Introduction
Atherosclerosis, which is characterized by the
formation of atherosclerotic plaques in large- and medium-sized
arteries, is the most common cause of mortality from cardiovascular
diseases in Western countries (1).
The pathological process of atherosclerosis is complex, and
numerous components of the metabolic, immune and vascular systems
are involved in the formation of atherosclerotic plaques (2,3).
However, the pathomechanism of atherosclerotic vascular disease has
yet to be fully elucidated.
Vascular endothelial cells (VECs) are found in the
inner layer of the vessel wall. They serve as a barrier between the
blood and the surrounding tissues, and serve important functions in
the cardiovascular system (4,5). The
association between endothelial dysfunction and the mechanism of
atherosclerosis has gained marked interest (6) and the present study investigates
endothelial dysfunction in relation to atherosclerosis.
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is
a Ca2+-independent secreted protein that belongs to the
PLA2 superfamily. It is produced by a wide range of
inflammatory and non-inflammatory cells, and circulates in plasma
bound mainly to low-density lipoprotein (LDL) and promotes vascular
inflammation (7–9). Despite the inflammatory potential, the
association of Lp-PLA2 with endothelial dysfunction has been
demonstrated in a number of epidemiological studies (10,11).
Yang et al (10) demonstrated
that circulating Lp-PLA2 is a strong predictor of coronary artery
endothelial dysfunction in humans. Local coronary production of
Lp-PLA2 is also associated with endothelial dysfunction in patients
with early coronary atherosclerosis (11). However, relatively few studies have
investigated the role of Lp-PLA2 in endothelial dysfunction and the
associated molecular mechanism in vitro.
AMP-activated protein kinase (AMPK) is an important
regulator of carbohydrate and lipid metabolism, and it appears to
be a therapeutic target for improving endothelial function in
patients with cardiovascular disease (12–14).
In the present study, the effects of Lp-PLA2 on
endothelial dysfunction in an in vitro cell model of
atherosclerosis were initially investigated. In addition, whether
AMPK mediates the effects of Lp-PLA2 on endothelial dysfunction was
also assessed.
Materials and methods
Study participants
The study enrolled 392 patients who had been
diagnosed with coronary artery disease (CAD) in Tianjin Chest
Hospital (Tianjin, China) and 66 healthy controls. All patients
signed a consent form agreeing to participate in the present study,
and the Ethics Committee of Tianjin Thoracic Hospital approved the
protocol. Among the patients with CAD, 10 had acute coronary
syndromes (ACS), 69 had acute myocardial infarction (AMI), 55 had
stable angina pectoris (SAP) and the remaining 258 patients had
unstable angina pectoris (UAP). The mean age of patients was 62±5
years, and the mean age of healthy controls was 59±6 years. Fasting
blood samples were drawn by vein-puncture in the morning.
Cell culture and treatments
Human umbilical vascular endothelium, (HUVECs;
catalogue no. CRL-1730; American Type Culture Collection (ATCC),
Manassas, VA, USA) were cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Invitrogen; Thermo
Fisher Scientific, Inc.). The cells were maintained at 37°C in a
humidified 5% CO2, 95% air atmosphere, and the cell
culture media was changed every 2–3 days. Oxidized low density
lipoprotein (oxLDL) was purchased from Yiyuan Biotech Co., Ltd.
(Guangzhou, China) and diluted in DMEM to 200 µg/ml to treat cells.
The Lp-PLA2 inhibitor, SB-435495 was obtained from GlaxoSmithKline
(Collegeville, PA, USA) and used at a concentration of 5 µM.
Compound C, an AMPK inhibitor, was purchased from Merck Millipore
(Darmstadt, Germany) and used at a concentration of 20 µM.
Measurement of nitric oxide (NO)
NO production of HUVECs was determined using a NO
Assay kit (Beyotime Institute of Biotechnology, Haimen, China)
according to the manufacturer's protocol. Cell culture supernatant
of HUVECs (50 µl), Griess Reagent (50 µl) and Griess Reagent (50
µl) were added to each well of the 96-well plates. The plates were
incubated at room temperature for 2 min, and the optical density
(OD) was measured at 540 nm using a microplate reader (MK3; Thermo
Fisher Scientific, Inc.). NO production was then calculated by
comparing the OD of the samples with the standard curve.
ELISA assay
Plasma levels of Lp-PLA2 were detected using a
commercial ELISA kit: Lp-PLA2 Assay kit (Kangerke Biotech Co.,
Ltd., Tianjin, China). The concentrations of endothelin 1 (ET-1),
intercellular adhesion molecule 1 (ICAM-1) and platelet/endothelial
cell adhesion molecule 1 (PECAM-1) in the cell culture supernatant
were determined using Human ET-1 ELISA kit (TW Reagent Company,
Shanghai, China), Human ICAM-1 ELISA kit (Huijia Biotechnology,
Xiamen, Fujian, China), and Human PECAM-1 ELISA kit (Meixuan
Biological Technology Co., Ltd, Shanghai, China). The microtiter
plate provided in each of the kits had been pre-coated with an
antibody specific to ET-1, ICAM-1 or PECAM-1. Standards (50 µl) or
samples (10 µl) were then added to the wells, followed by the
addition of horseradish peroxidase (HRP)-conjugated antibody, as
stated in manufacturer protocols of aforementioned ELISA kits.
Subsequent to incubation, a tetramethylbenzidine substrate solution
was added to each well and the enzyme-substrate reaction was
terminated by the addition of stop buffer. The color change was
measured at a wavelength of 450 nm (MK3). The concentration of the
samples was determined by comparing the OD of the samples to the
standard curve.
MTT assay
An MTT metabolic assay was considered to indicate
the changes in cell viability. The cells (1.5×103
cells/well) were plated into the 96-well plates and cultured at
37°C overnight. After treatment for 24, 48 and 72 h, 10 µl MTT
stock solution (0.5 mg/ml; Beyotime Institute of Biotechnology) was
added to each well, and the cells were incubated at 37°C for 4 h.
Next, 150 µl dimethyl sulfoxide was added to each well and mixed
thoroughly with a pipette. After incubation at 37°C for 10 min, the
absorbance at 570 nm was measured using a microplate reader
(MK3).
Western blot analysis
The cells were washed with phosphate-buffered saline
(PBS) and lysed in cell lysis buffer [20 mM Tris-HCl (pH 8.0), 150
mM NaCl, 50 mM NaF, 2 mM EDTA, 1 mM Na3VO4,
1% NP-40, 1 µg/ml Leupeptin, 1 µg/ml Pepstatin and 1 mM PMSF].
After centrifugation at 12,000 × g for 10 min at 4°C, the
supernatant was subjected to 10% SDS-PAGE and transferred to
polyvinylidene difluoride membranes (Merck Millipore). The
membranes were blocked in Tris-buffered saline containing 5% nonfat
milk and 0.05% Tween-20 at 4°C overnight. Thereafter, the blots
were probed with Lp-PLA2 rabbit polyclonal antibody (1:800;
ab96619; Abcam, Cambridge, MA, USA), AMPKα rabbit monoclonal
antibody (1:400; 2603), phospho-AMPKα (Thr172) rabbit monoclonal
antibody (1:400; 2535; Cell Signaling Technology, Inc., Danvers,
MA, USA) at 37°C for 2 h. After washing with PBS, the membranes
were incubated with HRP-conjugated donkey anti-rabbit IgG (1:2,000;
ab16284; Abcam) at 37°C for 1 h. Immunoreactive bands were detected
by an enhanced chemiluminescence western blotting kit (Pierce
Protein Biology; Thermo Fisher Scientific, Inc., Rockford, IL,
USA). ImageJ software (National Institutes of Health, Bethesda, MD,
USA) was used to calculate band density.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
software (SPSS IBM, Armonk, NY, USA). All data are representative
of a minimum of three independent experiments, and are expressed as
the mean ± standard deviation. Differences between two groups were
compared using the Student's t-test. One-way analysis of variance,
followed by a least significant difference test was used to compare
the differences among three groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
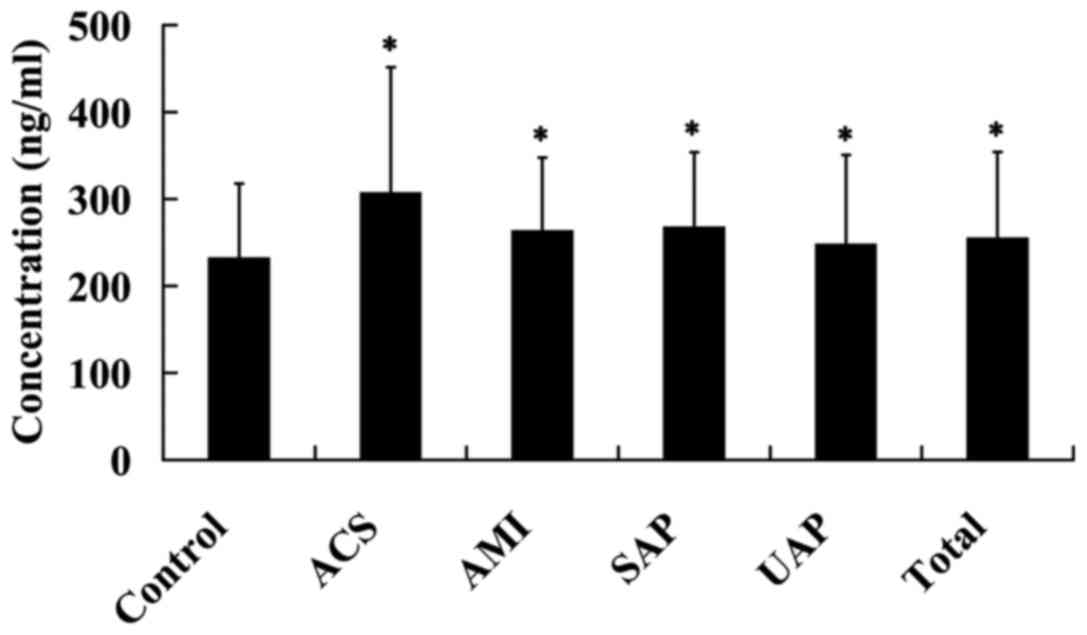
Plasma Lp-PLA2 levels were upregulated
in patients with CAD
The plasma Lp-PLA2 levels were detected in 392
patients with CAD and 66 healthy controls. It was found that,
compared with the control subjects, the plasma Lp-PLA2 levels were
significantly increased in patients with SAP, UAP, ACS and AMI, as
well as the total CAD (P<0.05; Fig.
1).
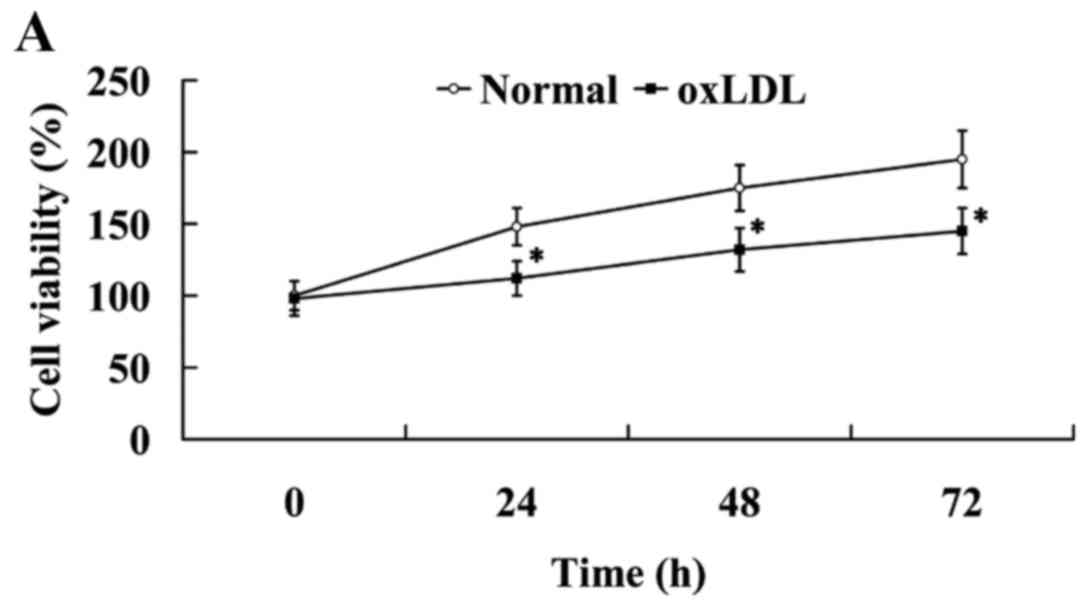
oxLDL induced endothelial dysfunction
in HUVECs
As shown in Fig. 2A,
oxLDL exerted an inhibitory effect on cell proliferation. Compared
with the cells in the normal group, cell viability was
significantly decreased in the oxLDL group (P<0.05).
The HUVECs were treated with 200 µg/ml oxLDL for 24
h, and then the cells were harvested for western blot analysis. The
expression levels of vasorelaxation factor NO and vasoconstrictor
ET-1 were determined by western blot analysis to assess endothelial
vasorelaxation capacity. It was observed that NO expression was
significantly decreased (P<0.05), while ET-1 expression was
significantly increased in HUVECs following treatment with oxLDL
(P<0.05; Fig. 2B).
The expression levels of ICAM-1 and PECAM-1 were
evaluated to determine the secretion of adhesion molecules by
HUVECs. As shown in Fig. 2C,
treatment with oxLDL significantly increased the expression levels
of ICAM-1 and PECAM-1 proteins in HUVECs (P<0.05).
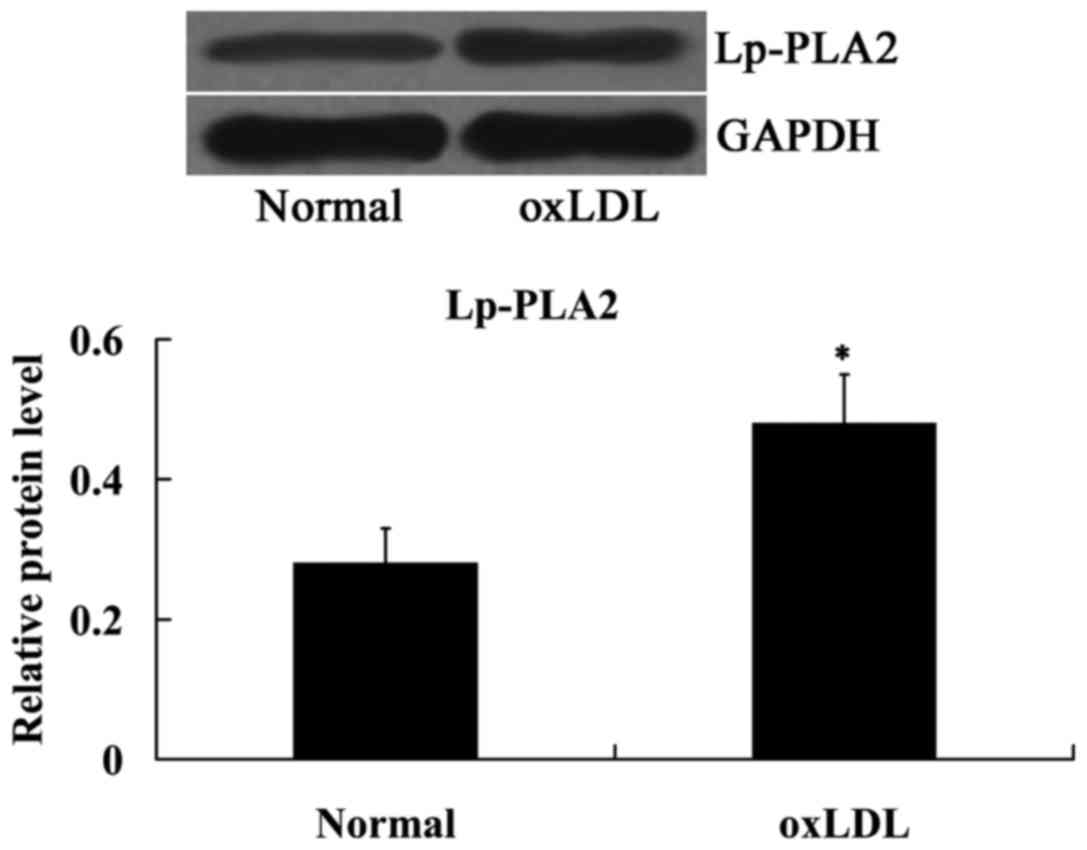
oxLDL induced Lp-PLA2 expression in
HUVECs
The effect of oxLDL on Lp-PLA2 expression was
determined using western blot analysis. As indicated in Fig. 3, compared with the normal cells,
Lp-PLA2 protein expression levels were significantly upregulated in
the cells treated with oxLDL (P<0.05).
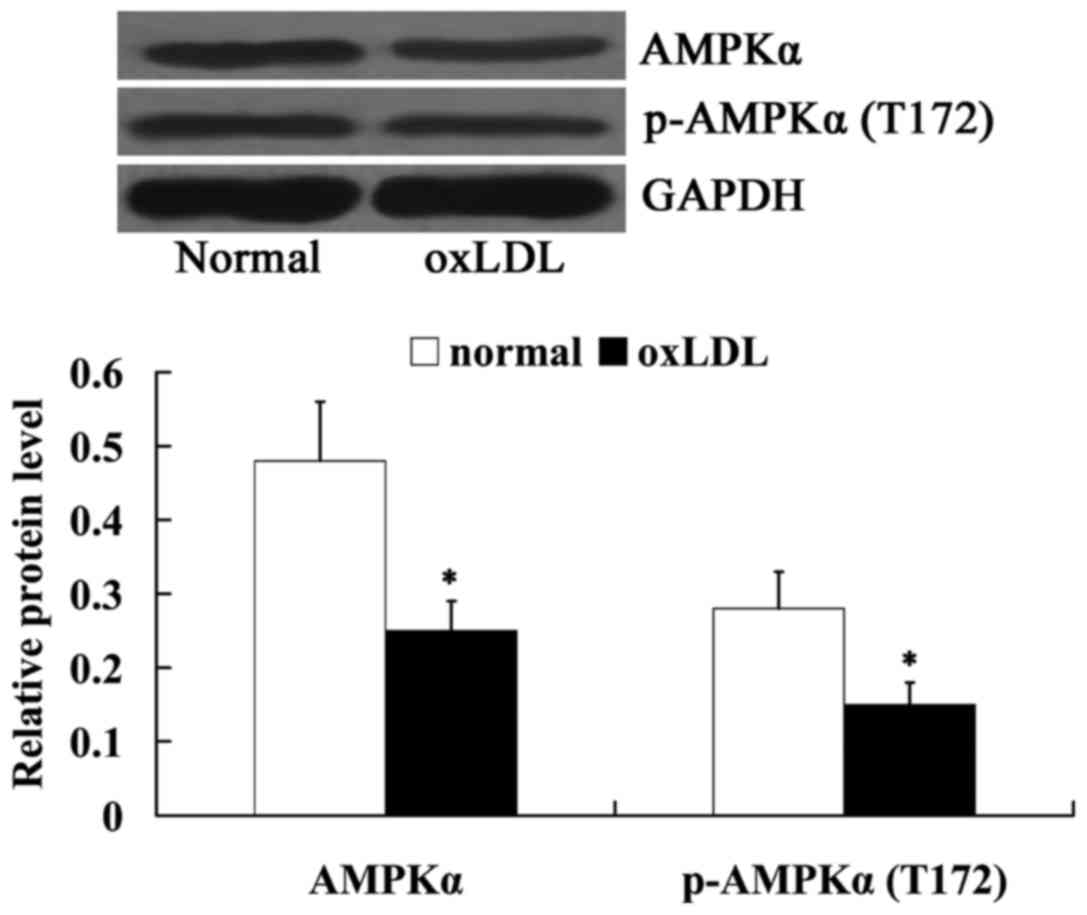
oxLDL inhibited AMPKα in HUVECs
The expression levels of AMPKα and
phosphorylated-AMPKα (T172) in oxLDL-treated HUVECs were also
measured using western blot analysis. Fig. 4 indicated that the relative protein
expression levels of AMPKα and phosphorylated-AMPKα (T172) were
decreased significantly in oxLDL-exposed HUVECs, compared with
normal cells (P<0.05).
Lp-PLA2 suppression protected against
endothelial dysfunction in oxLDL-exposed HUVECs
To investigate the effects of Lp-PLA2 on endothelial
dysfunction, Lp-PLA2 protein was suppressed in oxLDL-exposed HUVECs
by incubation with the Lp-PLA2 inhibitor SB-435495, then cell
viability, endothelial vasorelaxation capacity and the secretion of
adhesion molecules were investigated.
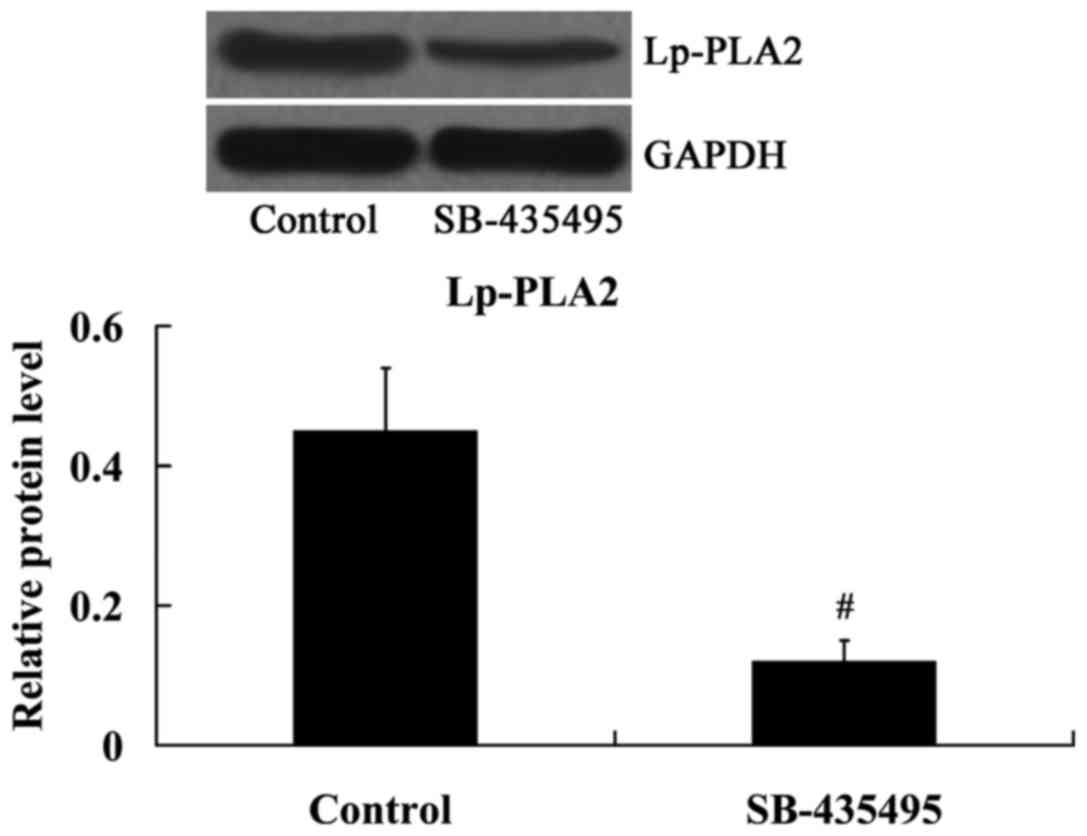
As expected, the results from the western blot
analysis demonstrated that the expression of Lp-PLA2 protein was
significantly inhibited by SB-435495 in oxLDL-exposed HUVECs
(P<0.01; Fig. 5).
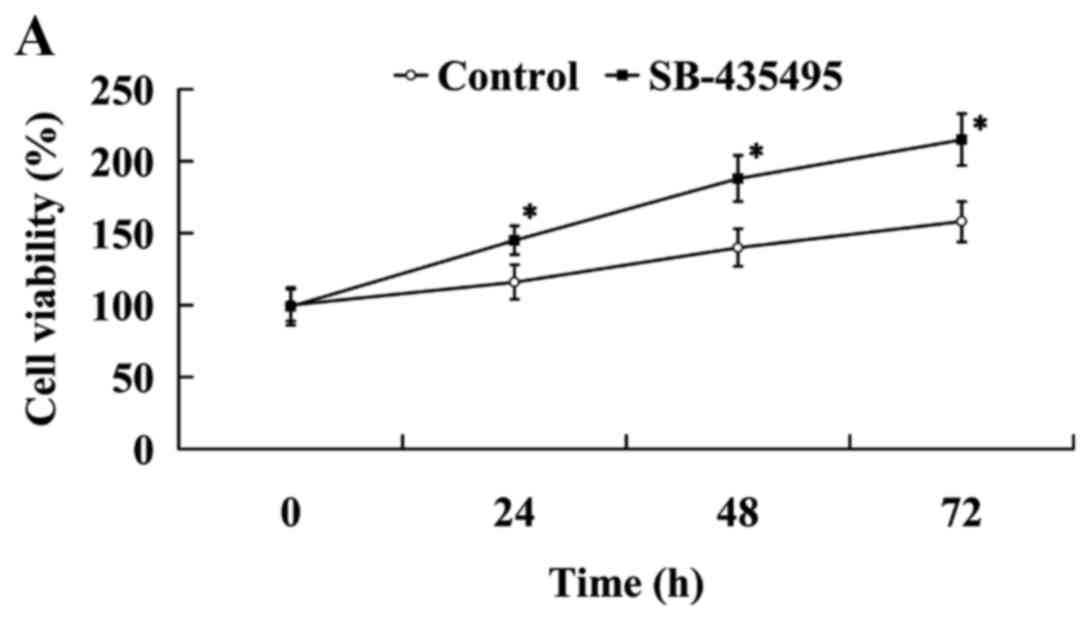
The results of the MTT assay revealed that cell
viability was significantly increased following treatment with
SB-435495 (P<0.05; Fig. 6A).
Western blot analysis results demonstrated that NO expression
levels were significantly increased, while ET-1 expression was
significantly decreased in the oxLDL-exposed HUVECs following
treatment with SB-435495 (P<0.05; Fig. 6B). The expression of adhesion
molecules ICAM-1 and PECAM-1 was significantly decreased as a
result of treatment with SB-435495 (P<0.05; Fig. 6C).
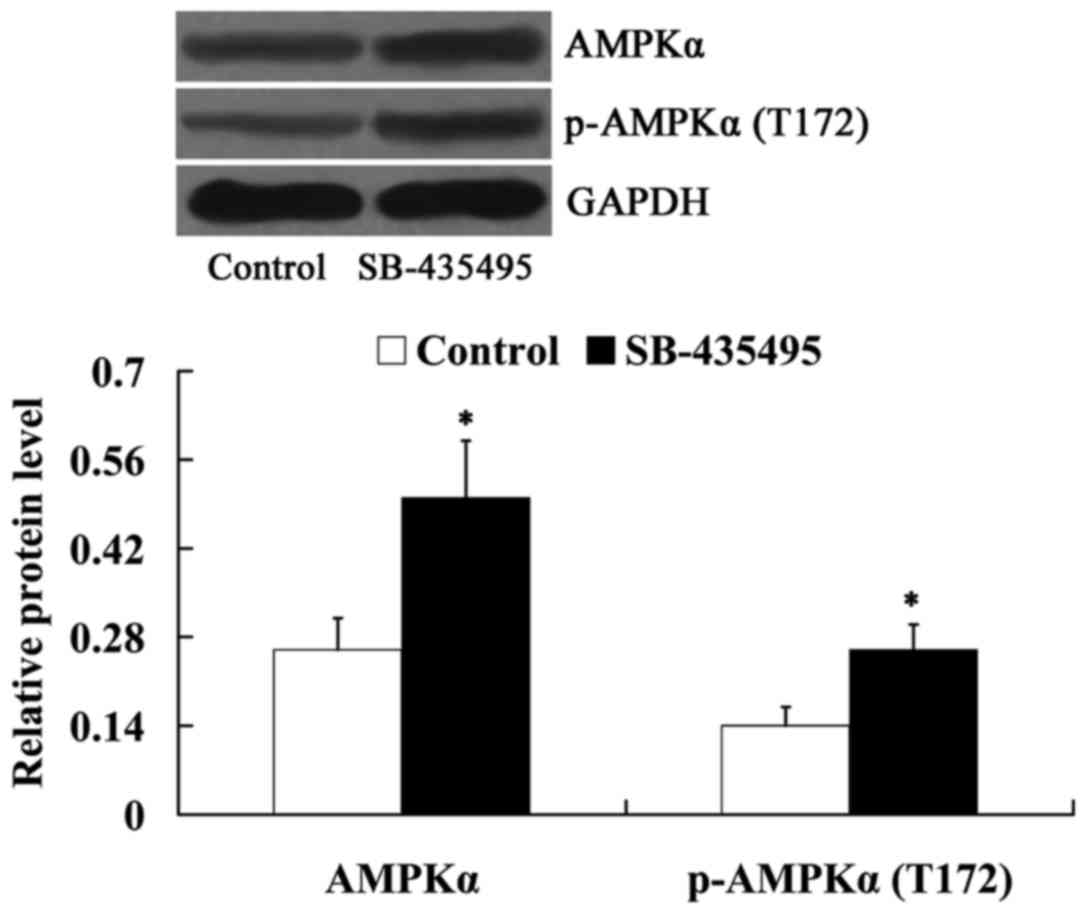
Lp-PLA2 suppression activated AMPKα in
oxLDL-exposed HUVECs
Subsequently, the expression levels of AMPKα and
phosphorylated-AMPKα (T172) in oxLDL-exposed HUVECs were detected
following incubation with SB-435495. It was found that Lp-PLA2
suppression led to increased expression levels of AMPKα and
phosphorylated-AMPKα (T172) in oxLDL-exposed HUVECs (P<0.05;
Fig. 7).
AMPK mediates the effects of Lp-PLA2
on endothelial dysfunction in oxLDL-exposed HUVECs
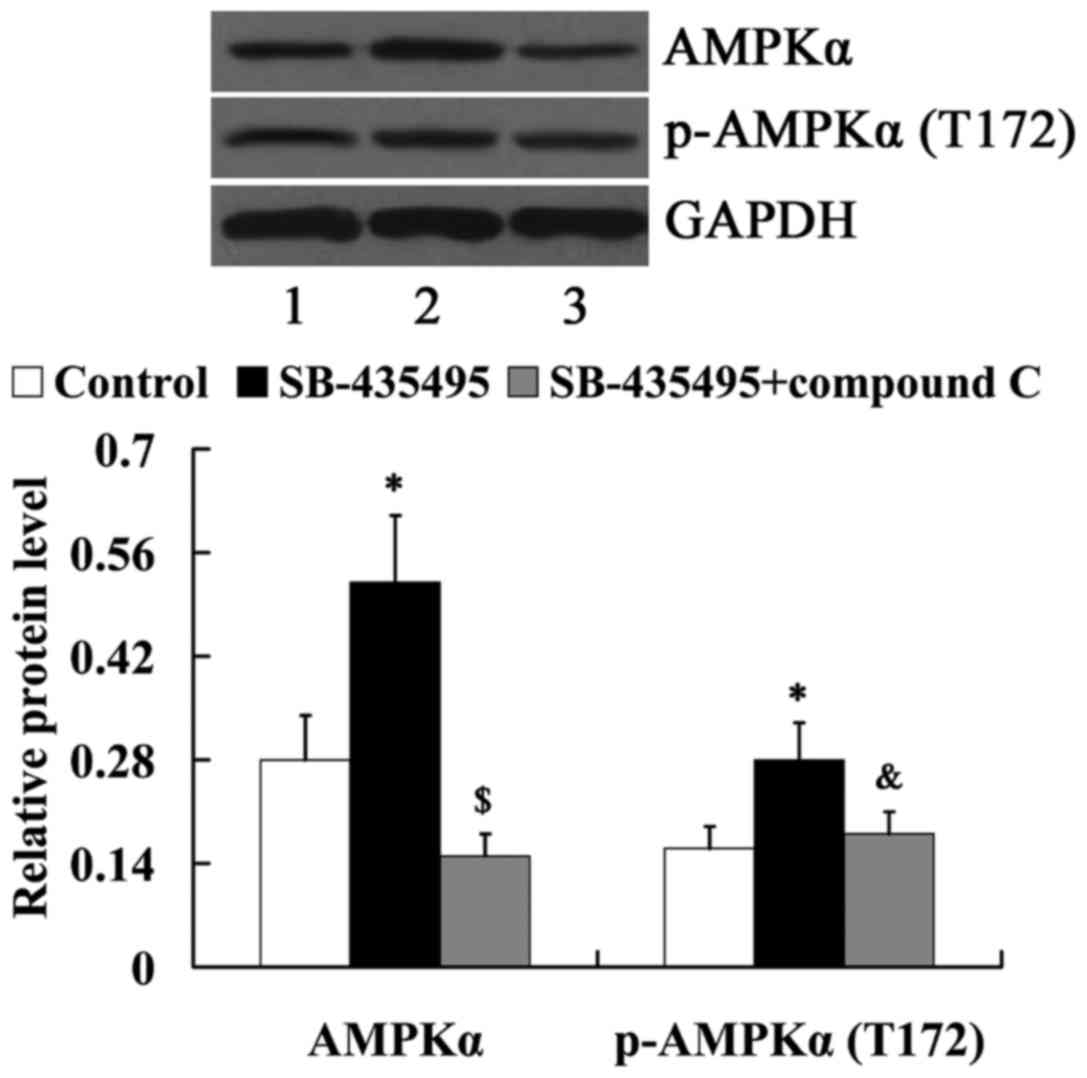
In order to determine whether AMPK mediates the
effects of Lp-PLA2 on endothelial dysfunction, cells were treated
with compound C to suppress AMPK expression. As demonstrated in
Fig. 8, compared with the SB-435495
group, the relative protein levels of AMPKα and
phosphorylated-AMPKα (T172) were significantly decreased in the
SB-435495 + compound C group (P<0.01 and P<0.05,
respectively).
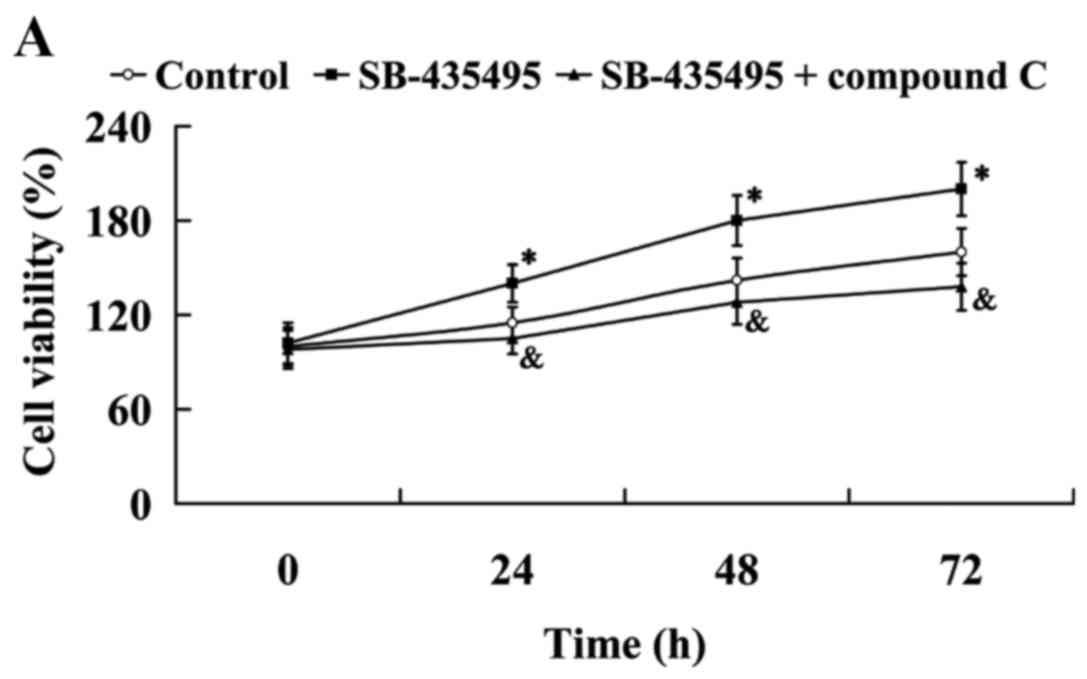
The MTT results showed that Lp-PLA2 suppression led
to significantly increased cell viability compared with the control
group (P<0.05); however, this effect was reversed by treatment
with compound C (P<0.05; Fig.
9A). Western blot analysis results revealed that the
significantly altered expression of NO and ET-1 by Lp-PLA2
suppression in oxLDL-exposed HUVECs was attenuated by compound C
(P<0.05; Fig. 9B). In addition,
it was found that the relative protein expression levels of ICAM-1
and PECAM-1 were significantly increased in the SB-435495 +
compound C group, compared with those in the SB-435495 group
(P<0.01; Fig. 9C).
Discussion
In the present study, a large number of CAD samples
were collected, including those from patients with SAP, UAP, ACS
and AMI. Atherosclerosis is the most common cause of stenosis of
the heart arteries and, as such, of angina pectoris, including SAP
and UAP (15). The instability of
atherosclerotic plaques forms the pathological basis of ACS
(16). Plaques can become unstable,
rupture and promote the formation of blood clots that may occlude
the artery, leading to AMI (17,18). The
current study demonstrated that plasma Lp-PLA2 expression levels
were significantly upregulated in patients with CAD, and this
result was consistent with a previous report (19). Subsequently, the current study
investigated the role of Lp-PLA2 in endothelial dysfunction in
atherosclerosis.
oxLDL is an important factor in the initiation and
development of atherosclerosis (20). In the present study, an in
vitro cell model of atherosclerosis was established by exposing
HUVECs to 200 µg/ml oxLDL for 24 h. Thus, the present study
provided the first in vitro evidence that Lp-PLA2 induction
results in endothelial dysfunction in experimental atherosclerosis,
and that AMPK was involved in mediating the effects of Lp-PLA2.
The endothelium has a central role in vascular
disease, to the extent that several clinical tests have been
conducted to evaluate the functional properties of normal and
activated endothelium (21,22). Endothelial dysfunction is defined as
an imbalance between vasodilating and vasoconstricting substances
that are produced by or act on the endothelium (23), in addition to the altered
anti-inflammatory and anticoagulant properties of the endothelium
(6,24). Endothelial dysfunction is associated
with numerous vascular diseases, including atherosclerosis, stroke,
myocardial ischemia and acute coronary syndrome (6). Clinical assessments of endothelial
function have enabled the detection of early disease,
quantification of risk, judgment of response to interventions, and
a reduction in the occurrence of later adverse events in patients.
Endothelial dysfunction is often regarded as a key early event in
the development of atherosclerosis (6). NO is the most important vasorelaxation
factor released by the endothelium. The decrease in NO production
could induce pathological conditions associated with endothelial
dysfunction, such as atherosclerosis (25,26).
ET-1 is another endothelium-derived regulatory protein that
functions as a potent vasoconstrictor, and is a marker of
endothelial dysfunction (27).
During the process of atherosclerosis, endothelial dysfunction
could be induced by the inflammatory cytokines, and consequently
led to the induction of cell adhesion molecules (28). ICAM-1 and PECAM-1 are cell adhesion
molecules expressed by several cell types, including endothelial
cells (29–31). The endothelial expression of ICAM-1
and PECAM-1 is increased in atherosclerotic lesions and is involved
in their progression (32,33). In the present study, it was found
that cell viability was inhibited by oxLDL treatment. Furthermore,
oxLDL treatment led to decreased production of NO and increased
secretion of ET-1 in HUVECs. In addition, the expression of cell
adhesion molecules, including ICAM-1 and PECAM-1, was induced by
oxLDL. Collectively, the aforementioned results demonstrated that
oxLDL caused endothelial dysfunction in HUVECs.
Lp-PLA2 is strongly associated with several
cardiovascular risk markers and cardiovascular events in the clinic
(34,35). A number of drugs and diet components
have been demonstrated to demonstrate the vascular protective
effect of Lp-PLA2 through influencing the enzyme concentration and
activity (35). Lp-PLA2 is an
important enzyme that can be found in human and rabbit
atherosclerotic plaques (36). A
large study that included 172 patients with no significant coronary
artery disease (<30% stenosis) reported that circulating Lp-PLA2
levels were elevated in patients with early atherosclerosis
(10). Compared with the patients
without evidence of atherosclerosis, local net production of
Lp-PLA2 is significantly increased in the patients with minimal
coronary atherosclerosis (11). A
study by Yang et al (10)
demonstrated the potential of circulating Lp-PLA2 as an independent
predictor of coronary endothelial dysfunction. In addition, local
coronary production of Lp-PLA2 is associated with local endothelial
function (11). In the present
study, in vitro assessments were initially performed to
investigate the role of Lp-PLA2 in endothelial dysfunction.
Consistent with previous studies (37–39), the
expression of Lp-PLA2 was induced by oxLDL. SB-435495, an inhibitor
of Lp-PLA2, was used to suppress Lp-PLA2 expression, and it was
subsequently identified that oxLDL-induced endothelial dysfunction
was reversed by Lp-PLA2 suppression. The aforementioned results
indicated that Lp-PLA2 induction could cause endothelial
dysfunction in experimental atherosclerosis.
AMPK has been identified as a sensor of cellular
energy and cellular redox status (40,41).
AMPK activation appears to be a shared molecular target in vascular
tissues, and the beneficial effects of certain agents are mediated
by the activation of AMPK in endothelial cells (12–14). The
phosphorylation of AMPKα at Thr172 is required for AMPK activation
(42). AMPK phosphorylation has been
demonstrated to reverse the decreases in cell viability caused by
oxLDL (43), and the activation of
AMPK activity in endothelial cells stimulates NO production
(44). AMPK also decreases ET-1
expression and secretion from endothelial cells (45). Pretreatment with an AMPK inhibitor
compound C significantly increased basal ET-1 mRNA and protein
expression levels. In addition, Cacicedo et al (46) reported that AMPK reduces tumor
necrosis factor-induced elevated expression of adhesion molecules
in endothelial cells. Cell adhesion molecules such as ICAM-1 and
PECAM-1 have been reported to be suppressed by AMPK activation
(14). It has also been reported
that the knockdown or mutation of Lp-PLA2 could induce apoptosis in
macrophages and Cos-7 cells, and a reduction in the activated Akt
level was involved in this phenomenon (47). AMPK is the upstream regulator of Akt
(48–50). In the present study, the association
between Lp-PLA2 and AMPK was initially investigated in an in
vitro cell model of atherosclerosis, and whether AMPK mediates
the effects of Lp-PLA2 on endothelial dysfunction was also
investigated. Western blot analysis results indicated a negative
role of Lp-PLA2 in the regulation of AMPK, which was evidenced by
the increased expression levels of AMPKα and phosphorylated-AMPKα
(Thr172) in oxLDL-exposed HUVECs following treatment with the
Lp-PLA2 inhibitor SB-435495. Furthermore, the present study found
that compound C, an AMPK inhibitor, was able to reverse the effects
of Lp-PLA2 on cell viability, endothelial vasorelaxation capacity
and the secretion of adhesion molecules in oxLDL-exposed HUVECs.
Thus, these results suggest that AMPK serves an important role in
mediating the effects of Lp-PLA2 on endothelial dysfunction.
In conclusion, the present study demonstrated the
role of Lp-PLA2 in endothelial dysfunction and the associated
mechanism in an in vitro cell model of atherosclerosis.
Lp-PLA2 expression was induced in oxLDL-exposed HUVECs. The
increased expression levels of Lp-PLA2 promotes endothelial
dysfunction by inhibiting cell viability and endothelial
vasorelaxation capacity, as well as inducing the secretion of
adhesion molecules protein, at least partially through the
downregulation of AMPK, thus contributing to the progression of
atherosclerosis. The present study provided a novel molecular
mechanism underlying the role of Lp-PLA2 in endothelial dysfunction
and atherosclerosis, and it may aid the improvement of treatments
for atherosclerotic vascular diseases.
Acknowledgements
The present study was supported by the Science and
Technology Foundation of Tianjin Municipal Health Bureau (grant no.
2011kz63).
References
|
1
|
Leys D: Atherothrombosis: A major health
burden. Cerebrovasc Dis. 11 Suppl 2:S1–S4. 2011. View Article : Google Scholar
|
|
2
|
Galkina E and Ley K: Immune and
inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol.
27:165–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ross R: Atherosclerosis-an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eskin SG, Ives CL, McIntire LV and Navarro
LT: Response of cultured endothelial cells to steady flow.
Microvasc Res. 28:87–94. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Langille BL and Adamson SL: Relationship
between blood flow direction and endothelial cell orientation at
arterial branch sites in rabbits and mice. Circ Res. 48:481–488.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonetti PO, Lerman LO and Lerman A:
Endothelial dysfunction: A marker of atherosclerotic risk.
Arterioscler Thromb Vasc Biol. 23:168–175. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karabina SA and Ninio E: Plasma
PAF-acetylhydrolase: An unfulfilled promise? Biochim Biophys Acta.
1761:1351–1358. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Venable ME, Zimmerman GA, McIntyre TM and
Prescott SM: Platelet-activating factor: A phospholipid autacoids
with diverse actions. J Lipid Res. 34:691–702. 1993.PubMed/NCBI
|
|
9
|
Snyder F: Platelet-activating factor and
its analogs: Metabolic pathways and related intracellular
processes. Biochim Biophys Acta. 1254:231–249. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang EH, McConnell JP, Lennon RJ, Barsness
GW, Pumper G, Hartman SJ, Rihal CS, Lerman LO and Lerman A:
Lipoprotein-associated phospholipase A2 is an independent marker
for coronary endothelial dysfunction in humans. Arterioscler Thromb
Vasc Biol. 26:106–111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lavi S, McConnell JP, Rihal CS, Prasad A,
Mathew V, Lerman LO and Lerman A: Local production of
lipoprotein-associated phospholipase A2 and lysophosphatidylcholine
in the coronary circulation: Association with early coronary
atherosclerosis and endothelial dysfunction in humans. Circulation.
115:2715–2721. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hardie DG: AMPK and raptor: Matching cell
growth to energy supply. Mol Cell. 30:263–265. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zou MH, Hou XY, Shi CM, Kirkpatick S, Liu
F, Goldman MH and Cohen RA: Activation of 5′-AMP-activated kinase
is mediated through c-Src and phosphoinositide 3-kinase activity
during hypoxia-reoxygenation of bovine aortic endothelial cells.
Role of peroxynitrite. J Biol Chem. 278:34003–34010. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suzuki K, Uchida K, Nakanishi N and
Hattori Y: Cilostazol activates AMP-activated protein kinase and
restores endothelial function in diabetes. Am J Hypertens.
21:451–457. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hombach V, Höher M, Kochs M, Eggeling T,
Schmidt A, Höpp HW and Hilger HH: Pathophysiology of unstable
angina pectoris-correlations with coronary angioscopic imaging. Eur
Heart J. 9 Suppl N:40–45. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Plutzky J: Inflammatory pathways in
atherosclerosis and acute coronary syndromes. Am J Cardiol.
88:10K–15K. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsujita K, Kaikita K, Soejima H, Sugiyama
S and Ogawa H: Acute coronary syndrome-initiating factors. Nihon
Rinsho. 68:607–614. 2010.(In Japanese). PubMed/NCBI
|
|
18
|
Dohi T and Daida H: Change of concept and
pathophysiology in acute coronary syndrome. Nihon Rinsho.
68:592–596. 2010.(In Japanese). PubMed/NCBI
|
|
19
|
Khuseyinova N, Imhof A, Rothenbacher D,
Trischler G, Kuelb S, Scharnagl H, Maerz W, Brenner H and Koenig W:
Association between Lp-PLA2 and coronary artery disease: Focus on
its relationship with lipoproteins and markers of inflammation and
hemostasis. Atherosclerosis. 182:181–188. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Steinberg D: Low density lipoprotein
oxidation and its pathobiological significance. J Biol Chem.
272:20963–20966. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ryu JH, Yu M, Lee S, Ryu DR, Kim SJ, Kang
DH and Choi KB: AST-120 improves microvascular endothelial
dysfunction in end-stage renal disease patients receiving
hemodialysis. Yonsei Med J. 57:942–949. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ceriello A, De Nigris V, Pujadas G, La
Sala L, Bonfigli AR, Testa R, Uccellatore A and Genovese S: The
simultaneous control of hyperglycemia and GLP-1 infusion normalize
endothelial function in type 1 diabetes. Diabetes Res Clin Pract.
114:64–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deanfield J, Donald A, Ferri C,
Giannattasio C, Halcox J, Halligan S, Lerman A, Mancia G, Oliver
JJ, Pessina AC, et al: Endothelial function and dysfunction. Part
I: Methodological issues for assessment in the different vascular
beds: A statement by the working group on endothelin and
endothelial factors of the european society of hypertension. J
Hypertens. 23:7–17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gokce N, Keaney JF Jr, Hunter LM, Watkins
MT, Menzoian JO and Vita JA: Risk stratification for postoperative
cardiovascular events via noninvasive assessment of endothelial
function: A prospective study. Circulation. 105:1567–1572. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shah V, Toruner M, Haddad F, Cadelina G,
Papapetropoulos A, Choo K, Sessa WC and Groszmann RJ: Impaired
endothelial nitric oxide synthase activity associated with enhanced
caveolin binding in experimental cirrhosis in the rat.
Gastroenterology. 117:1222–1228. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taguchi K, Matsumoto T and Kobayashi T:
G-protein-coupled receptor kinase 2 and endothelial dysfunction:
Molecular insights and pathophysiological mechanisms. J Smooth
Muscle Res. 51:37–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Böhm F and Pernow J: The importance of
endothelin-1 for vascular dysfunction in cardiovascular disease.
Cardiovasc Res. 76:8–18. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tuttolomondo A, Di Raimondo D, Pecoraro R,
Arnao V, Pinto A and Licata G: Atherosclerosis as an inflammatory
disease. Curr Pharm Des. 18:4266–4288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lawson C and Wolf S: ICAM-1 signaling in
endothelial cells. Pharmacol Rep. 61:22–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jackson DE: The unfolding tale of PECAM-1.
FEBS Lett. 540:7–14. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Albelda SM, Muller WA, Buck CA and Newman
PJ: Molecular and cellular properties of PECAM-1 (endoCAM/CD31): A
novel vascular cell-cell adhesion molecule. J Cell Biol.
114:1059–1068. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Collins RG, Velji R, Guevara NV, Hicks MJ,
Chan L and Beaudet AL: P-Selectin or intercellular adhesion
molecule (ICAM)-1 deficiency substantially protects against
atherosclerosis in apolipoprotein E-deficient mice. J Exp Med.
191:189–194. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zibara K, Chignier E, Covacho C, Poston R,
Canard G, Hardy P and McGregor J: Modulation of expression of
endothelial intercellular adhesion molecule-1, platelet-endothelial
cell adhesion molecule-1, and vascular cell adhesion molecule-1 in
aortic arch lesions of apolipoprotein E-deficient compared with
wild-type mice. Arterioscler Thromb Vasc Biol. 20:2288–2296. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Silva IT, Mello AP and Damasceno NR:
Antioxidant and inflammatory aspects of lipoprotein-associated
phospholipase A2 (Lp-PLA2): A review. Lipids Health Dis.
10:1702011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zalewski A and Macphee C: Role of
lipoprotein-associated phospholipase A2 in atherosclerosis:
Biology, epidemiology, and possible therapeutic target.
Arterioscler Thromb Vasc Biol. 25:923–931. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Häkkinen T, Luoma JS, Hiltunen MO, Macphee
CH, Milliner KJ, Patel L, Rice SQ, Tew DG, Karkola K and
Ylä-Herttuala S: Lipoprotein-associated phospholipase A(2),
platelet-activating factor acetylhydrolase, is expressed by
macrophages in human and rabbit atherosclerotic lesions.
Arterioscler Thromb Vasc Biol. 19:2909–2917. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang WY, Li J, Yang D, Xu W, Zha RP and
Wang YP: OxLDL stimulates lipoprotein-associated phospholipase A2
expression in THP-1 monocytes via PI3K and p38 MAPK pathways.
Cardiovasc Res. 85:845–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
De Keyzer D, Karabina SA, Wei W, Geeraert
B, Stengel D, Marsillach J, Camps J, Holvoet P and Ninio E:
Increased PAFAH and oxidized lipids are associated with
inflammation and atherosclerosis in hypercholesterolemic pigs.
Arterioscler Thromb Vasc Biol. 29:2041–2046. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pasini A Fratta, Stranieri C, Pasini A,
Vallerio P, Mozzini C, Solani E, Cominacini M, Cominacini L and
Garbin U: Lysophosphatidylcholine and carotid intima-media
thickness in young smokers: A role for oxidized LDL-induced
expression of PBMC lipoprotein-associated phospholipase A2? PLoS
One. 8:e830922013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zou MH and Wu Y: AMP-activated protein
kinase activation as a strategy for protecting vascular endothelial
function. Clin Exp Pharmacol Physiol. 35:535–545. 2000. View Article : Google Scholar
|
|
41
|
Li C: Genetics and molecular
biology-protein kinase C-zeta as an AMP-activated protein kinase
kinase kinase: The protein kinase C-zeta-LKB1-AMP-activated protein
kinase pathway. Curr Opin Lipidol. 19:541–542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hawley SA, Davison M, Woods A, Davies SP,
Beri RK, Carling D and Hardie DG: Characterization of the
AMP-activated protein kinase kinase from rat liver and
identification of threonine 172 as the major site at which it
phosphorylates AMP-activated protein kinase. J Biol Chem.
271:27879–27887. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guo H, Chen Y, Liao L and Wu W:
Resveratrol protects HUVECs from oxidized-LDL induced oxidative
damage by autophagy upregulation via the AMPK/SIRT1 pathway.
Cardiovasc Drugs Ther. 27:189–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Morrow VA, Foufelle F, Connell JM, Petrie
JR, Gould GW and Salt IP: Direct activation of AMP-activated
protein kinase stimulates nitric-oxide synthesis in human aortic
endothelial cells. J Biol Chem. 278:31629–31639. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Reiter CE, Kim JA and Quon MJ: Green tea
polyphenol epigallocatechin gallate reduces endothelin-1 expression
and secretion in vascular endothelial cells: Roles for
AMP-activated protein kinase, Akt, and FOXO1. Endocrinology.
151:103–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cacicedo JM, Yagihashi N, Keaney JF Jr,
Ruderman NB and Ido Y: AMPK inhibits fatty acid-induced increases
in NF-kappaB transactivation in cultured human umbilical vein
endothelial cells. Biochem Biophys Res Commun. 324:1204–1209. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Maeda T, Takeuchi K, Xiaoling P, P Zankov
D, Takashima N, Fujiyoshi A, Kadowaki T, Miura K, Ueshima H and
Ogita H: Lipoprotein-associated phospholipase A2 regulates
macrophage apoptosis via the Akt and caspase-7 pathways. J
Atheroscler Thromb. 21:839–853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ji L, Zhang X, Liu W, Huang Q, Yang W, Fu
F, Ma H, Su H, Wang H, Wang J, et al: AMPK-regulated and
Akt-dependent enhancement of glucose uptake is essential in
ischemic preconditioning-alleviated reperfusion injury. PLoS One.
8:e699102013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sag D, Carling D, Stout RD and Suttles J:
Adenosine 5′-monophosphate-activated protein kinase promotes
macrophage polarization to an anti-inflammatory functional
phenotype. J Immunol. 181:8633–8641. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Leclerc GM, Leclerc GJ, Fu G and Barredo
JC: AMPK-induced activation of Akt by AICAR is mediated by IGF-1R
dependent and independent mechanisms in acute lymphoblastic
leukemia. J Mol Signal. 5:152010. View Article : Google Scholar : PubMed/NCBI
|