Introduction
Pigs have similar coronary artery anatomical
features to humans, thus pigs are often used in coronary
intervention studies as an ischemia/reperfusion model through the
use of balloon obstruction (1),
coronary microemboli model (2) and
coronary slow flow model (3) through
microsphere injection. Pigs are also used in electrophysiological
and pacemaker studies such as in radiofrequency ablation (4) and cardiac resynchronization therapy
(CRT) research (5). Placing a
coronary sinus (CS) catheter or electrode in the coronary sinus
ostium is an essential technique in these studies. However, placing
CS catheters in swine remains a challenging technique for
researchers. Previous studies have collected metabolic and
hemodynamic measurements data through CS catheters with the
open-chest technique in pigs (6–11), and
have demonstrated that the aforementioned method islinked with
complicated operation procedures and heavy traumatic injury in
swine. A number of novel techniques have been developed to place
the CS catheter in swine. Hilbert et al (12) introduced a method utilizing
fluoroscopy and a high-density electroanatomical mapping catheter
to verify the CS anatomy to facilitate CS catheter placement.
Neizel et al (13)
reconstructed the cardiac venous system in pigs using magnetic
resonance imaging and performed magnetic resonance-guided
placements of CS catheters in swine. Nazarian et al
(14) used a flexible steerable
fiber optic infrared endoscope to visualize the CS ostium and
branches in closed-chest dogs. However, such methods require
high-end equipment, which is not available in all laboratories.
Therefore, in the present study, the efficacy of a simple CS
catheter placement method via the femoral vein in a closed-chest
pig model was assessed.
Materials and methods
Animals
A total of 16 male domestic pigs (3–4 months; 25±2
kg) were used in this study, which were purchased from the
Experimental Animal Center of Tongji Medical College, Huazhong
University of Science and Technology (Wuhan, China). Aspirin (2–3
mg/kg/day; Bayer China Co., Ltd., Shanghai, China) was mixed in the
food three days prior to experimental studies. Animals were
maintained in a conventional animal environment (21±2°C; 55±10%
humidity), with a 12-h light/dark cycle (lights off at 19:30 h and
no twilight period). Animals had free access to water and were fed
three times per day in accordance with the Guide for the Care and
Use of Laboratory Animals published by the US National Institute of
Health (Bethesda, MD, USA) (15).
Four animals were used in the pilot study for initial evaluation of
the surgical technique and 12 animals were used in the main study.
The study protocol was approved by the Tongji Medical College
Council of the Animal Care Committee of Huazhong University of
Science and Technology (Wuhan, China).
Preoperative preparation and
anesthesia
The pigs fasted for 12 h prior to operation but had
ad libitum access to drinking water until 4 h prior to
operation. Pigs were anesthetized by an intramuscular injection of
ketamine (15 mg/kg; Jiangsu Hengrui Medicine Co., Ltd.,
Lianyungang, China) combined with atropine (1 mg; Guangdong South
China Pharmaceutical Co., Ltd., Guangzhou, China), then fixed in a
supine position on the workstation. During the operation, 3–5 ml 3%
pentobarbital sodium solution (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) was injected via ear marginal vein on demand to
maintain the anesthesia state. Electrocardiogram and vital signs
were continuously monitored. Oxygen saturation (SO2) was
measured with a pulse oximeter attached to the ear of pigs.
Evaluation of the surgical
technique
In the pilot study, four pigs were used to evaluate
the feasibility of the surgical technique applied. A catheter was
inserted into the CS through the jugular vein of the pigs; however,
it took a long time to separate the neck vasculature due to the
complexity of the anatomical structures. Therefore, the method of
catheter insertion though the femoral vein was assessed during the
pilot study and main study. Following routine disinfection, the
muscle layer and the femoral sheath, femoral vein, arteria
cruralis and nerve were carefully separated. A 5F vascular
sheath (Cordis Corporation, Milpitas, CA, USA) was placed for
arterial and venous access. Anticoagulation was induced with 200
IU/kg heparin sodium (Jiangsu Wanbang Biopharmaceuticals Co., Ltd.,
Xuzhou, China). The arterial sheath can be used for pressure
monitoring, coronary angiography (16). The 5F pigtail catheter (Cordis
Corporation, Milpitas, CA, USA) was advanced into the right atrium
from the right femoral vein. Right atrium imaging was performed
[right anterior oblique (RAO) 30°, anterior-posterior (AP) angle)]
to observe the CS opening and record right atrium pressure.
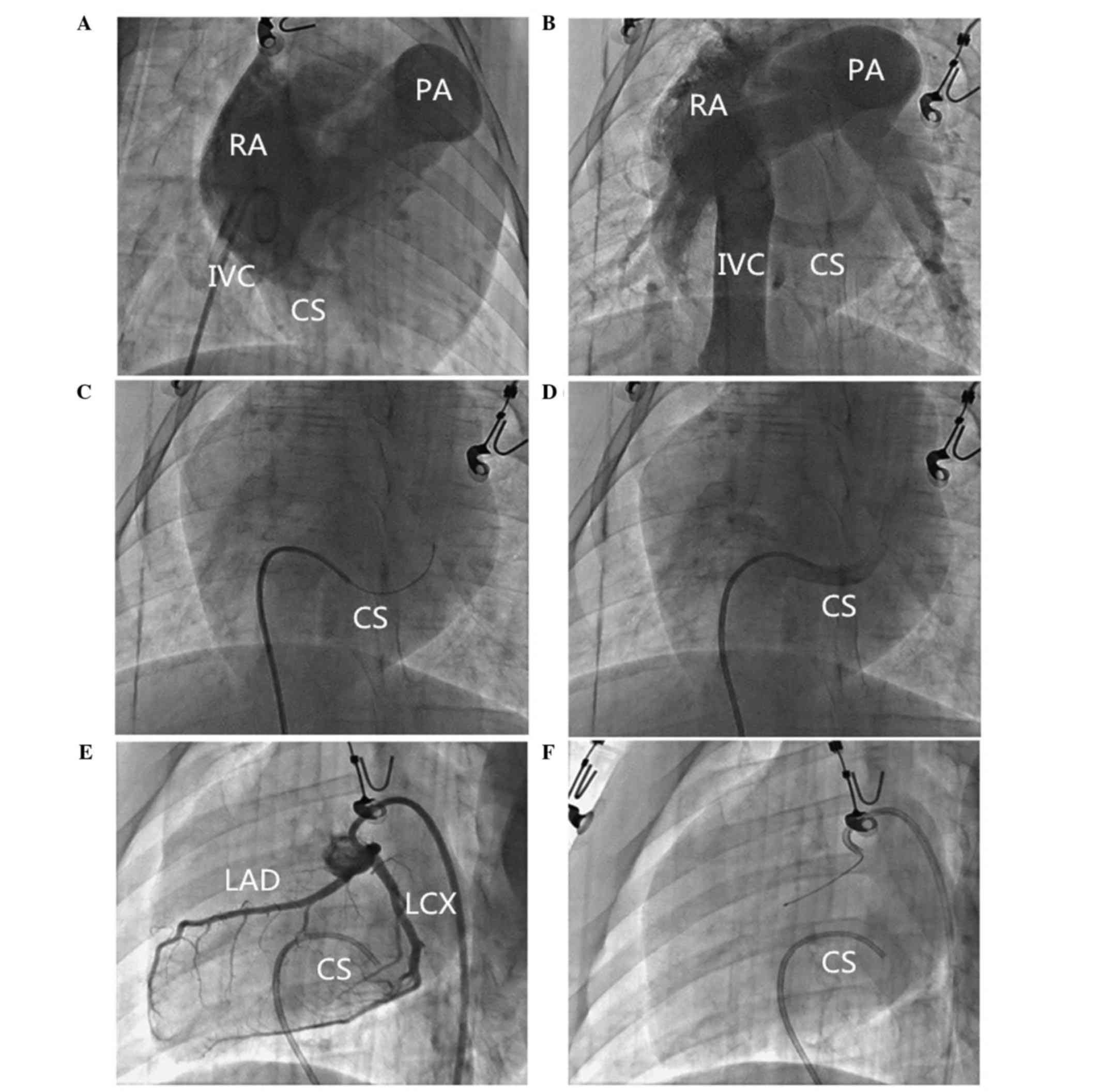
Imaging analysis of pig heart
Under the X-ray, the transparent triangle area in
the pig hearts could not be clearly visualized at RAO 30°, left
anterior oblique (LAO) 45°, AP angle, as it is in humans. In order
to observe the structure of the right atrium and the CS ostium, the
5F pigtail catheter was advanced to the lower part of right atrium
and radiography was performed (AP, RAO 30° angle) with a high
pressure injector (20 ml/sec; 300 kPa; Auto injector 120S; Nemoto
Co., Ltd., Tokyo, Japan). The CS ostium could clearly be detected
at AP or RAO 30°, and the contorts of CS could be clearly presented
at the AP position (Fig. 1A and
B).
Radiation dose assessment
All operations were performed by two experienced
researchers. Radiation exposure time was recorded and the radiation
dose received by the operator was detected using a Wearing X-ray
detector provided by the Wuhan Center for Disease Prevention and
Control.
Results
CS catheter placement method
In the main experiment, a 5F guide wire (RF*GA3513M;
Terumo Corporation, Tokyo, Japan) and Cobara catheter (RF*DB55008M;
Terumo Corporation, Tip curve L: Middle) were advanced into the
right atrium through the femoral vein and the guide wire was
retracted out of the catheter, which then spontaneously bent. CS
ostium was generally located one width of a rib under the middle of
long axis of the heart. Following gentle clockwise rotation of the
catheter to the left, the catheter could be easily and smoothly
placed into the CS ostium. Following successful placement, the
characteristic swing sign could be visualized. Contrast medium was
injected to confirm the successful placement of catheter inside the
CS (Fig. 1C and D). Afterwards, CS
blood sampling was performed (Fig. 1E
and F).
Reliability and safety evaluation
This method was associated with a short procedure
time (the time used on separation of the blood vessels was 15.5±5.8
min and the time of radiation exposure was 112±20 sec) and a
success rate of 100%. Only one pig experienced a hematoma after the
arterial sheath was pulled out. All swine recovered without serious
complications, including perforation of coronary vein and
pericardial tamponade. Additionally, this method had a low per
capita irradiation dose (1.56±0.32 µGy) at each operation.
Discussion
Cardiologists usually pay more attention to the
arterial system of coronary circulation, and less to the coronary
vein system. With the rapid development of interventional
techniques in recent years, there is an increased requirement of CS
placement of CS catheters, for example to determine the myocardial
metabolic product in CS. Moreover, placing electrodes to the CS
during electrophysiological examinations and left ventricular
electrode placement in CRT also requires the placement of a CS
catheter in selected patients. This technique is also necessary
during retrograde stem cell transplantation through the CS route
(17). It has been demonstrated that
simultaneous coronary artery and CS perfusion facilitate myocardial
preservation (18). As pigs are the
ideal experimental animal model when investigating the heart,
particularly in coronary intervention and electrophysiology
pacemaker research field, an effective method for placing a CS
catheter in swine is particularly important. The primary difficulty
is that the catheter may pop out and injure the CS if the catheter
is pushed directly without adjusting the direction. In some pigs,
pushing catheters to distal CS is difficult. The current study
demonstrated that if the guide wire can be fixed, and the direction
of catheter gently adjusted, the majority of catheters can be
advanced to the distal CS without injuring the CS. In addition, the
COBARA catheter used in the present study is a 5F peripheral vessel
operated catheter with soft tip; the probability of perforation is
thus low. Moreover, placing the CS catheter through the femoral
vein may reduce the operation time and avoid pneumothorax and
hemothorax complications. This method may also significantly reduce
the radiation dose the operator is exposed to.
Placing a catheter to the CS through the femoral
vein in miniature pigs may also be useful in electrophysiology
research in swine animal models. If an adjustable curved electrode
is used and the direction of the catheter is gently adjusted, the
CS electrode could successfully be advanced to the distal CS.
Placing the CS catheter through the femoral vein in
miniature pigs has the following advantages: i) Avoiding open chest
and other complex surgical procedures, thus reducing trauma and the
operating time; ii) does not require the support of endotracheal
intubation and breathing machine ventilation when animals are under
anesthetic; and iii) avoiding the jugular vein puncture-related
pneumothorax and hemothorax complications. The groin hematoma
complication observed in one of the pigs in the current study may
be avoided by prolonged local oppression.
In conclusion, the method of lacing a catheter into
the CS of swine assessed in the current study is simple, safe and
reliable; it may be beneficial for scientific researchers using
swine as animal models in related coronary studies.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81270266) and the
Nature Science Foundation of Hubei Province (grant nos. 2014CFB365
and 2015CFA081).
References
|
1
|
Kondo K, Shibata R, Unno K, Shimano M,
Ishii M, Kito T, Shintani S, Walsh K, Ouchi N and Murohara T:
Impact of a single intracoronary administration of adiponectin on
myocardial ischemia/reperfusion injury in a pig model. Circ
Cardiovasc Interv. 3:166–173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma J, Qian J, Ge J, Zeng X, Sun A, Chang
S, Chen Z and Zou Y: Changes in left ventricular ejection fraction
and coronary flow reserve after coronary microembolization. Arch
Med Sci. 8:63–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu L, Bai Y, Gu Y, Yu D, Peng S, Liu X,
Zhang M, Liu T and Hu S: Establishment of an experimental
angiographic slow coronary flow model by microsphere embolism in
swines. Int J Cardiol. 176:1123–1125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bessiere F, N'djin WA, Colas EC, Chavrier
F, Greillier P, Chapelon JY, Chevalier P and Lafon C:
Ultrasound-guided transesophageal high-intensity focused ultrasound
cardiac ablation in a beating heart: A pilot feasibility study in
pigs. Ultrasound Med Biol. 42:1848–1861. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rigol M, Solanes N, Fernandez-Armenta J,
Silva E, Doltra A, Duchateau N, Barcelo A, Gabrielli L, Bijnens B,
Berruezo A, et al: Development of a swine model of left bundle
branch block for experimental studies of cardiac resynchronization
therapy. J CardiovascTransl Res. 6:616–622. 2013.
|
|
6
|
Safranow K, Rzeuski R, Listewnik MJ,
Jakubowska K, Rać ME, Olszewska M and Chlubek D: Myocardial and
coronary sinus purines as indicators of pig heart energy metabolism
during reperfusion after extracorporeal circulation. Acta Physiol
Scand. 185:13–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karapanos NT, Wettstein PJ, Li Z, Huebner
M, Park SJ, Deschamps C and Cassivi SD: Does lung ischemia and
reperfusion have an impact on coronary flow? A quantitative
coronary blood-flow analysis with inflammatory cytokine profile.
Eur J Cardiothorac Surg. 41:154–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pantely GA, Bristow JD, Ladley HD and
Anselone CG: Effect of coronary sinus occlusion on coronary flow,
resistance, and zero flow pressure during maximum vasodilatation in
swine. Cardiovasc Res. 22:79–86. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Andersen FR, Sejersted OM and Ilebekk A: A
model for quantitative sampling of myocardial venous blood in the
pig. Acta Physiol Scand. 119:187–195. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harvey RC and Jones EF: A technique for
bioinstrumentation of the thorax of miniature swine. Lab Anim Sci.
32:94–96. 1982.PubMed/NCBI
|
|
11
|
McKenzie JE, Scandling DM, Ahle NW, Bryant
HJ, Kyle RR and Abbrecht PH: Effects of soman (pinacolyl
methylphosphonofluoridate) on coronary blood flow and cardiac
function in swine. Fundam Appl Toxicol. 29:140–146. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hilbert S, Kosiuk J, John S, Hindricks G
and Bollmann A: A guide to the porcine anatomy for the
interventional electrophysiologist. Fluoroscopy and high density
electroanatomical mapping. J Cardiovasc Transl Res. 8:67–75. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neizel M, Krämer N, Schütte A,
Schnackenburg B, Krüger S, Kelm M, Günther RW, Kühl HP and Krombach
GA: Magnetic resonance imaging of the cardiac venous system and
magnetic resonance-guided intubation of thecoronary sinus in swine:
A feasibility study. Invest Radiol. 45:502–506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nazarian S, Knight BP, Dickfeld TL, Zviman
MM, Jayanti VB, Amundson D, Hanlin J, Castleberry J, Smith MF,
Blankenship L, et al: Direct visualization of coronary sinus ostium
and branches with a flexible steerable fiberoptic infrared
endoscope. Heart Rhythm. 2:844–848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guide for the Care and Use of Laboratory
Animals, NIH Publication. (85-23)1996.
|
|
16
|
Ma J, Qian J, Chang S, Chen Z, Jin H, Zeng
M, Zou Y and Ge J: Left ventricular remodeling with
preservedfunction after coronary microembolization: The effect of
methylprednisolone. Eur J Med Res. 19:72014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Berry MF, Engler AJ, Woo YJ, Pirolli TJ,
Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE and
Sweeney HL: Mesenchymal stem cell injection after myocardial
infarction improves myocardial compliance. Am J Physiol Heart Circ
Physiol. 290:H2196-H22032006. View Article : Google Scholar
|
|
18
|
Tian G, Xiang B, Dai G, Sun J, Lindsay WG
and Deslauriers R: Simultaneous antegrade/retrograde cardioplegia
protects myocardium distal to a coronary occlusion: A study in
isolated pig hearts. Magn Reson Med. 46:773–780. 2001. View Article : Google Scholar : PubMed/NCBI
|