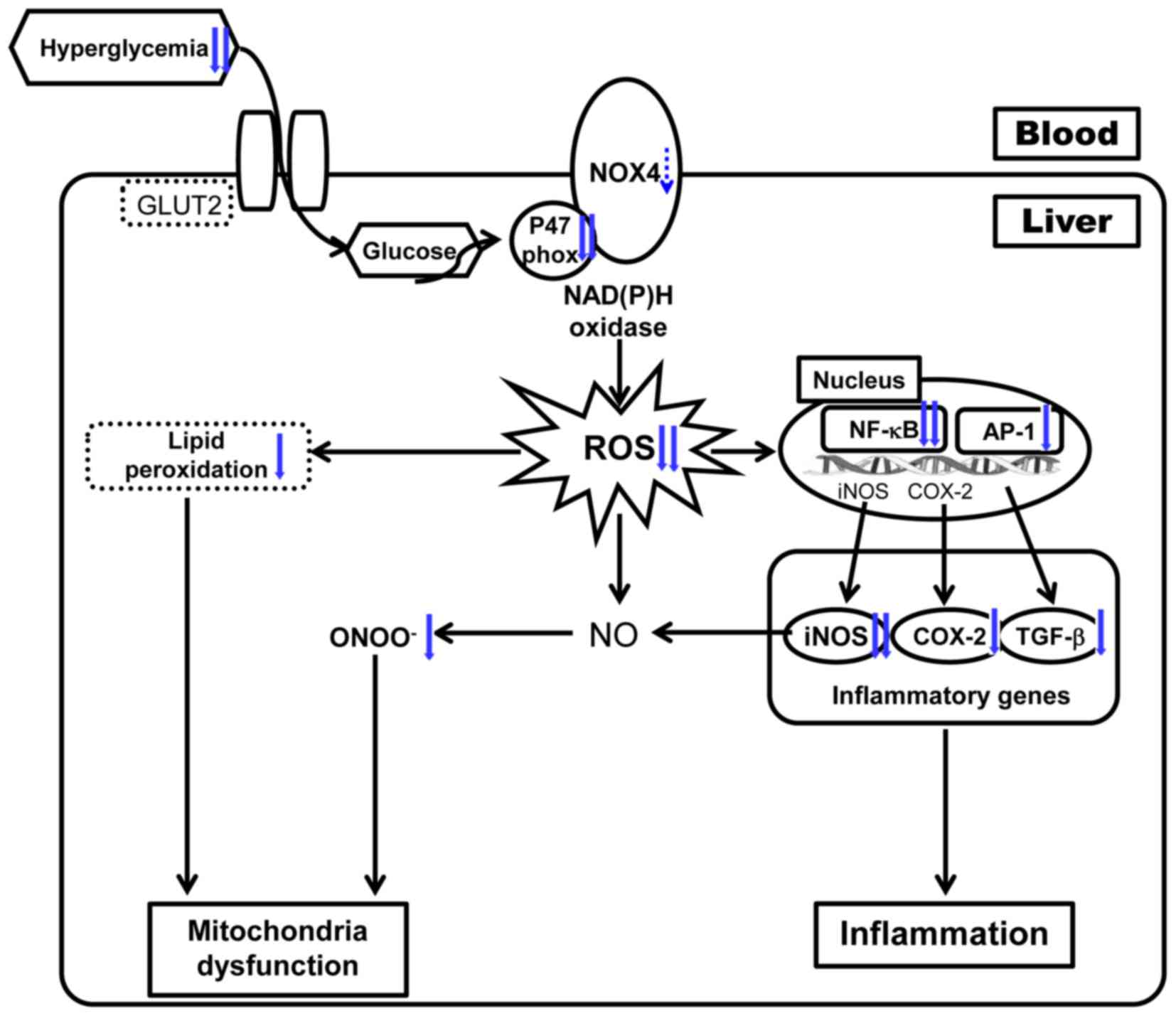
In conclusion, the results from this study suggest
that persicarin protects against type 1 diabetes by attenuating
oxidative stress and the inflammatory response under hyperglycemic
conditions, as shown in Fig. 6.
Thus, it is suggested that persicarin may be a potential
therapeutic agent for the treatment of diabetic complications
including hepatic damage and diabetic symptoms.
References
|
1
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Pract. 87:4–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
King H, Aubert RE and Herman WH: Global
burden of diabetes, 1995–2025: Prevalence, numerical estimates and
projections. Diabetes Care. 21:1414–1431. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harrison SA: Liver disease in patients
with diabetes mellitus. J Clin Gastroenterol. 40:68–76. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aalthira R and Jain V: Advances in
management of type 1 diabetes mellitus. World J Diabetes.
5:689–696. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baig NA, Herrine SK and Rubin R: Liver
disease and diabetes mellitus. Clin Lab Med. 21:193–207.
2001.PubMed/NCBI
|
|
6
|
Babior BM, Lambeth JD and Nauseef W: The
neutrophil NADPH oxidase. Arch Biochem Biophys. 397:342–344. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Manna SK, Mukhopadhyay A and Aggarwal BB:
Resveratrol suppresses TNF-induced activation of nuclear
transcription factors NF-kappa B, activator protein-1, and
apoptosis: Potential role of reactive oxygen intermediates and
lipid peroxidation. J Immunol. 164:6509–6519. 2002. View Article : Google Scholar
|
|
8
|
Park JC, Yu YB, Lee JH, Hattori M, Lee CK
and Choi JW: Persicarin effect of Oenanthe javanica on the hepatic
lipid peroxidation in bromobenzene-treated rats and its bioactive
component. J Med Plant Res. 62:488–490. 1996. View Article : Google Scholar
|
|
9
|
Han YQ, Huang ZM, Yang XB, Liu HZ and Wu
GX: In vivo and in vitro anti-hepatitis B virus activity of total
phenolics from Oenanthe javanica. J Ethnopharmacol. 118:148–153.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ku SK, Kim TH, Lee S, Kim SM and Bae JS:
Antithrombotic and profibrinolytic activities of
isorhamnetin-3-O-galactoside and hyperoside. Food Chem Toxicol.
53:197–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ji G, Yao X, Zang Z and Huang Z:
Antiarrhythmic effect of Oenanthe javanica (Bl.) DC. Injection.
Zhongguo Zhong Yao Za Zhi. 15:429–431, 448. 1990.(In Chinese).
PubMed/NCBI
|
|
12
|
Yang XB, Huang ZM, Cao WB, Zheng M, Chen
HY and Zhang JZ: Antidiabetic effect of Oenanthe javanica flavones.
Acta Pharmacol Sin. 21:239–242. 2000.PubMed/NCBI
|
|
13
|
Kim JY, Kim KH, Lee YJ, Lee SH, Park JC
and Nam DH: Oenanthe javanica extract accelerates ethanol
metabolism in ethanol-treated animals. BMB Rep. 42:482–485. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma CJ, Lee KY, Jeong EJ, Kim SH, Park J,
Choi YH, Kim YC and Sung SH: Persicarin from water dropwort
(Oenanthe javanica) protects primary cultured rat cortical cells
from glutamate-induced neurotoxicity. Phytother Res. 24:913–918.
2010.PubMed/NCBI
|
|
15
|
Kim JE, Lee DE, Lee KW, Son JE, Seo SK, Li
J, Jung SK, Heo YS, Mottamal M, Bode AM, et al: Isorhamnetin
suppresses skin cancer through direct inhibition of MEK1 and PI3-K.
Cancer Prevention Research (Phila). 4:582–591. 2011. View Article : Google Scholar
|
|
16
|
Ma G, Yang C, Qu Y, Wei H, Zhang T and
Zhang N: The flavonoid component isorhamnetin in vitro inhibits
proliferation and induces apoptosis in Eca-109 cells. Chem Biol
Interact. 167:153–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim TH, Ku SK and Bae JS: Persicarin is
anti-inflammatory mediator against HMGB1-induced inflammatory
responses in HUVECs and in CLP-induced sepsis mice. J Cell Physiol.
228:696–703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park JC, Young HS, Yu YB and Lee JH:
Isorhamnetin sulphate from the leaves and stems of Oenanthe
javanica in Korea. Planta Med. 61:377–378. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Momose T, Yano Y and Ohashi K: Organic
analysis. XLIV. A new deproteinizing agent for determination of
blood sugar. Chem Pharm Bull (Tokyo). 11:968–972. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ali SF, LeBel CP and Bondy SC: Reactive
oxygen species formation as a biomarker of methylmercury and
trimethyltin neurotoxicity. Neurotoxicology. 13:637–648.
1992.PubMed/NCBI
|
|
21
|
Mihara M and Uchiyama M: Determination of
malonaldehyde precursor in tissues by thiobarbituric acid test.
Anal Biochem. 86:271–278. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kooy NW, Royall JA, Ischiropoulos H and
Beckman JS: Peroxynitrite-mediated oxidation of dihydrorhodamine
123. Free Radic Biol Med. 16:149–156. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Komatsu S: Extraction of nuclear proteins.
Methods Mol Biol. 355:73–77. 2007.PubMed/NCBI
|
|
24
|
Park JC, Yu YB and Lee JH: Isolation of
steroids and flavonoids from the herb of Oenanthe javanica DC.
Korean J Pharmacogn. 24:244–246. 1993.
|
|
25
|
Okon UA, Owo DU, Udokang NE, et al: Oral
administration of aqueous leaf extract of Ocimum gratissimum
ameliorates polyphagia, polydipsia and weight loss in
streptozotocin-induced diabetic rats. Am J Med Med Sci. 2:45–49.
2012.
|
|
26
|
Punithavathi VR, Anuthama R and Prince PS:
Combined treatment with naringin and vitamin C ameliorates
streptozotocin-induced diabetes in male Wistar rats. J Appl
Toxicol. 28:806–813. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moraes IB, Manzan-Martins C, de Gouveia
NM, Calábria LK, Hiraki KR, Ada S Moraes and Espindola FS:
Polyploidy analysis and attenuation of oxidative stress in hepatic
tissue of STZ-induced diabetic rats treated with an aqueous extract
of Vochysia rufa. Evid Based Complement Alternat Med.
2015:3160172015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rencurel F, Waeber G, Antoine B,
Rocchiccioli F, Maulard P, Girard J and Leturque A: Requirement of
glucose metabolism for regulation of glucose transporter type 2
(GLUT2) gene expression in liver. Biochem J. 314:903–909. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Burcelin R, Eddouks M, Kande J, Assan R
and Girard J: Evidence that GLUT-2 mRNA and protein concentrations
are decreased by hyperinsulinaemia and increased by hyperglycaemia
in liver of diabetic rats. Biochem J. 288:675–679. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee SH, Heo SJ, Hwang JY, Han JS and Jeon
YJ: Protective effects of enzymatic digest from Ecklonia cava
against high glucose-induced oxidative stress in human umbilical
vein endothelial cells. J Sci Food Agric. 90:349–356. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aljofan M and Ding H: High glucose
increases expression of cyclooxygenase-2, increases oxidative
stress and decreases the generation of nitric oxide in mouse
microvessel endothelial cells. J Cell Physiol. 222:669–675.
2010.PubMed/NCBI
|
|
32
|
Sayed AA, Khalifa M and Abd el-Latif FF:
Fenugreek attenuation of diabetic nephropathy in alloxan-diabetic
rats: Attenuation of diabetic nephropathy in rats. J Physiol
Biochem. 68:263–269. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Friederich M, Hansell P and Palm F:
Diabetes, oxidative stress, nitric oxide and mitochondria function.
Curr Diabetes Rev. 5:120–144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sedeek M, Callera G, Montezano A, Gutsol
A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM
and Hébert RL: Critical role of Nox4-based NADPH oxidase in
glucose-induced oxidative stress in the kidney: Implications in
type 2 diabetic nephropathy. Am J Physiol Renal Physiol.
299:F1348–F1358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cos P, Ying L, Calomme M, Hu JP, Cimanga
K, Van Poel B, Pieters L, Vlietinck AJ and Berghe D Vanden:
Structure-activity relationship and classification of flavonoids as
inhibitors of xanthine oxidase and superoxide scavengers. J Nat
Prod. 61:71–76. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Obrosova IG: Diabetes and the peripheral
nerve. Biochim Biophys Acta. 1792:931–940. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Drel VR, Lupachyk S, Shevalye H, Vareniuk
I, Xu W, Zhang J, Delamere NA, Shahidullah M, Slusher B and
Obrosova IG: New therapeutic and biomarker discovery for peripheral
diabetic neuropathy: PARP inhibitor, nitrotyrosine and tumor
necrosis factor-{alpha}. Endocrinology. 151:2547–2555. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kuhad A and Chopra K: Attenuation of
diabetic nephropathy by tocotrienol: Involvement of NFκB signaling
pathway. Life Sci. 84:296–301. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Garcia-Mediavilla V, Crespo I, Collado PS,
Esteller A, Sánchez-Campos S, Tuñón MJ and González-Gallego J: The
anti-inflammatory flavones quercetin and kaempferol cause
inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and
reactive C-protein, and down-regulation of the nuclear factor kappa
B pathway in Chang Liver cells. Eur J Pharmacol. 557:221–229. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dubois RN, Abramson SB, Crofford L, Gupta
RA, Simon LS, Van De Putte LB and Lipsky PE: Cyclooxygenase in
biology and disease. FASEB J. 12:1063–1073. 1998.PubMed/NCBI
|
|
41
|
Llorens S and Nava E: Cardiovascular
diseases and the nitric oxide pathway. Curr Vasc Pharmacol.
1:335–346. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim SJ, Glick A, Sporn MB and Roberts AB:
Characterization of the promoter region of the human transforming
growth factor-beta1 gene. J Biol Chem. 264:402–408. 1989.PubMed/NCBI
|