Introduction
Hepatitis C virus (HCV) represents a global health
problem, particularly in developing countries such as Egypt, and is
a principal cause of progressive liver disease (1). Currently, 130–180 million people are
infected with HCV worldwide (2).
Recently, a number of reports have suggested that HCV-related
mortality rates will continue to rise for the next 20 years
(3,4). Acute HCV infection differs from chronic
infection in various aspects, including whether or not there is a
previous history of increase in alanine aminotransferase (ALT), the
duration of ALT elevation and the presence of symptoms, such as
jaundice (5). In acute infections,
HCV RNA may be detected in serum two weeks following exposure to
HCV; however, anti-HCV antibodies cannot often be identified until
2–3 months after exposure to the virus (6).
Anti-HCV treatment regimens aim to prevent disease
complications and decrease rates of HCV-associated mortality.
Chronic HCV infection develops slowly, which makes it difficult to
establish whether certain treatments prevent liver disease
complications. Biochemical assays [normalization of ALT and
aspartate aminotransferase (AST) levels in serum] and virological
assays [absence of viral RNA from serum as measured by a sensitive
polymerase chain reaction (PCR)-based assay] are employed to
measure short-term outcomes of HCV infection (7,8).
Western countries (9)
and Japan (10) have conducted major
clinical trials concerning antiviral therapy for the treatment of
chronic HCV infection. As the majority of published data addresses
patients infected with HCV genotypes 1, 2 and 3, there is an
established type of treatment and duration of anti-HCV therapy for
these patients (11). Ray et
al (12) demonstrated that an
Egyptian HCV epidemic comprised multiple lineages of genotypes 1
and 4, with the predominance of genotype 4; however, no regional
links in samples from 15 geographically diverse governorates in
Egypt could be identified that suggested a pattern of spread. This
was explained by the fact that multiple HCV strains were
disseminated simultaneously to the entire population. Few studies
have been published on the treatment of patients infected with HCV
genotype 4, and results from clinical trials of combination therapy
for these patients have not been encouraging (13–15).
Randomized controlled clinical trials of several agents are
required for the establishment of an appropriate treatment for HCV
genotype 4. Vaccination is currently used against HCV; however,
vaccines against some important pathogenic strains of HCV are not
available. The high cost of vaccinations results in patients using
traditional medicines as an alternative treatment for HCV
infection. One example of a traditional medicine is camel milk;
many traditional stories exist about camel milk utility as a
therapy for patients infected with hepatitis diseases, particularly
diseases caused by HCV.
The composition and physiology of camel milk is
different from the milk of ruminants (16,17).
Little fat (2%) is found in camel milk and lactose is present at a
concentration of 4.8%, meaning that camel milk is easily
metabolized by lactose-intolerant individuals (18). No β-lactoglobulin (19) and a different β-casein fragment
derived from a non-tryptic type of parent protein cleavage
(20) have been identified among
camel milk proteins. Additionally, camel milk is rich in iron,
calcium and vitamin C (16).
Two major families of proteins are identified in all
types of milk, including camel and cow milk: Caseins and whey
proteins (21). Whey proteins
include immunoglobulins, lactalbumin, lactoperoxidase, lysozyme and
lactoferrin (22). Camels produce a
novel type of antibody devoid of light chains, referred to as a
heavy chain antibody (23), in
addition to conventional antibodies that are composed of two heavy
and two light chains. Lactalbumin, when in a particular
conformational state, possesses antimicrobial properties (24). Lactoferrin (alone or in combination
with other milk proteins) exhibits antibacterial, antiviral,
antifungal, antiparasitic, immunomodulatory, anti-inflammatory and
even antineoplastic effects (25–29). It
has been demonstrated that camel milk has therapeutic effects on
cancer, jaundice, hepatitis B and C, diabetes, dropsy,
tuberculosis, spleen problems, anemia, piles, food allergies, high
cholesterol in the blood and asthma (30,31).
The present study aimed to examine how camels or
products derived from camels may improve people's lives or assist
in protecting the health of animals and people. In vitro
studies have delivered promising results supporting the use of
camel milk against HCV infection (22,26,27,32–42),
prompting the present in vivo investigation. The present
study aimed to determine whether or not camel milk has therapeutic
effects on HCV in infected Egyptian patients who did not take any
medication in the 6 months prior to participation in the current
study. In the present study, whole camel milk was examined as an
alternative medicine. The ability of camel milk to reduce viral
particle load, reduce the levels of antibodies against HCV in
patient sera and improve patient liver functions (ALT and AST) was
investigated.
Materials and methods
Patients
A total of 17 Egyptian patients (12 male and 5
female; aged 20–65 years) suffering from chronic hepatitis C
infection caused by HCV genotype 4, participated in the present
study. Clinical and laboratory evaluation of patients demonstrated
that they were HCV RNA-positive, with mild to moderate fibrosis and
raised levels of transaminases (ALT and AST). Patients were
randomly recruited from the Tropical and Biochemistry Outpatient
Clinic, Faculty of Medicine, Assiut University (Assiut, Egypt),
which is in South Egypt where the HCV genotype 4 is more endemic
than in North. Furthermore, the Assiut governorate is considered
the poorest governorate in Egypt. All participating patients did
not take medication against HCV within the 6 months prior to
initiation of milk consumption. Three healthy adults (two male and
one female) with normal hepatic serum parameters were recruited
from the same Outpatient Clinic at Assiut University and included
in the current study to serve as a control group. The patients
consumed their routine daily meals in addition to camel milk during
the experimental period. Patients were recommended to terminate
their routine bovine milk consumption 6 months prior to and during
the experiment. Patients consumed 250 ml camel milk every morning
under physician supervision in the hospital for 4 months. The
present study was conducted with approval from the Ethics Committee
of the Genetic Engineering and Biotechnology Research Institute and
Faculty of Medicine, Assiut University. Written informed consent
was provided by all subjects prior to participation in this study
and all patients will remain anonymous.
Camel milk
Fresh camel (Camelus dromedaries) milk was collected
from a camel farm (ALKHIR, Alexandria, Egypt) containing a large
herd of camels fed with natural herbs. Milk was collected in 250 ml
bottles and frozen to be transferred to the laboratories of the
Medical Biochemistry Department, Faculty of Medicine, Assiut
University. Milk was kept frozen until consumption by patients in
medical care rooms. Prior to milk collection, a sanitary inspection
was conducted, supervised by the farm veterinarian staff. Camels
and the obtained milk were inspected in this way twice a month at
the farm. All camels used for milk production and their milk, were
free from pathogenic and zoonotic microbes, and their mammary
glands were free from infection and mastitis.
Experimental design
The present study was an open case control,
randomized and parallel design investigation. Three types of assays
were performed for HCV diagnosis and treatment: Liver function
tests (ALT and AST); serologic tests that detected anti-HCV
specific antibodies; and molecular assessments that determined the
load of HCV RNA. Liver function tests were conducted twice for all
patients and control adults, both prior and subsequent to
consumption of camel milk. Levels of AST and ALT were evaluated by
conventional methods, using ALT/GPT and AST/GOT kits (catalogue
nos. 11832 and 11830, respectively, BioSystems, Barcelona, Spain).
Absorbance was measured at 340 nm using a Perkin-Elmer
spectrophotometer (PerkinElmer GmbH, Überlingen, Germany) in the
Medical Biochemistry Department, Faculty of Medicine, Assiut
University. Quantitative assessment of HCV RNA was performed using
Rotor-Gene Q (model no. R0708103; Corbett life Science; Qiagen,
Hilden, Germany). A positive response to camel milk treatment was
determined by the patient being negative for qualitative HCV
analysis or exhibiting a decreasing number of viral particles in
quantitative HCV RNA analysis.
Reverse transcription-quantitative PCR
(RT-qPCR) to evaluate antiviral activity of camel milk against
HCV
HCV RNA was isolated and extracted from HCV patient
and control sera using an INSTANT Virus RNA kit (AJ Roboscreen
GmbH, Leipzig, Germany). DNA may potentially remain in the final
eluted RNA following isolation, thus DNasI treatment was performed
in order to completely remove any trace DNA. One unit of RNAse-free
DNase I (New England Biolabs, Inc., Ipswich, MA, USA) was added to
1 µg RNA in an RNase-free tube containing 1 µl 10X reaction buffer
with MgCl2 and 10 µl DEPC-treated water, followed by
incubation for 30 min at 37°C. Subsequently, 1 µl 50 mM EDTA was
added and incubated for 10 min at 65°C in order to inactivate the
DNase I. Amplification of HCV RNA in samples and standards was
achieved and measured using a RoboGene HCV RNA Quantification kit
(cat. no. 0207200141; AJ Roboscreen GmbH, Leipzig, Germany) and a
Rotor-Gene real time PCR machine (model no. R0708103; Corbett Life
Science; Qiagen). The RoboGene HCV RNA Quantification kit includes
the following components: Extraction tubes coated with internal
control (IC) RNA and carrier nucleic acid, sample tubes coated with
amplification enhancer, quantification standard tubes of 8
different HCV RNA concentrations (ready-to-use reference curves),
reagent mix with HCV/IC specific primers and probes, Mg-sulfate (50
mM), RT-PCR enzyme mix, 2X reaction mix, and PCR grade water. qPCR
1X master mix was prepared by mixing 3.5 µl PCR grade water, 12.5
µl 2X reaction mix, 2 µl 50 mM Mg-sulfate solution, 1 µl 25X
reagent mix with HCV/IC specific primers and probes, and 1 µl
RT-PCR enzyme mix. The mix was then vortexed at 1,000 rpm for 3 sec
and centrifuged at 18,078 × g and 4°C for 5 sec. Twenty microliters
of 1X master mix were added to sample tubes and quantification
standard tubes. PCR grade water (5 µl) was added to sample tubes
serving as a no template control (NTC), as well as all
quantification standard tubes and 5 µl of isolated RNA were added
to the respective sample tubes. The RoboGene HCV RNA Quantification
kit detects all HCV genotypes using primers specific for a
subsequence of the HCV 5′ untranslated region (5′ UTR). Each sample
was tested in duplicate. Specific controls were included: i) IC to
control RNA extraction and to indicate for inhibitory effects on
detection; ii) two quantification standards in the kit to serve as
HCV positive control; iii) sera samples from 3 healthy adults to
serve as HCV negative control. qPCR cycling conditions were as
follows: 1X of reverse transcription at 55°C for 30 min, 1X of Taq
activation at 95°C for 2 min, 50X of melting at 95°C for 15 sec,
stem formation at 45°C for 15 sec, annealing at 57°C for 40 sec. A
quantification report was created by the Rotor-Gene Q Series
Software 1.7, Build 94 (Corbett Life Science; QIAGEN GmbH). HCV RNA
was evaluated based on the Cq values obtained for the
sample HCV RNA and a standard curve resulted from quantification
standard analysis and calibration coefficient specific for the
assay (43). All methods were
conducted according to the manufacturers' protocols.
Anti-HCV immunoglobulin (Ig) G
profile
ELISA plates (cat. no. 2592, Costar, Cambridge, USA)
were coated with 50 µl of HCV peptide 1, 2, 3, 4 or 5 at a
concentration of 5 µg/ml. Subsequent to an incubation period of 24
h at room temperature, the plates were washed 5 times with 0.12 M
NaCl and phosphate-buffered saline (PBS; pH 7.2). Subsequently, 100
µl blocking buffer [2% bovine serum albumin (BSA) in PBS] was added
to the ELISA plates, followed by incubation for 1 h at room
temperature. Following this, 50 µl hepatitis C patient sera (prior
and subsequent to camel milk drinking) diluted 1:100 in 2% BSA-PBS
were added. After 1 h incubation at room temperature, the plates
were washed five times, sequentially, with PBS and 50 µl
biotin-labeled goat-antihuman IgG1 (1:1,000; cat. no. B6775),
biotin-labeled goat-antihuman IgG2 (1:15,000; cat. no. B3398),
biotin-labeled goat-antihuman IgG3 (1:4,000; cat. no. B3523), or
biotin-labeled goat-antihuman IgG4 (1:15,000; cat. no. B3648;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) were added. The
plates were incubated for 1 h at room temperature. The plates were
washed five times to remove unbound antibodies and 50 µl
streptavidin-alkaline phosphatase-conjugate (Sigma-Aldrich; Merck
Millipore) diluted 1:4,000 with 2% BSA-PBS was subsequently added.
Following this, the plates were incubated for 1 h at room
temperature. Finally, para-Nitrophenyl phosphate (Sigma-Aldrich;
Merck Millipore) was added for color development and absorbance was
recorded immediately at a wavelength of 405 nm (44,45)
using an ELISA microtiter plate reader (MicroPlate Reader; Bio-Rad
Laboratories Inc., Hercules, CA, USA).
HCV peptide design and synthesis
ClustalW version 1.4 (46) was employed to analyze extracted
sequences from the Los Alamos HCV sequence database (47). Five peptide (P) sequences were
synthesized commercially (Anaspec Inc., San Jose, CA, USA) in the
following design: P1 from the HCV core protein
(DVKFPGGGQIVGGVYLLPRR); P2 from the HCV core protein
(GPRLGVRATRKTSERSQPRG); P3 from the HCV core protein
(IPKARRPEGRTWAQPGY); P4 from the HCV core protein
(IPKDRRSTGKSWGKPGY); and P5 from the HCV envelope 2 protein
(NLQLINTNGS). The peptides were synthesized in the amide form using
the standard solid phase synthesis involving 9-flurenylmethoxy
carbonyl chemistry (48) and
purified by high performance liquid chromatography according to the
manufacturer's instructions (49).
Peptides were synthesised and purified commercially (AstraZeneca,
Cambridge, UK).
Statistical analysis
Statistical analysis was performed on Microsoft
Office Excel 2003 v11.0 (Microsoft Corporation, Redmond, WA, USA)
using Student's t-test and McNemar's test (50). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patients
A total of 17 patients were enrolled in the study:
12 males (70.59%) and five females (29.41%). Prior to initiation of
the study, all patients suffered from generalized symptoms of
malaise, anorexia, fever, abdominal distension and arthralgia.
Additionally, 80% of patients suffered with jaundice, right
hypochondrial pain, ankle swelling, hematemesis and melena and
breast swelling (gynecomastia). Loss of libido, amenorrhea and
changes in secondary sex characteristics affected 50% of patients.
Within the 4 months of camel milk treatment, and after treatment,
generalized symptoms, including malaise, anorexia, fever,
athralgia, indigestion and changes in secondary sex
characteristics, were resolved in all patients. Jaundice, right
hypochondrial pain, ankle swelling, hematemesis, melena and
gynecomastia improved in 50% of patients who suffered from these
conditions at the beginning of the study.
AST and ALT sera assays
Results of liver function tests revealed a marked
decrease in ALT in 88.23% of patients and in AST in all patients
(Table I). Subsequent to camel milk
treatment, AST activity decreased in five patients to values within
the international normal range of 10–34 international units (IU)/l.
Camel milk treatment decreased AST activity in ten patients to mean
± standard deviation (SD) values between 37±3.94 and 53±1.81 IU/l,
which are still promising when considering the high levels of AST
activity exhibited prior to treatment, where mean ± SD values
ranged between 70±3.19 and 132±0.99 IU/l. Furthermore, in two
patients, AST activity decreased to 101±3.65 and 71±1.22 IU/l,
compared to 199±1.54 and 121±1.84 IU/l, respectively, prior to
treatment. However, this level of AST activity is high when
compared to the international normal range. Additionally, ALT
levels decreased in four patients subsequent to camel milk
treatment to values within the normal range (up to 41 IU/l). Camel
milk treatment decreased ALT levels in 11 patients to values
between 43±1.24 and 58±2.36 IU/l, which are encouraging compared to
the high levels of ALT observed prior to treatment (ranging from
80±1.65 to 161±2.11 IU/l). However, two patients did not respond to
treatment and exhibited elevated ALT levels subsequent to
treatment. The ratio of AST to ALT was <1 in 15 out of 17
patients (Table I).
 | Table I.Assaying of ALT and AST in patient
sera prior to and following camel milk treatment. |
Table I.
Assaying of ALT and AST in patient
sera prior to and following camel milk treatment.
|
|
| ALT (IU/l) | AST (IU/l) | AST/ALT ratio |
|---|
|
|
|
|
|
|
|---|
| Patient no. | Age (years) | Prior to
treatment | Following
treatment | Prior to
treatment | Following
treatment | Prior to
treatment | Following
treatment |
|---|
| 1 | 52 | 105±2.34 | 48±1.99 | 81±1.33 | 39±2.01 | 0.77 | 0.81 |
| 2 | 34 | 98±3.21 | 45±3.25 | 83±3.02 | 41±2.56 | 0.85 | 0.91 |
| 3 | 47 | 93±1.35 | 57±1.79 | 72±1.63 | 49±1.48 | 0.77 | 0.86 |
| 4 | 45 | 144±1.33 | 55±2.17 | 119±0.56 | 53±1.81 | 0.83 | 0.96 |
| 5 | 20 | 108±2.42 | 58±2.28 | 90±2.98 | 42±0.79 | 0.83 | 0.72 |
| 6 | 51 | 114±1.57 | 58±2.36 | 91±1.44 | 29±1.27 | 0.79 | 0.50 |
| 7 | 43 | 90±3.54 | 47±3.21 | 77±3.01 | 25±2.38 | 0.86 | 0.53 |
| 8 | 30 | 161±2.11 | 46±2.48 | 132±0.99 | 39±1.45 | 0.82 | 0.85 |
| 9 | 58 | 97±2.18 | 41±2.47 | 70±3.19 | 38±3.05 | 0.72 | 0.93 |
| 10 | 35 | 80±1.65 | 43±1.24 | 71±2.99 | 37±3.94 | 0.89 | 0.86 |
| 11 | 41 | 88±1.47 | 49±1.82 | 81±2.01 | 42±1.66 | 0.92 | 0.86 |
| 12 | 36 | 90±1.89 | 32±1.24 | 87±2.57 | 31±2.83 | 0.97 | 0.97 |
| 13 | 24 | 99±2.43 | 47±1.37 | 91±1.66 | 42±2.55 | 0.92 | 0.89 |
| 14 | 37 | 128±2.61 | 35±2.55 | 104±1.79 | 33±1.45 | 0.81 | 0.94 |
| 15 | 65 | 168±1.97 | 176±2.22 | 199±1.54 | 101±3.65 | 1.18 | 0.57 |
| 16 | 40 | 94±1.84 | 41±2.11 | 74±3.25 | 27±3.66 | 0.79 | 0.66 |
| 17 | 55 | 109±1.32 | 114±1.25 | 121±1.84 | 71±1.22 | 1.11 | 0.62 |
HCV RNA RT-qPCR results
The results demonstrated that, following daily
consumption of camel milk for four months, 13 patients (76.47%)
experienced marked decreases in HCV RNA levels in their sera
(Table II). One patient had
undetectable viremia (viral load=0) following camel milk treatment.
The remaining patients (23.53%) did not respond to the
treatment.
 | Table II.Determination of hepatitis C virus
particles in patient sera prior to and following camel milk
treatment. |
Table II.
Determination of hepatitis C virus
particles in patient sera prior to and following camel milk
treatment.
| Patient no. | Prior to treatment
(copies/ml) | Following treatment
(copies/ml) |
|---|
| Control (n=3) | Undetected | Undetected |
| 1 | 5,400,000 | 1,095,000 |
| 2 | 4,460,000 | 277,000 |
| 3 | 1,004,600 | 1,540,600 |
| 4 | 3,035,800 | 23,690 |
| 5 | 1,173,020 | 1,525,500 |
| 6 | 3,328,680 | 69,372 |
| 7 | 4,859,500 | 354,650 |
| 8 | 6,617,070 | 332,567 |
| 9 | 5,407,000 | 63,880 |
| 10 | 1,130,130 | 41,860 |
| 11 | 2,329,000 | 171,200 |
| 12 | 1,379,000 | 0 |
| 13 | 1,148,000 | 29,490 |
| 14 | 5,070,000 | 31,740 |
| 15 | 7,905,600 | 9,806,000 |
| 16 | 4,596,000 | 222,800 |
| 17 | 6,800,367 | 7,870,000 |
Anti-HCV IgG isotyping
Results demonstrated that anti-HCV antibody isotype
IgG1 exhibited significantly weaker (P<0.05) colour signals
(mean abs at 405 nm of IgG1 in 17 patients) subsequent to camel
milk treatment against all HCV peptides used (Table III). By contrast, anti-HCV antibody
isotypes IgG2, 3, and 4 exhibited significantly stronger
(P<0.05) colour signals (mean abs at 405 nm of IgG2, 3, and 4 in
17 patients) following camel milk treatment against all tested HCV
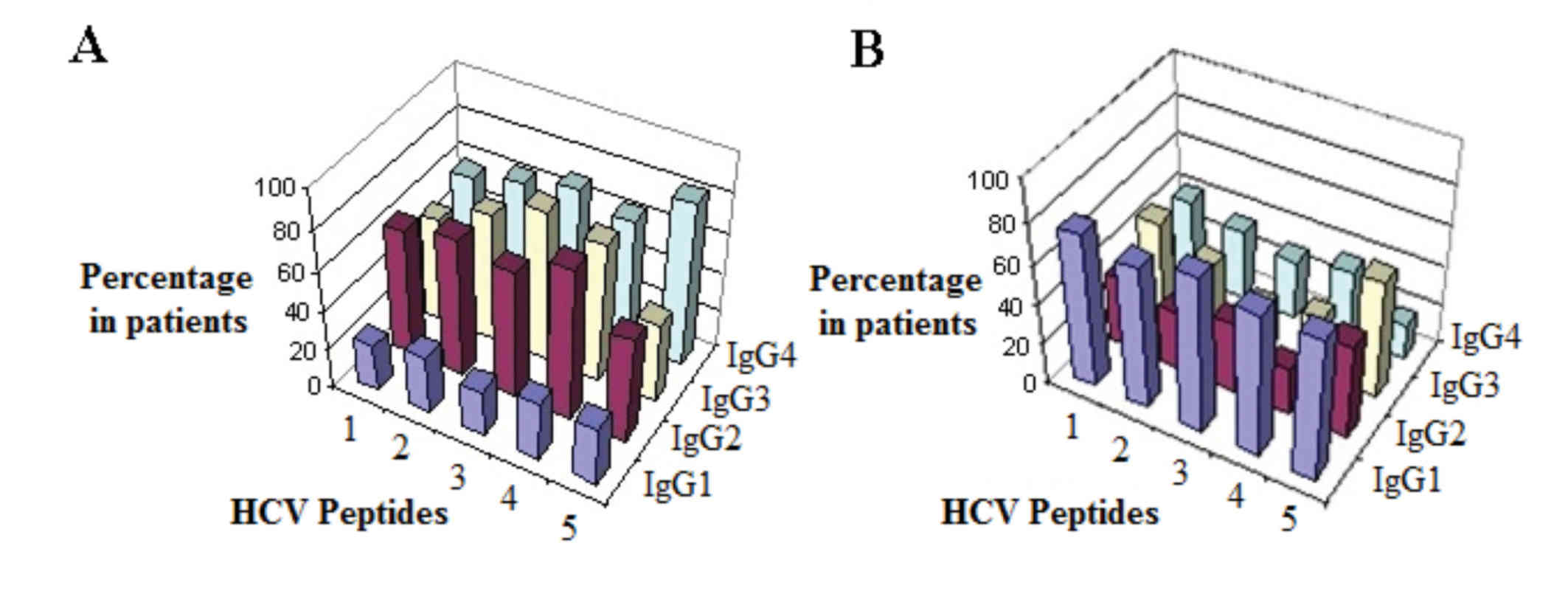
peptides. Fig. 1 presents the exact
percentage of patients that experienced either an increase or
decrease in color signals of anti-HCV antibody isotypes against all
tested HCV peptides, following camel milk treatment. A marked
reduction of IgG1 occurred in 70–76% of patients following camel
milk-treatment. By contrast, notable increases were demonstrated in
levels of IgG2, 3 and 4 in 52–76, 41–76 and 58–82% of patients,
respectively (Fig. 1).
 | Table III.Response to camel milk treatment
measured by determination of mean absorbance at 405 nm of 4 IgG
isotypes against five hepatitis C virus peptides. |
Table III.
Response to camel milk treatment
measured by determination of mean absorbance at 405 nm of 4 IgG
isotypes against five hepatitis C virus peptides.
|
| IgG1 | IgG2 | IgG3 | IgG4 |
|---|
|
|
|
|
|
|
|---|
|
| Prior to
treatment | Following
treatment | Prior to
treatment | Following
treatment | Prior to
treatment | Following
treatment | Prior to
treatment | Following
treatment |
|---|
| 1 | 0.53±0.06 |
0.29±0.09a | 0.26±0.12 |
0.58±0.10a | 0.31±0.11 |
0.61±0.16a | 0.30±0.15 |
0.74±0.15a |
| 2 | 0.54± 0.05 |
0.27±0.10a | 0.22±0.11 |
0.54±0.12a | 0.34±0.14 |
0.79±0.20a | 0.26±0.06 |
0.64±0.20a |
| 3 | 0.63±0.12 |
0.34±0.09a | 0.36±0.09 |
0.63±0.10a | 0.33±0.09 |
0.57±0.10a | 0.24±0.11 |
0.56±0.21a |
| 4 | 0.80±0.11 |
0.34±0.21a | 0.33±0.10 |
0.67±0.20a | 0.22±0.08 |
0.65±0.22a | 0.28±0.06 |
0.45±0.22a |
| 5 | 0.53±0.06 |
0.21±0.05a | 0.22±0.06 |
0.57±0.04a | 0.22±0.05 |
0.52±0.06a | 0.23±0.06 |
0.41±0.07a |
| Total | 0.61±0.11 |
0.29±0.05a | 0.28±0.06 |
0.60±0.05a | 0.28±0.05 |
0.63±0.10a | 0.26±0.03 |
0.56±0.13a |
Discussion
Studies suggest that Egypt has the highest HCV
prevalence rate globally (51–53),
possibly as a result of the use of unsterile injection tools during
public anti-schistosomiasis injection campaigns. Egyptians are
predominantly infected with HCV genotype 4 (subtype 4a), which has
a reduced response rate to standard interferon (IFN)-based therapy
compared with genotypes 1, 2, or 3 (54). The standard therapy for Egyptian HCV
patients is the use of IFN as a monotherapy (IFN-α2a, -α2b and
pegylated IFN) (55,56) or combined with ribavirin; however,
patients often use various alternative medicines (57–59).
Recent studies investigated the use of whey protein concentrate
(57), pasteurized camel milk
(58) and fresh camel milk (59) in the treatment of HCV genotype 4
infection; the results demonstrated significant decreases in viral
load, markers of active inflammation and ALT and AST activity
(57–59) following treatment. However, the study
by Mohamed et al (59) did
not clarify the camel milk source and how and where camel milk was
consumed by participants. Furthermore, the camel milk was used as a
treatment in combination with pegylated IFN/ribavirin in HCV-4
infected Egyptian patients.
One of the most popular alternative treatments for
HCV infection, based on traditional beliefs, is camel milk, which
is used among the main human dietary constituents in several parts
of the world. In the present study, the potential role of camel
milk components in viral inhibition and treatment of HCV infection
was investigated. A total of 17 patients (12 male and 5 female)
were treated by consumption of camel milk, without heating or the
addition of chemical substances. This treatment continued for four
months and each patient consumed 250 ml/day.
Following camel milk treatment, a marked improvement
was observed in the general fatigue and health (personal
observation) of the patients. Following 4 months treatment, serum
samples were collected from patients to evaluate the antiviral
activity of camel milk against HCV. Results demonstrated that 13
out of 17 patients responded to the treatment and exhibited reduced
levels of HCV RNA in their sera, and one patient had no detectable
HCV RNA in their serum following treatment. Four patients did not
respond to the treatment at all, which may suggest that they
required increased doses of camel milk. Results of liver function
tests revealed a significant reduction in ALT and AST activity in
15 out of 17 patients and in all patients, respectively, subsequent
to treatment. Results also demonstrated that the ratio of AST to
ALT was <1 in 15 out of 17 patients, which is in agreement with
the concept that in viral hepatitis the AST/ALT ratio is usually
<1 (60). The AST/ALT ratio was
>1 in two patients prior to treatment, which is an indication of
cirrhosis (61,62).
In the present study, following treatment, IgG1
exhibited weaker color signals against different HCV peptides
compared with other IgG subtypes in 70–76% of patients (Table III). Prior to treatment, chronic
hepatitis C patients with high viremia exhibited detectable IgG1.
The camel milk may, therefore, regulate the expression of Th1/Th2
immunity (63) towards the
Th1-dependent immunity profile (IgG2). This resembles the immunity
profile of chronic hepatitis B patients treated with camel milk
(64). This supports the view that
Th2 immunity in patients with chronic hepatitis C cannot regulate
viral clearance (65). In keeping
with previous findings concerning the use of camel milk to improve
tuberculosis, problems with the spleen, dropsy, asthma, anemia,
piles and jaundice (31), other lung
ailments and treatment of diabetic patients (66), the present study demonstrated that,
following treatment with camel milk, all generalized symptoms
(including malaise, anorexia, fever, athralgia, indigestion and
changes in the secondary sex characteristics) were resolved in all
HCV patients in the present study.
Camel milk contains several proteins, including IG
and lactoferrin, and other components with anti-viral and
immunomodulatory activities. In a previous study (34), it was observed that purified camel
polyclonal IgG exhibited in vitro inhibitory effects against
HCV. This is in accordance with the results from a study by Martin
et al (67), which
demonstrated similar inhibitory effects of recombinant camel heavy
chain variable domain antibody against HCV nonstructural
protease.
Previous studies (26,27,32–34) have
demonstrated that natural and recombinant camel lactoferrin exerts
strong antiviral activity against HCV in vitro. Camel
lactoferrin is more potent over sheep, bovine and human
lactoferrins against HCV cellular infectivity (36). The exact mechanisms by which
lactoferrin regulates its immune modulating functions are
incompletely understood; however, it has been demonstrated that
lactoferrin enhances and improves immune response, both directly
and/or indirectly, in response to a wide range of immune challenges
(28).
There are some limitations to acknowledge for the
present study. Firstly, the study included a small sample size due
to the difficulty of obtaining volunteers and scheduling follow-up
meetings. Future research should include a larger sample size.
Secondly, there was no local epidemiological information that could
be referred to or compared with throughout the study. Thirdly, the
study was only concerned with Egyptian patients infected with HCV
genotype 4; therefore, no knowledge was gained on genotypes 1, 2,
or 3, or on the effect of camel milk in non-Egyptian populations
infected with HCV.
In conclusion, the present study demonstrated that
whole camel milk has marked in vivo efficacy against HCV, by
reducing viral load in patient sera and converting the IgG isotype
profile to Th1 immunity. Therefore, the use of camel milk is
recommended for treating patients infected with HCV genotype 4;
however, it is necessary for the benefits of camel milk to be
investigated further.
Acknowledgments
The present study was financially supported, in
part, by Science & Technology Development Fund (grant no.
409).
References
|
1
|
Williams R: Global challenges in liver
disease. Hepatology. 44:521–526. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shepard CW, Finelli L and Alter MJ: Global
epidemiology of hepatitis C virus infection. Lancet Infect Dis.
5:558–567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deuffic-Burban S, Poynard T, Sulkowski MS
and Wong JB: Estimating the future health burden of chronic
hepatitis C and human immunodeficiency virus infections in the
United States. J Viral Hepat. 14:107–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ly KN, Hughes EM, Jiles RB and Holmberg
SD: Rising mortality associated with hepatitis C virus in the
united states, 2003–2013. Clin Infect Dis. 62:1287–1288. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chung RT: Acute hepatitis C virus
infection. Clin Infect Dis. 41 Suppl 1:S14–S17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghany MG, Strader DB, Thomas DL and Seeff
LB: American Association for the Study of Liver Diseases.
Diagnosis, management and treatment of hepatitis C An update.
Hepatology. 49:1335–1374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Manns MP, McHutchison JG, Gordon SC,
Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M
and Albrecht JK: Peginterferon alfa-2b plus ribavirin compared with
interferon alfa-2b plus ribavirin for initial treatment of chronic
hepatitis C: A randomised trial. Lancet. 358:958–965. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fried MW, Shiffman ML, Reddy KR, Smith C,
Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G,
Dhumeaux D, et al: Peginterferon alfa-2a plus ribavirin for chronic
hepatitis C virus infection. N Engl J Med. 347:975–982. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Poynard T, Marcellin P, Lee SS, Niederau
C, Minuk GS, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C and
Albrecht J: Randomized trial of interferon alpha2b plus ribavirin
for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo
for 48 weeks for treatment of chronic infection with hepatitis C
virus. International Hepatitis Interventional Therapy Group (IHIT).
Lancet. 352:1426–1432. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okanoue T, Itoh Y, Minami M, Sakamoto S,
Yasui K, Sakamoto M, Nishioji K, Murakami Y and Kashima K:
Interferon therapy lowers the rate of progression to hepatocellular
carcinoma in chronic hepatitis C but not significantly in advanced
stage: A retrospective study in 1148 patients. Viral Hepatitis
Therapy Study Group. J Hepatol. 30:653–659. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Booth JC, O'Grady J and Neukerger J: Thr
Royal College of Physicians of London and the British Society of
Gastroenterology: Clinical guidelines on the management of
hepatitis C. Gut. 49 Suppl 1:I1–I21. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ray SC, Arthur RR, Carella A, Bukh J and
Thomas DL: Genetic epidemiology of hepatitis C virus throughout
Egypt. J Infect Dis. 182:698–707. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shiha G and Salem S: Interferon alone or
in combination with ribavirin for the treatment of chronic
hepatitis C genotype IV. J Hepatol. 36:1292002. View Article : Google Scholar
|
|
14
|
El Makhzangy H, Esmat G, Said M, Elraziky
ME, Shouman S, Refai R, Rekacewicz C, Gad RR, Vignier N,
Abdel-Hamid M, et al: Response to pegylated interferon alfa-2a and
ribavirin in chronic hepatitis C genotype 4. J Med Virol.
81:1576–1583. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Esmat G and Fattah S Abdel: Evaluation of
a novel pegylated interferon alpha-2a (Reiferon Retard®)
in Egyptian patients with chronic hepatitis C-genotype 4. Digest
Liver Dis. 3:17–19. 2009.
|
|
16
|
Yagil R and van Creveld C: Medicinal use
of camel milk. Fact or Fancy?Proceedings of the 2nd International
Camelid Conference on Agro-economics of Camelids. Almaty;
Kazahstan: 2000
|
|
17
|
Yagil R and Combs SB: The Desert Camel:
Comparative Physiological AdaptationComparative Animal Nutrition.
5. Karger, Basel: 1985
|
|
18
|
Hanna J Over the hump. Jack Hanna's Animal
Adventures. TV series (USA) 2001 season. #2190. http://www.animaladventures.com
|
|
19
|
Merin U, Bernstein SD, Bloch-Damti N,
Yagil R, van Creveld C and Lindner P: A comparative study of milk
proteins in camel (Camelus dromedarius) and bovine colostrum.
Livestock Product Sci. 67:297–301. 2001. View Article : Google Scholar
|
|
20
|
Beg OU, von-Bahr-Lindstström H, Zaidi ZH
and Jörnvall H: Characterisation of camel milk protein rich proline
identifies a new beta casein fragment. Regul Pept. 15:55–61. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Evans EW: Uses of milk proteins in
formulated foodsDevelopments in Food Proteins. Hudson BJF: Applied
Science; London: 1982
|
|
22
|
EL-Fakharany EM, Serour EA, Abdelrahman
AM, Haroun BM and Redwan el-RM: Purification and characterization
of camel (Camelus dromedarius) milk amylase. Prep Biochem
Biotechnol. 39:105–123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hamers-Casterman C, Atarhouch T,
Muyldermans S, Robinson G, Hamers C, Songa E Bajyana, Bendahman N
and Hamers R: Naturally occurring antibodies devoid of light
chains. Nature. 363:446–448. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Håkansson A, Zhivotovsky B, Orrenius S,
Sabharwal H and Svanborg C: Apoptosis induced by a human milk
protein. Proc Natl Acad Sci. 92:8064–8068. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harmsen MC, Swart PJ, de Bethune MP,
Pauwels R, De Clercq E, The TH and Meijer DK: Antiviral effects of
plasma and milk proteins: Lactoferrin shows potent activity against
both human immunodeficiency virus and human cytomegalovirus
replication in vitro. J Infect Dis. 172:380–388. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Redwan el-RM and Tabll A: Camel
lactoferrin markedly inhibits hepatitis C virus genotype 4
infection of human peripheral blood leukocytes. J Immunoassay
Immunochem. 28:267–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
El-Fakharany EM, Tabll A, El-Wahab AA,
Haroun BM and Redwan EM: Potential activity of camel milk-amylase
and lactoferrin against hepatitis C virus infectivity in HepG2 and
lymphocytes. Hepatitis Monthly. 8:101–109. 2008.
|
|
28
|
Valenti P and Antonini G: Lactoferrin: An
important host defense against microbial and viral attack. Cell Mol
Life Sci. 62:2576–2587. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
El-Fakharany EM, Haroun BM, Ng TB and
Redwan EM: Oyster mushroom laccase inhibits hepatitis C virus entry
into peripheral blood cells and hepatoma cells. Protein Pept Lett.
17:1031–1039. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rao MB, Gupta RC and Dastur NN: Camels'
milk and milk products. Ind J Dairy Sci. 23:71–78. 1970.
|
|
31
|
Kaskous S: Importance of camel milk for
human health. Emir J Food Agric. 28:158–163. 2016.
|
|
32
|
Almahdy O, EL-Fakharany EM, EL-Dabaa E, Ng
TB and Redwan EM: Examination of the activity of camel milk casein
against hepatitis C virus (genotype-4a) and its apoptotic potential
in hepatoma and hela cell lines. Hepat Mon. 11:724–730. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liao Y, El-Fakharany E, Lönnerdal B and
Redwan EM: Inhibitory effects of native and recombinant full-length
camel lactoferrin and its N and C lobes on hepatitis C virus
infection of Huh7.5 cells. J Med Microbiol. 61:375–383. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
EL-Fakharany EM, Abedelbaky N, Haroun BM,
Sánchez L, Redwan NA and Redwan EM: Anti-infectivity of camel
polyclonal antibodies against hepatitis C virus in Huh7.5 hepatoma.
Virol J. 9:2012012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
EL-Fakharany EM, Haroun BM, Redwan NA,
Sánchez L and Redwan EM: Potential activity of camel milk proteins
on the cellular infectivity of hepatitis C virus. Virol J.
9:2012012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
El-Fakharany EM, Sánchez L, Al-Mehdar HA
and Redwan EM: Effectiveness of human, camel, bovine and sheep
lactoferrin on the hepatitis C virus cellular infectivity:
Comparison study. Virol J. 10:1992013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Redwan EM, Uversky VN, El-Fakharany EM and
Al-Mehdar H: Potential lactoferrin activity against pathogenic
viruses. C R Biol. 337:581–595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Albar AH, Almehdar HA, Uversky VN and
Redwan EM: Structural heterogeneity and multifunctionality of
lactoferrin. Curr Protein Pept Sci. 15:778–797. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Redwan EM, El-Fakharany EM, Uversky VN and
Linjawi MH: Screening the anti infectivity potentials of native N-
and C-lobes derived from the camel lactoferrin against hepatitis C
virus. BMC Complement Altern Med. 14:2192014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Almehdar HA, El-Fakharany EM, Uversky VN
and Redwan EM: Disorder in milk proteins: Structure, functional
disorder, and biocidal potentials of lactoperoxidase. Curr Protein
Pept Sci. 16:352–365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Redwan EM, Almehdar HA, EL-Fakharany EM,
Baig AWK and Uversky VN: Potential antiviral activities of camel,
bovine, and human lactoperoxidases against hepatitis C virus
genotype 4. RSC Adv. 5:60441–60452. 2015. View Article : Google Scholar
|
|
42
|
Redwan EM, El-Baky NA, Al-Hejin AM,
Baeshen MN, Almehdar HA, Elsaway A, Gomaa AB, Al-Masaudi SB,
Al-Fassi FA, Abu Zeid IE and Uversky VN: Significant antibacterial
activity and synergistic effects of camel lactoferrin with
antibiotics against methicillin-resistant Staphylococcus aureus
(MRSA). Res Microbiol. 167:480–491. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dogar MZ Hassan, Nazir T, Akram H,
Nasir-ud-Din A and Farooq M: Molecular study of human HCV RNA by
real time-polymerase chain reaction, viral kit and RoboGene
quantification. Cell Mol Biol. 61:22015.PubMed/NCBI
|
|
44
|
Redwan el-RM, Fahmy A, El Hanafy A, Abd
EL-Baky N and Sallam SM: Ovine anti-rabies antibody production and
evaluation. Comp Immunol Microbiol Infect Dis. 32:9–19. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
EL-Baky NA, Omar SH, EL-Badry H and Redwan
el-RM: Efficacy comparison of gel-based, membrane and glass array
techniques to detect human antibodies isotypes among the Egyptian
HCV-patients. Hum Antibodies. 17:63–71. 2008.PubMed/NCBI
|
|
46
|
Thompson JD, Higgins DG and Gibson TJ:
CLUSTAL W Improving the sensitivity of progressive multiple
sequence alignment through sequence weighting, position specific
gap penalties and weight matrix choice. Nucleic Acids Res.
22:4673–4680. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kuiken C, Yusim K, Boykin L and Richardson
R: The Los Alamos hepatitis C sequence database. Bioinformatics.
21:379–384. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fields GB and Noble RL: Solid phase
peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids.
Int J Pept Protein Res. 35:161–214. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Boysen RI and Hearn MT: Purification of
peptides from solid-phase peptide synthesis with RP-HPLC. CSH
Protoc. 2006:pdb.prot45482006.PubMed/NCBI
|
|
50
|
Conover WJ: Practical nonparametric
statistic. 2nd edition. John Wiley and Sons; New York: 1971
|
|
51
|
el-Zayadi A, Simmonds P, Dabbous H,
Prescott L, Selim O and Ahdy A: Response to interferon-alpha of
Egyptian patients infected with hepatitis C virus genotype 4. J
Viral Hepat. 3:261–264. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kamal SM, El Tawil AA, Nakano T, He Q,
Rasenack J, Hakam SA, Saleh WA, Ismail A, Aziz AA and Madwar MA:
Peginterferon {alpha}-2b and ribavirin therapy in chronic hepatitis
C genotype 4: Impact of treatment duration and viral kinetics on
sustained virological response. Gut. 54:858–866. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
El-Zanaty F and Way A: Egypt Demographic
and Health Survey 2008. Egyptian Ministry of Health. El-Zanaty and
Associates and Macro International. 4312009.
|
|
54
|
Zekri AK, Ashour MS, Hassan A, El-Din H
Alam, El-Shehaby AM and Abu-Shady MA: Cytokine profile in Egyptian
hepatitis C virus genotype-4 in relation to liver disease
progression. World J Gastroenterol. 11:6624–6630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
El-Baky NA, Omar SH and Redwan EM: The
anti-cancer activity of human consensus interferon-alpha
synthesized in cell-free system. Protein Expr Purif. 80:61–67.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mohammed Y, El-Baky NA and Redwan EM:
Expression, purification, and characterization of recombinant human
consensus interferon-alpha in Escherichia coli under λp(L)
promoter. Prep Biochem Biotechnol. 42:426–447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Elattar G, Saleh Z, EL-Shebini S, Farrag
A, Zoheiry M, Hassanein A, EL-Ghannam M, Shendy S, EL-Dabaa E and
Zahran N: The use of whey protein concentrate in management of
chronic hepatitis C virus-a pilot study. Arch Med Sci. 5:748–755.
2010. View Article : Google Scholar
|
|
58
|
Abbas S, Imran R, Nazir A, Qamar MF and
Sarfraz L: Effect of camel milk supplementation on blood parameters
and liver function of hepatitis patients. Am J Ethnomedicine.
1:129–146. 2014.
|
|
59
|
Mohamed WA, Schaalan MF and El-Abhar HS:
Camel milk: Potential utility as an adjunctive therapy to
Peg-IFN/RBV in HCV-4 infected patients in Egypt. Nutr Cancer.
67:1305–1313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Park GJH, Lin BP, Ngu MC, Jones DB and
Katelaris PH: Aspartate aminotransferases: Alanine
aminotransferases ratio in chronic hepatitis C infection: Is it a
predictor of cirrhosis? J Gastroenterol Hepatol. 15:386–390. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Rosalki SB and Mcintyre N: Biochemical
investigations in the management of liver diseaseOxford textbook of
clinical hepatology. 2nd edition. New York: Oxford university
press; pp. 503–521. 1999
|
|
62
|
Thapa BR and Walia A: Liver function tests
and their interpretation. Indian J Pediatr. 74:663–671. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Roitt V, Brostoff J and Male D:
Immunology. 6th edition. Goldsby, Kindt, Osborne, Kuby. Freeman
& Company; pp. 8.4–8.5. 2006
|
|
64
|
Saltanat H, Li H, Xu Y, Wang J, Liu F and
Geng XH: The influences of camel milk on the immune response of
chronic hepatitis B patients. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
25:431–433. 2009.(In Chinese). PubMed/NCBI
|
|
65
|
Gigi E, Raptopoulou-Gigi M, Kalogeridis A,
Masiou S, Orphanou E, Vrettou E, Lalla TH, Sinakos E and Tsapas V:
Cytokine mRNA expression in hepatitis C virus infection: TH-1
predominance in patients with chronic hepatitis C and TH1-TH2
cytokine profile in subjects with self-limited disease. J Viral
Hepat. 15:145–154. 2008.PubMed/NCBI
|
|
66
|
Breitling L: Insulin and anti-diabetes
activity of camel milk. J Camel Practice Research. 9:43–45.
2002.
|
|
67
|
Martin F, Volpari C, Steinkuhler C, Dimas
N, Brunetti M, Biasiol G, Altamura S, Cortese R, De Francesco R and
Sollazzo M: Affinity selection of a camelized V(H) domain antibody
inhibitor of hepatitis C virus NS3 protease. Protein Eng.
10:607–614. 1997. View Article : Google Scholar : PubMed/NCBI
|