Introduction
Over the past ten years, patients who underwent
coronary artery bypass grafting (CABG) often presented with
complications such as diffuse coronary diseases and multiple
comorbidities (1). For patients with
severe diffuse coronary disease, CABG combined with coronary
endarterectomy (CE) has been demonstrated to be an effective
approach for complete revascularization (1,2).
However, the short-term clinical outcomes from CE appears to be
worse compared with CABG alone (1).
Soylu et al, reported that CABG combined with CE markedly
increased perioperative myocardial infarction (MI) and
postoperative inflammation (2). In
addition to inflammation and MI, 30-day mortality is a severe
postoperative complication associated with CE (3). Furthermore, a 30-day mortality is also
a severe postoperative complication associated with CE (4). Therefore, the development of the effect
of CE and the addition of the subsequent optimal treatment has been
significant in coronary surgery field. Electrocautery is a routine
surgical option for various clinical requirements. Hence, the
design of CE combined with electrocautery has been emerging to
further distribute coronary disease treatment in this research. The
aim of this study is to investigate the mechanism and optimize the
therapeutic effect of electrocautery using a rabbit model of
abdominal aortic endarterectomy.
Materials and methods
Ethics statement
A total of 30 healthy male white New Zealand
rabbits, weighing between 2.5–3.6 kg, were purchased from the
Animal Center of Capital Medical University (Beijing, China). We
conducted animal experiments according to the Animal Management
Rules of the Ministry of Health of China and the Guidance for the
Care and Use of Laboratory Animals (National Institutes of Health).
All animals used for experiments were cared for according to these
guidelines. The present study was approved by the Ethics Committee
of the Animal Center of Beijing Anzhen Hospital.
Preliminary experiments for
establishment of electrocautery parameters
Five male rabbits housed at 25°C with 50% humidity
and with free access to food and water were used for preliminary
experiments. An operating microscope was used during surgery under
aseptic conditions. After fasting for 6 h, the rabbits were
anaesthetized with 3% pentobarbital sodium via ear vein injection.
Only those that presented with airway occlusion were placed on
ventilation. Chest movement and respiratory rates were closely
observed during surgery. The color of the oral mucosa, lip, and the
conjunctival membranes was evaluated to reflect blood oxygenation.
After airway occlusion developed, the airways of rabbits were
opened and connected to a ventilator (DW: 3000B; Nanjing Jiancheng
Co. Ltd., Nanjing, China). The surgical site over the abdominal
aorta was anaesthetized by spreading 2% lidocaine (5 ml) on the
skin. Next, the abdominal aorta was exposed and heparin was
injected via the ear vein at a dose of 700 IU/kg. We placed Bulldog
hemostatic clamps on the abdominal aorta to stop blood flow
temporarily. The model of abdominal aortic endarterectomy was
performed by denudation in the control and study groups as
previously described (5,6). To confirm the establishment of the
model of electrocautery, the blocked abdominal aorta was partially
incised and electrocautery via an electrocoagulation electrotome
was performed through the entire lumen with the following
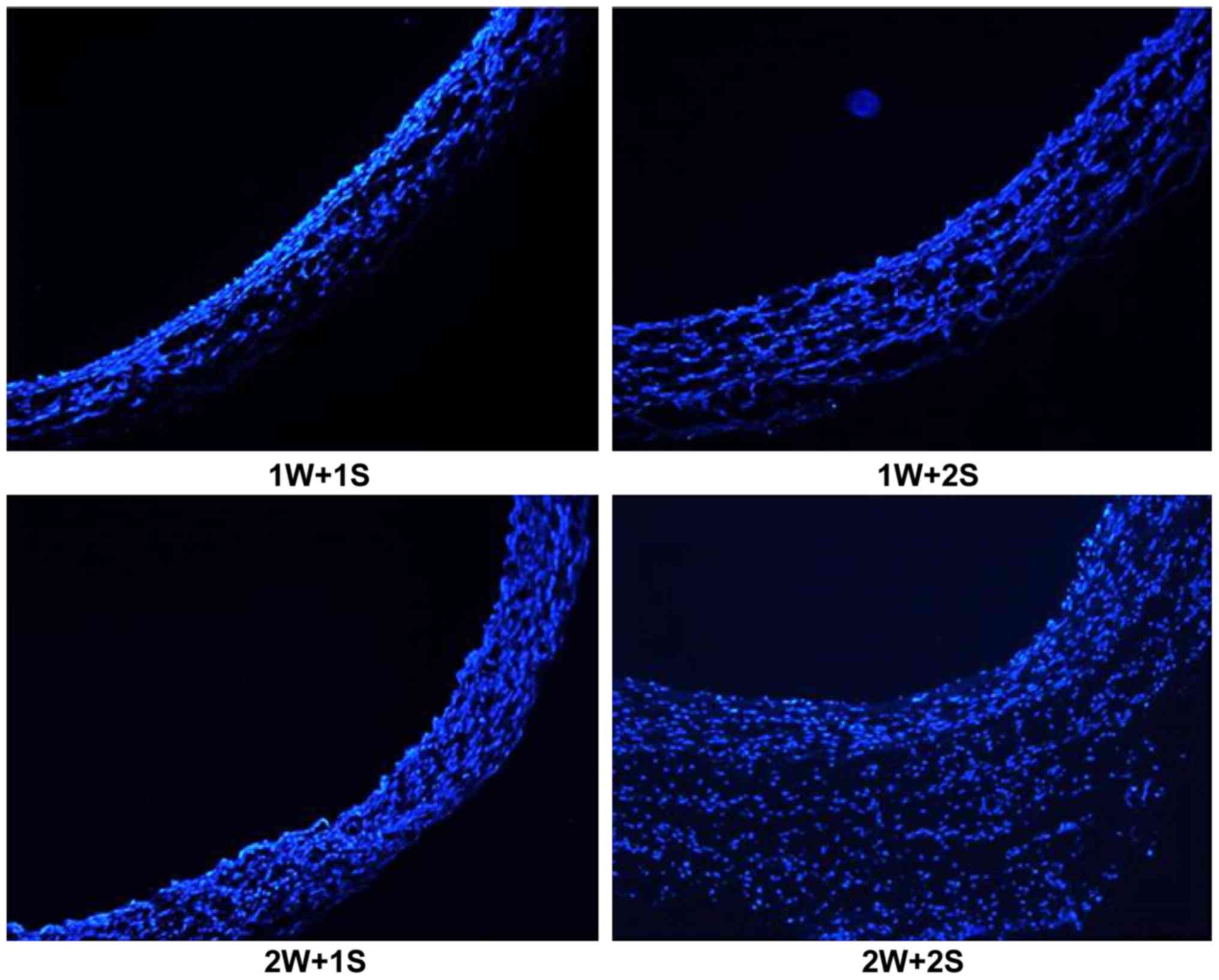
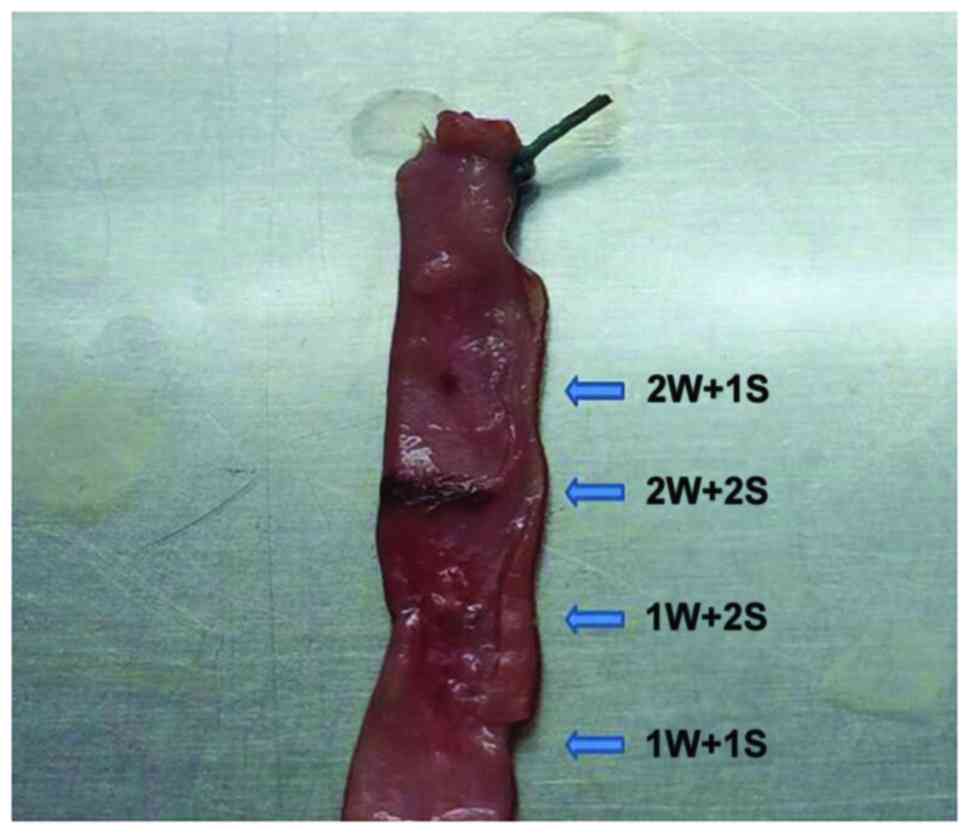
parameters: 1W+1S, 1W+2S, 2W+1S and 2W+2S (Fig. 1). The rest of the blocked aorta was
cut off and split lengthways. Next, electrocautery was performed
with each parameter for macroscopic observation (Fig. 1). The surgically treated vessels were
harvested, and frozen sections were prepared for immunofluorescence
staining to evaluate the results of electrocautery.
The rabbit model of abdominal aortic
endarterectomy + electrocautery
Thirty rabbits were randomly divided into three
groups as follows (n=10/group): the sham and control groups
(abdominal aortic endarterectomy by balloon catheter), and the
study group (abdominal aortic endarterectomy by balloon catheter
treated with electrocautery). The surgical procedures were
performed as described above with optimal electrocautery parameters
determined from the preliminary experiments. In the control and
study groups, the incisions were anastomosed with mattressed 8–0
prolene sutures (Johnson & Johnson, New Brunswick, NJ, USA).
The surgical procedures for arterial blocking were completed within
30 min to avoid lower limb ischemia. After completing anastomoses,
we released the hemostatic clamps at the abdominal aorta and then
carefully examined the quality of anastomoses. The wounds were then
closed. In the sham group, the abdominal incision was the operative
site. In all groups, penicillin (4 million IU) and heparin (700
IU/kg) were administered daily for the first 3 days after surgery.
Each rabbit received intragastric treatment with aspirin (12.5
mg/day) until euthanasia.
Vascular histology
Rabbits were euthanized 3 weeks after surgery. To
remove blood, animals were perfused with saline supplemented with
heparin. The surgically treated aortas from the control and study
groups were dissected and embedded in OCT. Vascular tissue sections
(7 µm) were prepared and stained with DAPI. Next, images were
obtained and analyzed with a Nikon Nie fluorescence inversion
microscope system (Nikon, Tokyo, Japan). The degree of endothelial
injury and vascular morphology regarding pathological changes were
determined.
Measurement of ultrasound parameters
in the abdominal aorta
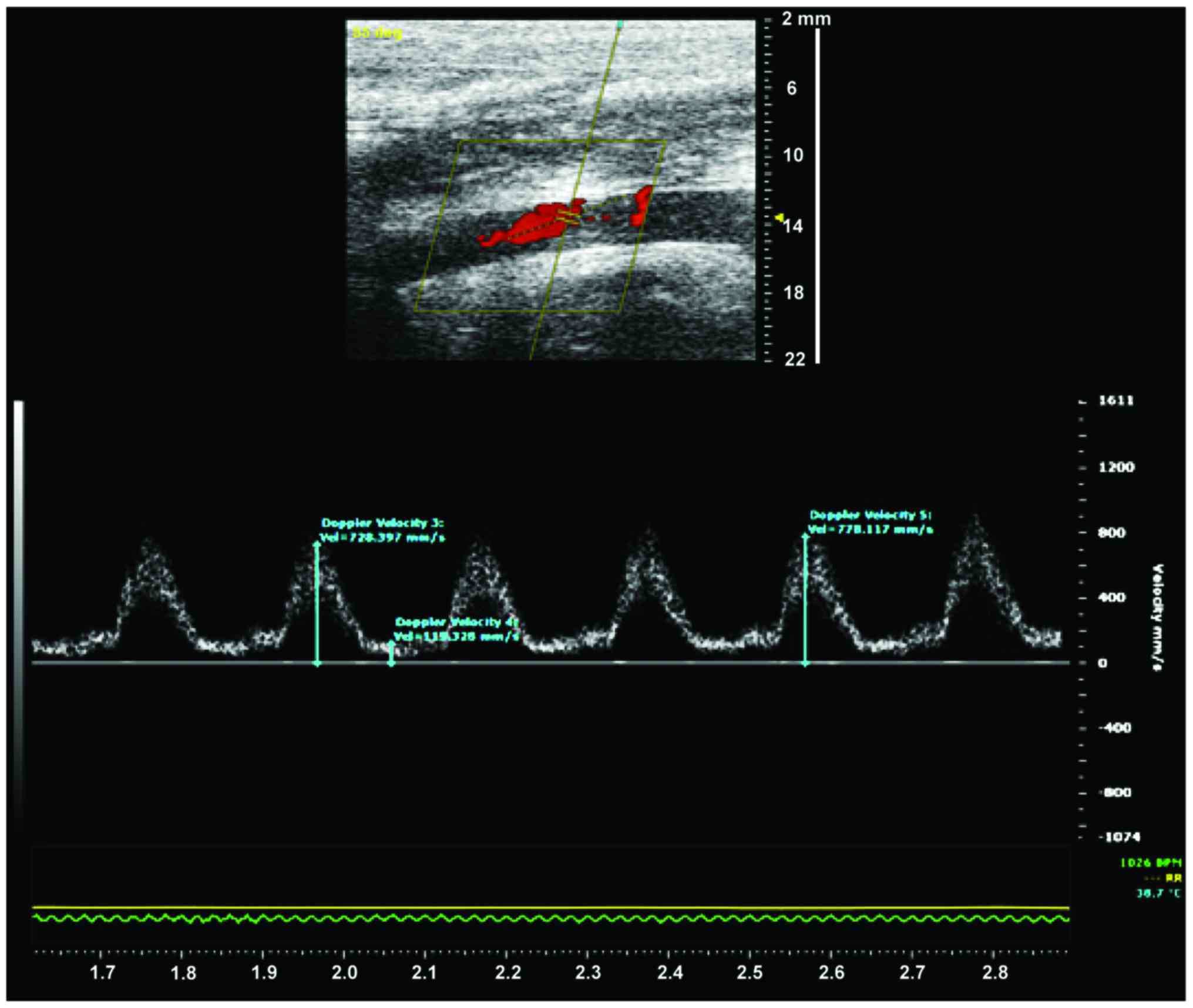
Blood flow velocity and arterial diameter were
measured on postoperative 1, 3, 7, and 20 days using a flow QC
meter (Vevo 2100; Fujifilm VisualSonics Inc., NY, USA). Using
pentobarbital sodium (3%), rabbits were anaesthetized and the
abdominal aorta was exposed. The probe was placed on the abdominal
aorta for measurement of vascular diameter and maximum blood flow
velocity (Fig. 2).
Rate of apoptosis of vascular
endothelial cells (ECs)
Vascular tissues harvested at the various time
points were digested with 2.5% trypsin for 10 min, and the reaction
was stopped by addition of FBS. The samples were centrifuged at 800
× g for 5 min. Next, tissues were incubated with 5 µl of annexin V
and propidium iodide (PI). Samples were then analyzed by flow
cytometry.
ELISA for interleukin-6 (IL-6) and
tumor necrosis factor-α (TNF-α)
Blood samples were collected from the ear vein and
centrifuged at 3,000 rpm for 15 min. ELISA was performed according
to the manufacturers instructions (R&D, NY, USA). A total of 50
µl of serum was used for the assay. Measurements were performed in
triplicate. OD values at 450 nm were measured using a plate reader
(Bio-Rad, NY, USA).
Quantitative polymerase chain reaction
(qRT-PCR)
We used a TRIzol kit from Life Technologies (Grand
Island, NY, USA) for isolation of total RNA from vessels. The
samples were treated with a DNase I kit as follows: 2 µg total RNA,
2 µl buffer (10X), and 2 µl DNase I were incubated for 10 min at
65°C. The conditions for cDNA synthesis were as follows: 65°C for 5
min and 4°C for 2 min. The PCR reaction contained 4 µl of 5X RT
Buffer (Takara, Bio, Inc., Otsu, Japan), 2 µl of 0.1 M DTT, 1 µl of
10 mM dNTPs, 1 µl of HiFi-MMLV enzyme mix, and 2 µl of primers. ROX
plus and SYBR Premix Ex Taq II were used for RT-PCR. The thermal
profile was as follows: 95°C for 5 sec (30 cycles), and 60°C for 40
sec (45 cycles). The primer sequences are shown in Table I.
 | Table I.The sequence of PCR primers. |
Table I.
The sequence of PCR primers.
| Gene | DNA sequence
(5–3) | Product size
(bp) |
|---|
| TNF-α | U:
ACCCTCACACTCAGATCATCTTCT | 422 |
|
| D:
CAGATTGACCTCAGCGCTGAGTTG |
|
| IL-6 | U:
GTCTATACCACTTCACAAGTCGGA | 441 |
|
| D:
TTGGATGGTCTTGGTCCTTAGCCA |
|
| α-actin | U:
GAAATCGTGGGTGACATCAAA | 478 |
|
| D:
ACTCATCGTACTCCTGCTTGCTGA |
|
Statistical analysis
Data were analyzed using SPSS version 17.0 software
(SPSS, Inc., Chicago, Il, USA). All data are presented as mean ±
standard deviation. Comparisons between groups were by independent
t-test. Comparisons among groups were by analysis of variance
(ANOVA) followed by an LSD test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Determination of optimal parameters
for electrocautery
The rabbits were euthanized in preliminary
experiments and the abdominal aortas after treatment were examined
by immunofluorescence staining of frozen sections. Combined with
visual comparison, immunofluorescence staining showed that with the
1W+1S power and activation time setting, vascular morphology was
not affected. In addition, samples treated with the 1W+1S setting
had smooth vascular lumens, which was the optimal effect of
electrocautery in the rabbit model (Fig.
3). Increasing the power and activation time settings lead to
more impaired results. In some vessels, electrocautery caused
obvious thermal damage to the vascular structure, according to the
comparisons by visual examination and immunofluorescence (Fig. 4).
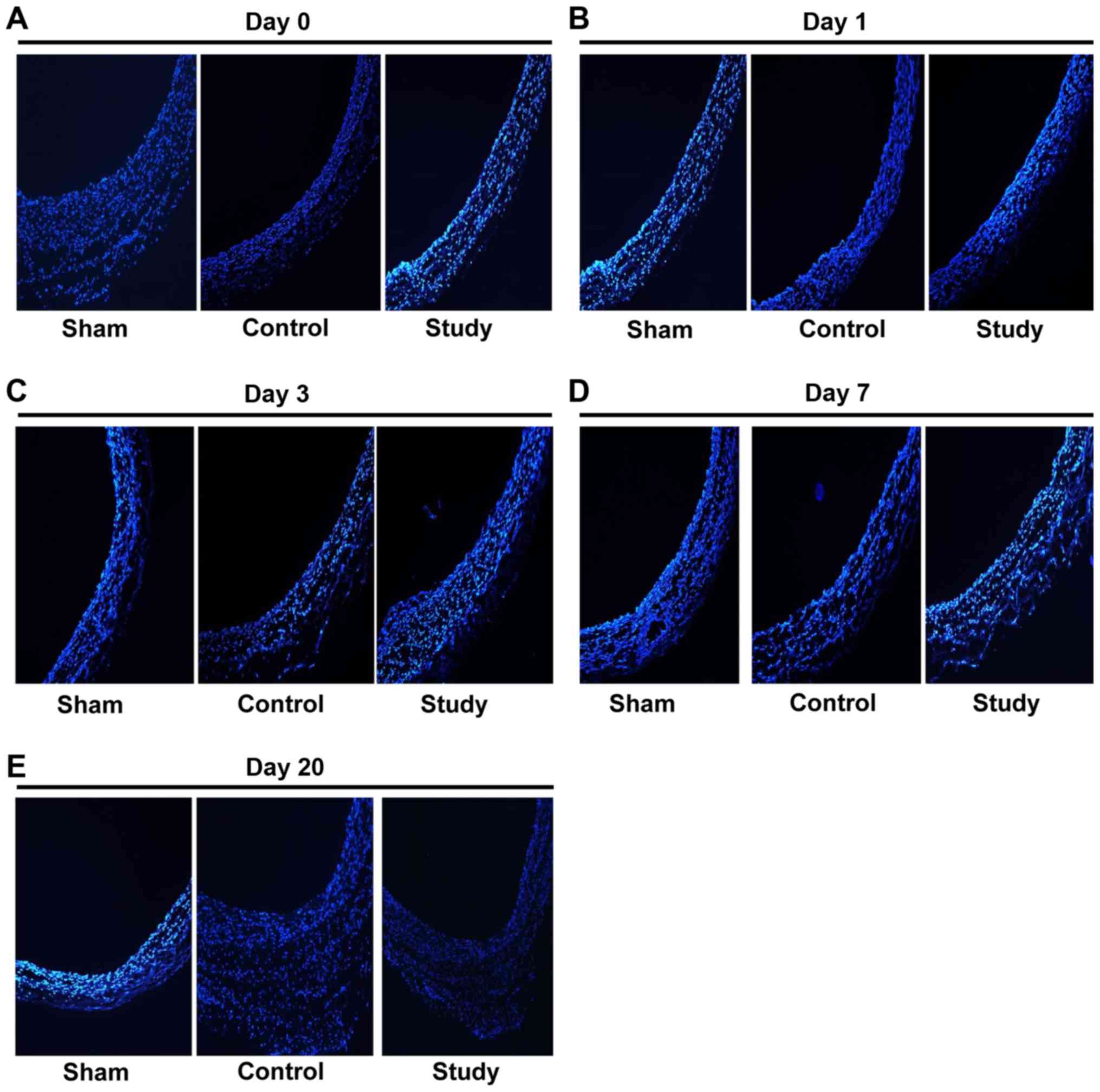
Endarterectomy + electrocautery
reduces intimal injury of the abdominal aorta
Aortic tissues were examined histologically to
clarify the mechanism of the effect of electrocautery on blood flow
after surgery. Immunofluorescence showed that the lumens of
abdominal aortas in endarterectomy + electrocautery group were
significantly smoother than those of the control group (Fig. 5A-E). These findings demonstrated that
endarterectomy + electrocautery reduces intimal injury of the
abdominal aorta by making the vascular lumen smooth without
protuberance.
Endarterectomy + electrocautery
improves postoperative blood flow
All rabbits survived and recovered well after
surgery. None required ventilation because of airway occlusion.
There was no significant difference in blood flow velocity after
surgery between the sham and study groups (808±39.4 vs. 793±27.8
mm/sec, P>0.05), whereas the velocity of blood flow in the
control group was significantly lower than in the study group
(728±31.5 vs. 793±27.8 mm/sec, P<0.05), suggesting successful
establishment of the rabbit model of endarterectomy +
electrocautery. Interestingly, 20 days after surgery, the velocity
of blood flow in the control group decreased significantly.
However, blood flow in the study group was significantly higher
than in the control group (660±31.7 vs. 758±23.9 mm/sec,
P<0.05). Furthermore, vessel diameter was significantly lower in
the control group compared with the study group 20 days after
surgery (3.4±0.4 vs. 4.0±0.7 mm, P<0.05). Taken together, these
results indicate that endarterectomy+electrocautery improves
postoperative blood flow.
Endarterectomy + electrocautery
reduces the rate of apoptosis of vascular ECs
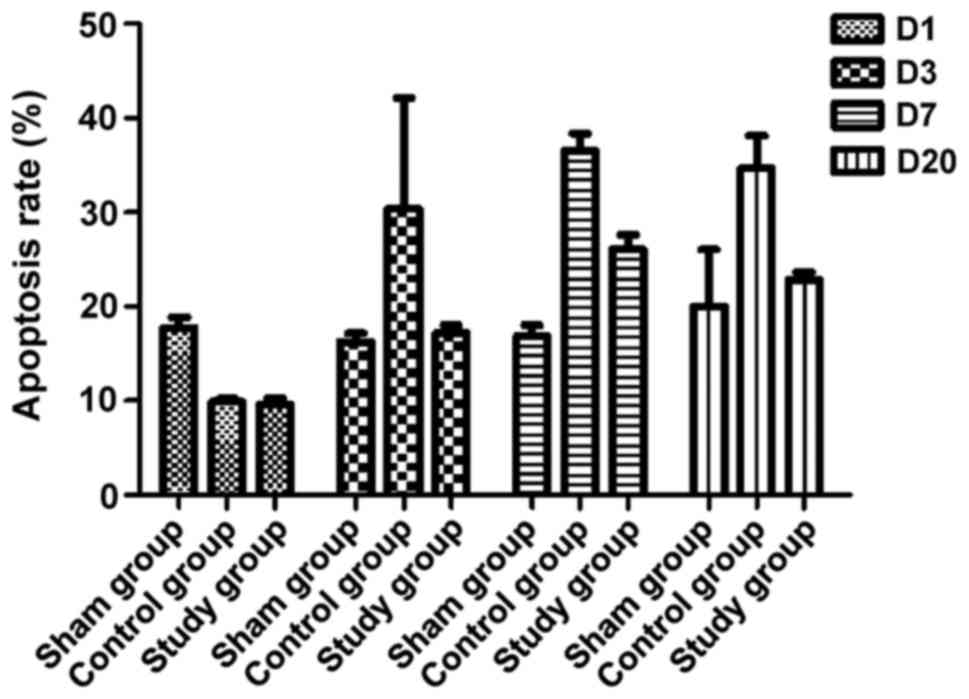
Flow cytometric analysis of ECs demonstrated that
both the study and control groups had higher rates of apoptosis
than the sham group (P<0.05, Fig.
6), while the range of the rise in the study group was
significantly lower than that in the control group over time
(P<0.05, Fig. 6). These results
further suggested that endarterectomy + electrocautery attenuates
apoptosis of ECs postoperatively. Therefore, the
electrocautery-mediated effects on blood flow after surgery appears
to be associated with decreased intimal injury of the abdominal
aorta and decreased rate of apoptosis of vascular ECs.
Endarterectomy + electrocautery
reduces TNF-α and IL-6 expression and inflammation
To further explore the mechanism related to the
favorable effects of electrocautery, the vascular levels of TNF-α
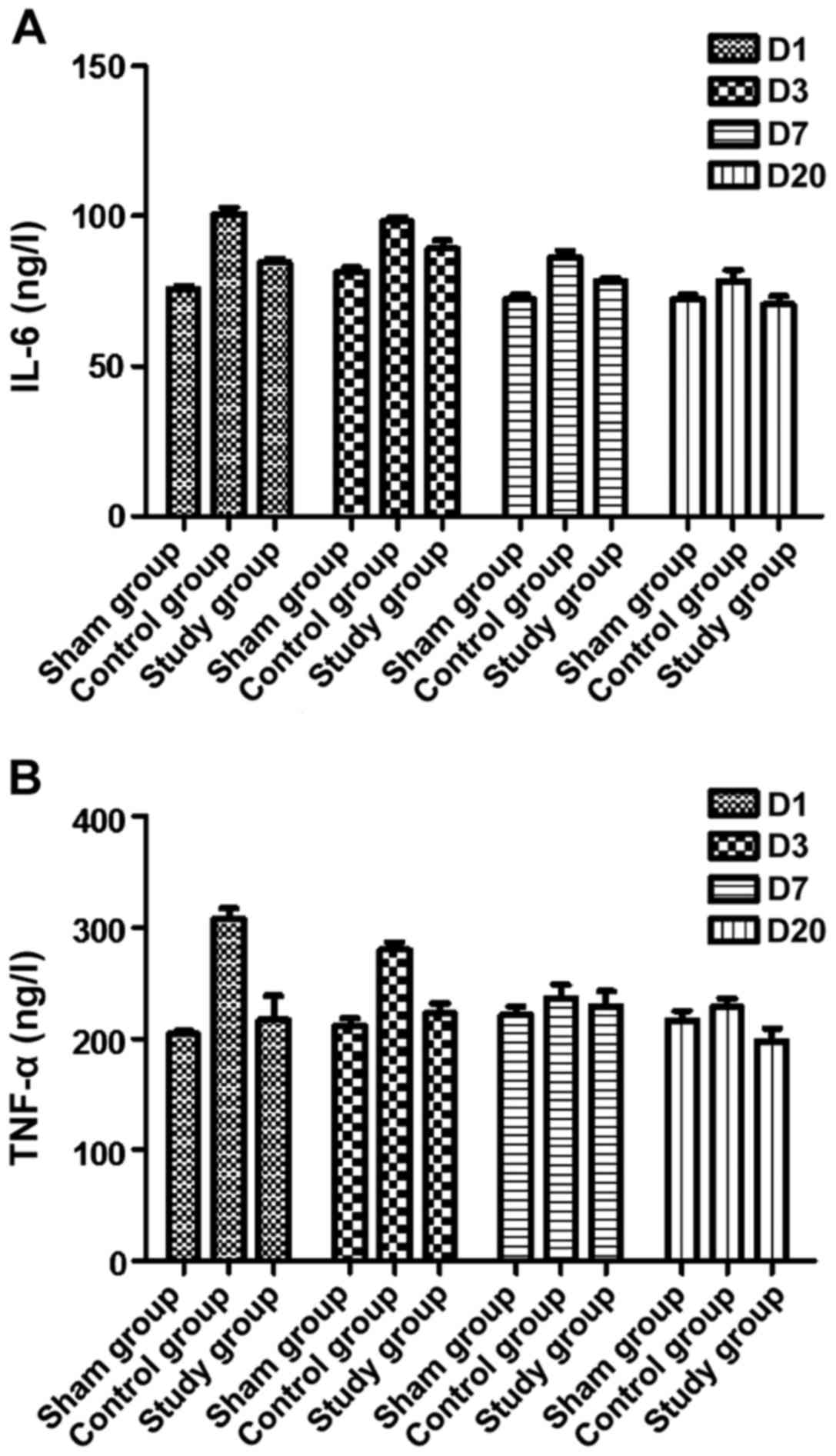
and IL-6 were measured by ELISA and qRT-PCR. Our ELISA results
showed that the levels of TNF-α and IL-6 in serum were
significantly increased in the control group, while they decreased
gradually compared with the sham group. In the study group, the
postoperative levels of TNF-α and IL-6 were similar to normal serum
levels, and they remained unchanged over time compared with the
sham group (P<0.05, Fig. 7).
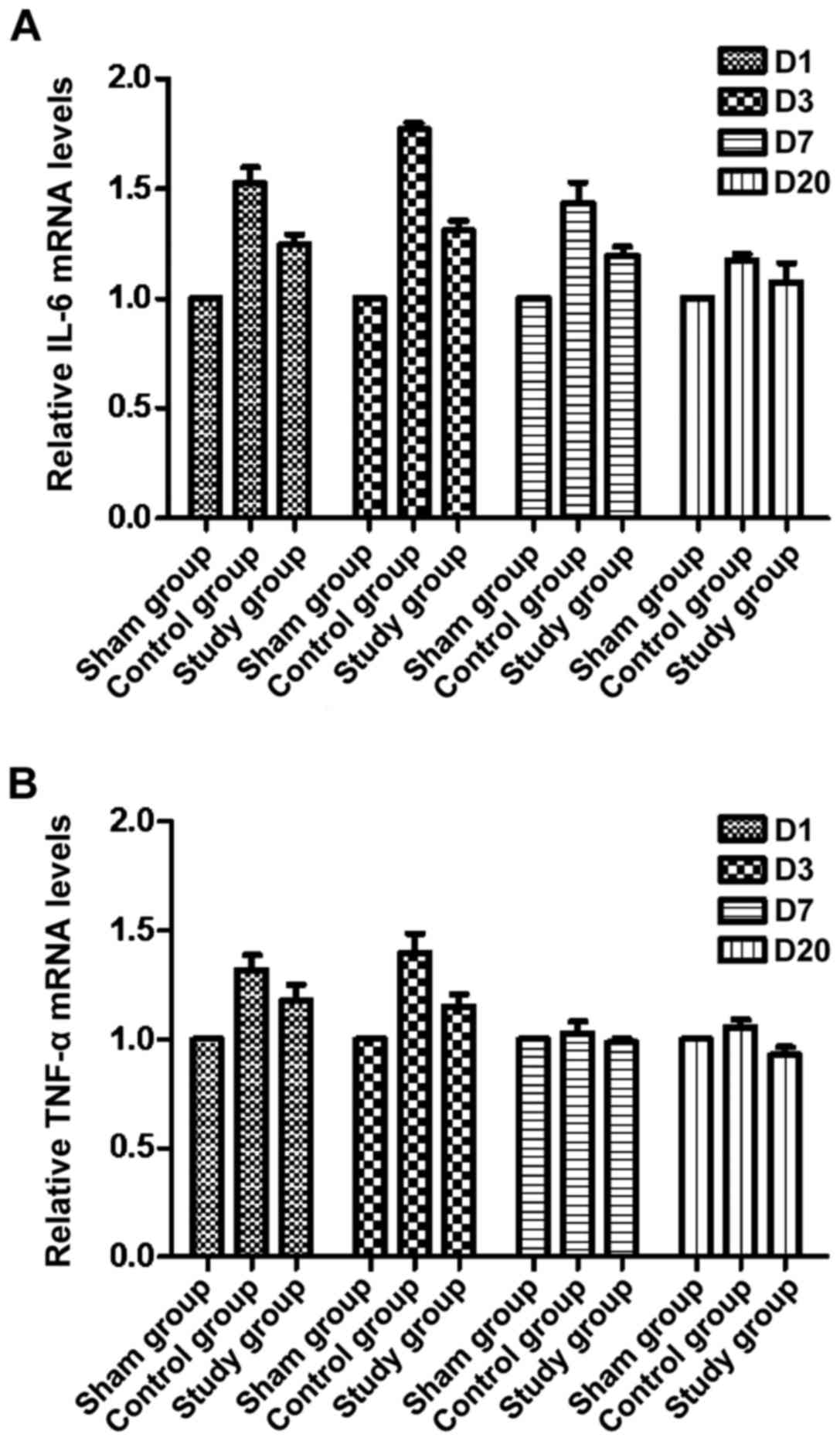
Excessive inflammation was shown to be related to intimal
hyperplasia. Electrocautery significantly reduced TNF-α and IL-6
expression in vessel tissue in the control group, and their
expression decreased gradually compared with the sham group. Their
expression was maintained at almost normal levels postoperatively,
and there were no significant differences over time (P<0.05,
Fig. 8). These results indicate that
electrocautery alleviated inflammation in rabbits.
Discussion
CE is widely applied to treat diffuse coronary
artery disease (7). Previous
evidence suggested that the long-term value of CE combined with
off-pump CABG was promising (8).
However, recent studies arrived at different conclusions. A study
suggested that the rate of mortality of patients who underwent CABG
combined with CE was significantly higher than those who were
treated by CABG or CE alone (9). The
fact that patients who require CE treatment are commonly at a
higher risk of perioperative MI and death may contribute to the
higher rates of morbidity and mortality associated with CE
(2).
CE-related increases in inflammation and thrombosis
may be associated with embolization and thrombogenesis from
dislodged atheromatous material (2).
CE commonly injures vascular endothelium and causes mechanical
injury to the coronary vessels. Endothelial dysfunction may result
in increased thrombogenesis and atheroembolization, and reduction
in graft flow, which consequently leads to thrombosis and
postoperative inflammation. The release of inflammatory factors
(such as IL-6 and TNF-α) was enhanced following the extent of
endothelial damage. To accommodate shear stress and high wall
stress, arteries without an intima undergo postoperative
remodeling. The remodeling is characterized by endothelial
proliferation and is affected by platelet aggregation. The process
of thrombogenesis may contribute to vascular occlusion or stenosis,
leading to bypass graft failure (10). Furthermore, endothelial proliferation
may balance the remodeling process by covering the exposed coarse
vessel wall induced by mechanical injury from endarterectomy.
Immediate responses occur after acute traumatic
damage to control injury and trigger the recovery process,
including the release of inflammatory factors. TNF-α is an
important inflammatory mediator that can activate cell signaling
and induce the release of IL-6 (11). The activation and aggregation of
neutrophils are increased by IL-6, and are used as sensitive
indexes of vascular endothelial injury and inflammation (12). Our study showed that serum TNF-α and
IL-6 in the control group reached peak levels on approximately
postoperative day 7, and decreased to normal levels on day 20. In
the study group, the levels increased slightly and decreased to
normal on day 7. The IL-6 and TNF-α gene expression showed similar
trends. Therefore, monitoring the variation in the levels of IL-6
and TNF-α can help estimate the degree of postoperative injury in
animal models or clinical trials.
Electrocautery with electrocoagulation electrotomes
is emerging for use in various clinical procedures, as a
self-modified strategy to prevent excessive inflammation and
thrombosis (13). However, technical
challenges associated with the power and activation time of
electrocautery limit the applications in animals and in the clinic
(14). Although the application of
endarterectomy on arteries appears to exert protective effects via
endothelial coverage and smoothing of coarse vessel lumens, high
power and activation time settings may cause severe thermal and
excessive damage in animal models (13). The aim of the preliminary experiments
was to limit the range of electrocautery within the adventitia.
Only flattening effects occurred.
During the early phase of post-endarterectomy
adaptation, the media and adventitia are exposed to high wall shear
stress and the intense pulsatile stretch force from the arterial
circulation. Marked increases in mechanical stretch on coarse
vascular lumens resulting from exposure to the arterial circulation
has been demonstrated to induce apoptosis of ECs in a sheep model
of open carotid endarterectomy (5).
This mechanical stretch-induced vascular injury may contribute to
the subsequent excessive inflammatory response and rapid EC
apoptosis, which can ultimately result in stenosis and thrombosis
(6). Brown et al (15) found that vessel wall injury induced
changes in blood flow dynamics in a rat model of carotid
endarterectomy. The electrocautery likely caused a tissue
flattening effect in the abdominal aorta after endarterectomy to
prevent mechanism-induced shear stress injury. However, the
duration of the electrocautery use in rabbits is unknown.
Electrocautery can be considered a double-edged sword in that it
causes both severe thermal injury and vascular lumen improvement
after abdominal aortic endarterectomy. Therefore, we established
the protocol in rabbits with appropriate power and activation time.
Our study showed that intravascular electrocautery in the abdominal
aorta significantly improved blood flow 20 days after surgery
compared with the isolated endarterectomy group. The beneficial
effects of endarterectomy + electrocautery on blood flow appeared
to be associated with smooth vascular lumens and reduced
inflammation. Compared with isolated application of endarterectomy,
endarterectomy + electrocautery has substantial advantages. It can
not only be managed without side effects such as thermal injury,
but can also ameliorate inflammation after surgery, thus,
protecting vessels from vascular restenosis (6). In this study, we found that
intravascular application of electrocautery significantly reduced
the serum expression of TNF-α and vascular expression of IL-6. In
addition, electrocautery was quite safe. In this study, obvious
electrocautery-related injury responses in rabbits were not
observed, although there are several reports of side effects from
electrocautery in different clinical studies and animal models
(13,16).
In our study, to evaluate the effects of
endarterectomy + electrocautery on the abdominal aorta in a rabbit
model, we explored the levels of pro-inflammatory factors, measured
arterial flow parameters, and examined graft histology 20 days
after surgery. More et al (17) evaluated changes in the vessel wall in
a rabbit model of balloon angioplasty and showed that thickened
endothelium was observed at day 7 and peaked at 4 weeks. Therefore,
20 days after surgery appears to be the ideal time point for
assessing the effects of electrocautery on arterial flow and
histology. Given that our results showed that electrocautery
reduced inflammation and improved vessel flow 20 days after surgery
in the model of abdominal aortic endarterectomy, we believe that
the clinical use of endarterectomy + electrocautery in CABG is a
promising approach. In the rabbit model, electrocautery can only
partially alleviate postoperative intravascular resistance. The
long-term effects of electrocautery on arterial remodeling remain
unknown. Additionally, a pig model of artery bypass grafting
appears to be more appropriate for the observation of long-term
effects because of the high similarity in coronary circulation
between humans and pigs. Currently, we are using a pig model to
explore the effects of electrocautery on remodeling of the coronary
artery. However, the activation time and precise power of
electrocautery in animals varies depending on the size, weight, and
species.
In conclusion, we found that intravascular
application of electrocautery reduced thrombosis and inflammation 4
weeks post-artery bypass grafting in a rabbit model. The favorable
effects appeared to be associated with smoothed vascular lumens,
decreased inflammation, and decreased EC apoptosis. Although the
short-term effects of intravascular application of electrocautery
appear to be promising, the long-term effects of electrocautery on
arterial remodeling and the clinical value of electrocautery in CE
require further exploration.
Acknowledgements
This study was supported by the project of Basic and
Clinical Research Cooperation From Capital Medical University (no.
16JL05) and the Yangfan Project of Clinical Technical Innovation
From the Hospital Authority of Beijing (no. XM201312).
References
|
1
|
Sirivella S, Gielchinsky I and Parsonnet
V: Results of coronary artery endarterectomy and coronary artery
bypass grafting for diffuse coronary artery disease. Ann Thorac
Surg. 80:1738–1744. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soylu E, Harling L, Ashrafian H, Casula R,
Kokotsakis J and Athanasiou T: Adjunct coronary endarterectomy
increases myocardial infarction and early mortality after coronary
artery bypass grafting: A meta-analysis. Interact Cardiovasc Thorac
Surg. 19:462–473. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soylu E, Harling L, Ashrafian H, Casula R,
Kokotsakis J and Athanasiou T: Adjunct coronary endarterectomy
increases myocardial infarction and early mortality after coronary
artery bypass grafting: a meta-analysis. Interact Cardiovasc Thorac
Surg. 19:462–473. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Atik FA, Dallan LA, De Oliveira SA, Lisboa
LA, Platania F, Cabral RH and Jatene AD: Myocardial
revascularization with coronary endarterectomy. Stratification of
risk factors for early mortality. Arq Bras Cardiol. 75:269–280.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bezon E, Khalifa AA, Le Gal G, Choplain
JN, Mansourati J and Barra JA: Use of arterial patch to improve
re-endothelialization in a sheep model of open carotid
endarterectomy. An incentive to use internal thoracic artery as an
on-lay patch following coronary endarterecomy? Interact Cardiovasc
Thorac Surg. 8:543–547. 2009.PubMed/NCBI
|
|
6
|
Liang JJ, Xue W, Lou LZ, Liu C, Wang ZF,
Li QG and Huang SH: Correlation of restenosis after rabbit carotid
endarterectomy and inflammatory cytokines. Asian Pac J Trop Med.
7:231–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bailey CP, May A and Lemmon WM: Survival
after coronary endarterectomy in man. J Am Med Assoc. 164:641–646.
1957. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vohra HA, Kanwar R, Khan T and Dimitri WR:
Early and late outcome after off-pump coronary artery bypass graft
surgery with coronary endarterectomy: A single-center 10-year
experience. Ann Thorac Surg. 81:1691–1696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tiruvoipati R, Loubani M, Lencioni M,
Ghosh S, Jones PW and Patel RL: Coronary endarterectomy: Impact on
morbidity and mortality when combined with coronary artery bypass
surgery. Ann Thorac Surg. 79:1999–2003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu SQ, Ruan YY, Tang D, Li YC, Goldman J
and Zhong L: A possible role of initial cell death due to
mechanical stretch in the regulation of subsequent cell
proliferation in experimental vein grafts. Biomech Model
Mechanobiol. 1:17–27. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wainwright CL, Miller AM and Wadsworth RM:
Inflammation as a key event in the development of neointima
following vascular balloon injury. Clin Exp Pharmacol Physiol.
28:891–895. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holub Z, Jabor A, Sprongl L, Kliment L,
Fischlová D and Urbánek S: Inflammatory response and tissue trauma
in laparoscopic hysterectomy: Comparison of electrosurgery and
harmonic scalpel. Clin Exp Obstet Gynecol. 29:105–109.
2002.PubMed/NCBI
|
|
13
|
Fiorelli A, Accardo M, Carelli E, Del
Prete A, Messina G, Reginelli A, Berritto D, Papale F, Armenia E,
Chiodini P, et al: Harmonic technology versus neodymium-doped
yttrium aluminium garnet laser and electrocautery for lung
metastasectomy: An experimental study. Interact Cardiovasc Thorac
Surg. 23:47–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Robinson AM, Fishman AJ, Bendok BR and
Richter CP: Functional and physical outcomes following use of a
flexible CO2 laser fiber and bipolar electrocautery in close
proximity to the rat sciatic nerve with correlation to an in vitro
thermal profile model. BioMed Res Int. 2015:2802542015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brown AT, Chen H, Davis JA, Qureshi I,
Cruz CP, Poirier LA, Eidt JF and Moursi MM: Plasma homocysteine
measurements after carotid artery manipulation and clamping in a
rat CEA model. J Vasc Surg. 40:796–802. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uluyol S, Karakaya NE, Gur MH, Kilicaslan
S, Kantarcioglu EO, Yagiz O and Arslan IB: Radiofrequency thermal
ablation versus bipolar electrocautery for the treatment of
inferior turbinate hypertrophy: Comparison of efficacy and
postoperative morbidity. Int Arch Otorhinolaryngol. 20:2–5.
2016.PubMed/NCBI
|
|
17
|
More RS, Rutty G, Underwood MJ, Brack MJ
and Gershlick AH: A time sequence of vessel wall changes in an
experimental model of angioplasty. J Pathol. 172:287–292. 1994.
View Article : Google Scholar : PubMed/NCBI
|