Introduction
Cardiovascular diseases (CVDs) associated morbidity
and mortality is continuously evolving worldwide (1–3). The
term CVD encompasses all diseases of the heart and circulatory
system, including stroke, myocardial infarction (MI) and a variety
of ischemic diseases. Although the number of people living with CVD
has fallen in recent years, there is still need for the development
of novel therapeutic strategies to relieve the burden on health
services around the world.
Endothelial cells (ECs) play a major role in
maintenance of vascular integrity, particular with relation to
angiogenesis and wound repair post-injury, as well as in
vasculogenesis during in vivo embryo development (4). Further, miRNAs have been implicated in
EC function and proliferation, as well as in the regulation of
angiogenesis and vasculogenesis. Global reduction of miRNAs, via
conditional knockdown of the miRNA processing Dicer using siRNAs
in vitro, altered the expression of several important
regulators of endothelial biology and angiogenesis including
vascular endothelial growth factor receptor 2 (VEGFR2; KDR) and
endothelial nitric oxide synthase (eNOS), as well reducing
proliferation and tubule formation capacity (5). In vivo, Dicer silencing was also
shown to reduce postnatal angiogenesis in response to angiogenic
cytokines, such as VEGF (5).
Combined transient silencing of both Dicer and Drosha processing
enzymes reduced the sprout forming and angiogenic properties of
ECs, although only silencing of Dicer had significant effects on
angiogenic potential in vivo (6). Several miRNAs have been identified to
play a role in the regulation of function, proliferation and growth
of vascular ECs (7). These include
miR-126, miR-10a, the Let-7 cluster, the pro-angiogenic miR-17-92
cluster and the anti-angiogenic miR-221 and miR-222. miRNAs
identified to play key roles in the regulation of angiogenesis may
be important therapeutic targets in the treatment of a range of
ischemic diseases, as well as in the regulation of angiogenesis
during cancer and tumor progression.
miR-126 in vascular integrity
One of the most studied and extensively
characterized EC miRNAs is miR-126. Early miRNA profiling studies
found that miR-126 is enriched in tissues with a high vascular
component, and expression patterns in zebrafish also showed the
expression of miR-126 confined to the vascular system (8). Further, a study revealed miR-126 is
expressed in primary human umbilical vein ECs (HUVECs), as well as
in a number of EC lines (9).
Moreover, miR-126 has been reported to be highly enriched miRNA in
ECs generated from differentiating mESC (10). Generation of miR-126-null mice
resulted in ~40% embryonic lethality, exhibiting a large number of
vascular defects, including severe systemic oedema, haemorrhage and
vessel rupture, and knockdown in zebrafish using
pri-miR-126-specific morpholinos resulted in comprised blood vessel
integrity, haemorrhages and compromised endothelial tube formation.
Taken together, this data suggested a role for miR-126 in the
maintenance of endothelial and vascular integrity during
development. Knockdown of miR-126 also resulted in a decrease in
angiogenesis during ex vivo and in vivo assays. To
exert its angiogenic affects, miR-126 was also shown to augment MAP
kinase pathway activation in response to angiogenic factors such as
FGF and VEGF in vitro, with knockdown of the miRNA resulting
in a reduction in FGF and VEGF-mediated migration, possibly through
defective reorganisation of the actin cytoskeleton (10). It was subsequently demonstrated that
miR-126 exerts its angiogenic effects through the targeting of the
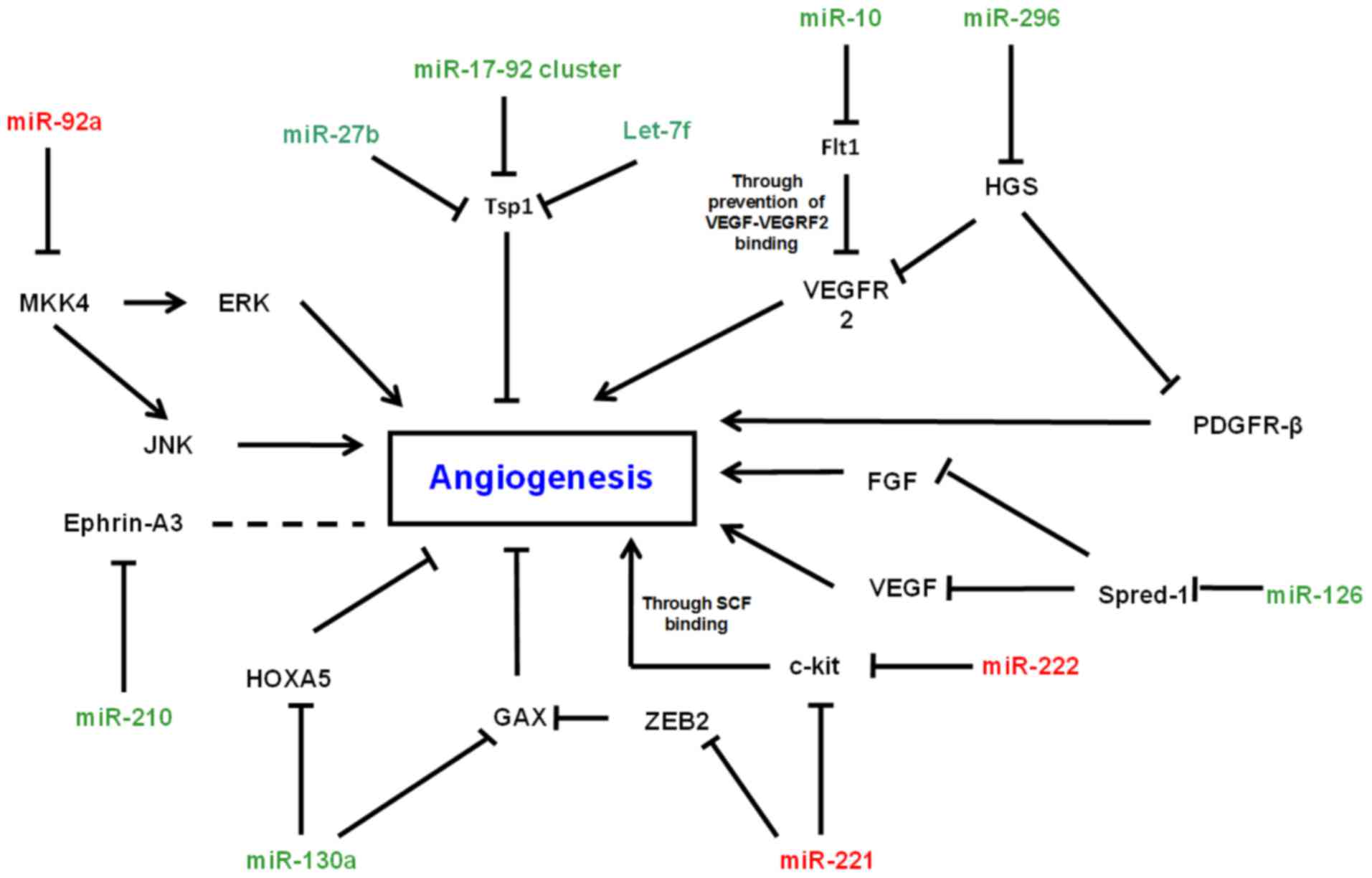
sprouty-related protein Spred-1 (Fig.
1). A role for miR-126 in the regulation of vascular
inflammation has also been elucidated, via its targeting and
repression of vascular cell adhesion molecule-1 (VCAM-1), an
intercellular adhesion molecule expressed by ECs. VCAM-1 expression
is upregulated during vascular inflammation, where it functions to
mediate the ratio of EC:leukocyte adhesion. Reduced expression of
miR-126, in tumour necrosis factor-α (TNF-α) stimulated ECs,
increases the expression of VCAM-1, in turn enhancing leukocyte
adherence and, ultimately, vascular inflammation (9).
miR-126 is located within intron 7 of epidermal
growth factor-like domain 7 (Egfl7), a gene which is highly
expressed in ECs, and the expression of both strands of the miR-126
stem loop (miR-126-3p and −5p) mirrors the expression of Egfl7
(11). This suggested that miR-126
is processed from the pre-mRNA-Egfl7 transcript. A 5′ region
upstream of the Egfl7/miR-126 locus was then found to regulate
expression of miR-126, and in silico analysis subsequently
revealed two Ets binding sites (EBS) within this region. The Ets
factors are a family of transcription factors, and several members,
including Ets-1 and −2, have been shown to play important roles in
vasculo- and angiogenesis (12).
Ets-1 and −2 were found to bind to the Egfl7/miR-126 5′ region to
activate transcription in ECs, therefore suggesting that these
transcription factors play an important role in the regulation of
miR-126 expression and, therefore, the regulation of it angiogenic
affects (11).
miR-17-92 cluster in vascular integrity
The miR-17-92 cluster, consisting of miR-17, −18a,
−19a/b, −20a and −92a, has also been extensively studied in the
context of ECs and angiogenesis. Originally, it was discovered that
this cluster plays a role in tumor angiogenesis. The above cluster
of miRNAs have been observed to be upregulated also in colonocytes.
In this system the miRNAs target the anti-angiogeneic
thrombospondin-1 (Tsp1) and the related protein connective tissue
growth factor (CTGF), to cause an increase in angiogenesis, and
cells transduced with the miR-17-92 cluster formed larger,
better-perfused tumors (13). On the
other hand a study by Doebele et al showed that the
overexpression of individual members of the cluster, specifically
miR-17, −18a, −19a and −20a, resulted in reduction in angiogenic
sprouting. However, same inhibitors of the similar miRNAs caused an
increase in angiogenesis in vitro, suggesting an
anti-angiogenic role for this cluster (14). This ties in with previous work
showing that miR-92a targets several proangiogenic proteins,
including integrin subunit α5, to exert an anti-angiogenic effect,
with overexpression of this miRNA blocking angiogenesis in
vivo and in vitro (15).
More recently, it was shown that histone deacetylase 9 (HDAC9)
displays a proangiogenic affect, regulated through the
transcriptional repression of the miR-17-92 cluster (16). Taken together, the data suggest a
varied role for the miR-17-92 cluster in the context of EC function
and angiogenesis.
miR-210 in vascular integrity
Reduced miR-210 expression has been shown to inhibit
EC growth and induce apoptosis, suggesting a proangiogenic role for
this miRNA. In hypoxic conditions, it was found that the level of
miR-210 was increased in HUVECs when compared to cells in normoxic
conditions (17). In normoxic ECs
in vitro, overexpression of miR-210 simulated the formation
of capillary-like structures on Matrigel, and VEGF-mediated
migration. Knockdown of this miRNA inhibited these same functions,
and also inhibited cell growth and caused increased apoptosis.
In silico analysis revealed Ephrin-A3 (EFNA3), a gene
post-transcriptionally downregulated by hypoxia, as a potential
target for miR-210, and luciferase assays using the 3′-UTR of the
EFNA3 mRNA confirmed this. Downregulation of this gene was proven
to be necessary for miR-210-regulated angiogenesis. Lou et
al further elucidated the mechanism of miR-210 and its role in
angiogenesis by investigating the role of this microRNA in
regulating angiogenesis in response to brain ischemic injury.
miR-210 was significantly upregulated in adult rat ischemic brain
cortexes in vivo, where the expression of Notch-1 signaling
molecules, which facilitate the migration of ECs, were also
increased. Using lentiviral-mediated overexpression, it was shown
that miR-210 activated the Notch signaling pathway, facilitating
the migration of ECs inducing in vitro capillary formation
(18). This has also been shown in
the normal adult mouse brain, where overexpression of miR-210,
using stereotactic injection of a lentiviral vector, increased
neurogenesis and angiogenesis, associated with a local increase in
the levels of VEGF (19). Overall,
data suggest that miR-210 could be used as a therapy, or a
therapeutic target for the modulation of angiogenesis in the
treatment of ischemic diseases. Indeed, miR-210 has been used as a
therapy in vivo to enhance wound healing, and tissue repair,
processes in which angiogenesis plays a key role (20).
miR-10 in vascular integrity
Interestingly, it was also suggested that miR-10
played a role in angiogenesis, after it was discovered that
heparin, an anti-thrombotic drug, impairs angiogenesis through the
inhibition of miR-10, therefore inducing upregulation of its mRNA
target Hoxd10 (21). Indeed, further
investigation showed an increase in the expression of miR-10a in
CD144+ VEGFR2+ ECs generated from mESCs when compared to CD144-
VEGFR2- non-ECs (22). Knockdown of
all four miR-10 isoforms (a-d) during zebrafish development
resulted in an impairment in angiogenesis and the development of
the intersegmental vessels, whereas overexpression lead to enhanced
angiogenic potential in vivo. In vitro experiments
demonstrated the direct targeting of the 3′-UTR of Flt1, also known
as VEGFR1, and its soluble splice variant sFlt1 by miR-10, with
miR-10 depletion leading to an increase in Flt-1 expression both
in vivo and in vitro. Flt-1 binds VEGF and is thought
to function to inhibit angiogenesis by sequestering VEGF to prevent
binding to VEGFR2 (22).
miR-221 and miR-222
Just as important as miRNAs with potential
proangiogenic functions, the miRNAs with anti-angiogenic functions
may also be useful targets in the control of angiogenesis in a
disease setting, such as in the regulation of tumor angiogenesis
during cancer, as well as in cardiovascular and ischemic diseases.
Similarly to those involved in the positive regulation of
angiogenesis, anti-angiogenic miRNAs function via the targeting of
an array of angiogenic-associated mRNAs (Fig. 1).
Located intergenically, miR-221 and miR-222 are
transcribed as a cluster, and have been shown to share an identical
seed sequence. Both of these miRNAs have been described as
anti-angiogenic, and were shown to regulate c-kit, the receptor for
stem cell factor (SCF), at the protein level (23). SCF is an angiogenic factor, which has
been shown to promote survival, migration and capillary formation
of HUVECs in vitro (24).
Overexpression of miR-221 and miR-222 caused a reduction in
survival, migration and capillary formation in vitro
mediated through reduction in the levels of c-kit. Another study
showed that miR-221 and miR-222 regulate the expression of eNOS,
with overexpression of these miRNAs inhibiting the increase in eNOS
expression observed after Dicer knockdown (5).
It has also been shown that miR-221 indirectly
upregulates a potential master regulator of EC angiogenic phenotype
namely GAX, so as to inhibit angiogenesis (25). It was hypothesised that this
upregulation was mediated through the downregulation of an
intermediate protein, and this was subsequently discovered to be
ZEB2, a zinc-finger nuclear factor which primarily acts as a
transcriptional repressor (26).
Other notable pro- and anti-angiogenic
miRNAs
Similarly to miRNAs already mentioned, other
angiogenesis-regulating miRNAs also function via the direct or
indirect targeting of growth factors and their receptors. One such
miRNA is the proangiogenic miR-296, which was found to be
upregulated when human brain microvascular ECs were treated with
angiogenic factors, and also shown to be expressed at higher levels
in primary highly angiogenic ECs derived from human brain tumors,
when compared to normal brain ECs (27).
In this context miR-296 was found to target
hepatocyte growth factor-regulated tyrosine kinase substrate (HGS),
a protein involved in the mediation of the degradation of a number
of growth factor receptors, including platelet-derived growth
factor receptor β and VEGFR2, therefore increasing the levels of
signaling by angiogenic factors (28).
Moreover, miR-15b and −16, along with miR-20a and
−20b, are also thought to exert their antiangiogenic effects via
the targeting of VEGF (29).
Although the study was performed in a human nasopharyngeal
carcinoma cell line, all four of these miRNAs were downregulated in
response to hypoxia, in contrast to the concomitant upregulation of
VEGF. These data suggested a role for the four miRNAs in the
regulation of angiogenesis, and this has been supported by evidence
in a number of other studies (30,31). In
the same study miR-378 has been noted to regulate the expression of
VEGF (29). Further, studies showed
a proangiogenic role for miR-378, through its propensity to cause
an increase in cell survival and a decrease in apoptosis, in a
similar way to miR-210 (32).
MiR-100, a miRNA expressed in both ECs and vascular smooth muscle
cells (VSMCs), was also found to be downregulated during hypoxia,
much like miR-15 and miR-16. In vivo, miR-100 was
significantly downregulated after the induction of hind limb
ischemia in mice, and it was found to control angiogenic sprouting
and the proliferation of vascular cells in vitro (33). Mammalian target of rapamycin (mTOR),
a gene previously demonstrated to be involved in angiogenesis and
proliferation of ECs in response to hypoxia (34), was found to be a direct target of
miR-100 (4) and this was confirmed
in the context of ECs, indicating a role for this miRNA in the
negative regulation of EC angiogenesis. More recently, this has
also been shown in the context of graft-versus-host disease (GvHD),
whereby miR-100 antagonism increased neovascularisation and,
therefore, increased disease severity (35).
A global knock out of miRNA processing enzymes in
human ECs also lead to the identification of miR-27b and Let-7f as
possible proangiogenic miRNAs (6).
Conclusion
It was concluded from the above studies that miRNAs
have significant role in the control of vascular EC function and
angiogenic phenotype. This information could further help in
development of novel therapies, not only for the treatment of
ischemic diseases, but also for angiogenesis regulation in the
context of cancer.
References
|
1
|
British Heart Foundation, . Cardiovascular
Disease Statistics 2014. British Heart Foundation; London: pp.
1–59. 2014
|
|
2
|
Sawicka M, Janowska J and Chudek J:
Potential beneficial effect of some adipokines positively
correlated with the adipose tissue content on the cardiovascular
system. Int J Cardiol. 222:581–589. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kooij KW, Schouten J, Wit FW, van der Valk
M, Kootstra NA, Stolte IG, van der Meer JT, Prins M, Grobbee DE,
van den Born BJ, et al: Difference in aortic stiffness between
treated middle-aged HIV type 1-infected and uninfected individuals
largely explained by traditional cardiovascular risk factors, with
an additional contribution of prior advanced immunodeficiency. J
Acquir Immune Defic Syndr. 73:55–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang FZ, Weber F, Croce C, Liu CG, Liao X
and Pellett PE: Human cytomegalovirus infection alters the
expression of cellular microRNA species that affect its
replication. J Virol. 82:9065–9074. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suárez Y, Fernández-Hernando C, Pober JS
and Sessa WC: Dicer dependent microRNAs regulate gene expression
and functions in human endothelial cells. Circ Res. 100:1164–1173.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuehbacher A, Urbich C, Zeiher AM and
Dimmeler S: Role of Dicer and Drosha for endothelial microRNA
expression and angiogenesis. Circ Res. 101:59–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jakob P and Landmesser U: Role of
microRNAs in stem/progenitor cells and cardiovascular repair.
Cardiovasc Res. 93:614–622. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wienholds E, Kloosterman WP, Miska E,
Alvarez-Saavedra E, Berezikov E, De Bruijn E, Horvitz HR, Kauppinen
S and Plasterk RH: MicroRNA expression in zebrafish embryonic
development. Science. 309:310–311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harris TA, Yamakuchi M, Ferlito M, Mendell
JT and Lowenstein CJ: MicroRNA-126 regulates endothelial expression
of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA.
105:pp. 1516–1521. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fish JE, Santoro MM, Morton SU, Yu S, Yeh
RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY and Srivastava D:
miR-126 regulates angiogenic signaling and vascular integrity. Dev
Cell. 15:272–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Harris TA, Yamakuchi M, Kondo M, Oettgen P
and Lowenstein CJ: Ets-1 and Ets-2 regulate the expression of
microRNA-126 in endothelial cells. Arterioscler Thromb Vasc Biol.
30:1990–1997. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dejana E, Taddei A and Randi AM: Foxs and
Ets in the transcriptional regulation of endothelial cell
differentiation and angiogenesis. Biochim Biophys Acta.
1775:298–312. 2007.PubMed/NCBI
|
|
13
|
Dews M, Homayouni A, Yu D, Murphy D,
Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, et
al: Augmentation of tumor angiogenesis by a Myc-activated microRNA
cluster. Nat Genet. 38:1060–1065. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Doebele C, Bonauer A, Fischer A, Scholz A,
Reiss Y, Urbich C, Hofmann WK, Zeiher AM and Dimmeler S: Members of
the microRNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic
function in endothelial cells. Blood. 115:4944–4950. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bonauer A, Carmona G, Iwasaki M, Mione M,
Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, et
al: MicroRNA-92a controls angiogenesis and functional recovery of
ischemic tissues in mice. Science. 324:1710–1713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaluza D, Kroll J, Gesierich S, Manavski
Y, Boeckel JN, Doebele C, Zelent A, Rössig L, Zeiher AM, Augustin
HG, et al: Histone deacetylase 9 promotes angiogenesis by targeting
the antiangiogenic microRNA-17-92 cluster in endothelial cells.
Arterioscler Thromb Vasc Biol. 33:533–543. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fasanaro P, D'Alessandra Y, Di Stefano V,
Melchionna R, Romani S, Pompilio G, Capogrossi MC and Martelli F:
MicroRNA-210 modulates endothelial cell response to hypoxia and
inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol
Chem. 283:15878–15883. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lou YL, Guo F, Liu F, Gao FL, Zhang PQ,
Niu X, Guo SC, Yin JH, Wang Y and Deng ZF: miR-210 activates notch
signaling pathway in angiogenesis induced by cerebral ischemia. Mol
Cell Biochem. 370:45–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeng L, He X, Wang Y, Tang Y, Zheng C, Cai
H, Liu J, Wang Y, Fu Y and Yang GY: MicroRNA-210 overexpression
induces angiogenesis and neurogenesis in the normal adult mouse
brain. Gene Ther. 21:37–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Usman MA, Nakasa T, Shoji T, Kato T,
Kawanishi Y, Hamanishi M, Kamei N and Ochi M: The effect of
administration of double stranded MicroRNA-210 on acceleration of
Achilles tendon healing in a rat model. J Orthop Sci. 20:538–546.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen X, Fang J, Lv X, Pei Z, Wang Y, Jiang
S and Ding K: Heparin impairs angiogenesis through inhibition of
microRNA-10b. J Biol Chem. 286:26616–26627. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hassel D, Cheng P, White MP, Ivey KN,
Kroll J, Augustin HG, Katus HA, Stainier DY and Srivastava D:
MicroRNA-10 regulates the angiogenic behavior of zebrafish and
human endothelial cells by promoting vascular endothelial growth
factor signaling. Circ Res. 111:1421–1433. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Poliseno L, Salmena L, Zhang J, Carver B,
Haveman WJ and Pandolfi PP: A coding-independent function of gene
and pseudogene mRNAs regulates tumour biology. Nature.
465:1033–1038. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsui J, Wakabayashi T, Asada M,
Yoshimatsu K and Okada M: Stem cell factor/c-kit signaling promotes
the survival, migration, and capillary tube formation of human
umbilical vein endothelial cells. J Biol Chem. 279:18600–18607.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y, Banda M, Speyer CL, Smith JS,
Rabson AB and Gorski DH: Regulation of the expression and activity
of the antiangiogenic homeobox gene GAX/MEOX2 by ZEB2 and
microRNA-221. Mol Cell Biol. 30:3902–3913. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Grunsven LA, Michiels C, van de Putte
T, Nelles L, Wuytens G, Verschueren K and Huylebroeck D:
Interaction between Smad-interacting protein-1 and the corepressor
C-terminal binding protein is dispensable for transcriptional
repression of E-cadherin. J Biol Chem. 278:26135–26145. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Würdinger T, Tannous BA, Saydam O, Skog J,
Grau S, Soutschek J, Weissleder R, Breakefield XO and Krichevsky
AM: miR-296 regulates growth factor receptor overexpression in
angiogenic endothelial cells. Cancer Cell. 14:382–393. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ewan LC, Jopling HM, Jia H, Mittar S,
Bagherzadeh A, Howell GJ, Walker JH, Zachary IC and Ponnambalam S:
Intrinsic tyrosine kinase activity is required for vascular
endothelial growth factor receptor 2 ubiquitination, sorting and
degradation in endothelial cells. Traffic. 7:1270–1282. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hua Z, Lv Q, Ye W, Wong CK, Cai G, Gu D,
Ji Y, Zhao C, Wang J, Yang BB, et al: MiRNA-directed regulation of
VEGF and other angiogenic factors under hypoxia. PLoS One.
1:e1162006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun CY, She XM, Qin Y, Chu ZB, Chen L, Ai
LS, Zhang L and Hu Y: miR-15a and miR-16 affect the angiogenesis of
multiple myeloma by targeting VEGF. Carcinogenesis. 34:426–435.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xue G, Yan HL, Zhang Y, Hao LQ, Zhu XT,
Mei Q and Sun SH: c-Myc-mediated repression of miR-15-16 in hypoxia
is induced by increased HIF-2α and promotes tumor angiogenesis and
metastasis by upregulating FGF2. Oncogene. 34:1393–1406. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee DY, Deng Z, Wang CH and Yang BB:
MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis
by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci USA.
104:pp. 20350–20355. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grundmann S, Hans FP, Kinniry S, Heinke J,
Helbing T, Bluhm F, Sluijter JP, Hoefer I, Pasterkamp G, Bode C, et
al: MicroRNA-100 regulates neovascularization by suppression of
mammalian target of rapamycin in endothelial and vascular smooth
muscle cells. Circulation. 123:999–1009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Humar R, Kiefer FN, Berns H, Resink TJ and
Battegay EJ: Hypoxia enhances vascular cell proliferation and
angiogenesis in vitro via rapamycin (mTOR)-dependent signaling.
FASEB J. 16:771–780. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leonhardt F, Grundmann S, Behe M, Bluhm F,
Dumont RA, Braun F, Fani M, Riesner K, Prinz G, Hechinger AK, et
al: Inflammatory neovascularization during graft-versus-host
disease is regulated by αv integrin and miR-100. Blood.
121:3307–3318. 2013. View Article : Google Scholar : PubMed/NCBI
|