Introduction
A variety of serious infection factors induce
endotoxic and septic shock, which are common clinical pathological
processes (1,2). A previous study has demonstrated that
intravenous injection of lipopolysaccharide (LPS) attenuated both
norepinephrine (NE)-induced vascular contraction and
acetylcholine-induced vasodilation in the aortas of rats (3). Liang et al (4) also indicated that LPS treatment
decreased the vascular reactivity of superior mesenteric arteries
to a α1AR agonist (phenylephrine) after 2 h in rabbits. These
results demonstrated that endotoxic shock induced hypovascular
reactivity during the late stage, which is an important factor of
microcirculation disturbance and tissue hypoperfusion, and further
leads to multiple organ injuries and dysfunction (1,2). A
number of studies have indicated that resveratrol (Res) has a
variety of biological activities (5,6).
Previous studies have also revealed that Res alleviated the
endotoxic shock-induced structural damage and dysfunction of vital
organs, including the lung, kidney and liver (7–9).
Mechanisms of Res have been demonstrated to be associated with
reducing oxidative stress and inflammation and improving
microcirculation perfusion status (8–13).
However, whether or not Res enhances vascular reactivity following
endotoxic shock remains unclear. Tseng et al (14) indicated that LPS-induced
hyporeactivity of isolated mesenteric arteries is associated with
the decreased phosphorylation of Rho kinase (ROCK) and suppression
of Ras homolog gene family, member A (RhoA) activities in smooth
muscle of arteries. However, an increase of RhoA activity may
compensate for vascular reactivity in early endotoxaemia induced by
LPS injection, and suppression of RhoA activity may eventually lead
to vascular hyporeactivity in late endotoxaemia (15). In conclusion, the RhoA has an
important role in LPS-induced vascular hyporeactivity. Therefore,
the present study hypothesized that Res treatment alleviates
LPS-induced vascular hyporeactivity via the activation of the
RhoA-ROCK-myosin light chain phosphatase (MLCP) pathway. To assess
this hypothesis, the current study observed the role and mechanism
of Res on vascular reactivity subjected to LPS challenge using a
microvascular tension measurement technique. The current study may
provide novel experimental evidence for the treatment of vascular
hyporeactivity induced by endotoxic shock.
Materials and methods
Animals
A total of 72 healthy, male and specific
pathogen-free BALB/c mice (weight, 23–27 g; age, 3 months) were
purchased from the National Institutes for Food and Drug Control
[Beijing, China; SCXK (jing) 2009–0017], and housed in clear
plastic cages at a temperature of 23±1°C and humidity of 40–50%,
with a 12-h light/dark cycle and access to water and food as
indicated. Mice were randomly divided into four groups, as follows:
Sham, Sham + Res, LPS and LPS + Res (n=18 per group). A total of 6
mice in each group were used for the observation of survival time,
6 mice in each group were used for the investigation of pressor
response to NE in vivo and the remaining 6 mice in each
group were used for the investigation of vascular reactivity ex
vivo. Prior to the experiment, the mice were fasted for 12 h
and were allowed ad libitum access to water. All
experimental procedures involving animals were reviewed and
approved by the Institutional Animal Care and Use Committee of
Hebei North University (Zhangjiakou, China) and conformed to the
National Institutes of Health guidelines. All efforts were made to
minimize the suffering of the animals during the experiment.
Model preparation and survival
assessment
Mice were anesthetized with 1% pentobarbital sodium
(70 mg/kg; Merck KGaA, Darmstadt, Germany; H8060) in a volume of
0.16–0.19 ml. Under the guidance of a stereomicroscope (SZ61;
Olympus Corporation, Shanghai, China), surgery on the neck was
performed in order to separate the right jugular vein from the
surrounding tissues. Following a 30-min stabilization period, LPS
administration (0.5%, 5 mg/kg Escherichia coli; O111:B4;
Sigma-Aldrich; Merck KGaA) was performed via the right jugular vein
in the LPS and LPS + Res groups. The same amount of saline (NaCl),
instead of LPS, was administered in the sham and sham + Res groups.
Subsequently, jugular vein ligation, wound suture and disinfection
with iodine were performed in sequence. After 1 h of LPS or saline
administration, an intramuscular injection of Res (30 mg/kg,
Sigma-Aldrich; Merck KGaA) was administered to mice in the LPS +
Res and sham + Res groups. The dosage of Res used in the present
study was in accordance with previous studies (16,17). The
vehicle, dimethyl sulfoxide, was administered instead of Res to the
mice in the sham and LPS groups. Subsequently, the survival
assessment was performed. Because the mice in the LPS and LPS + Res
groups were all sacrificed within 24 h, therefore, in this current
study, survival time and survival rate of 24 h were recorded in
each group (n=6) and the observed end-point was 24 h. Surviving
mice in the sham and sham + Res groups were anesthetized at 24 h
with 1% pentobarbital sodium (70 mg/kg) via intraperitoneal
injection and sacrificed using cervical dislocation. The full
duration of this experiment was 24 h, while subsequent experiments
were completed.
Pressor response to NE in vivo
In addition to the aforementioned treatment,
following anesthetization and administration of intravenous heparin
(500 U/kg, 1 ml/kg; RongSheng Pharmaceutical Co., Ltd., Jiaozuo,
China), the mice were subjected to femoral surgery in order to
separate the right femoral artery from the surrounding tissues. A
minimally heparinized microcatheter was subsequently introduced
into the right femoral artery to monitor mean arterial pressure
(MAP) during the experiment, using the PowerLab biological signal
collection and processing system (ML818; ADInstruments Australia,
Bella Vista, NSW, Australia). At 6 h after LPS or saline
administration, NE (4.2 µg/kg) was injected into the jugular vein.
Changes in MAP at pre- and post-administration of NE were recorded
and the maximal difference of MAP (ΔMAP) was presented as an index
of vascular reactivity in vivo (18,19) (n=6
per group). Subsequently, the mice were sacrificed by cervical
dislocation. The present experiment continued 7 h from
anesthetization to sacrifice of mice, and there was no animal
sacrificed during the experiment.
Vascular reactivity ex vivo
A total of 24 mice received treatment (n=6 from each
group) at the same observed end-point as described. At 6 h after
LPS or corresponding sham administration, under anesthesia as
described above, a laparotomy was performed prior to cervical
dislocation and a loop of intestine with mesenteric tissues was
removed and placed into chilled physiological saline solution (PSS;
in mmol/l: 118.99 NaCl, 4.69 KCl, 1.17 MgSO4, 25.0
NaHCO3, 1.20 KH2PO4, 2.50
CaCl2, 0.03 EDTA·Na2 and 5.5 glucose at pH 7.3–7.4),
which was continuously bubbled with 95% O2/5%
CO2. Suitable mesentery arteries were identified and
excised under the operating microscope. Four segments of 2 mm in
length were prepared and transferred to an isolated vessel chamber
of wire myograph system (620M; Danish Myo Technology A/S, Aarhus N,
Denmark) filled with chilled PSS. Each vascular ring was cannulated
onto two 2.5-cm lengths of stainless steel wire (diameter, 40 µm)
in the raw; namely, no traction and tension. Chambers were mounted
onto a wire myograph. Following a 5-min stabilization period, the
passive tension (preload) calculated with the standardized program
was initially set using the myograph system. Normalization was
performed by stretching the segment stepwise and measuring the
micrometer and force readings. These data are converted into values
of internal circumference (µm) and wall tension T (mN/mm),
respectively. Subsequently, the bath temperature was slowly
increased to 37°C within 20 min and the vessel was equilibrated for
30 min in PSS, which was continuously bubbled with 95%
O2/5% CO2, with the solution being changed
and the transducer re-zeroed at the end of the equilibration
period. When the temperature reached 37°C, the vessel underwent
normalization and activation. The vessel was activated with NE
(1×10−3 mol/l) and a high potassium solution (2.70 NaCl,
120.0 KCl, 0.45 MgSO4, 25.0 NaHCO3, 1.20
KH2PO4, 2.50 CaCl2 and 5.5 glucose
mmol/l at pH 7.3–7.4). Following 3 to 5 washes with PSS solution
for 10 min, NE was administered to all samples at final
concentrations of 1×10−7, 3×10−7,
1×10−6, 3×10−6, 1×10−5 and
3×10−5 mol/l using a concentration accumulation method
with an interval of 80 sec, respectively, as in previous studies
(18,19). The difference of tension and baseline
induced by NE was recorded using the PowerLab system as the
vascular tension response. Following the experiment, the
dose-response line was constructed using SigmaPlot software,
version 11.0 (Systat Software, Inc., San Jose, CA, USA). Effective
concentration at 50% (EC50) and negative log of molar concentration
(pD2) were used to identify the -log 50% effective concentration of
the dose-response line using the curve-fitting method.
Dose-response line, maximum tension (Emax), and pD2 were presented
as indices of vascular tension reactivity. The full duration of
micro-vascular reactivity observation was 3 h.
When the contractile response to NE was measured in
PSS, the vessels were incubated with tool reagents of RhoA, ROCK
and MLCP in an isolated vessel chamber with PSS bubbled with 95%
O2/5% CO2 for 10 min, and then the
contractile response to NE was measured again to determine the role
of these signal molecules in vascular reactivity. Briefly, two
segments of microvascular vessels from the sham group were treated
with RhoA agonist U-46619 (Alexis, Inc., Lausen, Switzerland; final
concentration, 5×10−7 mol/l) or MLCP inhibitor okadaic
acid (OA; Enzo, Inc., New York, NY, USA; final concentration,
1×10−6 mol/l) for 10 min to measure the contractile
response to NE. Following 3 to 5 washes with PSS, the two segment
vessels were treated with RhoA inhibitor C3 transferase
(Cytoskeleton, Inc., Denver, CO, USA; final concentration, 60 µg/l)
or ROCK inhibitor Y-27632 (Alexis, Inc.; final concentration,
6×10−6 mol/l) for 10 min for the measurement of the
response to NE. Vessels from the sham + Res group were treated with
the same procedure as the sham group. Only one segment of
microvascular vessel from the LPS group was treated with OA and
measured for a response to NE, after 3 to 5 washes with PSS. The
segment was then treated with OA and Y-27632 together and the
response to NE was measured. The other segment of microvascular
vessel from the LPS group was treated with U-46619 and U-46619 plus
Y-27632 for the measurement of the response to NE. Similarly, the
vascular reactivity to NE of the LPS + Res group was measured
following treatment with Y-27632, Y-27632 plus U-46619, C3
transferase and C3 transferase plus U-46619 for the measurement of
the response to NE.
Measurement of p-RhoA, p-Mypt1 and
MLCP
At 6 h after LPS or corresponding treatment, the
mesenteric arteries were separated under an operating microscope,
and stored at −75 to −80°C. Subsequently, the vascular tissue was
pulverized using a MagNA Lyser automatic tissue homogenizer (Roche
Diagnostics International AG, Rotkreuz, Switzerland) with 50 µl
cell lysis buffer (Beyotime Biotechnology Research Institute,
Shanghai, China), and was centrifuged at 850 × g at 0–4°C for 10
min using the 3–30K high-speed refrigerated Laborzentrifugen (Sigma
Laborzentrifugen GmbH, Osterode am Harz, Germany). Phosphorylated
(p-)RhoA, p-myosin phosphatase target subunit 1 (Mypt1) and MLCP
levels were examined using the mouse-specific ELISA kits (Jiangsu
Hope, Inc., Zhenjiang, China; for p-RhoA, HBT-0587M; p-Mypt1,
HBT-0684M; and MLCP, HBT-0622M) in accordance with the
manufacturer's protocol. Antibodies for p-RhoA (ab41435; 1:2,000),
p-Mypt1 (ab59203; 1:4,000), and MLCP (ab59235; 1:4,000) were
purchased from Abcam (Cambridge, MA), and used for the ELISA
analysis. Protein concentration of the vascular homogenate was
measured using a bicinchoninic acid protein assay kit (Applygen
Technologies Inc., Beijing, China), according to the manufacturer's
instructions, for the normalization of protein levels.
Statistical analysis
Data in this study were expressed as mean + or ±
standard deviation or mean ± standard error as indicated. For the
comparison of more than two conditions, a one-way analysis of
variance (ANOVA) with Turkey's post hoc test or repeated-measures
ANOVA with Bonferroni post hoc test were used. For survival time,
Kaplan-Meier analysis was used, followed by a Log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of Res treatment on the
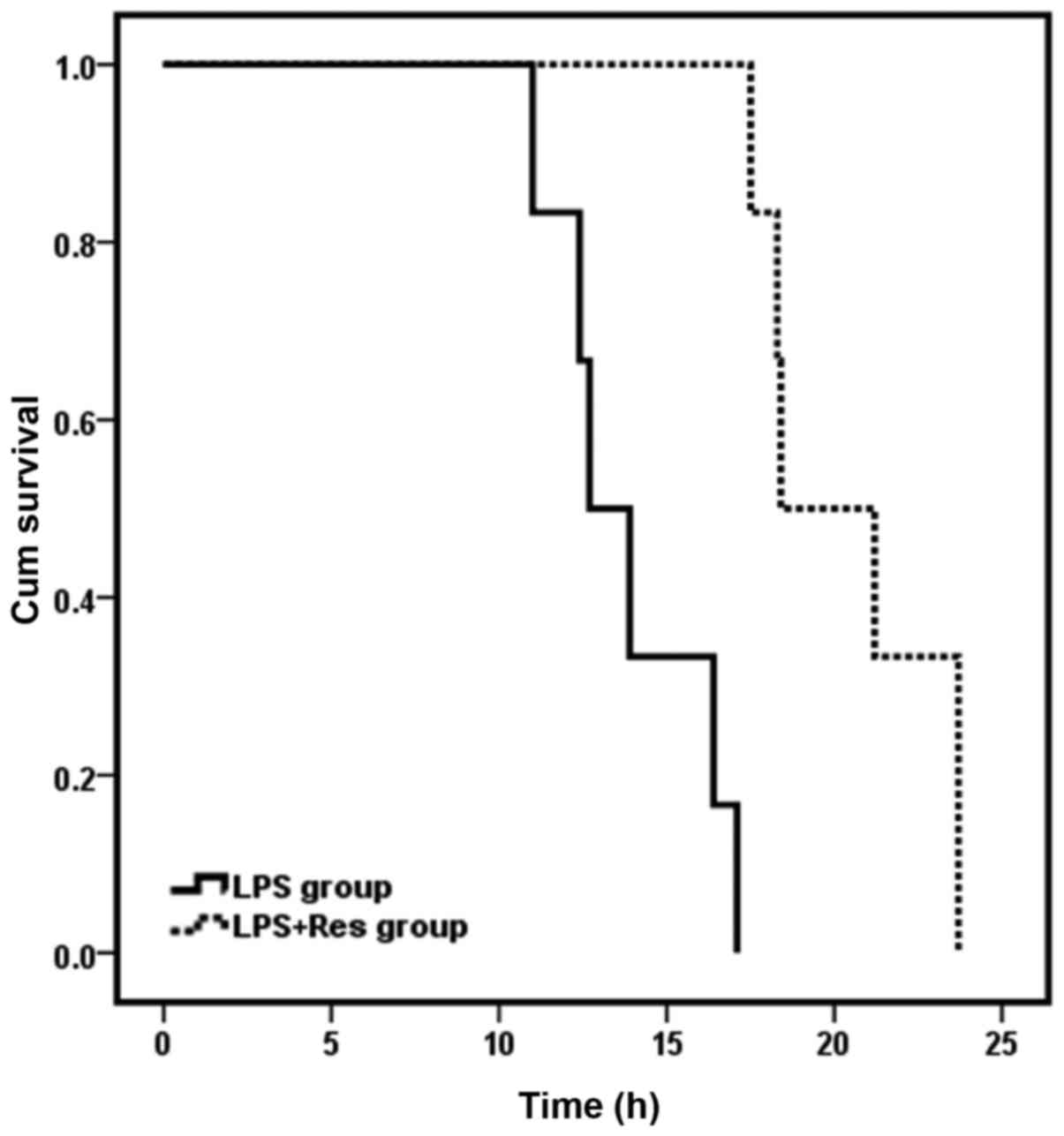
survival time of mice subjected to LPS challenge
Observation of survival time was initiated at the
point of intravenous injection of NaCl/LPS. Mice in the sham and
sham + Res groups survived for 24 h and the 24 h survival rate was
100% for these groups. Survival time of mice in the LPS + Res group
was 20.45±2.81 h, which was significantly longer than the LPS group
(13.89±2.37 h; P<0.05). However, the 24-h survival rate for the
LPS and LPS + Res groups was 0%. The survival curve of the LPS and
LPS + Res groups is presented in Fig.
1.
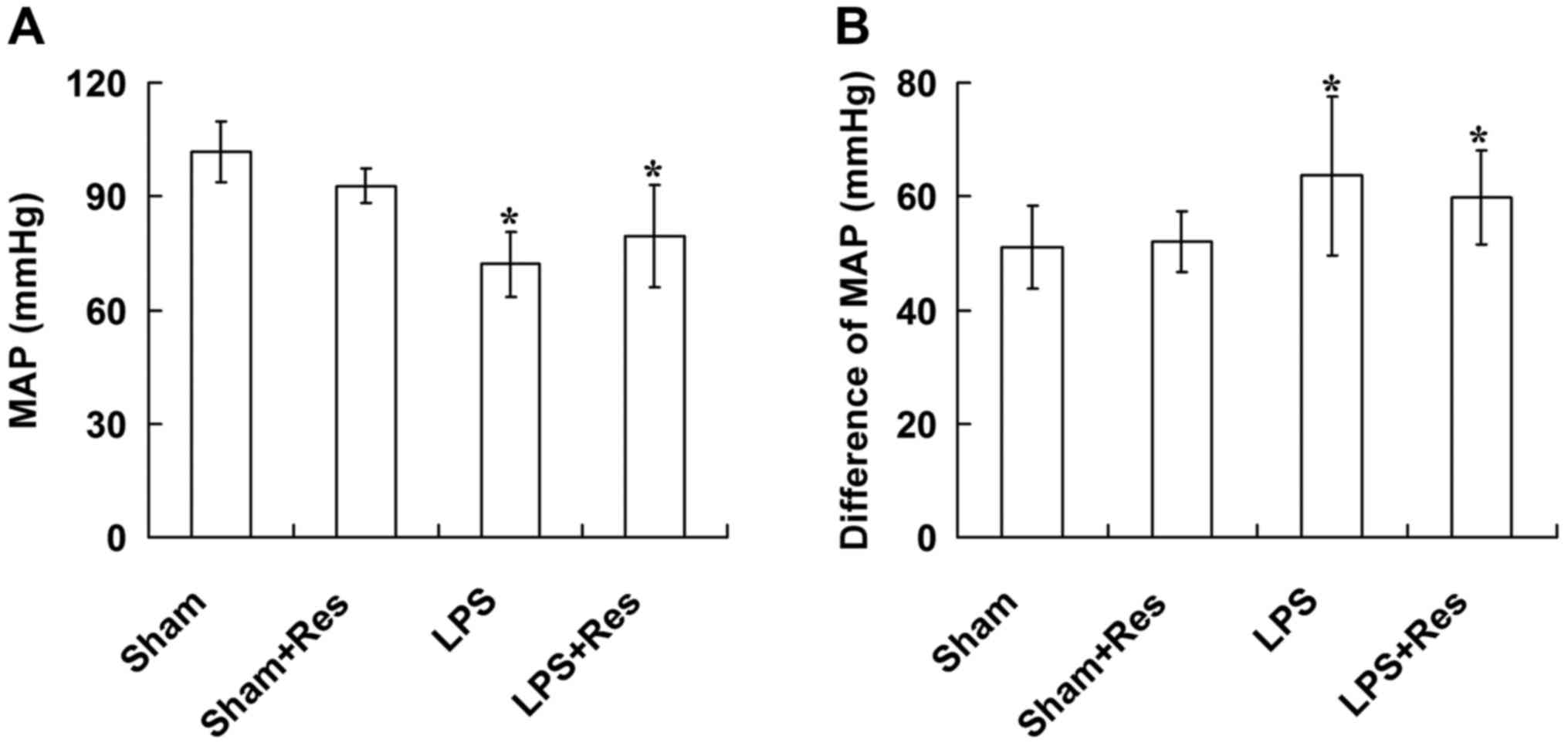
Effect of Res treatment on MPA and
ΔMAP levels of mice subjected to LPS challenge
In the LPS and LPS + Res groups, MAP was
significantly decreased and ΔMAP was significantly increased when
compared with that of the sham and sham + Res groups, 6 h after
intravenous injection of NaCl/LPS (P<0.05; Fig. 2). There were no significant
differences in the MAP and ΔMAP levels between the sham and sham +
Res groups or the LPS and LPS + Res groups (P>0.05; Fig. 2).
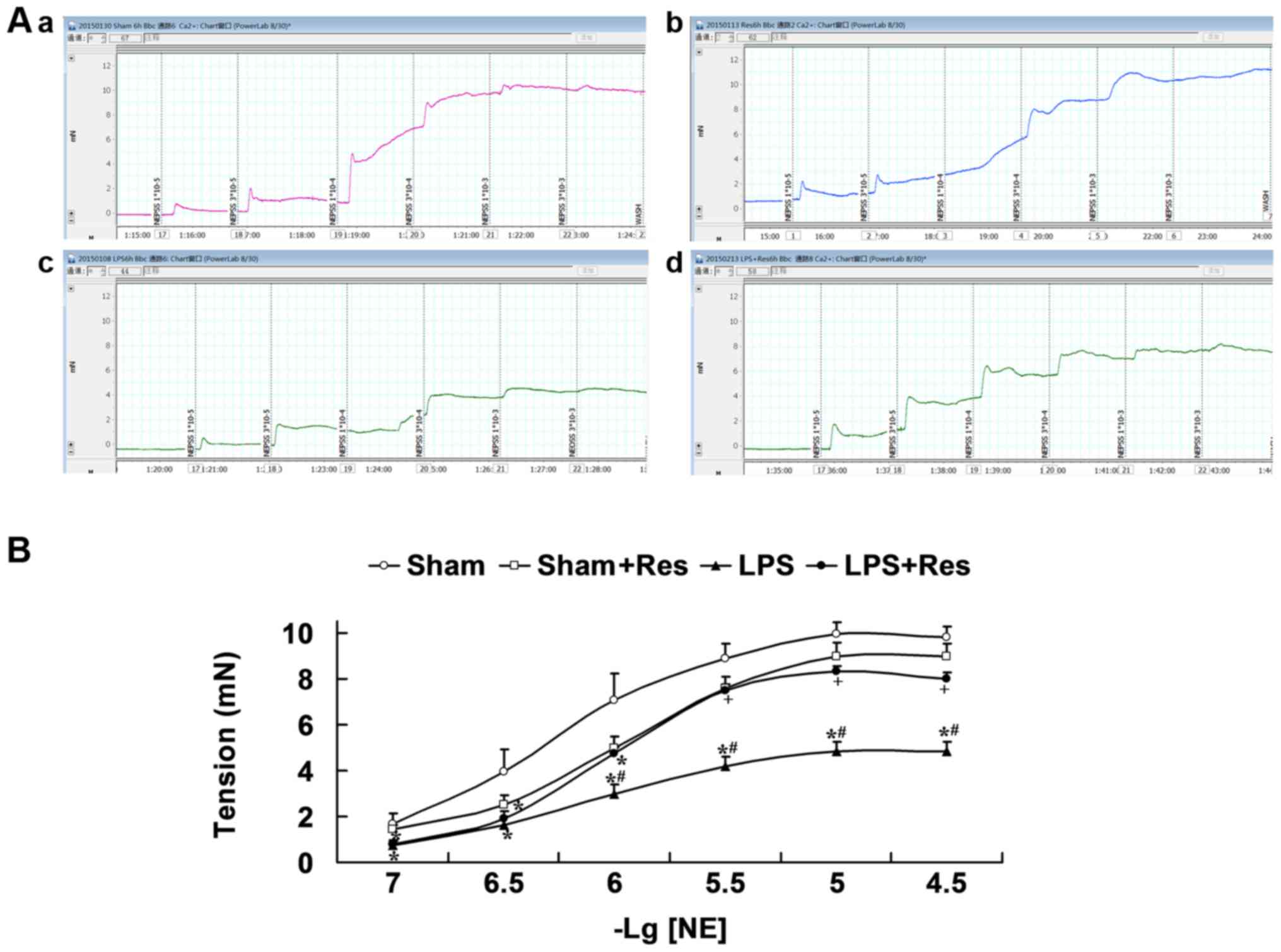
Effect of Res treatment on the
vascular reactivity of mice subjected to LPS challenge
Fig. 3 and Table I indicate that there were no
significant differences in the response of mouse arteriole rings to
NE in the sham and sham + Res groups (P>0.05), with the
exception of the 1×10−6 mol/l concentration between the
sham and sham + Res groups. The response of mouse arteriole rings
to NE in the LPS group was significantly decreased when compared
with that of the sham group for 3×10−5,
1×10−5, 3×10−6, 1×10−6,
3×10−7 and 1×10−7 mol/l, and the sham + Res
group for 3×10−5, 1×10−5, 3×10−6
and 10−6 mol/l (P<0.05). Emax of the LPS group was
significantly decreased compared with that of the sham and sham +
Res groups (P<0.05). Concentration-response curves of arteriole
rings to NE in the LPS + Res group were shifted markedly to the
left, the tension reactivity induced by NE at 3×10−5,
1×10−5 and 3×10−6 mol/l and the Emax were
significantly enhanced compared with that of the LPS group
(P<0.05).
 | Table I.Effects of Res on the Emax and
pD2 of micro-vessels to norepinephrine in mice following
LPS intravenous injection. |
Table I.
Effects of Res on the Emax and
pD2 of micro-vessels to norepinephrine in mice following
LPS intravenous injection.
| Group | Emax, mN | pD2 |
|---|
| Sham | 10.03±1.16 | 4.20±0.28 |
| Sham + Res | 9.12±1.41 |
3.95±0.14a |
| LPS |
4.89±1.10a,b | 4.05±0.19 |
| LPS + Res |
8.30±0.59a,c | 4.04±0.08 |
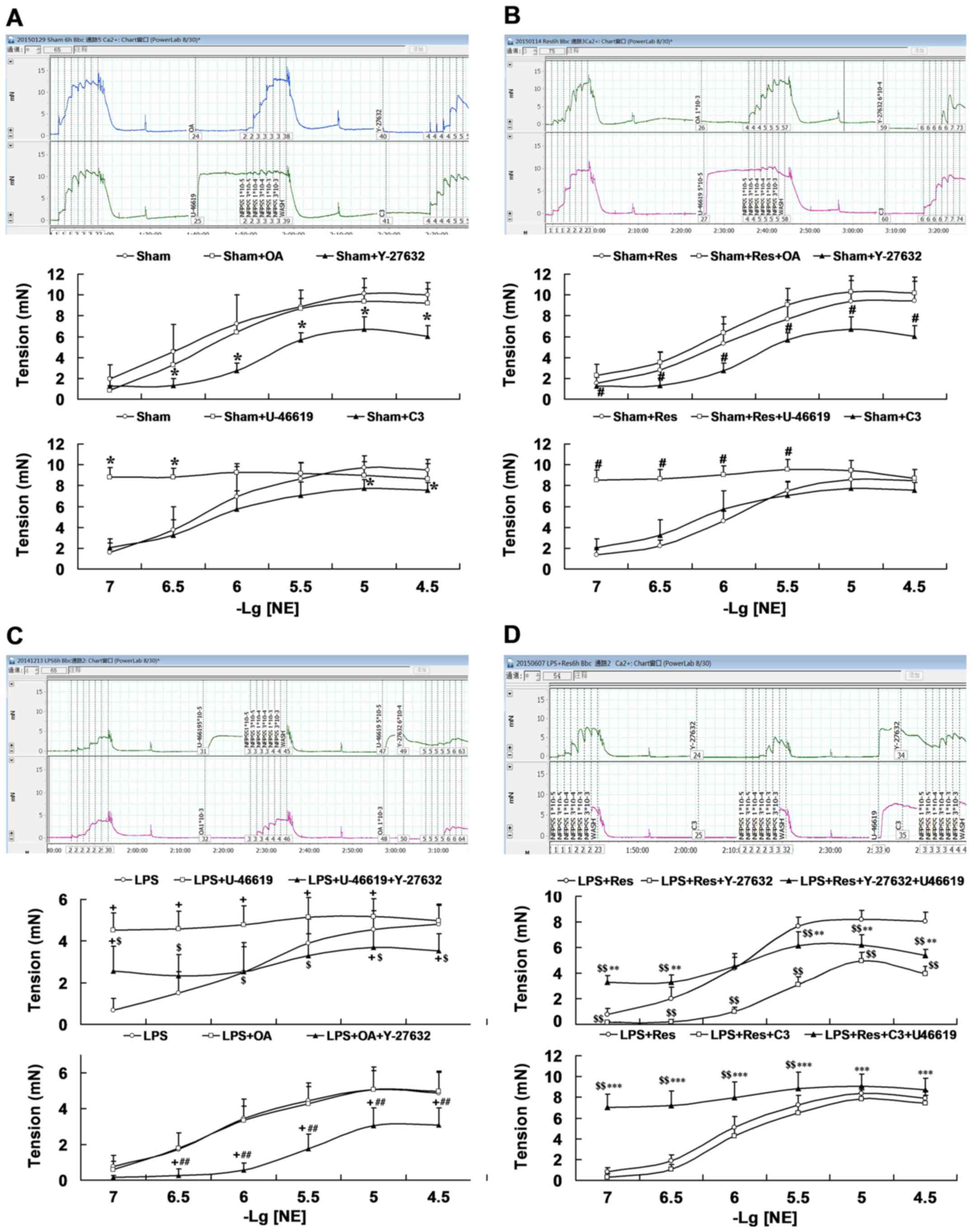
Role of the RhoA-ROCK-MLCP signal
pathway in Res treatment improved the vascular reactivity in vitro
of mice subjected to LPS challenge
Results from different tool agents on the arteriole
rings reactivity to NE in the sham mice indicated that the RhoA
agonist U-46619 increased the response of arteriole rings (at
3×10−7 and 1×10−7 mol/l of NE), but exhibited
no significant difference in Emax, and that both the RhoA inhibitor
C3 transferase (at 3×10−5 and 1×10−5 mol/l of
NE) and ROCK inhibitor Y-27632 (at 3×10−5,
1×10−5, 3×10−6, 1×10−6 and
3×10−7 mol/l of NE) significantly decreased the response
(P<0.05; Fig. 4A; Table II). Emax and Y-26732 also
significantly decreased the response of PD2 of arteriole rings
(P<0.05; Fig. 4A; Table II). However, the MLCP inhibitor OA
had no significant effect (P>0.05). Similarly, U-46619 treatment
increased the response (at 3×10−7 and 1×10−7
mol/l of NE) and Y-27632 incubation significantly decreased the
response (at 3×10−5, 1×10−5,
3×10−6, 1×10−6 and 3×10−7 mol/l of
NE) of Emax and the PD2 of arteriole rings compared with the
sham+Res group (P<0.05; Fig. 4B;
Table II).
 | Table II.Effects of agonists or inhibitors of
RhoA-RhoA kinase-myosin light chain phosphatase pathway on the Emax
and pD2 of micro-vessels to norepinephrine. |
Table II.
Effects of agonists or inhibitors of
RhoA-RhoA kinase-myosin light chain phosphatase pathway on the Emax
and pD2 of micro-vessels to norepinephrine.
| Group | Emax, mN | pD2 |
|---|
| Sham | 9.73±1.14 | 4.16±0.32 |
| Sham + U-46619 | 9.55±0.78 | 3.96±0.41 |
| Sham + C3 |
7.78±0.86a | 4.15±0.27 |
| Sham | 10.21±1.28 | 4.25±0.30 |
| Sham + OA | 9.50±1.27 | 4.22±0.12 |
| Sham + Y-26732 |
6.73±1.19a |
3.79±0.11a |
| Sham + Res | 8.67±1.00 | 3.94±0.11 |
| Sham + Res +
U-46619 | 9.57±0.95 | 3.76±0.51 |
| Sham + Res +
C3 | 8.06±1.50 | 4.08±0.12 |
| Sham + Res | 9.58±1.91 | 3.98±0.22 |
| Sham + Res +
OA | 10.42±1.52 | 4.01±0.06 |
| Sham + Res +
Y-26732 |
6.68±2.03b |
3.54±0.20b |
| LPS | 4.82±1.00 | 3.92±0.29 |
| LPS + U-46619 | 5.27±0.90 | 3.90±0.17 |
| LPS + U-46619 +
Y-27632 |
3.75±0.92c,d | 3.68±0.27 |
| LPS | 5.09±1.24 | 4.10±0.16 |
| LPS + OA | 5.09±1.07 | 4.19±0.18 |
| LPS + OA +
Y-27632 |
3.25±0.88c,d |
3.56±0.21c,d |
| LPS + Res | 8.38±0.61 | 4.07±0.09 |
| LPS + Res + C3 | 7.86±0.86 | 4.01±0.17 |
| LPS + Res + C3 +
U-46619 |
9.20±1.26e | 3.88±0.23 |
| LPS + Res | 8.25±0.70 | 4.02±0.13 |
| LPS + Res +
Y-27632 |
4.93±0.68f |
3.63±0.11f |
| LPS + Res + Y-27632
+ U-46619 |
6.38±0.93e,f |
3.95±0.20e |
As presented in Fig.
4C and Table II, RhoA agonist
U-46619 increased the response of arteriole rings harvested from
the LPS challenged mice (at 1×10−5, 3×10−6,
1×10−6, 3×10−7, and 1×10−7 mol/l
of NE; P<0.05), which was significantly reduced following ROCK
inhibitor Y-27632 co-incubation (at 3×10−5,
1×10−5, 3×10−6, 1×10−6,
3×10−7 and 1×10−7 mol/l of NE; P<0.05),
along with decreased Emax. The MLCP inhibitor, OA, had no
significant effect on arteriole rings from LPS-challenged mice.
However, the combination of OA and Y-27632 significantly decreased
the response to NE (at 3×10−5, 1×10−5,
3×10−6, 1×10−6 and 3×10−7 mol/l),
Emax and PD2 of the arteriole rings harvested from LPS
challenged mice and, following treatment with OA (P<0.05;
Fig. 4C).
As indicated in Fig.
4D and Table II, ROCK inhibitor
Y-27632 significantly decreased the response to NE
(3×10−5, 1×10−5, 3×10−6,
1×10−6, 3×10−7 and 1×10−7 mol/l),
Emax and PD2 of arteriole rings harvested from mice of
the LPS+Res group (P<0.05). RhoA agonist U-46619 suppressed the
effect of Y-27632 on vascular response at any concentration of NE,
leading to significant increases at concentrations of
3×10−7 and 1×10−7 mol/l and Emax, and
significant decreases at concentrations of 3×10−5,
1×10−5 and 3×10−6 mol/l when compared with
that of the LPS + Res group (P<0.05). Furthermore, RhoA
inhibitor C3 transferase exhibited an inhibiting trend on the
response of arteriole rings harvested from mice of the LPS + Res
group, but no significant statistical differences were observed.
U-46619 plus C3 transferase co-incubation significantly increased
the response and Emax of arteriole rings harvested from mice of the
LPS + Res group compared with those incubated with C3 transferase
(3×10−5, 1×10−5, 3×10−6,
1×10−6, 3×10−7 and 1×10−7 mol/l)
or not (at 3×10−6, 1×10−6, 3×10−7
and 1×10−7 mol/l; P<0.05; Fig. 4D).
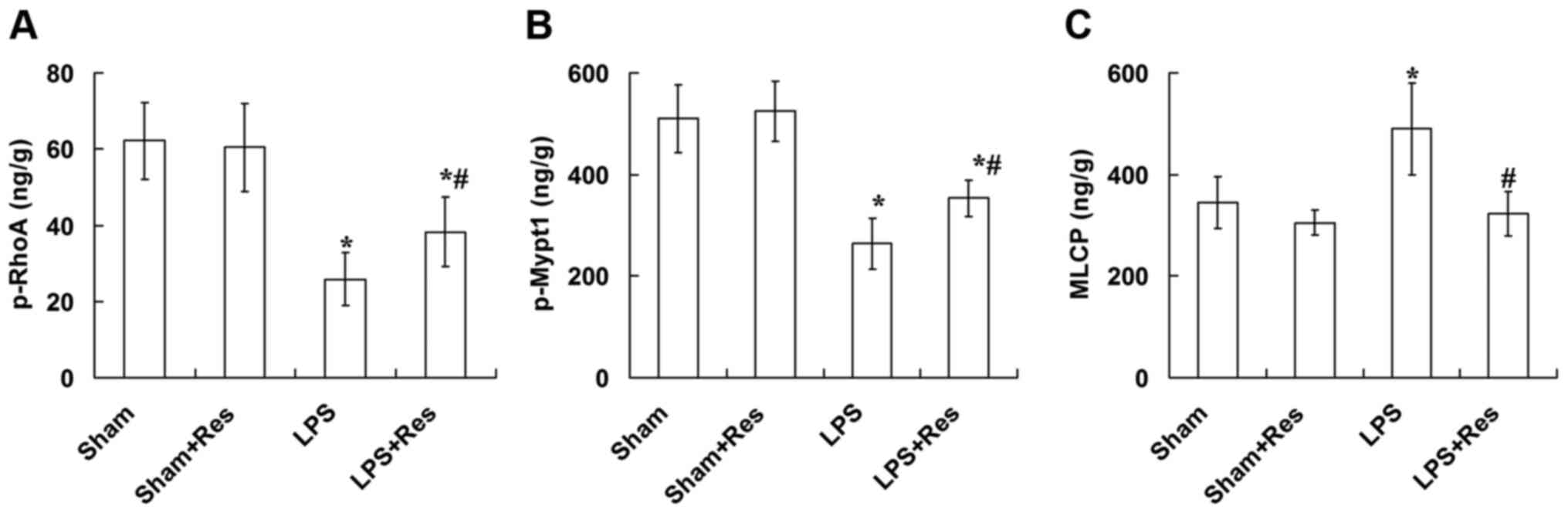
Effect of Res treatment on the p-RhoA,
p-Mypt1 and MLCP levels in vascular tissue of mice subjected to LPS
challenge
As presented in Fig.
5, the LPS challenge significantly decreased the p-RhoA and
p-Mypt1 levels and significantly increased the MLCP level in
vascular tissue (P<0.05), and these adverse effects were
eradicated following treatment with Res. However, the p-RhoA and
p-Mypt1 levels in the LPS + Res group were significantly decreased
compared with the sham and sham + Res groups (P<0.05).
Discussion
Previous breakthroughs indicate that endotoxemia is
characterized by vascular hyporeactivity, which contributes to
microcirculation failure and organ injury (3,4).
Therefore, vascular reactivity has been a focus of previous studies
(18,19); the present study demonstrated that
post-treatment with Res significantly ameliorated LPS-induced
vascular hyporeactivity, which was associated with the
RhoA-ROCK-MLCP pathway. However, no marked effects of Res treatment
on MAP and pressor response in LPS challenge mice were
observed.
A number of methods have been used to establish
endotoxic shock models, including the intravenous (20–22) or
intraperitoneal injection of LPS or gram-negative bacteria
suspension (23–26) and cecal ligation and puncture
(27–30), with each model representing the
common clinical patient of endotoxic shock from different
pathogenic factors. In the present study, the LPS challenge model
was established through intravenous LPS injection, which reflects
the shedding of LPS from gram-negative bacteria transposition into
circulation, which results in endotoxemia. Based on the LPS
challenge model, the present study investigated the effect of
post-treatment of Res on survival time and revealed that Res
treatment prolonged the survival time of mice. This result
suggested that Res treatment inhibits the effect of the LPS
challenge. The survival time and rate identified in the present
study were similar to those indicated in previous research
(31). In this previous study,
intraperitoneal injection of highly purified LPS (5 mg/kg) resulted
in a high mortality rate of 100% in wild-type C57 mice within 24 h
post-injection. Because the mice in the LPS and LPS + Res groups
were all sacrificed within 24 h, the present study only compared
the survival time between the LPS and LPS + Res groups, and did not
observe the survival time of the Sham and Sham + Res group. The
results of MAP indicated that LPS challenge induced a typical blood
pressure curve of endotoxic shock, which briefly decreases in the
early stage, then rises up before gradually declining. However, Res
treatment was not observed to exhibit an evidently perfecting
effect on MAP. A previous study reported that the ameliorating
effect of post-treatment with Res on LPS-induced acute kidney
injury was weaker when compared with Res pre-treatment (32). These previous findings suggested that
the lack of a marked effect on MAP was associated with the
opportunity of Res administration 1 h following LPS challenge. The
results of the current study also demonstrated that the early
application of Res treatment may be favorable for patients with
sepsis.
Vascular reactivity is typically assessed through
observing the pressor response of the whole animal to NE in
vivo and the contractile response of artery rings to NE ex
vivo (4). Thus, the current
study measured the effect of Res on the pressor response of
intravenous LPS-challenged mice and the contractile response to NE
of microvessel rings from intravenous LPS-challenged mice. However,
the results from in vivo and ex vivo experiments in
the present study are inconsistent. The pressor response of mice to
NE increased 6 h after the onset of intravenous infusion of LPS,
whereas the contractile response to NE of isolated microvessel
rings decreased. Res post-treatment increased the contractile
response to NE of microvessel rings, but had no significant effect
on the pressor response of mice.
Previous studies have demonstrated that an
intravenous injection of LPS with a dose of 10 mg/kg reproduced
vascular hyporeactivity to NE in vivo (15,33), but
no marked change was observed in the activity of NE following
intraperitoneal injection of LPS with a dose of 8.5 mg/kg (34). Therefore, the present study
hypothesized that the effect of LPS induced an increased pressor
response to NE and may be associated with the lower dose of 5 mg/kg
LPS. In addition, with the exception of the contractile apparatus
and proteins in vascular smooth muscle cells (VSMCs), the pressor
response in vivo is associated with a series of
neuro-humoral factors, including a vasoconstrictive effect induced
by sympathetic nervous excitement, catecholamines, angiotensin and
vasopressin and a vasodilative effect of parasympathetic nervous
hyper function, prostaglandins and nitric oxide (35). Thus, LPS injection may induce the
sympathomimetic action by contributing to the vessel contraction
and increase in pressor response. The detailed mechanism of
LPS-induced increase in pressor response to NE requires further
investigation.
In general, the contractile tension of isolated
vessels to vasoconstrictor substances is associated with the
contractile apparatus and proteins in VSMCs, in addition to the
function and content of adrenergic receptors (36–38).
Therefore, LPS challenge-induced hyporeactivity of isolated vessels
in the present study was caused by the decreased contractile
function of VSMCs, which may be associated with the damage of
contractile apparatus, the dysfunction of membrane ion channels and
the desensitization of adrenal receptors. These results suggested
that Res treatment improved the reactivity of isolated vessels to
NE and was associated with the aforementioned targets. Furthermore,
the present study also hypothesized that the discordance of pressor
response and isolated vascular reactivity to NE may be associated
with the weaker effect of LPS decreasing isolated vascular
reactivity compared with sympathomimetic action. This may explain
why the Res treatment did not affect the pressor response.
Therefore, the mechanism by which Res treatment improved the
isolated vascular reactivity of LPS-treated mice may be associated
with the change of vasoconstrictor substances in mesenteric
arteries or VSMCs.
Previous studies demonstrated that LPS-induced
vascular hyporeactivity was associated with the suppression of
small guanosine triphosphate-binding proteins, including RhoA, ROCK
and non-muscular isoform of myosin light chain kinase activities
(15,39,40).
However, the RhoA/ROCK pathway is involved in the hemorrhagic
shock-induced vascular hyporeactivity (41–44). The
current study observed the role of the RhoA/ROCK pathway in Res
treatment enhancing vascular reactivity following LPS challenge,
through the isolated vascular rings treated by tool reagents of
RhoA, ROCK and MLCP ex vivo. The results of the present
study indicated that the RhoA agonist and MLCP inhibitor eradicated
the vascular hyporeactivity caused by challenge with LPS. The ROCK
inhibitor may antagonize the effects of RhoA agonist and MLCP
inhibitor, increasing vascular reactivity and the effect of ROCK
inhibitor may be reversed by the RhoA agonist, U-46619. In
addition, the effect of Res treatment enhancing vascular reactivity
on LPS challenged mice was suppressed by ROCK inhibitor incubation,
which was further eradicated by the RhoA agonist. Furthermore, RhoA
inhibitor C3 transferase did not significantly inhibit the
enhancing role of Res treatment, which was further increased by
U-46619 plus C3 transferase co-incubation.
To assess the association between Res-treatment
enhancing vascular reactivity and the RhoA-ROCK-MLCP signal
pathway, the present study measured the effect of Res treatment on
protein levels of p-RhoA, p-Mypt1 (which is a substrate of ROCK and
reflects the activity of ROCK) and MLCP in the mesenteric arteries.
The results of the present study indicated that after LPS
treatment, Res treatment increased the level of RhoA and p-Mypt1
and decreased the level of MLCP. Combined with the results of the
ex vivo experiment; while the inhibiting effect of the ROCK
inhibitor Y-27632 on Res treatment was suppressed by the RhoA
agonist U-46619 treatment and the increasing effect of U-46619 on
LPS-induced vascular hyporeactivity were eradicated by Y-27632
incubation, the present findings suggested that the mechanism by
which LPS challenge was eliciting vascular hyporeactivity is
associated with the suppression of the RhoA-ROCK-MLCP signal
pathway. Furthermore, the vascular protective effect of Res
treatment for LPS challenge is also associated with this signal
pathway.
A number of studies have indicated that the
RhoA-ROCK pathway has various pathophysiologic roles in the process
of cell death or apoptosis (45),
reactive oxygen species generation (46), cytoskeleton remodeling (47) and vasoconstriction (15,42).
However, the mechanism of activation or suppression for the
RhoA-ROCK pathway remains unknown. The detailed mechanism by which
Res activates the RhoA-ROCK-MLCP pathway following LPS challenge
also requires further research.
In addition, Liang et al (4) reported that the suppression of vascular
α1 adrenergic receptors expression was involved in LPS-induced
vascular hyporeactivity. Therefore, whether or not Res treatment
may enhance vascular α1 adrenergic receptors expression requires
further investigation. A number of studies have provided evidence
that mitochondrial dysfunction is involved in hemorrhagic
shock-induced vascular hyporeactivity (48,49). Res
treatment may attenuate the mitochondrial dysfunction of VSMCs
induced by serious hemorrhagic shock, enhance ATP levels, reduce
the content of lipid peroxides and improve the vascular reactivity
of hemorrhagic shock animals (50,51).
Therefore, whether the LPS challenge elicited mitochondrial injury
of VSMCs and whether Res improves vascular reactivity may be
associated with its mitochondrial protective effect remains to be
elucidated.
In conclusion, the present findings demonstrated
that Res attenuates the vascular hyporeactivity and survival time
of LPS challenged mice, but no significant effects were observed
regarding MAP and pressor response for the whole animal. The
inconsistent results of pressor response in vivo and
contractile response of isolated vascular rings to NE may be
associated with the LPS-induced sympathomimetic effect, but the
mechanism requires further investigation. The present study
presents a novel underlying role of Res on LPS-induced sepsis and
further investigation of the mechanism by which Res elevates
vascular reactivity may provide novel insights for the associated
diseases in clinical practice.
References
|
1
|
Maloney PJ: Sepsis and septic shock. Emerg
Med Clin North Am. 31:583–600. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Angus DC and van der Poll T: Severe sepsis
and septic shock. N Engl J Med. 369:840–851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsao CM, Chen SJ, Tsou MY and Wu CC:
Effect of propofol on vascular reactivity in thoracic aortas from
rats with endotoxemia. J Chin Med Assoc. 75:262–268. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liang JL, Yang GM, Li T and Liu LM:
Interleukin 1β attenuates vascular α1 adrenergic receptors
expression following lipopolysaccharide-induced endotoxemia in
rabbits: Involvement of JAK2-STAT3 pathway. J Trauma Acute Care
Surg. 76:762–770. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piotrowska H, Kucinska M and Murias M:
Biological activity of piceatannol: Leaving the shadow of
resveratrol. Mutat Res. 750:60–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuršvietienė L, Stanevičienė I,
Mongirdienė A and Bernatonienė J: Multiplicity of effects and
health benefits of resveratrol. Medicina (Kaunas). 52:148–155.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kolgazi M, Sener G, Cetinel S, Gedik N and
Alican I: Resveratrol reduces renal and lung injury caused by
sepsis in rats. J Surg Res. 134:315–321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu W, Lu Y, Yao J, Li Z, Chen Z, Wang G,
Jing H, Zhang X, Li M, Peng J and Tian X: Novel role of
resveratrol: Suppression of high-mobility group protein box 1
nucleocytoplasmic translocation by the upregulation of sirtuin 1 in
sepsis-induced liver injury. Shock. 42:440–447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang HX, Duan GL, Wang CN, Zhang YQ, Zhu
XY and Liu YJ: Protective effect of resveratrol against
endotoxemia-induced lung injury involves the reduction of
oxidative/nitrative stress. Pulm Pharmacol Ther. 27:150–155. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sebai H, Sani M, Aouani E and
Ghanem-Boughanmi N: Cardioprotective effect of resveratrol on
lipopolysaccharide-induced oxidative stress in rat. Drug Chem
Toxicol. 34:146–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sebai H, Sani M, Yacoubi MT, Aouani E,
Ghanem-Boughanmi N and Ben-Attia M: Resveratrol, a red wine
polyphenol, attenuates lipopolysaccharide-induced oxidative stress
in rat liver. Ecotoxicol Environ Saf. 73:1078–1083. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao Q, Jing C, Tang X, Yin Y, Han X and Wu
W: Protective effect of resveratrol on acute lung injury induced by
lipopolysaccharide in mice. Anat Rec (Hoboken). 294:527–532. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Holthoff JH, Wang Z, Seely KA, Gokden N
and Mayeux PR: Resveratrol improves renal microcirculation,
protects the tubular epithelium, and prolongs survival in a mouse
model of sepsis-induced acute kidney injury. Kidney Int.
81:370–378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tseng TL, Chen MF, Liu CH, Pang CY, Hsu YH
and Lee TJ: Induction of endothelium-dependent constriction of
mesenteric arteries in endotoxemic hypotensive shock. Br J
Pharmacol. 173:1179–1195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liao MH, Shih CC, Tsao CM, Chen SJ and Wu
CC: RhoA/Rho-kinase and nitric oxide in vascular reactivity in rats
with endotoxaemia. PLoS One. 8:e563312013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smeding L, Leong-Poi H, Hu P, Shan Y,
Haitsma JJ, Horvath E, Furmli S, Masoom H, Kuiper JW, Slutsky AS,
et al: Salutary effect of resveratrol on sepsis-induced myocardial
depression. Crit Care Med. 40:1896–1907. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lai X, Pei Q, Song X, Zhou X, Yin Z, Jia
R, Zou Y, Li L, Yue G, Liang X, et al: The enhancement of immune
function and activation of NF-κB by resveratrol-treatment in
immunosuppressive mice. Int Immunopharmacol. 33:42–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu LM, Ward JA and Dubick MA:
Hemorrhage-induced vascular hyporeactivity to norepinephrine in
select vasculatures of rats and the roles of nitric oxide and
endothelin. Shock. 19:208–214. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao ZG, Niu CY, Wei YL, Zhang YP, Si YH
and Zhang J: Mesenteric lymph return is an important contributor to
vascular hyporeactivity and calcium desensitization after
hemorrhagic shock. Shock. 38:186–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li A, Dong L, Duan ML, Sun K, Liu YY, Wang
MX, Deng JN, Fan JY, Wang BE and Han JY: Emodin improves
lipopolysaccharide-induced microcirculatory disturbance in rat
mesentery. Microcirculation. 20:617–628. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Sun K, Liu YY, Zhang YP, Hu BH,
Chang X, Yan L, Pan CS, Li Q, Fan JY, et al: Ginsenoside Rb1
ameliorates lipopolysaccharide-induced albumin leakage from rat
mesenteric venules by intervening in both trans- and paracellular
pathway. Am J Physiol Gastrointest Liver Physiol. 306:G289–G300.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pei H, Zhang Y, Wu H, Ren L, Jia X, Zhang
Y and Chen M: Relationship between iNOS expression and apoptosis in
cerebral tissue, and the effect of sini injection in endotoxin
shock rats. J Tradit Chin Med. 33:486–491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Y, Liu B, Fukudome EY, Lu J, Chong W,
Jin G, Liu Z, Velmahos GC, Demoya M, King DR and Alam HB:
Identification of citrullinated histone H3 as a potential serum
protein biomarker in a lethal model of lipopolysaccharide-induced
shock. Surgery. 150:442–451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu C, Zhang GF, Song SW, Cai GJ, Liu WH,
Miao CY and Su DE: Effects of ketanserin on endotoxic shock and
baroreflex function in rodents. J Infect Dis. 204:1605–1612. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Madonna R, Jiang J and Geng YJ: Attenuated
expression of gelsolin in association with induction of aquaporin-1
and nitric oxide synthase in dysfunctional hearts of aging mice
exposed to endotoxin. Int J Immunopathol Pharmacol. 25:911–922.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Noworyta-Sokołowska K, Górska A and
Gołembiowska K: LPS-induced oxidative stress and inflammatory
reaction in the rat striatum. Pharmacol Rep. 65:863–869. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park EJ, Jang HJ, Tsoyi K, Kim YM, Park
SW, Kim HJ, Lee JH and Chang KC: The heme oxygenase-1 inducer
THI-56 negatively regulates iNOS expression and HMGB1 release in
LPS-activated RAW 264.7 cells and CLP-induced septic mice. PLoS
One. 8:e762932013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao T, Li Y, Liu B, Liu Z, Chong W, Duan
X, Deperalta DK, Velmahos GC and Alam HB: Novel pharmacologic
treatment attenuates septic shock and improves long-term survival.
Surgery. 154:206–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang K, Nan C, Fei D, Meng X, Liu W, Zhang
W, Jiang L and Zhao M, Pan S and Zhao M: Heme oxygenase 1 modulates
thrombomodulin and endothelial protein C receptor levels to
attenuate septic kidney injury. Shock. 40:136–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Olguner CG, Koca U, Altekin E, Ergür BU,
Duru S, Girgin P, Taşdöğen A, Gündüz K, Güzeldağ S, Akkuş M and
Micili SC: Ischemic preconditioning attenuates lipid peroxidation
and apoptosis in the cecal ligation and puncture model of sepsis.
Exp Ther Med. 5:1581–1588. 2013.PubMed/NCBI
|
|
31
|
Buchholz BM, Chanthaphavong RS, Billiar TR
and Bauer AJ: Role of interleukin-6 in hemopoietic and
non-hemopoietic synergy mediating TLR4-triggered late murine ileus
and endotoxic shock. Neurogastroenterol Motil. 24:658–669, e294.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen L, Yang S, Zumbrun EE, Guan H,
Nagarkatti PS and Nagarkatti M: Resveratrol attenuates
lipopolysaccharide-induced acute kidney injury by suppressing
inflammation driven by macrophages. Mol Nutr Food Res. 59:853–864.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fatehi-Hassanabad Z, Muller B,
Andriantsitohaina R, Furman BL, Parratt JR and Stoclet JC:
Influence of indomethacin on the haemodynamic effects of
lipopolysaccharide in rats. Fundam Clin Pharmacol. 10:258–263.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boffa JJ and Arendshorst WJ: Maintenance
of renal vascular reactivity contributes to acute renal failure
during endotoxemic shock. J Am Soc Nephrol. 16:117–124. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shaikh WA, Patel M, Shah HD, Nimbalkar AS,
Patel N and Singh SK: Arterial blood pressure is inversely
associated with vascular sympathetic reactivity (isometric handgrip
exercise) in Gujarati Indian adolescents. Indian J Physiol
Pharmacol. 58:271–274. 2014.PubMed/NCBI
|
|
36
|
Bing RJ, Termin A, Conforto A, Dudek R and
Hoffmann MJ: Membrane function and vascular reactivity. Biosci Rep.
13:61–67. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ponnoth DS and Mustafa S Jamal: Adenosine
receptors and vascular inflammation. Biochim Biophys Acta.
1808:1429–1434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guzmán-Gutiérrez E, Arroyo P, Salsoso R,
Fuenzalida B, Sáez T, Leiva A, Pardo F and Sobrevia L: Role of
insulin and adenosine in the human placenta microvascular and
macrovascular endothelial cell dysfunction in gestational diabetes
mellitus. Microcirculation. 21:26–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liang JL, Yang GM, Li T and Liu LM:
Effects of interleukin-1β on vascular reactivity after
lipopolysaccharide-induced endotoxic shock in rabbits and its
relationship with PKC and Rho kinase. J Cardiovasc Pharmacol.
62:84–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Recoquillon S, Carusio N, Lagrue-Lakhal
AH, Tual-Chalot S, Filippelli A, Andriantsitohaina R and Martinez
MC: Interaction in endothelium of non-muscular myosin light-chain
kinase and the NF-κB pathway is critical to
lipopolysaccharide-induced vascular hyporeactivity. Clin Sci
(Lond). 129:687–698. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu Y, Yang G, Xiao X, Liu L and Li T: Bkca
opener, NS1619 pretreatment protects against shock-induced vascular
hyporeactivity through PDZ-Rho GEF-RhoA-Rho kinase pathway in rats.
J Trauma Acute Care Surg. 76:394–401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao Z, Si Y, Zhang Y, Du S, Zhang L and
Niu C: Postshock mesenteric lymph drainage ameliorates vascular
reactivity and calcium sensitivity through RhoA. J Surg Res.
186:304–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li T, Fang Y, Yang G, Xu J, Zhu Y and Liu
L: Effects of the balance in activity of RhoA and Rac1 on the
shock-induced biphasic change of vascular reactivity in rats. Ann
Surg. 253:185–193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu Y, Li T, Tang XF, Chen K and Liu L:
Effects of ischemic preconditioning on vascular reactivity and
calcium sensitivity after hemorrhagic shock and their relationship
to the RhoA-Rho-kinase pathway in rats. J Cardiovasc Pharmacol.
57:231–239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Okumura N, Fujii K, Kagami T, Makiko N,
Kitahara M, Kinoshita S and Koizumi N: Activation of the Rho/Rho
kinase signaling pathway is involved in cell death of corneal
endothelium. Invest Ophthalmol Vis Sci. 57:6843–6851. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Akbar H, Duan X, Saleem S, Davis AK and
Zheng Y: RhoA and Rac1 GTPases differentially regulate
agonist-receptor mediated reactive oxygen species generation in
platelets. PLoS One. 11:e01632272016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bang J, Jang M, Huh JH, Na JW, Shim M,
Carlson BA, Tobe R, Tsuji PA, Gladyshev VN, Hatfield DL and Lee BJ:
Deficiency of the 15-kDa selenoprotein led to cytoskeleton
remodeling and non-apoptotic membrane blebbing through a RhoA/ROCK
pathway. Biochem Biophys Res Commun. 456:884–890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lei Y, Peng X, Liu L, Dong Z and Li T:
Beneficial effect of cyclosporine A on traumatic hemorrhagic shock.
J Surg Res. 195:529–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fang Y, Li T, Fan X, Zhu Y and Liu L:
Beneficial effects of activation of PKC on hemorrhagic shock in
rats. J Trauma. 68:865–873. 2010.PubMed/NCBI
|
|
50
|
Wang X, Song R, Bian HN, Brunk UT, Zhao M
and Zhao KS: Polydatin, a natural polyphenol, protects arterial
smooth muscle cells against mitochondrial dysfunction and lysosomal
destabilization following hemorrhagic shock. Am J Physiol Regul
Integr Comp Physiol. 302:R805–R814. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang X, Song R, Chen Y, Zhao M and Zhao
KS: Polydatin - a new mitochondria protector for acute severe
hemorrhagic shock treatment. Expert Opin Investig Drugs.
22:169–179. 2013. View Article : Google Scholar : PubMed/NCBI
|