Introduction
Approximately 8 in 1,000 live births are diagnosed
with a congenital heart defect (1)
and many require surgical intervention to ensure survival beyond
the neonatal period. Recent advances in medical management,
transcatheter intervention, surgical procedures and cardiopulmonary
bypass, have significantly increased the survival rate of such
children. Up to 50% of children with congenital heart disease (CHD)
experience neurodevelopmental and behavioral problems including
seizures, cognitive impairment, delays in speech, language,
visual-motor and visual-spatial skills, attention
deficit/hyperactivity disorder and learning disabilities (2–5).
The etiology of the neurobehavioral problems in
children with CHD is complex, with contributions of innate factors
including genotype that cannot be modified and acquired factors
that potentially may be (6). These
acquired factors are secondary to chronic and acute hypoxia,
hypoperfusion, hematocrit, reperfusion injury, and surgery with
cardiopulmonary bypass and cross-clamp, and have a cumulative
effect (7,8). The developing fetus with congenital
heart defects may be exposed to hypoxia and abnormal cerebral
perfusion in utero and these insults on the brain may
continue during the neonatal period as a result of the failing
heart (9,10). The mechanisms of brain injury are
poorly understood but may involve multiple causative factors
including maternal infection, inflammation, hypoxia and
hypoperfusion, as observed in the development of periventricular
leukomalacia (PVL), characterized by cerebral white matter damage
(11–13). PVL has been documented by brain
magnetic resonance imaging (MRI) in 16% of children with congenital
heart disease and this incidence increases to 50% in children
following cardiac surgery with cardiopulmonary bypass (CPB)
(14). The duration of CPB,
complexity of the anatomical defect and surgical repair, and the
occurrence of cyanosis contribute to the postoperative systemic
inflammatory response. This involves activated complements and
lymphocytes, monocytes/macrophages, endothelial cells and myocytes
expressing cytokines, such as tumor necrosis factor (TNF)-α and
interleukins (ILs) (15–20). Postoperative systemic inflammatory
response syndrome is a complication occurring in pediatric
patients, particularly neonates undergoing corrective or palliative
heart surgery and remains a challenge during the postoperative
management of these patients (21–25).
Inflammation during early brain development has been implicated in
white matter damage and has been associated with cerebral palsy,
autism and neuropsychological disorders (26–29). In
experimental models, TNF-α induced myelin degeneration and
oligodendrocyte apoptosis, and transgenic animals overexpressing
TNF-α in the CNS exhibited demyelination and vacuolization
(30,31). White matter injury is a complication
of neonatal cardiac surgery and may occur in response to systemic
inflammation; however, this association remains unclear and may be
of relevance regarding patients undergoing highly complex cardiac
surgeries (32,33).
The blood-brain and blood-cerebrospinal fluid (CSF)
barriers restrict the passage of proteins into and out of the
brain. Research on the blood-brain barrier (BBB) in experimental
models has demonstrated that prolonged systemic inflammation in the
early post-natal period leads to a transient increase in the
permeability of blood vessels in the brain to serum proteins
(34). Similar to that observed with
ischemic or traumatic brain injury, it has been postulated that
cardiopulmonary bypass with concomitant inflammation and activation
of complements can lead to loss of integrity of the BBB and
blood-CSF barrier (35–38).
Previous studies have determined that there is an
association between the severity of the inflammatory response to
heart surgery and an increased need for medical intervention or
length of stay at hospitals (20,21,39–43). The
current study hypothesized that newborns with CHD and in whom
circulating pro-inflammatory mediators and complements were
elevated, would have measureable concentrations of CNS-derived
proteins, indicative of a compromised BBB and CNS injury. Serum
markers of brain injury (43)
include the high molecular weight (200 kDa) neurofilament protein
(NF-H) that is abundant in neurons and concentrated in axons. The
highly phosphorylated form of NF (pNF-H) is resistant to
proteolysis and when released from damaged axons remains largely
intact; thus, its presence in the blood is indicative of neuronal
damage (44,45). Other biomarkers measured included a
brain-specific isoform of enolase known as neuron specific enolase
(NSE) (46) and S100, a 20 kDa
protein belonging to the S100/calmodulin/troponin C superfamily of
calcium-binding proteins that forms heterodimers (S100A1B) and
homodimers (S100BB) in CNS glial cells and peripheral Schwann cells
(47,48).
Patients and methods
Patient demographics and diagnostic
classifications
The current prospective study conducted at the Cohen
Children's Medical Center of New York at Northwell Health
(Manhasset, NY, USA) was approved by the Northwell Health
Institutional Review Board and conducted in accordance with the
Declaration of Helsinki. Written informed consent was obtained from
parents on admission of patients to the hospital. A total of 23
patients with congenital heart disease requiring surgical repair
with cardiopulmonary bypass were enrolled in the present study
between January 2009 and August 2010. Full patient demographics are
listed in Table I. The lesions
consisted of hypoplastic left heart syndrome (n=8), transposition
of the great arteries (n=5), co-arctation of the aorta (n=3),
tricuspid atresia/pulmonary atresia (n=1), truncus arteriosus
communis (n=1), aortopulmonary window (n=1), endocardial cushion
defect (n=1), ventricular septal defect (n=1), anomalous right
pulmonary artery (n=1) and total anomalous pulmonary venous
connection (n=1). Two patients harbored known chromosomal
abnormalities, including one with DiGeorge syndrome (22q11
deletion). Criteria for exclusion were body weight <1,600 grams,
patent ductus arteriosus ligations, heart surgery without CPB,
newborns of mothers with autoimmune disease and suspected
congenital or postnatal infections.
 | Table I.Patient demographics and
peri-operative variables. |
Table I.
Patient demographics and
peri-operative variables.
| Variable | Mean ± SD | Median | Min-max |
|---|
| All patients
(n=23) |
|
|
|
| Male % (n) | 48 (11) |
|
|
| Gestational age,
weeks | 39±1.4 |
39.4 | 35.2–41.0 |
| Birth weight,
kg | 3.235±0.410 | 3.345 | 2.381–3.750 |
| Age at surgery,
days | 7.6±5 |
6.0 | 3–20 |
| Weight at surgery,
kg | 3.2±0.4 |
3.2 | 2.5–3.8 |
| Body mass index,
kg/m2 | 12.83±1.49 |
12.57 | 10.97–18.12 |
| Pre-operative
data |
|
|
|
|
SaO2 (%) | 87±11 |
90 | 60–99 |
| Hb
(g/dl) | 12.5±2.5 |
12.3 | 8.6–18.0 |
|
PaO2 (median value,
mmHg) | 48±29 |
40 | 25–154 |
|
PaCO2 (highest
value. mmHg) | 50±22 |
45 | 30–142 |
|
HCO3−
(lowest value, mEq/l) | 20.3±4.3 |
21.0 | 5–27 |
| pH | 7.27±0.16 |
7.31 | 6.71–7.42 |
| Lactate
(mg/dl) | 5.3±4.3 |
3.7 | 1.4–21.0 |
| MAP
(mmHg) | 48±4 |
48 | 40–56 |
| Intra-operative
data |
|
|
|
| Bypass
time, min | 141±49 |
139 | 44–240 |
|
Cross-clamp time, min | 71±32 |
72 | 21–14 |
| Postoperative data
(within 48 h) |
|
|
|
|
FiO2
requirement | 0.48±0.18 |
0.50 | 0.21–1.00 |
| Hb
(lowest value, g/dl) | 11.3±1.7 |
11.0 | 8.3–15.3 |
|
PaO2 (lowest value,
mmHg) | 74±37 |
64 | 29–160 |
|
PaCO2 (median
value, mmHg) | 49±8 |
50 | 35–60 |
| pH | 7.36±0.09 |
7.37 | 7.07–7.50 |
| Lactate
(highest value, mg/dl) | 8.0±6.6 |
6.0 | 1.9–25.0 |
|
SaO2 at discharge
% | 90±10 |
96 | 66–100 |
| MAP
(mmHg) | 52±5 |
50 | 45–66 |
|
Mechanical ventilation,
days | 10.0±15.9 |
5.0 | 1.0–69.0 |
|
Intensive care unit stay,
days | 13.3±14.5 |
9.0 | 4.0–69.0 |
|
Hospital stay, days | 20.8±13.5 |
17.0 | 7.0–69.0 |
Surgery, anesthesia and postoperative
care
The conduction of surgical procedures including
cardiopulmonary bypass and anesthesia followed our institutional
standard practices. Sixteen of the 23 patients underwent CPB with
deep hypothermic circulatory arrest with an average circulatory
arrest time of 20±16 min. Eleven of these 16 patients were treated
by ice cooling of the head. Twenty-one patients required blood
pressure medication intra-operatively and all patients received
methylprednisolone. Postoperative patient management in the
pediatric intensive care unit (PICU) followed the standard
institutional protocol. Decisions regarding the medical management
of each patient were based on clinical assessment made by attending
PICU physicians who were unaware of the study protocol and
analysis.
Clinical data acquisition and sample
collection
Patient clinical data were recorded, including
pre-operative variables such as birth weight, gestational age,
APGAR score at birth, head circumference, abnormal chromosomal
findings, abnormal head ultrasound findings, need for respiratory
support including intubation and fraction of inspired oxygen
(FiO2) requirement, pre-operative systemic saturation,
arterial pO2 and pCO2, lowest pre-operative
hematocrit, degree of acidosis, lactate levels, mean arterial
pressures, urine output, presence of infection, presence of
seizures, administration of vaccines or Rhogam, need for blood
transfusion, need of prostin infusion or inotropes, or balloon
atrial septostomy. Intra-operative data were recorded, including
weight and age at perfusion, length of cardiopulmonary bypass and
aortic cross-clamp time, administration of intra-op steroids and
degree of cooling. Postoperative variables were also measured and
included presence of arrhythmia, medication including inotropes and
diuretics, mean arterial blood pressure (MAP), degree of acidosis,
blood lactate levels, hematocrit, pO2 and
pCO2, FiO2 requirement, duration of
mechanical ventilation, duration of PICU care and hospitalization,
systemic oxygen saturation at discharge and any neurological
findings such as seizures, neurodevelopmental risk evaluation score
or brain imaging results. Brain imaging protocols included brain
ultrasound, computed tomography, electroencephalogram (EEG) or MRI
during routine postoperative care of the patient.
Blood samples from patients were drawn from central
intravenous catheters preoperatively and 2, 24 and 48 h following
CPB. Blood was allowed to clot at room temperature for 4 h and
subsequently centrifuged at 1,000 × g for 15 min at room
temperature. Serum was stored at −70°C until analysis. Serum
samples were also obtained from cord blood collected from 26
healthy term births that were donated to the Tissue Donation
Program of Northwell Health but which were not used for stem cell
collection due to insufficient cell counts. Data from these samples
were used as normative newborn values.
Analyses of serum samples
Commercially available ELISA or EIA kits were used
to measure serum concentrations of pNF-H (cat. no. RD191138300R;
BioVendor LLC, Asheville, NC, USA), human NSE (cat. no. 0050; Alpha
Diagnostic International, San Antonio, TX, USA), S-100B (cat. no.
708-85; Fujirebio Diagnostics, Inc., Göteborg, Sweden), C5a and
sC5b-9 (cat. no. A021 & A020; Quidel Corp., San Diego, CA,
USA). The Meso Scale Discovery multiplex assay kit (cat. no.
K15008C-2; MSD, Rockville, MD, USA) was used for simultaneous
measurement of cytokines IL-6, IL-8, IL-12p70, IL-1β, TNF-α,
interferon (INF)-γ, and IL-10 in each serum sample.
Immunoblot analysis
The specificity of the anti-pNF-H antibody used in
the ELISA was verified via immunoblot analysis comparing mouse
brain tissue with patient plasma samples. Murine brain lysate was
prepared as previously described (49). For immunoblot analysis, serum samples
(12 µl) from 2 randomly selected patients were diluted in Laemmli
buffer and separated by SDS-PAGE on 10% polyacrylamide gels,
transferred to nitrocellulose membranes and incubated at 4°C
overnight with monoclonal antibody against pNF-H (cat no.
IMG-5018A-2; 1:1,000; Novus Biologicals, LLC, Littleton, CO, USA).
Signal from horseradish peroxidase-conjugated secondary antibody
(cat. no. 170-6516; 1:5,000; Bio-Rad Laboratories, Inc., Hercules,
CA, USA) was developed using chemiluminescence reagent (Western
Lightning Electrochemiluminescence Pro; cat. no. NEL120001EA;
Perkin-Elmer, Inc., Waltham, MA, USA) and detected by exposure to
x-ray film. A sample of murine brain lysate (20 µg protein) was
used to positively identify the antibody reactive pNF-H protein
band.
Statistical analysis
Comparisons among variables were made using one-way
analysis of variance or Kruskal-Wallis test, as appropriate, for
continuous-type measures and the χ2 or Fisher's exact
test for categorical outcomes. Healthy controls were compared to
patients using the Mann-Whitney test for continuous measures and
the χ2 or Fisher's exact test for categorical outcomes.
Spearman correlations were calculated to determine the strength of
correlation between serum biomarkers. A mixed models approach to
repeated measures analysis of variance was conducted to determine
if the patterns of change across time with respect to each of the
biomarker levels differed between groups. Bonferroni-like adjusted
pairwise comparisons (P<0.01) were used post-hoc to determine
which time points differed from one another. Statistical analysis
was performed using SAS V9.3 (SAS Institute Inc., Cary, NC,
USA).
Results
Study patients and perioperative
data
Table I lists patient
demographics, mean (± standard deviation) and median values
(min-max range) of specific pre-, intra- and postoperative
physiological measures and outcome variables that were used for
statistical analyses.
Role of cyanosis
A total of 70% (16/23) of patients were cyanotic
prior to surgery, and they received surgery at a younger age
compared with the seven acyanotic patients (5.9±3.2 vs. 11.4±6.4
days, respectively). The cyanotic patients had significantly longer
bypass times (160±49 vs. 100±15 min; P=0.007) and total cross clamp
times (75±38 vs. 40±17 min; P=0.012) indicative of greater
complexity of surgical cardiac repair. When patients prior to
surgery were grouped as cyanotic or acyanotic, no statistically
significant differences in serum biomarkers either prior to or
following surgery were measured. Therefore, data from all patients
were combined for the statistical analyses presented herein.
Brain biomarkers prior to and
following cardiopulmonary bypass surgery
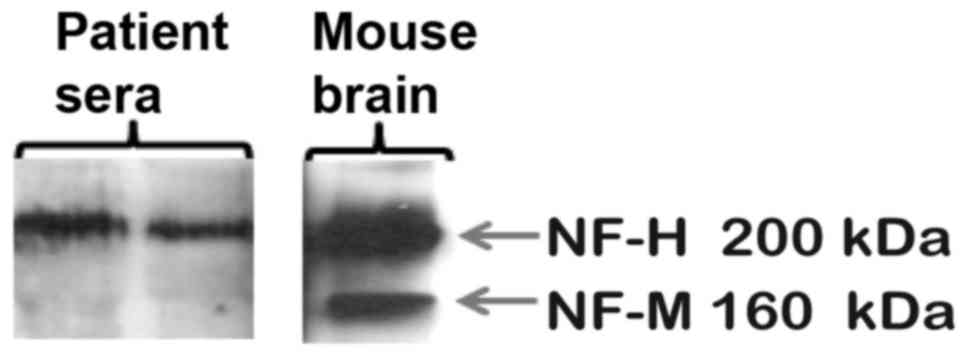
The presence of pNF-H in the serum of newborns with
CHD has not been previously reported. Therefore, the specificity of
the detection antibody used in the ELISA assay was tested by
immunoblot analysis of patient sera and mouse brain tissue known to
express pNF-H. The antibody recognized both high- and
medium-molecular weight NF proteins in mouse brain and a
corresponding 200 kDa protein in two patient serum samples, thus
confirming its specificity (Fig. 1).
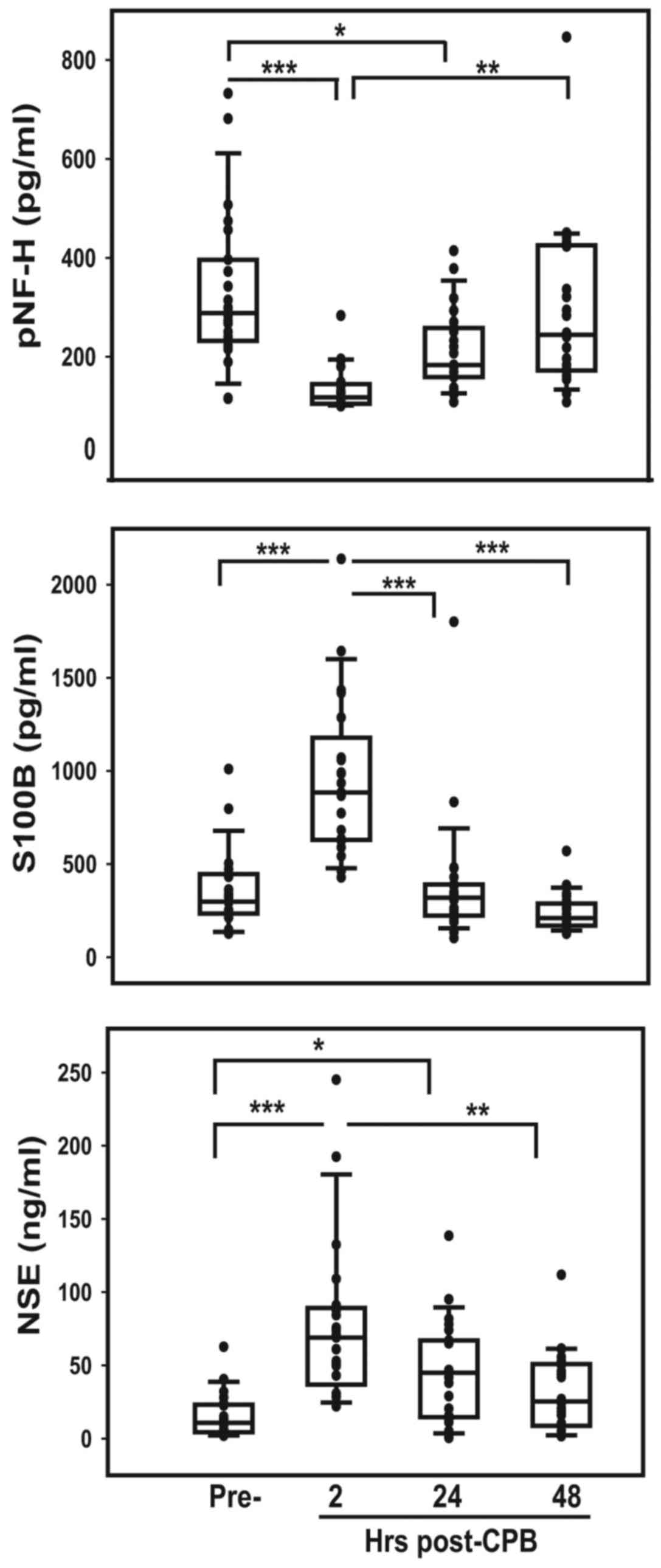
pNF-H concentration in the pre-surgical samples of patients was
2.4-fold higher (P<0.0001) than that measured in cord blood from
healthy term births (Table II). The
concentration of pNF-H in serum obtained 2 h after CPB was
significantly reduced compared with pre-surgery (P<0.0001) but
then accumulated during the subsequent period, reaching
pre-surgical levels 48 h following CPB (Fig. 2). S100B, a protein expressed in glial
cells, was also significantly higher in patient sera prior to
surgery compared with normal cord blood (P<0.00001; Table II) and increased significantly 2 h
following CPB (P<0.0001), further supporting potential CNS
injury in these patients (Fig. 2).
Both the rapid increase of S100B following CPB and subsequent rapid
decrease in 24 h likely reflects its reported short half-life (~60
min). The amount of NSE in patient sera prior to surgery did not
differ significantly from that in normal cord blood (Table II); however, it increased
significantly 2 h post-CPB (P<0.0001) and then slowly decreased
to pre-CPB levels over the subsequent 48 h, reflecting a longer
reported serum half-life of ~30 h (Fig.
2).
 | Table II.Serum concentrations of cytokines and
brain-derived biomarkers. |
Table II.
Serum concentrations of cytokines and
brain-derived biomarkers.
| Serum biomarker
(concentration) | Normal cord blood
Mean ± SD (n=20) | CHD patients
pre-surgery Mean ± SD (n=23) |
|---|
| pNF-H (pg/ml) | 136.8±53.9 |
328.7±155.7d |
| S100B (pg/ml) | 126.3±113.6 |
358.0±210.2d |
| NSE (ng/ml) | 20.27±9.93 | 15.50±15.17 |
| C5A (ng/ml) | 5.62±4.40 |
12.87±8.40b |
| sC5B9 (ng/ml) | 79.0±48.1 |
652.2±491.1d |
| INF-γ (pg/ml) | 0.18±0.13 | 0.40±0.39 |
| IL-10 (pg/ml) | 0.85±0.41 |
6.61±7.03d |
| IL-12p70
(pg/ml) | 0.15±0.26 |
0.59±1.38a |
| IL-1β (pg/ml) | 0.66±1.12 | 1.70±3.86 |
| IL-6 (pg/ml) | 1.86±1.08 |
11.01±17.99c |
| IL-8 (pg/ml) | 6.61±1.94 |
36.49±48.62d |
| TNF-α (pg/ml) | 4.43±1.12 |
14.02±8.01d |
Inflammation in neonates and response
to surgery
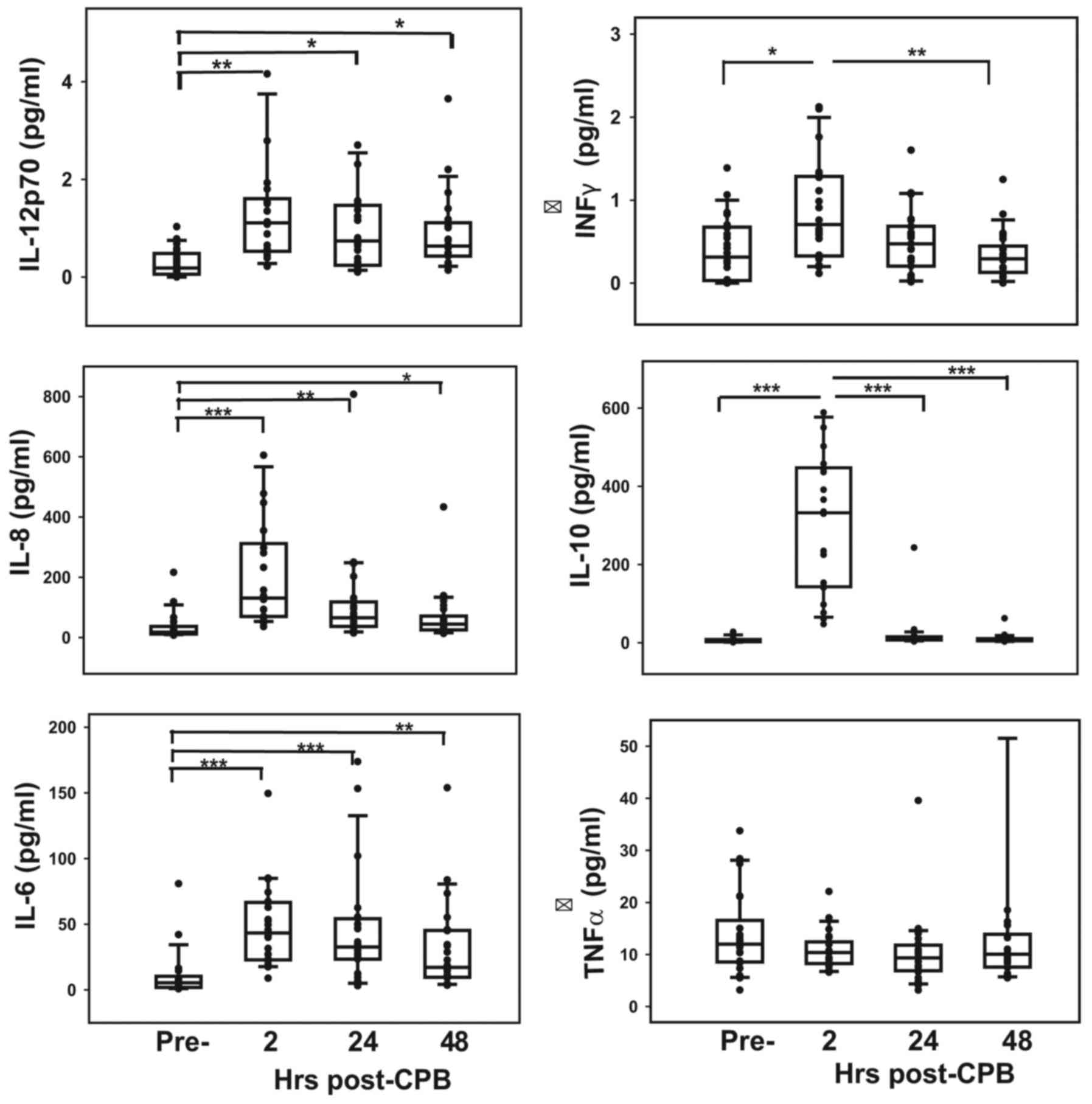
Concentrations of cytokines, apart from INF-γ and
IL-1β, were significantly lower in normal cord blood than in the
sera of patients prior to surgery (P<0.05) (Table II). The responses of these
inflammatory mediators to surgery are presented in Fig. 3. Serum levels of all ILs and INF-γ
peaked immediately following CPB (P<0.01) and then decreased
over the following 48 h (Fig. 3),
although levels of IL-1β increased slightly but not significantly
over 48 h post-CPB (data not shown). Serum TNF-α levels remained
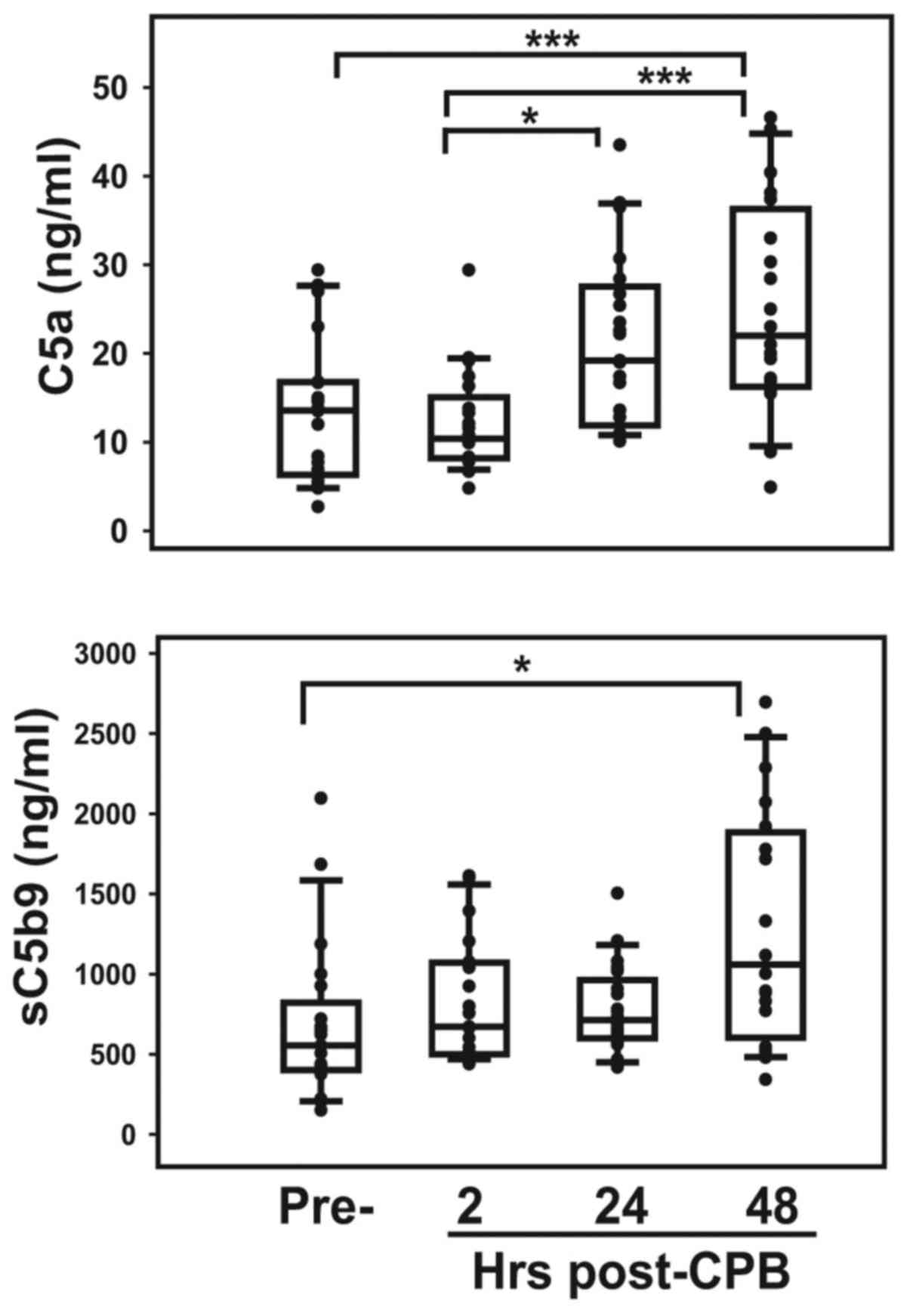
unchanged throughout the pre- and postoperative periods (Fig. 3). Complement activation, specifically
the serum level of soluble C5b9, which potentially measures
endothelial injury, was higher (P<0.0001) in patients prior to
surgery than in normal cord blood (Table II). Serum C5a and sC5b9
concentrations increased progressively following surgery, with
levels at 48 h being significantly higher than pre-surgical values
(P<0.01; Fig. 4).
Correlation between brain-derived
biomarkers and inflammatory cytokines prior to surgery
The systemic inflammatory response to heart surgery
with CPB in neonates has been well documented (15–21).
However, immune function in neonates with CHD prior to heart
surgery and its potential role on the clinical course of these
patients is less clear. In the present study, Spearman correlation
analysis demonstrated significant positive associations between the
age of patients at surgery and pre-surgical serum levels of the
cytokines (P<0.05; Table III).
The age-dependent increases in serum IL-12p70, INF-γ TNF-α and
IL-10 may be indicative of immune activation of T helper (Th) 1
immune response in these neonates. By contrast, the levels of the
interleukins IL-1β, IL-6 and IL-8 were not correlated with patient
age.
 | Table III.Correlations between serum biomarkers
and patient age. |
Table III.
Correlations between serum biomarkers
and patient age.
|
| TNF-α | INF-γ | IL-10 | IL-12p70 |
|---|
| Age at blood
draw/surgery | 0.500a | 0.638c | 0.491a | 0.656c |
| IL-12p70 | 0.544b | 0.737d | 0.576b | – |
| IL-8 | 0.660c | ns | ns | ns |
| sC5b9 | 0.450a | ns | ns | ns |
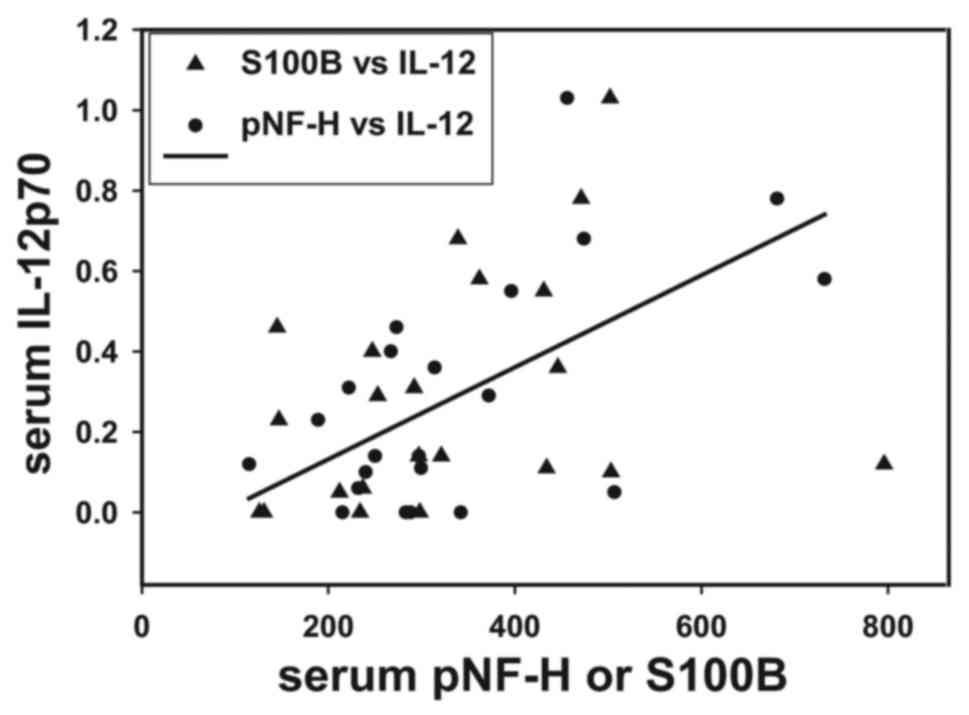
To support the hypothesis that inflammatory
mediators serve a role in BBB permeability, it was observed that
pre-surgical serum IL-12p70 concentrations correlated directly with
levels of the brain proteins pNF-H and S100B (r=0.442, P<0.05;
Fig. 5). Fig. 5 presents individual patient values
for these biomarkers and a linear regression line drawn between
serum pNF-H and IL-12p70.
It was examined whether pre-operative blood pressure
and/or inotropic support of patients correlated with inflammatory
biomarkers. A total of 15 patients treated with diuretics had
significantly higher serum TNF-α, IL-10, IL12p70, IL1β and IL-6
levels compared with untreated patients (P<0.05; Table IV). Seven of these 15 patients also
received dopamine and 8 patients (4 receiving both diuretics and
dopamine) had elevated blood creatinine levels compared with the
normal range for neonates (data not shown), suggesting that
patients who were hemodynamically unstable exhibited an augmented
inflammatory response. Furthermore, 12 patients requiring
ventilator support had significantly lower serum concentrations of
the anti-inflammatory cytokine IL-10, compared with those who were
not mechanically ventilated (P<0.05; Table IV). Of the other pre-operative
variables recorded, arterial blood pO2 correlated
inversely with serum pNF-H, suggesting a potential causal
relationship between hypoxemia and increased CNS injury (Table V).
 | Table IV.Effects of pre-operative treatments
on serum cytokine concentrations. |
Table IV.
Effects of pre-operative treatments
on serum cytokine concentrations.
|
|
| Serum cytokines
(pg/ml) |
|---|
|
|
|
|
|---|
| Treatment
variables | n | TNF-α | IL-10 | IL-12p70 | IL-1β | IL-6 |
|---|
| Ventilator
support |
|
|
|
|
|
|
|
(+) | 12 | ns |
3.72±2.88a | ns | ns | ns |
|
(−) | 10 | ns | 10.15±8.93 | ns | ns | ns |
| Diuretics |
|
|
|
|
|
|
|
(+) | 15 |
16.83±8.14a |
8.73±7.93a |
0.39±0.31a | 1.60±
2.73a |
14.84±21.58a |
|
(−) | 8 | 8.41±3.60 | 2.99±2.65 | 0.11±0.10 | 0.30±0.22 | 4.09±4.82 |
 | Table V.Correlations between pre- or
postoperative physiologic measurements and serum biomarkers. |
Table V.
Correlations between pre- or
postoperative physiologic measurements and serum biomarkers.
|
| Serum brain
biomarkers, complement, cytokines |
|---|
|
|
|
|---|
| Variables | pNF-H | S100B | NSE | C5a | sC5b9 | TNF-α | IL-10 | IL-12p70 | IL-8 |
|---|
| PRE-OP |
|
|
|
|
|
|
|
|
|
|
pO2 (median value
on ABG) | −0.493a | ns | ns | ns | ns | ns | ns | ns | ns |
|
pCO2 (highest value
on ABG) | ns | ns | ns | ns | ns | +0.448a | ns | ns | ns |
| POSTOP |
|
|
|
|
|
|
|
|
|
| MAP
(median daily value) | ns | ns | ns | +0.551b | ns | +0.407a | ns | ns | ns |
|
pO2 (lowest daily
value ABG) | ns | ns | ns | ns | −0.520b | ns | −0.437a | ns | −0.430a |
|
pCO2 (median daily
value ABG) | +0.426a | ns | ns | +0.423a | ns | ns | +0.449a | ns | ns |
| Lactate
(maximum daily value) | ns | ns | +0.631a | ns | −0.500a | ns | +0.553b | +0.727c | +0.475a |
|
FiO2
requirement | ns | ns | ns | ns | ns | +0.425a | +0.483a | ns | ns |
| O2
saturation at discharge | ns | −0.584b | −0.489a | ns | +0.663c | ns | −0.611b | ns | −0.556b |
| Length of
intubation | ns | ns | ns | ns | ns | ns | ns | +0.442a | +0.445a |
Postoperative outcomes and serum
biomarkers
Postoperative outcome variables demonstrating
statistically significant correlations with serum brain biomarkers,
cytokines and complements are presented in Table V. Correlations were determined
between physiological measurements recorded during the first or
second 24 h period post-CPB and blood samples obtained at 24 or 48
h. MAP during the first 2 days post-CPB correlated positively with
serum TNF-α and C5a. Measurements indicative of tissue hypoxia
including arterial blood gases (pO2, pCO2)
and lactate, and increased requirement for oxygen inhalation
(FiO2) correlated significantly with inflammatory
responses and complement activation. Levels of the brain biomarkers
pNF-H and NSE correlated directly with blood pCO2 and
lactate, respectively (both P<0.05) during the first 24 and 48 h
post-CPB, suggesting that hypoxia may have contributed to BBB
permeability and release of these brain proteins. Serum levels of
S100B and NSE, as well as IL-8 and IL-10, were inversely correlated
with blood oxygen saturation at hospital discharge (P<0.01,
P<0.05, P<0.01 and P<0.01, respectively) suggesting that
systemic inflammation following surgery was an important
determinant of clinical course (Table
V). The postoperative duration of mechanical ventilation
(length of intubation) was directly correlated with serum levels of
IL-12p70 and IL-8 measured 48 h post-CPB (both P<0.05; Table V).
Although only a small number of patients (six)
without chromosomal abnormalities exhibited anomalies in brain
imaging during their hospital course, they had significantly higher
serum levels of IL-12p70 (1.21±0.58 vs. 0.77±0.86 pg/ml, P<0.05)
and NSE (50.64±32.19 vs. 23.52±20.86 ng/ml, P<0.05) following
surgery compared with the 15 patients without any suspicion of CNS
pathology or clinical findings.
Discussion
In the present study, it was hypothesized that
newborns with CHD and specifically those with tissue hypoxia,
experience systemic inflammation as a consequence of the disease,
which results in dysfunction of the BBB and subsequent injury to
the CNS as measured by the presence of circulating CNS-derived
proteins. A number of studies have linked elevated levels of
circulating pro-inflammatory cytokines, including TNF-α and IL-6
that are derived from peripheral immune cells or sites of
organ/tissue injury, to BBB failure (50,51).
Evidence suggests that brain microvascular endothelial cell damage
may occur due to cytokine-induced reactive oxidative species
produced by NADPH oxidases or by altered function of endothelial
junction proteins (52,53) and that hypoxia-induced cytokine
production may be an initiating event (54). Brain biomarkers, including pNF-H,
S100B and NSE were detectable in patient serum prior to surgery and
the concentrations of pNF-H and S100B were ~2.5-fold higher in
patients than in normal cord blood. Both pNF-H and S100B levels
were directly correlated with serum IL-12p70 and the correlation
between pNF-H and IL-8 trended towards significance. These data
support a potential causal link between pro-inflammatory mediators
and BBB dysfunction with neuronal injury in these neonates.
Furthermore, pre-operative arterial pO2 correlated
inversely with serum pNF-H, while the strongest correlation with
pre-operative arterial pCO2 was with TNF-α, suggesting
that hypoxia and acidosis in neonatal patients with CHD may be an
initiating event for the peripheral inflammatory response with a
direct effect on CNS injury. This conclusion is further supported
by results obtained from the 24–48 h postoperative period, during
which serum pNF-H correlated significantly with arterial
pCO2, while NSE correlated directly with serum lactate.
Furthermore, NSE and S100B were inversely associated with arterial
oxygen saturation at discharge.
Modified ultrafiltration that allows for
hemoconcentration and recovery of circuit blood following CPB, has
been shown to remove certain inflammatory cytokines and components
of the complement system from the circulation (55). Serum concentrations of IL-6, IL-10,
TNF-α and C5b9 prior to and immediately following CPB in the
present study are similar to those published in a study by Berdat
et al (56) that demonstrated
that various ultrafiltration methods were effective at removing
such mediators to a varying extent. Despite ultrafiltration, levels
of the inflammatory mediators NSE and S100B, increased
significantly immediately following CPB, reflecting their
release/secretion and accumulation. Serum concentration of pNF-H, a
large 200 kDa protein, decreased following CPB compared to
pre-surgical levels, suggesting that it had been effectively
removed by ultrafiltration. One explanation for these changes could
be either volume expansion or hemoconcentration as a result of the
CPB procedure. However, the results of the current study suggest
that the temporal differences in serum concentrations of pNF-H, NSE
and S100B possibly depended on their individual half-lives. The
short half-life of S100B (90 min) predicted its rapid rise and fall
immediately following CPB (<24 h), whereas the relatively longer
half-life of NSE (~30 h) was reflected in its slower decline with
serum concentrations at 24 h remaining significantly higher than
pre-surgery. By contrast, the stable hyperphosphorylated NF-H
protein with a half-life >3 days, was slow to rise in serum and
took 48 h to reach concentrations similar to pre-surgery. Previous
studies have demonstrated that pNF is elevated in serum for weeks
following spinal cord or mild brain injury and in children with
febrile seizures (45,57). Although the kinetics of these
molecules in serum remain largely unknown, these data support the
hypothesis that the BBB remains permeable in the initial h
following CPB but begins to close within the first postoperative
day. Abu-Sultaneh et al (48)
demonstrated a significant correlation between peak serum S100B
following CPB with cerebral oxygen desaturation during surgery, as
measured by near-infrared spectroscopy. Furthermore, it has been
demonstrated that cerebral oxygen saturation is correlated with
neurodevelopmental outcomes and brain MRI at one year of age
(58). While brain biomarkers,
including S100B and NSE, have been studied in children in response
to cardiac surgery with CPB, to the best of our knowledge this
study isthe first to measure pNF-H in the serum of newborns with
CHD undergoing open-heart surgery with CPB.
Newborns with complex CHD have an increased risk of
white matter injury, reduced brain growth and delayed brain
development (6,59) and those requiring corrective
surgeries with CPB during the neonatal period experience an
inflammatory response (2,13,14,21,60,61).
Although inflammation during the perinatal period has been
determined to affect brain development in the pre-term infant
(28,29), the significance of inflammation on
the long-term neurocognitive development of children with CHD who
have undergone surgical cardiac repair remains unclear and its
significance is controversial (7,33). Upon
examination of various pre-operative treatments and postoperative
physiological outcome measurements, it was determined that patients
receiving diuretics and/or dopamine had significantly higher serum
concentrations of many cytokines, which cannot be explained solely
by changes in intravascular/extracellular fluid volumes. In the
postoperative period, levels of IL-10 and IL-8 correlated with
arterial hypoxemia and increased with blood lactate levels and
inhaled O2 requirements. Other cytokines, including
TNF-α and IL-12p70, similarly correlated with these post-surgical
parameters but with varying strengths of association. Thus, taken
together these data support an augmented systemic inflammatory
response to tissue hypoxia and acidosis, with an increased need for
mechanical ventilation with higher oxygen requirements in the
postoperative period, suggesting that these neonates may be at
higher risk of CNS injury.
In light of reports that activation of complement
leads to loss of integrity of the BBB and blood-CSF barrier, the
serum content of C5a was measured to assess activation of the
complement system and serum sC5b-9 to assess potential cellular
damage mediated by complement. Activation of the complement system
has been observed in intracranial inflammation following traumatic
brain injury with upregulation of soluble terminal complement
complex sC5b-9 in spinal cord fluid that correlated with degree of
BBB dysfunction (36). C5a and C3a
are known leukocyte chemotactic factors that can induce the release
of lysosomal enzymes and oxygen-derived free radicals, and can
stimulate cytokine production and release (62). In the present study, serum C5a and
sC5b9 were significantly higher in patients who had red blood cell
transfusion prior to surgery and sC5b9 correlated directly with
serum levels of TNF-α and IL-1β. In the postoperative period C5a
and sC5b9 correlated with various parameters indicative of arterial
hypoxemia, elevated blood pressure and increased length of hospital
stay. To the best of our knowledge, this is the first report
demonstrating complement activation in this setting.
Pagowska-Klimek et al (63)
have recently reported that cardiac surgery with CPB activated the
lectin pathway of complement activation and this response was
significantly greater in pediatric patients presenting with
post-bypass systemic inflammation. Further study is warranted in
assessing the role of complement activation in brain injury in
infants with CHD, particularly regarding novel therapeutic advances
directed at the complement system to treat autoimmune diseases that
may be repurposed for treatment of newborns with CHD (64–66).
It was observed that there was a significant
correlation between patient age at surgery (between 3 and 20 days)
and pre-surgical serum levels of TNF-α, IL-12p70, INF-γ and IL-10.
The strongest correlation was between patient age at surgery and
levels of IL-12 and INF-γ, which are Th1 cytokines. Newborns
exhibit a bias toward a Th2 cell-dominated cytokine response,
including the release of IL-4, IL-6 and IL-10; however, with
increasing age and maturation of dendritic cells and their
production of IL-12 in response to INF-γ, a shift to a Th1 cell
response occurs (67,68). The predominance of Th2 cytokine
production in the fetus and neonate serves to dampen innate immune
responses, protecting the newborn from Th1-induced affects
(69,70). The capacity of mononuclear cells to
secrete a number of inflammatory cytokines increases with age from
2 months through one year to adulthood; however, monocytes from
normal cord blood, which is essentially newborn blood, have
increased capacity in response to pathogens to secrete TNF-α, IL-6,
IL-10 and INF-γ (but not IL-12) at levels observed in adults
(71,72). Similarly, the observation that serum
concentrations of TNF-α, INF-γ and IL-12p70 increased in neonates
as they lived longer with their disease prior to heart surgery
suggests that the production of Th1 cytokines was in response to
disease-induced effects rather than normal maturation of immunity
towards Th1 cell polarization. Gestational age and weight have been
reported to be important determinants of the neonate's immune
response to infection (73). In the
current study, mean gestational age was 39 weeks and no neonates
were small for their gestational age, suggesting that these
newborns had the capacity to elicit an age-appropriate innate
immune response. Therefore, the current study concludes that term
newborns with congenital heart disease may be able to elicit an
age-appropriate immune response; however, this response to their
disease process may promote an excessive pro-inflammatory Th1
response potentially leading to end-organ damage, including
increased permeability of the BBB and neuronal injury. New avenues
of investigation, including studies examining the response of
isolated peripheral blood mononuclear cells from neonates with CHD
to various pathogenic and danger signals may advance understanding
of innate immunity in this unique patient group and improve
approaches to their medical care.
Limitations of the current study are the small
sample size and absence of data from age-matched normal neonates
aged between 3 and 30 days. Cord blood was analyzed from healthy
term deliveries to provide surrogate normative newborn values.
Although none of the study patients were small for gestational age
or born pre-maturely, prospective collection of cord blood from
these subjects would be preferable. A further limitation is the
inclusion of congenital heart disease pathologies with varying
degrees of severity and complexity. Despite these limitations,
consistent immune responses were observed in this patient
population, lending support to the significance of the results.
In summary, the results of the present study
demonstrate that neonates with congenital heart disease requiring
surgical repair/palliation during the first month of life have
elevated serum levels of pNF-H, a CNS-derived axonal protein that
suggests brain injury with loss of BBB integrity. Concentrations of
complement and pro-inflammatory cytokines in the patients' serum
were found to be higher than normal cord blood, and these
biomarkers of inflammation were significantly correlated with pre-
and post-operative measurements of tissue hypoxia/acidosis, and an
increased need for mechanical ventilation with higher oxygen
requirements, suggesting that these neonates may be at higher risk
of CNS injury.
Acknowledgements
The present study was funded in part by the National
Institutes of Health, National Center for Research Resources, The
Feinstein Institute for Medical Research at Northwell Health
(grant. no. M01RR018535).
References
|
1
|
Go AS, Mozaffarian D, Roger VL, Benjamin
EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al:
Heart disease and stroke statistics-2014 update: A report from the
american heart association. Circulation. 129:e28–e292. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller SP, McQuillen PS, Hamrick S, Xu D,
Glidden DV, Charlton N, Karl T, Azakie A, Ferriero DM, Barkovich AJ
and Vigneron DB: Abnormal brain development in newborns with
congenital heart disease. N Engl J Med. 357:1928–1938. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sherlock RL, McQuillen PS and Miller SP:
aCCENT: Preventing brain injury in newborns with congenital heart
disease: Brain imaging and innovative trial designs. Stroke.
40:327–332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marino BS, Lipkin PH, Newburger JW,
Peacock G, Gerdes M, Gaynor JW, Mussatto KA, Uzark K, Goldberg CS,
Johnson WH Jr, et al: Neurodevelopmental outcomes in children with
congenital heart disease: Evaluation and management: A scientific
statement from the American heart association. Circulation.
126:1143–1172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gaynor JW, Stopp C, Wypij D, Andropoulos
DB, Atallah J, Atz AM, Beca J, Donofrio MT, Duncan K, Ghanayem NS,
et al: Neurodevelopmental outcomes after cardiac surgery in
infancy. Pediatrics. 135:816–825. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tabbutt S, Gaynor JW and Newburger JW:
Neurodevelopmental outcomes after congenital heart surgery and
strategies for improvement. Curr Opin Cardiol. 27:82–91. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsia TY and Gruber PJ: Factors influencing
neurologic outcome after neonatal cardiopulmonary bypass: What we
can and cannot control. Ann Thorac Surg. 81:S2381–S2388. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gaynor JW, Wernovsky G, Jarvik GP,
Bernbaum J, Gerdes M, Zackai E, Nord AS, Clancy RR, Nicolson SC and
Spray TL: Patient characteristics are important determinants of
neurodevelopmental outcome at one year of age after neonatal and
infant cardiac surgery. J Thorac Cardiovasc Surg. 133:1344–1353,
1353 e1-3. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Licht DJ, Wang J, Silvestre DW, Nicolson
SC, Montenegro LM, Wernovsky G, Tabbutt S, Durning SM, Shera DM,
Gaynor JW, et al: Preoperative cerebral blood flow is diminished in
neonates with severe congenital heart defects. J Thorac Cardiovasc
Surg. 128:841–849. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Donofrio MT, Duplessis AJ and
Limperopoulos C: Impact of congenital heart disease on fetal brain
development and injury. Curr Opin Pediatr. 23:502–511. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dammann O and Leviton A: Maternal
intrauterine infection, cytokines, and brain damage in the preterm
newborn. Pediatr Res. 42:1–8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kadhim H, Tabarki B, Verellen G, De Prez
C, Rona AM and Sébire G: Inflammatory cytokines in the pathogenesis
of periventricular leukomalacia. Neurology. 56:1278–1284. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mahle WT, Tavani F, Zimmerman RA, Nicolson
SC, Galli KK, Gaynor JW, Clancy RR, Montenegro LM, Spray TL,
Chiavacci RM, et al: An MRI study of neurological injury before and
after congenital heart surgery. Circulation. 106 12 Suppl
1:I109–I114. 2002.PubMed/NCBI
|
|
14
|
Galli KK, Zimmerman RA, Jarvik GP,
Wernovsky G, Kuypers MK, Clancy RR, Montenegro LM, Mahle WT, Newman
MF, Saunders AM, et al: Periventricular leukomalacia is common
after neonatal cardiac surgery. J Thorac Cardiovasc Surg.
127:692–704. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Casey LC: Role of cytokines in the
pathogenesis of cardiopulmonary-induced multisystem organ failure.
Ann Thorac Surg. 56 5 Suppl:S92–S96. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Steinberg JB, Kapelanski DP, Olson JD and
Weiler JM: Cytokine and complement levels in patients undergoing
cardiopulmonary bypass. J Thorac Cardiovasc Surg. 106:1008–1016.
1993.PubMed/NCBI
|
|
17
|
Gessler P, Pfenninger J, Pfammatter JP,
Carrel T and Dahinden C: Inflammatory response of neutrophil
granulocytes and monocytes after cardiopulmonary bypass in
pediatric cardiac surgery. Intensive Care Med. 28:1786–1791. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alcaraz AJ, Manzano L, Sancho L, Vigil MD,
Esquivel F, Maroto E, Reyes E and Alvarez-Mon M: Different
proinflammatory cytokine serum pattern in neonate patients
undergoing open heart surgery. Relevance of IL-8. J Clin Immunol.
25:238–245. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kozik DJ and Tweddell JS: Characterizing
the inflammatory response to cardiopulmonary bypass in children.
Ann Thorac Surg. 81:S2347–S2354. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mahle WT, Matthews E, Kanter KR, Kogon BE,
Hamrick SE and Strickland MJ: Inflammatory response after neonatal
cardiac surgery and its relationship to clinical outcomes. Ann
Thorac Surg. 97:950–966. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Allan CK, Newburger JW, McGrath E, Elder
J, Psoinos C, Laussen PC, Del Nido PJ, Wypij D and McGowan FX Jr:
The relationship between inflammatory activation and clinical
outcome after infant cardiopulmonary bypass. Anesth Analg.
111:1244–1251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heying R, Wehage E, Schumacher K, Tassani
P, Haas F, Lange R, Hess J and Seghaye MC: Dexamethasone
pretreatment provides antiinflammatory and myocardial protection in
neonatal arterial switch operation. Ann Thorac Surg. 93:869–876.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kubicki R, Grohmann J, Siepe M, Benk C,
Humburger F, Rensing-Ehl A and Stiller B: Early prediction of
capillary leak syndrome in infants after cardiopulmonary bypass.
Eur J Cardiothorac Surg. 44:275–281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Graham EM, Atz AM, McHugh KE, Butts RJ,
Baker NL, Stroud RE, Reeves ST, Bradley SM, McGowan FX Jr and
Spinale FG: Preoperative steroid treatment does not improve markers
of inflammation after cardiac surgery in neonates: Results from a
randomized trial. J Thorac Cardiovasc Surg. 147:902–908. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Floh AA, Nakada M, La Rotta G, Mah K,
Herridge JE, Van Arsdell G and Schwartz SM: Systemic inflammation
increases energy expenditure following pediatric cardiopulmonary
bypass. Pediatr Crit Care Med. 16:343–351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Patterson PH: Maternal infection: Window
on neuroimmune interactions in fetal brain development and mental
illness. Curr Opin Neurobiol. 12:115–118. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luciana M: Cognitive development in
children born preterm: Implications for theories of brain
plasticity following early injury. Dev Psychopathol. 15:1017–1047.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
O'Shea TM, Shah B, Allred EN, Fichorova
RN, Kuban KC, Dammann O and Leviton A; ELGAN Study Investigators, :
Inflammation-initiating illnesses, inflammation-related proteins,
and cognitive impairment in extremely preterm infants. Brain Behav
Immun. 29:104–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hagberg H, Mallard C, Ferriero DM,
Vannucci SJ, Levison SW, Vexler ZS and Gressens P: The role of
inflammation in perinatal brain injury. Nat Rev Neurol. 11:192–208.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Selmaj KW and Raine CS: Tumor necrosis
factor mediates myelin and oligodendrocyte damage in vitro. Ann
Neurol. 23:339–346. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Taupin V, Renno T, Bourbonnière L,
Peterson AC, Rodriguez M and Owens T: Increased severity of
experimental autoimmune encephalomyelitis, chronic
macrophage/microglial reactivity, and demyelination in transgenic
mice producing tumor necrosis factor-alpha in the central nervous
system. Eur J Immunol. 27:905–913. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li X, Robertson CM, Yu X, Cheypesh A, Dinu
IA and Li J: Early postoperative systemic inflammatory response is
an important determinant for adverse 2-year
neurodevelopment-associated outcomes after the Norwood procedure. J
Thorac Cardiovasc Surg. 148:202–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Desai NK, Hamrick SE, Strickland MJ,
Matthews E, McMaster L and Mahle WT: White matter injury and the
inflammatory response following neonatal cardiac surgery. Pediatr
Cardiol. 36:942–949. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stolp HB, Dziegielewska KM, Ek CJ, Potter
AM and Saunders NR: Long-term changes in blood-brain barrier
permeability and white matter following prolonged systemic
inflammation in early development in the rat. Eur J Neurosci.
22:2805–2816. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chenoweth DE, Cooper SW, Hugli TE, Stewart
RW, Blackstone EH and Kirklin JW: Complement activation during
cardiopulmonary bypass: Evidence for generation of C3a and C5a
anaphylatoxins. N Engl J Med. 304:497–503. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stahel PF, Morganti-Kossmann MC, Perez D,
Redaelli C, Gloor B, Trentz O and Kossmann T: Intrathecal levels of
complement-derived soluble membrane attack complex (sC5b-9)
correlate with blood-brain barrier dysfunction in patients with
traumatic brain injury. J Neurotrauma. 18:773–781. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lassiter HA: The role of complement in
neonatal hypoxic-ischemic cerebral injury. Clin Perinatol.
31:117–127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Okamura T, Ishibashi N, Zurakowski D and
Jonas RA: Cardiopulmonary bypass increases permeability of the
blood-cerebrospinal fluid barrier. Ann Thorac Surg. 89:187–194.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ashraf SS, Tian Y, Zacharrias S, Cowan D,
Martin P and Watterson K: Effects of cardiopulmonary bypass on
neonatal and paediatric inflammatory profiles. Eur J Cardiothorac
Surg. 12:862–868. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McMahon CK, Klein I and Ojamaa K:
Interleukin-6 and thyroid hormone metabolism in pediatric cardiac
surgery patients. Thyroid. 13:301–304. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Madhok AB, Ojamaa K, Haridas V, Parnell
VA, Pahwa S and Chowdhury D: Cytokine response in children
undergoing surgery for congenital heart disease. Pediatr Cardiol.
27:408–813. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Merchant S, Nadaraj S, Chowdhury D,
Parnell VA, Sison C, Miller EJ and Ojamaa K: Macrophage migration
inhibitory factor in pediatric patients undergoing surgery for
congenital heart repair. Mol Med. 14:124–130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Toman E, Harrisson S and Belli T:
Biomarkers in traumatic brain injury: A review. J R Army Med Corps.
162:103–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shaw G, Yang C, Ellis R, Anderson K,
Mickle J Parker, Scheff S, Pike B, Anderson DK and Howland DR:
Hyperphosphorylated neurofilament NF-H is a serum biomarker of
axonal injury. Biochem Biophys Res Commun. 336:1268–1277. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hayakawa K, Okazaki R, Ishii K, Ueno T,
Izawa N, Tanaka Y, Toyooka S, Matsuoka N, Morioka K, Ohori Y, et
al: Phosphorylated neurofilament subunit NF-H as a biomarker for
evaluating the severity of spinal cord injury patients, a pilot
study. Spinal Cord. 50:493–496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schmitt B, Bauersfeld U, Schmid ER,
Tuchschmid P, Molinari L, Fanconi S and Bandtlow C: Serum and CSF
levels of neuron-specific enolase (NSE) in cardiac surgery with
cardiopulmonary bypass: A marker of brain injury? Brain Dev.
20:536–539. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lardner D, Davidson A, McKenzie I and
Cochrane A: Delayed rises in serum S100B levels and adverse
neurological outcome in infants and children undergoing
cardiopulmonary bypass. Paediatr Anaesth. 14:495–500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Abu-Sultaneh S, Hehir DA, Murkowski K,
Ghanayem NS, Liedel J, Hoffmann RG, Cao Y, Mitchell ME, Jeromin A,
Tweddell JS and Hoffman GM: Changes in cerebral oxygen saturation
correlate with S100B in infants undergoing cardiac surgery with
cardiopulmonary bypass. Pediatr Crit Care Med. 15:219–228. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Faust TW, Chang EH, Kowal C, Berlin R,
Gazaryan IG, Bertini E, Zhang J, Sanchez-Guerrero J, Fragoso-Loyo
HE, Volpe BT, et al: Neurotoxic lupus autoantibodies alter brain
function through two distinct mechanisms. Proc Natl Acad Sci USA.
107:pp. 18569–18574. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rochfort KD and Cummins PM: The
blood-brain barrier endothelium: A target for pro-inflammatory
cytokines. Biochem Soc Trans. 43:702–706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mir IN and Chalak LF: Serum biomarkers to
evaluate the integrity of the neurovascular unit. Early Hum Dev.
90:707–711. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Forster C, Burek M, Romero IA, Weksler B,
Couraud PO and Drenckhahn D: Differential effects of hydrocortisone
and TNFalpha on tight junction proteins in an in vitro model of the
human blood-brain barrier. J Physiol. 586:1937–1949. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rochfort KD, Collins LE, Murphy RP and
Cummins PM: Downregulation of blood-brain barrier phenotype by
proinflammatory cytokines involves NADPH oxidase-dependent ROS
generation: Consequences for interendothelial adherens and tight
junctions. PLoS One. 9:e1018152014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
McAdams RM and Juul SE: The role of
cytokines and inflammatory cells in perinatal brain injury. Neurol
Res Int. 2012:5614942012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jönsson H, Johnsson P, Bäckström M, Alling
C, Dautovic-Bergh C and Blomquist S: Controversial significance of
early S100B levels after cardiac surgery. BMC Neurol. 4:242004.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Berdat PA, Eichenberger E, Ebell J,
Pfammatter JP, Pavlovic M, Zobrist C, Gygax E, Nydegger U and
Carrel T: Elimination of proinflammatory cytokines in pediatric
cardiac surgery: Analysis of ultrafiltration method and filter
type. J Thorac Cardiovasc Surg. 127:1688–1696. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Matsushige T, Inoue H, Fukunaga S,
Hasegawa S, Okuda M and Ichiyama T: Serum neurofilament
concentrations in children with prolonged febrile seizures. J
Neurol Sci. 321:39–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kussman BD, Wypij D, Laussen PC, Soul JS,
Bellinger DC, DiNardo JA, Robertson R, Pigula FA, Jonas RA and
Newburger JW: Relationship of intraoperative cerebral oxygen
saturation to neurodevelopmental outcome and brain magnetic
resonance imaging at 1 year of age in infants undergoing
biventricular repair. Circulation. 122:245–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
von Rhein M, Buchmann A, Hagmann C, Dave
H, Bernet V, Scheer I, Knirsch W and Latal B; Heart and Brain
Research Group, : Severe congenital heart defects are associated
with global reduction of neonatal brain volumes. J Pediatr.
167:1259–1263.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Licht DJ, Shera DM, Clancy RR, Wernovsky
G, Montenegro LM, Nicolson SC, Zimmerman RA, Spray TL, Gaynor JW
and Vossough A: Brain maturation is delayed in infants with complex
congenital heart defects. J Thorac Cardiovasc Surg. 137:529–537.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ortinau C, Beca J, Lambeth J, Ferdman B,
Alexopoulos D, Shimony JS, Wallendorf M, Neil J and Inder T:
Regional alterations in cerebral growth exist preoperatively in
infants with congenital heart disease. J Thorac Cardiovasc Surg.
143:1264–1270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Barnum SR: Complement: A primer for the
coming therapeutic revolution. Pharmacol Ther. Dec 1–2016.(Epub
ahead of print). PubMed/NCBI
|
|
63
|
Pagowska-Klimek I, Świerzko AS, Michalski
M, Glowacka E, Szala-Poździej A, Sokolowska A, Moll M, Krajewski
WR, Romak J and Cedzyński M: Activation of the lectin pathway of
complement by cardiopulmonary bypass contributes to the development
of systemic inflammatory response syndrome after paediatric cardiac
surgery. Clin Exp Immunol. 184:257–263. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Holers VM, Tomlinson S, Kulik L, Atkinson
C, Rohrer B, Banda N and Thurman JM: New therapeutic and diagnostic
opportunities for injured tissue-specific targeting of complement
inhibitors and imaging modalities. Semin Immunol. 28:260–267. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zuercher AW, Spirig R, Baz Morelli A and
Käsermann F: IVIG in autoimmune disease-Potential next generation
biologics. Autoimmun Rev. 15:781–785. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kolev MV, Tediose T, Sivasankar B, Harris
CL, Thome J, Morgan BP and Donev RM: Upregulating CD59: A new
strategy for protection of neurons from complement-mediated
degeneration. Pharmacogenomics J. 10:12–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zaghouani H, Hoeman CM and Adkins B:
Neonatal immunity: Faulty T-helpers and the shortcomings of
dendritic cells. Trends Immunol. 30:585–591. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Diesner SC, Förster-Waldl E, Olivera A,
Pollak A, Jensen-Jarolim E and Untersmayr E: Perspectives on
immunomodulation early in life. Pediatr Allergy Immunol.
23:210–223. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Marodi L: Innate cellular immune responses
in newborns. Clin Immunol. 118:137–144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Levy O and Wynn JL: A prime time for
trained immunity: Innate immune memory in newborns and infants.
Neonatology. 105:136–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yerkovich ST, Wikström ME, Suriyaarachchi
D, Prescott SL, Upham JW and Holt PG: Postnatal development of
monocyte cytokine responses to bacterial lipopolysaccharide.
Pediatr Res. 62:547–552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Upham JW, Lee PT, Holt BJ, Heaton T,
Prescott SL, Sharp MJ, Sly PD and Holt PG: Development of
interleukin-12-producing capacity throughout childhood. Infect
Immun. 70:6583–6585. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Strunk T, Currie A, Richmond P, Simmer K
and Burgner D: Innate immunity in human newborn infants:
Prematurity means more than immaturity. J Matern Fetal Neonatal
Med. 24:25–31. 2011. View Article : Google Scholar : PubMed/NCBI
|