Introduction
Diabetes mellitus is a chronic metabolic disease
caused by a disorder of insulin secretion that results in the
development of insulin resistance in target tissues. This disease
that causes complications, such as metabolic disorders and multiple
organ damage syndrome, is becoming a major threat to human health,
with ~350 million cases in 2014 alone (1–3).
Hyperglycemia is a major contributor to oxidative stress and
reactive oxygen species (ROS) production (4,5).
Increased levels of ROS resulting from hyperglycemia may disrupt
the insulin signaling cascade, stimulating the development of
insulin resistance (6,7). There has been a growing interest in
dietary supplements and/or herbal medicines, which may improve the
management of blood glucose due to their perceived safety and
efficacy (8,9). A number of plants have been found with
potential anti-diabetic capacity, including Alpinia
oxyphylla (10). This has been
used in China for centuries as both a food and medicinal substance
(11) and is widely used as a tonic,
aphrodisiac and anti-polyuria according to the Chinese
Pharmacopoeia (12). A.
oxyphylla extracts (AOEs) are rich in polyphenols,
polysaccharides, protocatechuic acid and labdane diterpene
glycosides (13). These extracts
possess potent antioxidant activity, which inhibit nitric oxide
production and the biosynthesis of prostaglandin (14). Therefore, they have the potential to
be developed as a therapeutic to treat diabetes mellitus.
Phosphatase and tensin homolog (PTEN), is a
phosphoinositide phosphatase that negatively regulates the insulin
signaling pathway. It has been demonstrated that inhibiting PTEN
expression normalizes blood glucose concentrations and improves
insulin tolerance in db-/db- mice (15). PTEN overexpression in 3T3-L1
adipocytes inhibited serine-threonine kinase protein kinase B (Akt)
activation and glucose uptake. By contrast, reduced PTEN expression
enhanced insulin-stimulated Akt and glycogen synthase kinase-3α
activity (16). These results
suggest that PTEN may be a potential target to treat diabetes.
The primary objective of the present study was to
determine the effects of AOE on blood glucose, insulin and lipid
levels in a type II diabetic animal model, C57BIKsj db-/db-. The
effects of administering 100, 300 and 500 mg/kg of extract of A.
oxyphylla to db-/db- mice were determined by measuring changes
in the body weight and blood glucose concentrations in the mice.
Furthermore, the effects of 500 mg/kg AOE on glucose tolerance,
plasma insulin concentration, plasma lipid profiles, liver lipid
profiles, albumin and osmotic diuresis were determined.
Materials and methods
Animals and treatment
All experiments were performed on 4-week old male
C57BL/Ks DB/DB (normal mice, 12.6±1.8 g) and db-/db- mice (type II
diabetic animal model, 17.8±1.9 g). A total of 60 mice were
purchased from the Model Animal Research Center of Nanjing
University (MARC; Nanjing, China). Mice were left to acclimatize
for 1 week prior to the experimental period. The room that animals
were housed in was maintained under a constant 12-h light-dark
cycle with a temperature of 23±3°C and relative humidity of 70±10%
throughout the experimental period. All mice were housed in group
cages with two animals per cage and were given free access to
standard pellets and water. Animal experiments were carried out in
strict accordance with the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health and all protocols and
studies were approved by the Ethics Committee of Hainan Medical
College for Animal Care and Use (Hainan, China). For the care and
use of animals utilized in this research, animals were monitored
twice a week and none of animals succumbed or exhibited severe
illness that would require early euthanasia during the whole
experiment. Early sacrifice would have been performed if one of the
following criteria were met: Loss of >20% body weight, a wound
that cannot be improved following medication or the development of
neurological symptoms in animals stopping them from being able to
feed themselves. For anesthesia and sacrifice, mice were treated
with 2–3% isoflurane inhalation and 3% CO2 inhalation,
respectively.
Preparation of the plant extract
The ripe fruit of A. oxyphylla were purchased
from a market specializing in herbs (Herb Market, Haikou, China) in
February 2014. The plant was authenticated by Dr Qiang Liu of the
Department of Pharmacognosy, Hainan Medical college (Haikou,
China). A. oxyphylla was extracted with 640 ml water for 16
h at 90°C. This process was conducted twice. The plant extract was
then lyophilized and stored at room temperature until use in
experiments I and II. The dry yield was 8% (w/w).
2,2-diphenyl-1-picrylhydrazyl (DPPH)
assay
The ability of the prepared extracts to scavenge the
DPPH radicals was determined by the method described by Wang et
al (17). Briefly, 10 µl AOE of
the aforementioned different concentrations was added to 290 µl
methanol solution of DPPH (0.1 mM). The solution was mixed well and
then left at room temperature for 30 min in the dark. The
absorbance of the resulting solution was read at 519 nm using an
Eon™ Microplate spectrophotometer (Biotek Instruments, Inc.,
Winooski, VT, USA). Ascorbic acid was employed as a reference and
the radical scavenging activity was calculated as a percentage: of
DPPH discoloration using the following equation.
DPPHradicalscavenging(%)=Acontrol–AsampleAcontrol×100%
where Asample is the absorbance of
the solution when the extract/reference has been added at a
particular level and Acontrol is the absorbance
of the DPPH solution without the extract added. All analyses were
run in triplicate. The IC50 values calculated denote the
concentration of a sample required to decrease the absorbance at
519 nm by 50%.
Experiment I
A total of 30 mice that purchased from MARC were
divided into five groups with six animals in each group. DB/DB
group mice and db-/db-H2O group (db-/db- mice) were
administered placebo (saline) only, while groups db-/db-AOE100,
db-/db-AOE300, and db-/db-AOE500 were administered 100, 300 and 500
mg/kg AOE, respectively, via the intragastric route, once a day for
8 weeks (~0.2 ml in volume). The effect of AOE on db-/db- mice was
determined by changes in body weight assessed by weighing once a
week, while non-fasting blood glucose concentrations measurements
were analyzed from tail blood taken once a week.
Experiment II
Another group of 30 4-week-old male mice also
purchased from MARC, which included 10 DB/DB (12.1±1.6 g) and 20
db-/db- mice (17.3±1.8 g). These mice were divided into 3 groups,
with 10 animals in each group and were housed in the same
conditions as described previously. DB/DB mice group and
db-/db-H2O group were administered placebo (saline)
only, whereas the db-/db-AOE500 group was administered 500 mg/kg
AOE via the intragastric route once a day for 8 weeks (~0.2 ml in
volume). At the end of the 8-week period, following fasting
overnight, each animal was weighed. Individual mice were placed in
metabolic cages to obtain 24 h urine collections. Then, the animals
were sacrificed using 3% CO2 inhalation, and blood and
tissue samples were collected for analysis. Blood samples were
collected from the hepatic portal vein into a tube for EDTA
anticoagulation and centrifuged (1,000 × g for 15 min at
4°C) to separate the plasma. The plasma was then frozen at −70°C
for biochemical analysis. The liver and kidneys were excised,
weighed and homogenized in a 3:1 v/w of 0.25 M sucrose, 10 mM
HEPES, 1 mM EDTA (pH 7.5) buffer. Samples were homogenized for 30
sec at 6.45 m/s in an Omni Bead Ruptor (OMNI International IM,
Kennesaw, GA, USA). Protein concentrations in each sample were
determined using a Bradford protein assay kit (Tiangen Biotech Co.,
Ltd., Beijing, China).
Glucose tolerance test
Following 6 weeks AOE administration, the blood
glucose concentration of mice in experiment II was measured by the
oral glucose tolerance test (OGTT). Blood samples were collected
via the tail vein after an overnight fast and measured for fasting
blood glucose concentration. The animals were then administered
glucose (1.0 g/kg) solution orally. After 2 h, blood samples were
again collected via the tail vein and measured to determine 2 h
postprandial blood glucose concentration.
Measurement of plasma concentration
levels of glucose, triglycerides and total cholesterol
Commercial kits for glucose (F006), triglycerides
(F001-1) and cholesterol (F002-1) were all purchased from the
Nanjing Jiancheng Bioengineering Institute (Nanjing, China) and
used to measure all parameters, according to the manufacturer's
instructions. Glucose concentration was assayed by the glucose
oxidase method (18) and total
cholesterol and triglyceride levels in plasma were tested using the
enzymatic colorimetric method (19,20).
Measurement of hepatic concentrations
of reduced glutathione (GSH), malondialdehyde (MDA), peroxidase
(POD) and superoxide dismutase (SOD)
These parameters were measured using Commercial kits
for GSH (A006-2), MDA (A003-1), POD (A084-3) and SOD (A001-1) were
all purchased from the Nanjing Jiancheng Bioengineering Institute
(Nanjing, China) and used to measure all parameters, according to
the manufacturer's instructions. GSH concentration was assayed
using a chromogenic assay. Briefly, 0.5 ml cold EDTA tissue
homogenate (0.02 mol/l) was added to 0.2 mol/l Tris buffer (pH 8.2)
and 0.1 ml 5,5′-dithiobis-(2-nitrobenzoic acid). Samples were
centrifuged at 1,350 × g for 10 min at room temperature. The
absorbance of the clear supernatant was measured at 412 nm using a
Eon™ Microplate spectrophotometer (Biotek Instruments, Inc.). MDA
concentration was assayed using a chromogenic assay. This assay
measures free and protein-bound MDA without undue interference from
the other lipid peroxidation products (21). The standard curves for the 0–20
µmol/l range were prepared for each assay using the chromogen
supplied in the kits. POD was measured by monitoring oxidation of
16 mM guaiacol in 50 mM potassium phosphate buffer (pH 6.5),
following addition of 10 µl 10% H2O2 in a 3
ml volume. POD activity was measured as the absorbance increase at
470 nm. SOD activity was measured using the Beauchamp and Fridovich
method (22). The total reaction
mixture consisting of phosphate buffer (0.5 M, pH 7.4),
post-mitochondrial supernatant, xanthine (1 mM) and NBT (57 µΜ) was
incubated for 15 min at room temperature and the reaction was
initiated by addition of xanthine oxidase (50 mU). The reaction
rate was measured by recording the change in the absorbance at 550
nm.
Measurement of urine concentration of
creatinine and BUN
These parameters were measured using Commercial kits
for creatinine (C011-2) and BUN (C013-2) were purchased from the
Nanjing Jiancheng Bioengineering Institute (Nanjing, China), and
used to measure concentrations of creatinine and BUN, according to
the manufacturer's instructions. The creatinine concentration was
determined by the sarcosine oxidase method (23) and BUN concentration was determined
using the Urease method (24).
Urine albumin assay
Urine albumin was measured using an Albumin Mouse
ELISA kit (ab108792), which was purchased from Abcam (Cambridge,
UK). Absorbance was read using an automated microplate ELISA reader
(Biotek Instruments, Inc.) and concentrations were calculated by
the standard curve run on each assay plate. All samples were
measured in duplicate.
Western blot analysis
Western blot analysis was performed on tissue
extracts from the liver and kidney. Antibodies against PTEN
(ab32199) and β-actin (ab129348) were purchased from Abcam.
Homogenate (30 µg) was separated by 10% SDS-PAGE and transferred to
polyvinylidene difluoride membranes. Membranes were blocked with
Thermo Scientific SuperBlock (TBS) Blocking Buffer (cat. no. 37535;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) overnight at room
temperature. Membranes were then incubated with either anti-PTEN
(1:1,000; ab32199, Abcam) or anti-β-actin (1:5,000; ab129348,
Abcam) antibodies for 2 h. Membranes were subsequently incubated
with horseradish peroxidase-labeled goat anti-rabbit immunoglobulin
G (1;4,000; ab150088, Abcam) for 1.5 h. All membranes were
visualized using the Amersham ECL Prime Western Blotting Detection
Reagent enhanced chemiluminscence (RPN2232, GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA) and exposure to ECL Hyperfilm
(GE Healthcare Bio-Sciences). All western blot analyses were
performed at least three times.
Statistical analysis
Results are presented as the mean ± standard
deviation. Data were analyzed by the Statistical Product and
Service Solutions (SPSS) program ver. 16 (SPSS, Inc., Chicago, IL,
USA). Comparisons between two groups were analyzed by Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Free radical scavenging activity of
AOE
The DPPH radical is widely used for the assessment
of radical scavenging (25). The
soluble free radical DPPH is known to be a good hydrogen abstractor
that yields DPPH-H as a by-product (26). The antioxidant activities of AOE and
vitamin C (a positive control) (15)
were measured based on the scavenging activities for a stable DPPH
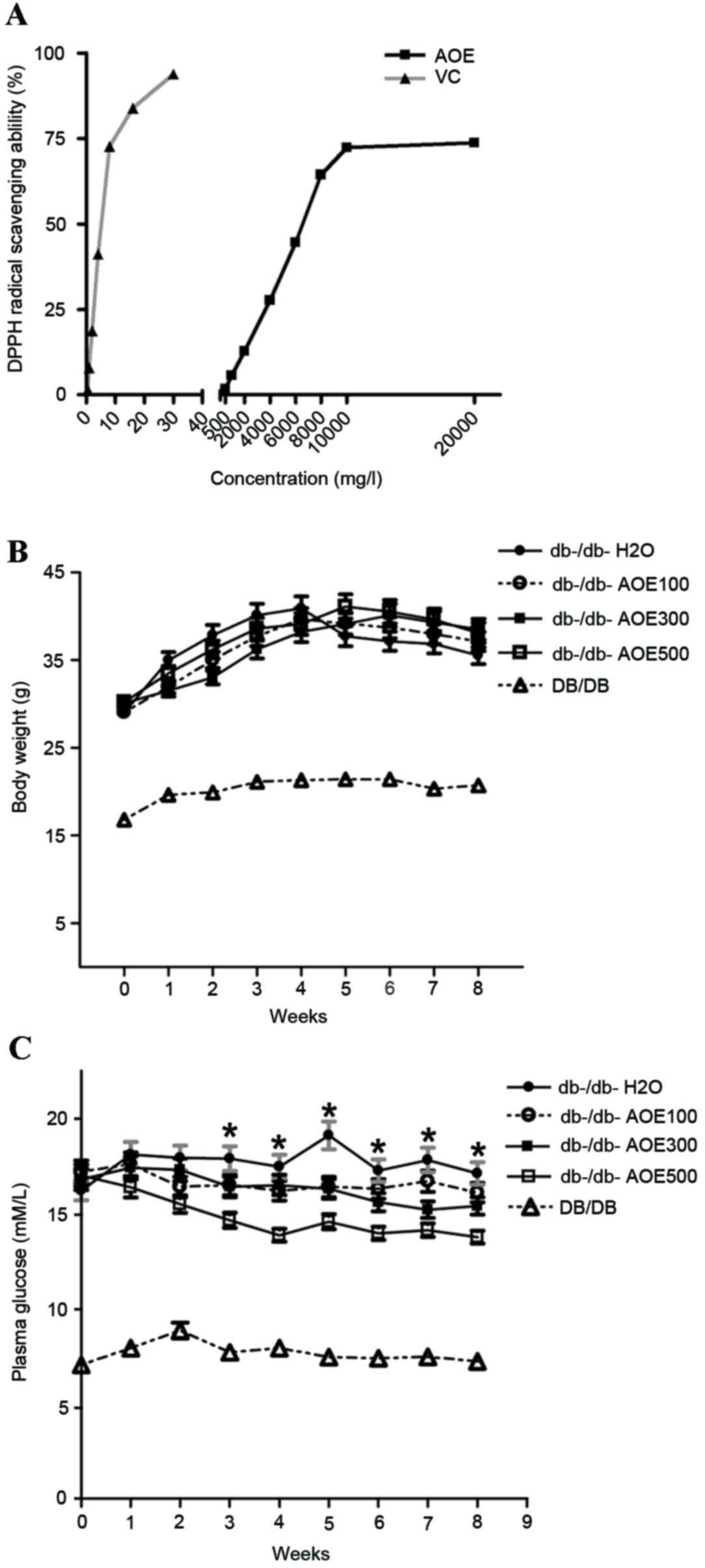
radical as presented in Fig. 1A. On
increasing the doses of AOE from 500 mg/l to 20 g/l and that of
vitamin C from 0.5 to 30 mg/l, the values of the DPPH scavenging
activity were found to be 1.72% (500 mg/l), 5.63% (1,000 mg/l),
12.74% (2,000 mg/l), 27.63% (4,000 mg/l), 44.61% (6,000 mg/l),
64.34% (8,000 mg/l), 72.35% (10,000 mg/l) and 73.75% (20,000 mg/l)
for AOE and 1.37% (0.5 mg/l), 8.23% (1 mg/l), 17.94% (2 mg/l),
40.62% (4 mg/l), 69.53% (8 mg/l), 80.32% (15 mg/l) and 90.71% (30
mg/l) for vitamin C. The half maximal effective concentration
(EC50) values of AOE for the scavenging of DPPH radicals
were 6,543.2 mg/l (AOE) and 5.2 mg/ml (vitamin C). These results
suggest that 10 g/l AOE exhibits the optimum antioxidant capacity
to scavenge DPPH free radicals.
Effects of AOE on body weight and
blood glucose
Changes in body weight in DB/DB and db-/db- mice,
and following AOE administration over 8 weeks, did not differ
significantly among any of the groups (Fig. 1B). Plasma glucose levels decreased
significantly [12% (P<0.05) in AOE100, 18% (P<0.05) in AOE300
and 28% (P<0.05) in AOE500] compared with the
db-/db-H2O group in a dose-dependent manner (Fig. 1C). The results demonstrated that the
highest dose of AOE, 500 mg/kg was most effective in decreasing
blood glucose levels.
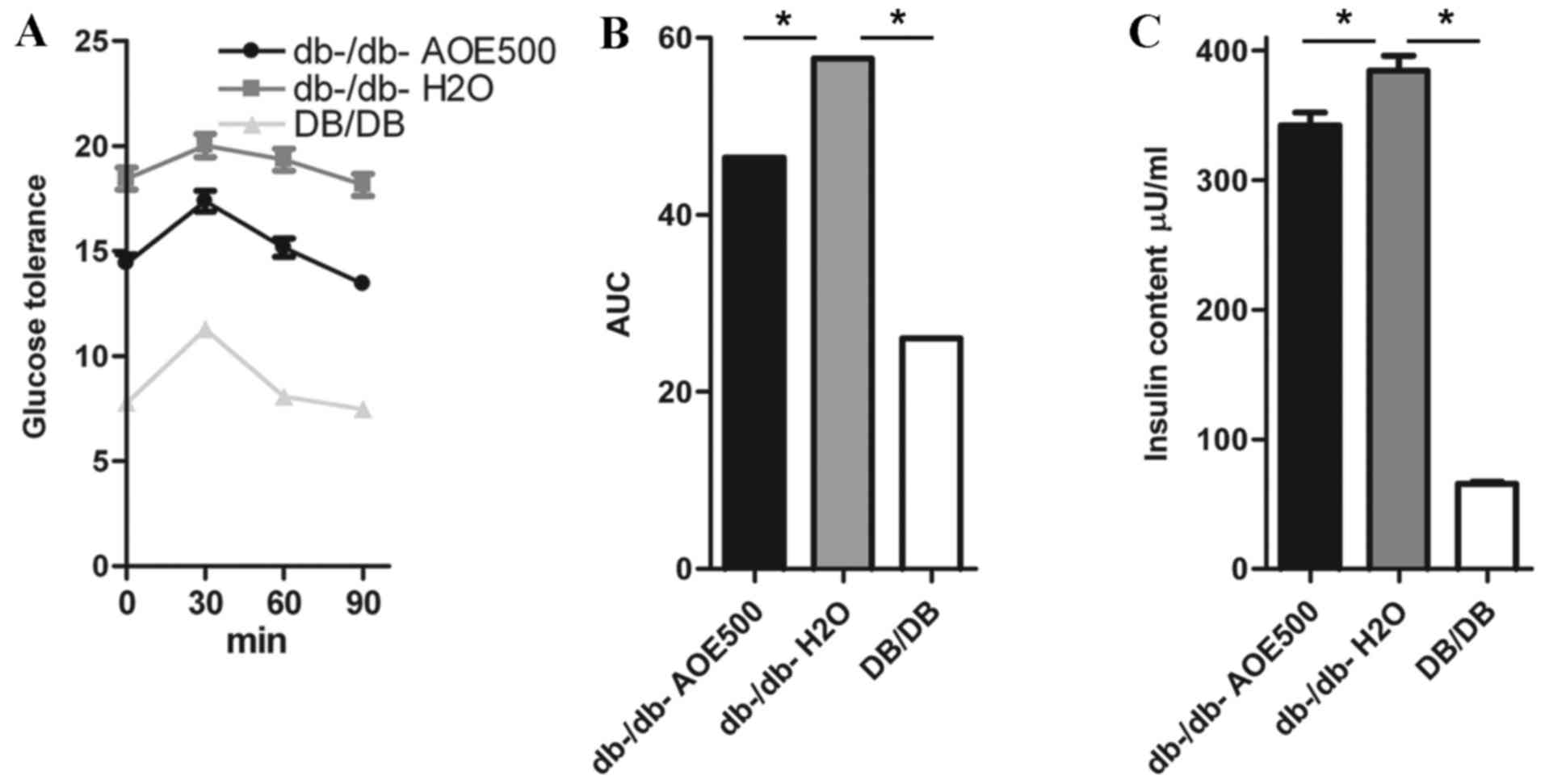
Effect of AOE on OGTT
OGTTs were performed to determine the effect of a
single oral dose of AOE on glucose tolerance in db-/db- mice
(Fig. 2A and B). Glucose challenge
dramatically increased the blood glucose concentration in
db-/db-H2O group mice, whereas AOE500 mice exhibited
significantly suppressed blood glucose concentration 30, 60 and 90
min following the glucose load (Fig.
2A). When the area under the curve was compared between the
groups, that of the DB/DB mice was only 39% of
db-/db-H2O group (P<0.05), whereas the AOE500 group
showed 19.4% reduction in blood glucose levels compared with
db-/db-H2O group (P<0.05; Fig. 2B).
Effects of AOE on the plasma insulin
concentration
The effect of AOE on plasma lipid concentration was
studied following AOE administration for 8 weeks in db-/db- mice to
reveal the mechanism of the A. oxyphylla effect. The plasma
insulin concentration in the db-/db-H2O group was found
to be significantly higher than in DB/DB mice and db-/db-AOE500
group (both P<0.05; Fig. 2C).
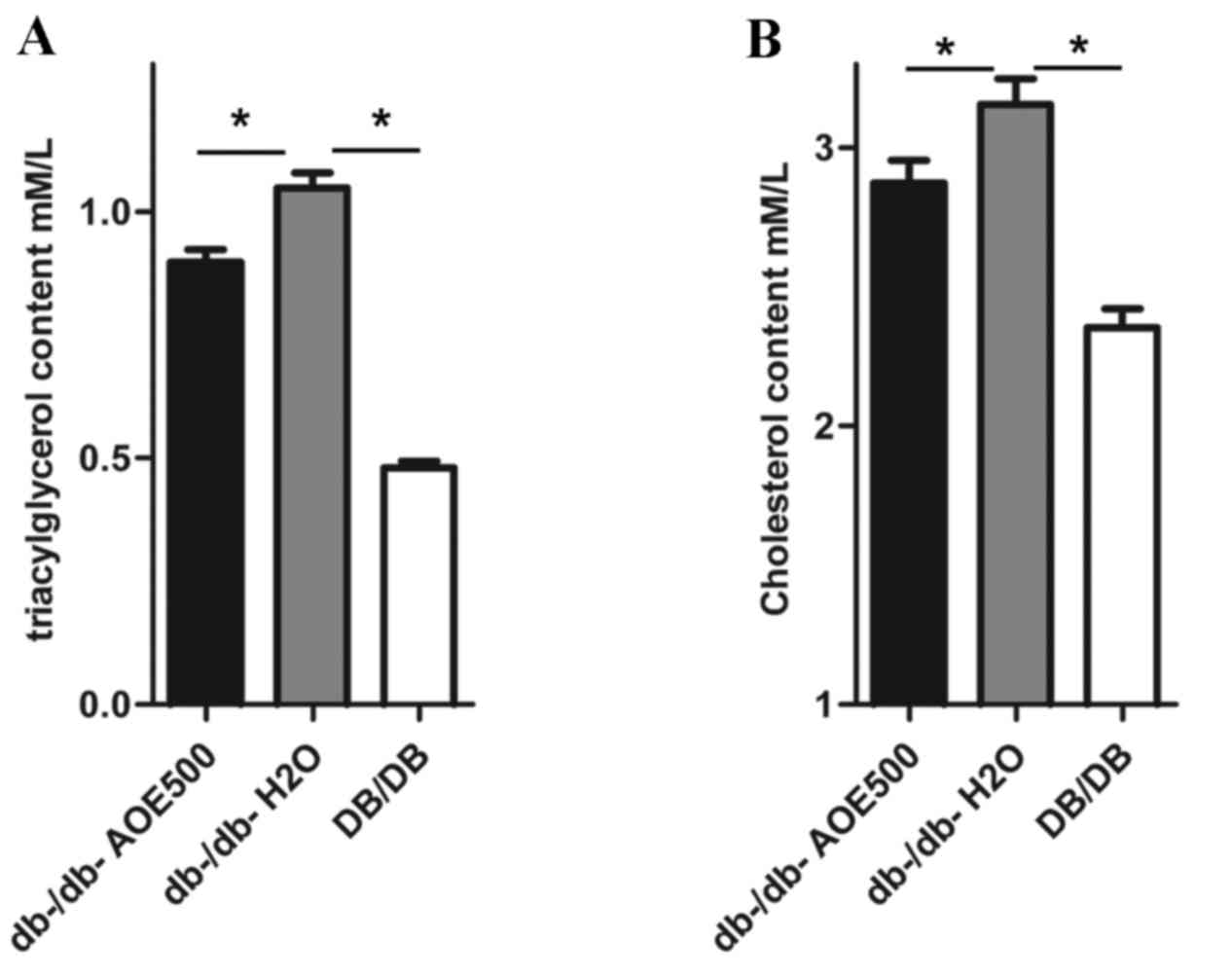
Effects of AOE on dyslipidemia
The effect of AOE500 on plasma lipid concentration
revealed significant differences in most of the lipid profiles
between the A. oxyphylla-treated and db-/db-H2O
group mice (Fig. 3A and B). The
plasma concentration of triglyceride and cholesterol was
significantly increased in db-/db-H2O mice compared with
DB/DB mice. However, the plasma concentrations of triglyceride and
cholesterol in the AOE-treated db-/db- mice were 15.0% (P<0.05)
and 10% (P<0.05) lower, respectively, when compared with levels
in the db-/db-H2O mice.
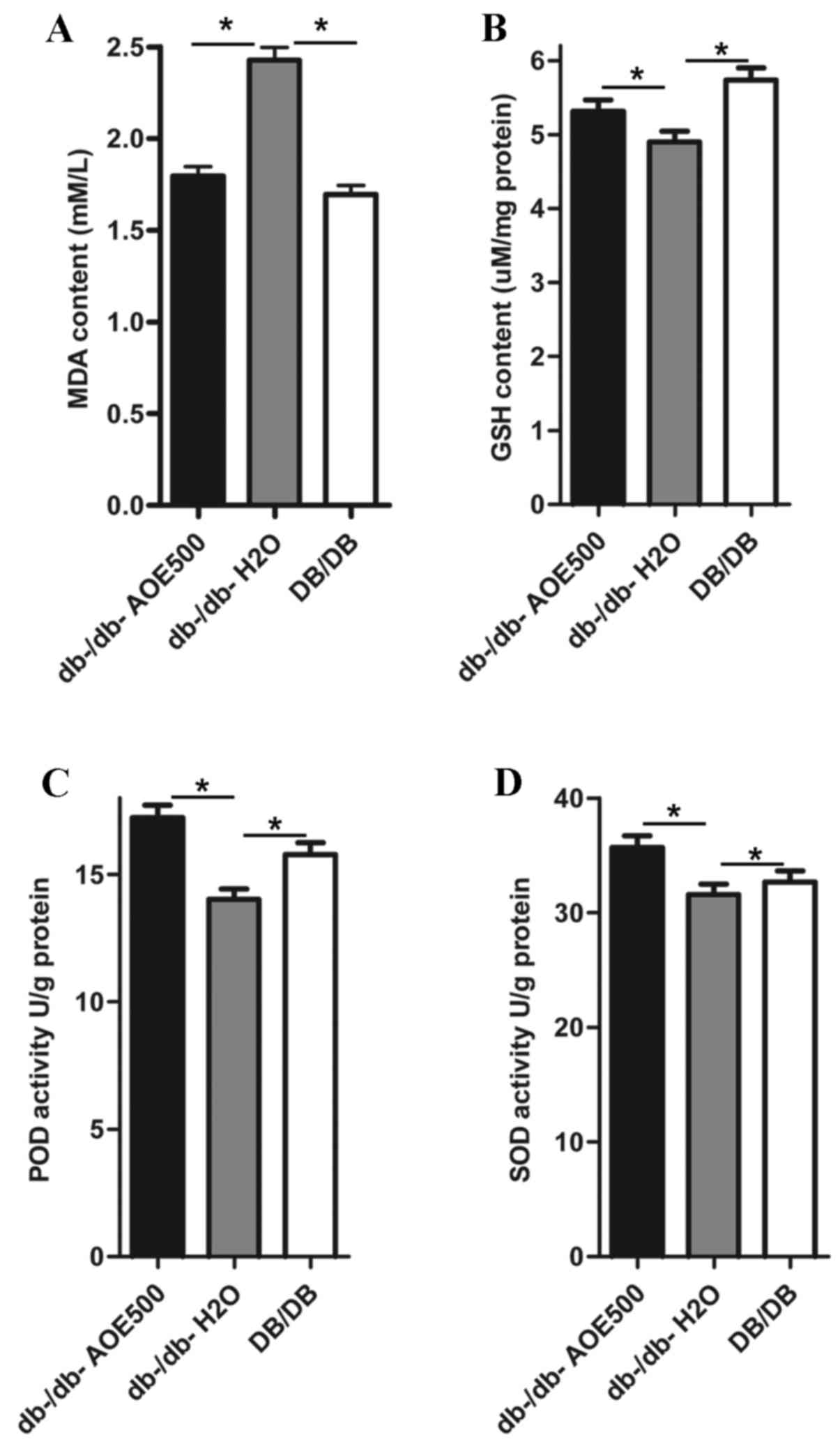
Effect of AOE on lipid peroxide
concentrations and antioxidant enzyme activity
The effects of AOE500 on concentrations of lipid
peroxides and activity of antioxidant enzymes in the liver are
shown in Fig. 4. The concentration
of hepatic thiobarbituric acid reactive substance (TBARS) in the
db-/db-H2O group was significantly increased compared
with that of DB/DB mice (P<0.05). There was a significant
decrease in the MDA concentration of AOE500 treated db-/db- mice
(P<0.05; Fig. 4A). As presented
in Fig. 4B, the GSH content was
significantly decreased in db-/db-H2O group mice
(P<0.05) and partially recovered following AOE500 treatment
(P<0.05). Activities of superoxide dismutase (SOD) and
peroxidase (POD) in the liver of the db-/db-H2O group
were inhibited compared with those of DB/DB group (both P<0.05).
Meanwhile, the activities of SOD and POD in the liver of the AOE500
group were partly restored compared with db-/db-H2O
group (both P<0.05; Fig. 4C and
D).
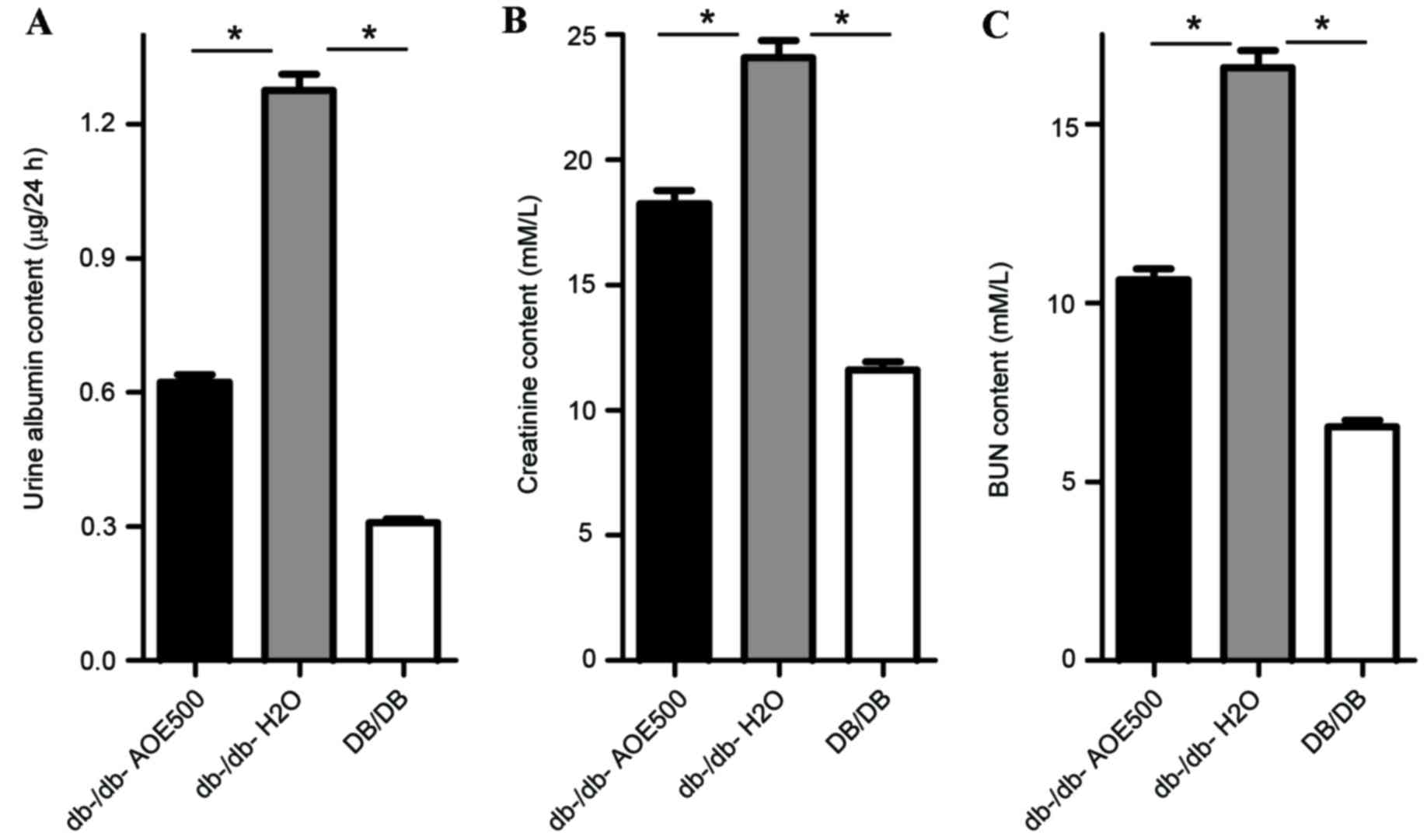
Effects of AOE on renal function
To assess the effect of AOE on renal function, urine
albumin, creatinine and BUN were measured. The results indicate
that urine albumin (P<0.05), creatinine (P<0.05) and BUN
(P<0.05) were all significantly increased in
db-/db-H2O group mice compared with DB/DB mice (Fig. 5). Urine albumin excretion was
0.62±0.17 mg/24 h in AOE500 mice, significantly lower than that of
the db-/db-H2O group mice (1.27±0.31 mg/24 h, P<0.05;
Fig. 5A), although still higher than
that of DB/DB group (0.32±0.04 mg/24 h, P<0.05). The
concentrations of creatinine and BUN were also significantly
reduced following AOE500 treatment (P<0.05), while the
BUN-to-Creatinine ratio was decreased. These results indicate that
AOE500 treatment improved renal function (Fig. 5B and C).
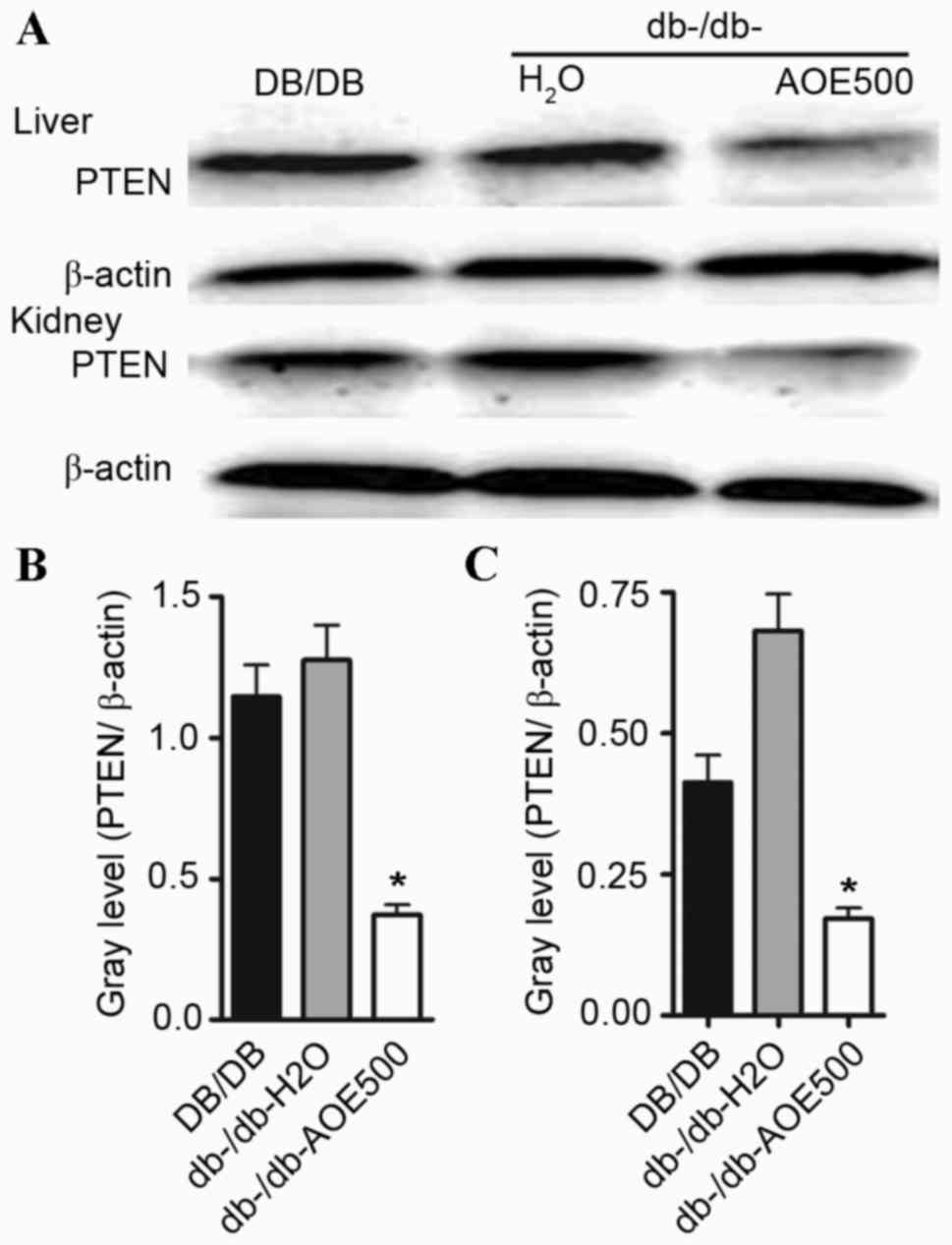
Effects of AOE on the PTEN
expression
In order to detect whether AOE500 impairs diabetic
nephropathy development via downregulation of PTEN, PTEN protein
expression was examined by western blot analysis. The present study
showed that PTEN protein expression was enhanced both in the liver
and renal tissue of db-/db-H2O group mice. However, PTEN
protein expression was significantly decreased (P<0.05)
following AOE500 treatment in the db-/db- mice (Fig. 6).
Discussion
A. oxyphylla is rich in eudesmane
sesquiterpenes, diterpenes, flavonoids and diarylheptanoids, the
components found to possess potent antioxidant properties (27). It has been reported that the extract
of A. oxyphylla fruit exhibits concentration-dependent
antioxidant capacity (17). AOE
serves a neuroprotective role by attenuating oxidative stress,
including increasing the activity of glutathione peroxidase,
decreasing levels of MDA and decreasing the neuronal damage and
apoptosis that occur in the frontal cortex and hippocampus in mice
(28,29). The active phenolic components
yakuchinone A and yakuchinone B that exist in A. oxyphylla
exert antiproliferative activity on mouse skin tumor and HL-60
cells, and have anti-inflammatory and antioxidant capacity in
vitro. (30) In addition,
yakuchinone A exhibit anti-adipocyte differentiation capacity
(31). The diarylheptanoids isolated
from the fruits of A. oxyphylla have potent antioxidant
activities in the DPPH assay (32)
and eudesmane sesquiterpenes may inhibit nitric oxide production in
lipopolysaccharide-induced and interferon-gamma-induced murine
macrophages (14,33). Furthermore, protocatechuic acid from
A. oxyphylla may protect against hydrogen peroxide-induced
oxidative pheochromocytoma cell death (34). In the present study, the ability of
the prepared extract of A. oxyphylla to scavenge DPPH
radicals was determined. The tested AOE showed a promising effect
on DPPH scavenging in a concentration-dependent manner. Compared
with the extracts, ascorbic acid showed higher radical scavenging
ability with 37.3 mg/ml IC50. Although ethanol extracts
are reported to have the highest DPPH radical scavenging effect
(35), the most common edible form
of A. oxyphylla is the boiled form. Thus, the present study
tested the anti-diabetic effect of the liquid extract of A.
oxyphylla.
Previous studies have shown that hyperglycemia
causes oxidative stress, and ROS overproduction serves a role in
impairing glucose-stimulated insulin secretion and increasing
β-cell apoptosis (36). Under normal
circumstances, ROS maintains an optimal oxidative balance for
appropriate biological cell function by antioxidants, including
GSH, vitamin C and vitamin E, as well as antioxidant enzymes, such
as SOD, POD and catalase. However, in type II diabetes mellitus
(T2DM), there are insufficient endogenous antioxidant defenses to
balance the increased ROS production. In the present study, db-/db-
mice showed decreased GSH concentration, inhibited SOD and POD
activities and enhanced MDA levels when compared with the DB/DB
mice. In accordance with this finding, Ihara et al (36) reported that the concentration of
protein carbonyls and lipid hydroperoxides increases in the kidneys
of db-/db- mice, and that oxidative stress serves an important role
in the progression of early diabetic nephropathy. The present study
found that AOE-treated db-/db- mice treated with AOE exhibited
partially restored antioxidative capacity. Following AOE treatment,
the primary non-enzyme antioxidant GSH concentration was
significantly higher and antioxidase SOD and POD activity were also
increased, whereas lipid peroxidation MDA concentration was
decreased in db-/db- mice. AOE anti-oxidative capacity may underlie
the beneficial effects of AOE in diabetes.
Previous studies have demonstrated that insulin
resistance, the inability of cells to efficiently respond to
stimulation by insulin, precedes the onset of T2DM by many years
(37,38). In the current study, db-/db- mice
exhibited higher insulin concentrations and reduced OGTT compared
with DB/DB mice. These results are in accordance with those from
previous studies, highlighting that the decreased insulin
sensitivity in db-/db- mice results in poor glucose regulation.
Following long-term AOE treatment, insulin concentration was
significantly decreased and notably, db-/db- AOE500 mice exhibited
better OGTT than that of db-/db-H2O mice. These results
suggest that AOE may mediate db-/db- mice insulin sensitivity to
ameliorate the symptoms of diabetes.
At a later stage of T2DM, certain patients develop a
progressive increase in the urinary albumin excretion rate,
creatinine and BUN, which has been identified as diabetic
nephropathy (39,40). The development of renal failure in
diabetic nephropathy is due to oxidative stress or inefficient
antioxidant systems (41). Oxidative
stress may disrupt renal sodium regulation and lead to hypertension
(42). The present study
demonstrated that AOE decreased urine albumin excretion and reduced
the increase of plasma creatinine and BUN that occurred in db-/db-
diabetic mice, indicating that AOE may protect the kidney glomeruli
against diabetic nephropathy.
PTEN overexpression may act as an originator or
promoter of diabetic nephropathy. Oxidative stress is one of the
activators that regulates the increase of nuclear PTEN expression
and additionally inhibits PTEN nuclear export (43). Long-term oxidative stress may induce
diabetes by upregulating PTEN expression. Furthermore, the partial
knockdown of PTEN ameliorates ROS-induced insulin resistance
(44). The suppression of PTEN
expression produces a marked improvement in blood glucose
concentration and insulin sensitivity in diabetic mice (15). Inhibitors of PTEN may therefore serve
as target proteins in future drug screens. Interestingly, AOE
significantly impaired the PTEN protein level in parallel to the
reduced glucose level, and attenuated oxidative stress. Further
studies should be conducted to characterize the anti-diabetic
component of AOE.
In conclusion, the present study demonstrated that
AOE exhibited significant amelioration in hyperglycemia and
hyperlipidemia by reducing blood glucose concentration and
oxidative stress, increasing plasma insulin levels, improving renal
function and impairing PTEN expression in the type II diabetic
C57BL/KsJ db-/db- mice. Therefore, A. oxyphylla may be
developed as a novel medicine or functional dietary food supplement
to act against diabetes.
Acknowledgements
The authors wish to thank the Model Animal Research
Center of Nanjing University for supplying C3H and G6PDx mice. The
present study was funded by the National Natural Science Foundation
of China (grant nos. 81473618 and 81360586), the Scientific
Research Fund of Hainan Education Department (grant no.
HNKY2014-51) and Science Foundation for Fostering Talents of Hainan
Medical College (grant no. HY2013-06).
Glossary
Abbreviations
Abbreviations:
|
AOE
|
Alpinia oxyphylla extract
|
|
ROS
|
reactive oxygen species
|
|
PTEN
|
phosphatase and tensin homolog
|
|
OGTT
|
oral glucose tolerance test
|
|
TBARS
|
thiobarbituric acid reactive
substance
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
|
POD
|
peroxidase
|
|
BUN
|
blood urea nitrogen
|
References
|
1
|
Dunkley AJ, Bodicoat DH, Greaves CJ,
Russell C, Yates T, Davies MJ and Khunti K: Diabetes prevention in
the real world: Effectiveness of pragmatic lifestyle interventions
for the prevention of type 2 diabetes and of the impact of
adherence to guideline recommendations: A systematic review and
Meta-analysis. Diabetes Care. 37:922–933. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liakos A, Karagiannis T, Athanasiadou E,
Sarigianni M, Mainou M, Papatheodorou K, Bekiari E and Tsapas A:
Efficacy and safety of empagliflozin for type 2 diabetes: A
systematic review and meta-analysis. Diabetes Obes Metab.
16:984–993. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
da Rocha Fernandes J, Ogurtsova K,
Linnenkamp U, Guariguata L, Seuring T, Zhang P, Cavan D and
Makaroff LE: IDF Diabetes Atlas estimates of 2014 global health
expenditures on diabetes. Diabetes Res Clin Pract. 117:48–54. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nowotny K, Jung T, Hohn A, Weber D and
Grune T: Advanced glycation end products and oxidative stress in
type 2 diabetes mellitus. Biomolecules. 5:194–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abdali D, Samson SE and Grover AK: How
effective are antioxidant supplements in obesity and diabetes? Med
Princ Pract. 24:201–215. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gurzov EN, Tran M, Fernandez-Rojo MA,
Merry TL, Zhang X, Xu Y, Fukushima A, Waters MJ, Watt MJ,
Andrikopoulos S, et al: Hepatic oxidative stress promotes
insulin-STAT-5 signaling and obesity by inactivating protein
tyrosine phosphatase N2. Cell Metab. 20:85–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Zhang W, Chen H, Liao N, Wang Z,
Zhang X and Hai C: High selenium impairs hepatic insulin
sensitivity through opposite regulation of ROS. Toxicol Lett.
224:16–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Evert AB, Boucher JL, Cypress M, Dunbar
SA, Franz MJ, Mayer-Davis EJ, Neumiller JJ, Nwankwo R, Verdi CL,
Urbanski P and Yancy WS Jr: Nutrition therapy recommendations for
the management of adults with diabetes. Diabetes Care. 37 Suppl
1:S120–S143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Afolayan AJ and Wintola OA: Dietary
supplements in the management of hypertension and diabetes-a
review. Afr J Tradit Complement Altern Med. 11:248–258. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patel D, Kumar R, Laloo D and Hemalatha S:
Natural medicines from plant source used for therapy of diabetes
mellitus: An overview of its pharmacological aspects. Asian Pacific
Journal of Tropical Disease. 2:239–250. 2012. View Article : Google Scholar
|
|
11
|
Commission CP: Pharmacopoeia of the
People's Republic of China. 1. China Medical Science and Technology
Press; Beijing: 2010
|
|
12
|
Liu A, Zhao X, Li H, Liu Z, Liu B, Mao X,
Guo L, Bi K and Jia Y: 5-Hydroxymethylfurfural, an antioxidant
agent from Alpinia oxyphylla Miq. Improves cognitive impairment in
Aβ1-42 mouse model of Alzheimer's disease. Int Immunopharmacol.
23:719–725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Wang D and Chen W: Application on
Alpinia oxyphylla extraction as food preservative on mango. Food
Sci Technol. 4:309–314. 2015.(In Chinese).
|
|
14
|
Qing ZJ, Yong W, Hui LY, Yong LW, Long LH,
Ao DJ and Xia PL: Two new natural products from the fruits of
Alpinia oxyphylla with inhibitory effects on nitric oxide
production in lipopolysaccharide-activated RAW264.7 macrophage
cells. Arch Pharm Res. 35:2143–2146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Butler M, McKay RA, Popoff IJ, Gaarde WA,
Witchell D, Murray SF, Dean NM, Bhanot S and Monia BP: Specific
inhibition of PTEN expression reverses hyperglycemia in diabetic
mice. Diabetes. 51:1028–1034. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakashima N, Sharma PM, Imamura T,
Bookstein R and Olefsky JM: The tumor suppressor PTEN negatively
regulates insulin signaling in 3T3-L1 adipocytes. J Biol Chem.
275:12889–12895. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang CZ, Yuan HH, Bao XL and Lan MB: In
vitro antioxidant and cytotoxic properties of ethanol extract of
Alpinia oxyphylla fruits. Pharm Biol. 51:1419–1425. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lott JA and Turner K: Evaluation of
Trinder's glucose oxidase method for measuring glucose in serum and
urine. Clin Chem. 21:1754–1760. 1975.PubMed/NCBI
|
|
19
|
Fossati P and Prencipe L: Serum
triglycerides determined colorimetrically with an enzyme that
produces hydrogen peroxide. Clin Chem. 28:2077–2080.
1982.PubMed/NCBI
|
|
20
|
Richmond W: Preparation and properties of
a cholesterol oxidase from Nocardia sp. And its application to the
enzymatic assay of total cholesterol in serum. Clin Chem.
19:1350–1356. 1973.PubMed/NCBI
|
|
21
|
Janero DR: Malondialdehyde and
thiobarbituric acid-reactivity as diagnostic indices of lipid
peroxidation and peroxidative tissue injury. Free Radic Biol Med.
9:515–540. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beauchamp C and Fridovich I: Superoxide
dismutase: Improved assays and an assay applicable to acrylamide
gels. Anal Biochem. 44:276–287. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fossati P, Prencipe L and Berti G: Enzymic
creatinine assay: A new colorimetric method based on hydrogen
peroxide measurement. Clin Chem. 29:1494–1496. 1983.PubMed/NCBI
|
|
24
|
Foster LB and Hochholzer JM: A
single-reagent manual method for directly determining urea nitrogen
in serum. Clin Chem. 17:921–925. 1971.PubMed/NCBI
|
|
25
|
Dinis TC, Maderia VM and Almeida LM:
Action of phenolic derivatives (acetaminophen, salicylate, and
5-aminosalicylate) as inhibitors of membrane lipid peroxidation and
as peroxyl radical scavengers. Arch Biochem Biophys. 315:161–169.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du Toit R, Volsteedt Y and Apostolides Z:
Comparison of the antioxidant content of fruits, vegetables and
teas measured as vitamin C equivalents. Toxicology. 166:63–69.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miao Q, Kong W, Zhao X, Yang S and Yang M:
GC-FID coupled with chemometrics for quantitative and chemical
fingerprinting analysis of Alpinia oxyphylla oil. J Pharm Biomed
Anal. 102:436–442. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi SH, Zhao X, Liu B, Li H, Liu AJ, Wu B,
Bi KS and Jia Y: The effects of sesquiterpenes-rich extract of
Alpinia oxyphylla Miq. On amyloid-β-induced cognitive impairment
and neuronal abnormalities in the cortex and hippocampus of mice.
Oxid Med Cell Longev. 2014:4518022014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi SH, Zhao X, Liu AJ, Liu B, Li H, Wu B,
Bi KS and Jia Y: Protective effect of n-butanol extract from
Alpinia oxyphylla on learning and memory impairments. Physiol
Behav. 139:13–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Surh Y: Molecular mechanisms of
chemopreventive effects of selected dietary and medicinal phenolic
substances. Mutat Res. 428:305–327. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin RJ, Yen CM, Chou TH, Chiang FY, Wang
GH, Tseng YP, Wang L, Huang TW, Wang HC, Chan LP, et al:
Antioxidant, anti-adipocyte differentiation, antitumor activity and
anthelmintic activities against Anisakis simplex and Hymenolepis
nana of yakuchinone A from Alpinia oxyphylla. BMC Complement Altern
Med. 13:2372013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bian QY, Wang SY, Xu LJ, Chan CO, Mok DK
and Chen SB: Two new antioxidant diarylheptanoids from the fruits
of Alpinia oxyphylla. J Asian Nat Prod Res. 15:1094–1099. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu J, Ji C, Zhang Y, Su J, Li Y and Tan N:
Inhibitory activity of eudesmane sesquiterpenes from Alpinia
oxyphylla on production of nitric oxide. Bioorg Med Chem Lett.
22:1660–1663. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shui Guan, Bao YM, Bo Jiang and An LJ:
Protective effect of protocatechuic acid from Alpinia oxyphylla on
hydrogen peroxide-induced oxidative PC12 cell death. Eur J
Pharmacol. 538:73–79. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang CZ, Yuan HH, Bao XL and Lan MB: In
vitro antioxidant and cytotoxic properties of ethanol extract of
Alpinia oxyphylla fruits. Pharm Biol. 51:1419–1425. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ihara Y, Toyokuni S, Uchida K, Odaka H,
Tanaka T, Ikeda H, Hiai H, Seino Y and Yamada Y: Hyperglycemia
causes oxidative stress in pancreatic beta-cells of GK rats, a
model of type 2 diabetes. Diabetes. 48:927–932. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Szendroedi J, Phielix E and Roden M: The
role of mitochondria in insulin resistance and type 2 diabetes
mellitus. Nat Rev Endocrinol. 8:92–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun M, Huang X, Jiang L, Yan Y, Li B,
Zhong W, Chen J, Zhang Y, Wang Z, Li J and Xie M: Characterization
of β-cell function and insulin resistance in overweight Chinese
adolescents with normal glucose tolerance. Exp Ther Med. 6:547–551.
2013.PubMed/NCBI
|
|
39
|
Huang B, Wang Z, Park JH, Ryu OH, Choi MK,
Lee JY, Kang YH and Lim SS: Anti-diabetic effect of purple corn
extract on C57BL/KsJ db-/db- mice. Nutr Res Pract. 9:22–29. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Parving HH, Oxenbøll B, Svendsen PA,
Christiansen JS and Andersen AR: Early detection of patients at
risk of developing diabetic nephropathy. A longitudinal study
ofurinary albumin excretion. Acta Endocrinol (Copenh). 100:550–555.
1982.PubMed/NCBI
|
|
41
|
Elks CM, Mariappan N, Haque M, Guggilam A,
Majid DS and Francis J: Chronic NF-{kappa}B blockade reduces
cytosolic and mitochondrial oxidative stress and attenuates renal
injury and hypertension in SHR. Am J Physiol Renal Physiol.
296:F298–F305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Banday AA and Lokhandwala MF:
Transcription factor Nrf2 protects renal dopamine D1 receptor
function during oxidative stress. Hypertension. 62:512–517. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chang CJ, Mulholland DJ, Valamehr B,
Mosessian S, Sellers WR and Wu H: PTEN nuclear localization is
regulated by oxidative stress and mediates p53-dependent tumor
suppression. Mol Cell Biol. 28:3281–3289. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Birnbaum Y, Nanhwan MK, Ling S, Perez-Polo
JR, Ye Y and Bajaj M: PTEN upregulation may explain the development
of insulin resistance and type 2 diabetes with high dose statins.
Cardiovasc Drugs Ther. 28:447–457. 2014. View Article : Google Scholar : PubMed/NCBI
|