Introduction
Scleral tunnel incision is used in the 23G minimally
invasive vitrectomy and has the advantage of scar closure. However,
surgery may be complicated by low intraocular pressure (IOP), which
is caused by intraoperative leakage in scleral incision. Sutureless
surgery avoids irritation of ocular tissues and is, therefore, the
most popular method to prevent IOP. To close the incision of the
sclera and conjunctiva, fibrin glue was used, first in a 20G
vitrectomy (1), and subsequently in
23G and 25G vitrectomies (2). No
incision leakage, adverse effects, or low IOP were observed during
the postoperative follow-up. However, the clinical use of fibrin
glue is limited in China due to its blood-borne origins (2).
In the present study, we tested Suncon medical
adhesive as a replacement for fibrin glue. Medical adhesive is a
new approach for closing surgical incisions. It reduces operation
times, requires no postoperative suture removal, and attenuates
postoperative foreign body sensation. The Suncon medical adhesive
is one of the homologues of α-cyanoacrylate and can be used with
some modifications as a rapid medical adhesive. It prevents scar
tissue formation, promotes tissue healing, has hemostatic and
bactericidal effects, and relieves pain. Adhesion time is 6–14 sec,
and protective film forms during 5–7 days elapsing from adhesion to
spontaneous detachment. The Suncon medical adhesive has other
advantages as well: i) Sufficient time to perform the operation
before coagulation, ii) sufficient adhesive force for closing the
incision after coagulation, iii) mild post-operative inflammatory
reactions, iv) free circulation of fluids which prevents tissue
necrosis, v) stable physical and chemical properties, and vi)
disappearance of adhered incision site.
The Suncon medical adhesive is effective in eyelid
laceration (3) and in patients with
corneal perforation of <3 mm (4),
and has been usable for transparent corneal notch (5). However, the usefulness of Suncon
medical adhesive for 23G minimally invasive vitrectomy (e.g.,
potentially leaking scleral incision or retinal toxicity) has yet
to receive proper attention.
To test its suitability for 23G minimally invasive
vitrectomy and exclude potential toxicity to retina, we utilized
the Suncon medical adhesive in an animal model of this
intervention. The results showed that Suncon medical adhesive is
well-tolerated by retina when used at volumes of 0.05 ml and can
thus be a suitable alternative to fibrin glue.
Materials and methods
Laboratory animals and reagents
We used 18 healthy male and female Japanese white
rabbits that did not have oculopathy. The animals were purchased
from the Laboratory Animal Center of Xuzhou Medical College
(Xuzhou, China). Conventional housing and diet were provided for
one week before the experiment to maintain the body weight at
2.5–3.0 kg. This study was approved by the Animal Ethics Committee
of Animal Center of Xuzhou Medical College.
Suncon medical adhesive was purchased from the
Beijing Suncon Science and Technology Development Co., Ltd.
(Beijing, China). The Retiscan Electrophysiology Examination System
was from Roland Inc. (Waiblingen, Germany), while contact lens and
needle electrodes were obtained from the Beijing Gaoshi Yuanwang
Science and Technology Co., Ltd. (Beijing, China).
Interventions
The rabbits were anaesthesized by intravenous
(auricular vein) injection of 3% pentobarbital sodium at a dose of
1 ml/kg. In each rabbit, one eye was chosen as a treatment eye, and
this eye received an intravitreal injection of 0.05 ml of the
Suncon medical adhesive. Another eye served as the control eye. The
Suncon medical adhesive was aspirated with a 1-ml sterile syringe
and injected intravitreally in the treatment eye at 3 mm behind the
upper limbus of the sclera. The depth of insertion was 0.7 cm in
the vertical direction of the eye center. Suncon medical adhesive
was slowly injected into the vitreous body, after which the needle
was removed and pressure was applied with a cotton bud.
Chloramphenicol eye drops were then administered into this eye. The
control eye received intravitreal injection of 0.05 ml of normal
saline.
Outcome measures
The conjunctiva, sclera, cornea, anterior chamber
and lens were observed with a slit lamp before the intravitreal
injection of Suncon medical adhesive, and on days 1, 7, 14, 21 and
28 after the injection. In addition, the vitreous body and retina
were examined with an indirect ophthalmoscope (Barui Medical
Equipment Co., Beijing, China). The electroretinogram (ERG)
examination was performed 28 days after the intravitreal
injections. The ERG pattern was selected with Reti port32 (Barui
Medical Equipment Co.), and the position of each wave was selected,
and b-wave amplitude of rod cell response (Rod-R), maximum mixing
response (Max-R) and cone cell response (Cone-R),
P2-wave amplitude of oscillatory potentials (Ops), and
mean amplitude of 30 Hz scintillation response were recorded. The
stimulator was GanzfeldQ450, and the flash source was white
light-emitting diode.
After examinations, the animals were euthanized. The
eyeballs were removed and immersed in the eyeball fixation solution
(150 ml of 80% ethanol, 60 ml of formalin, 15 ml of glacial acetic
acid, and 1 g of crystallized picric acid), dehydrated, embedded in
paraffin, sliced, stained with hematoxylin and eosin, and subjected
to light microscopy.
Statistical analysis
Statistical analysis was performed with SPSS 16.0
statistical software (SPSS, Inc., Shanghai, China). Quantitative
data are shown as mean ± standard deviation, and the t-test was
used for intergroup comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
Inflammatory reaction
No inflammatory reaction (e.g., keratic precipitates
or anterior chamber flare) were observed on a slit lamp examination
in either treatment or control eyes.
ERG
Table I shows the
mean amplitude of ERG patterns in treatment and control eyes. The
differences in the b-wave of Rod-R, Max-R and Cone-R,
P2-wave amplitude of Ops, and mean amplitude of 30 Hz
scintillation response were not statistically significant between
eyes subjected to the Suncon medical adhesive or saline.
 | Table I.ERG results. |
Table I.
ERG results.
| Variables | Rod-R (µV) | Max-R (µV) | Cone-R (µV) | Ops (µV) | 30 Hz (µV) |
|---|
| Control eyes | 114.478±12.157 | 227.528±20.268 | 158.632±12.402 | 11.540±1.187 | 87.088±7.053 |
| Treatment eyes | 120.756±9.679 | 244.896±16.645 | 160.933±9.919 | 12.808±2.590 | 82.712±6.750 |
Light microscopy examination
Layers of retina were normal, albeit the retinal
inner and outer limiting membranes were observed with difficulties.
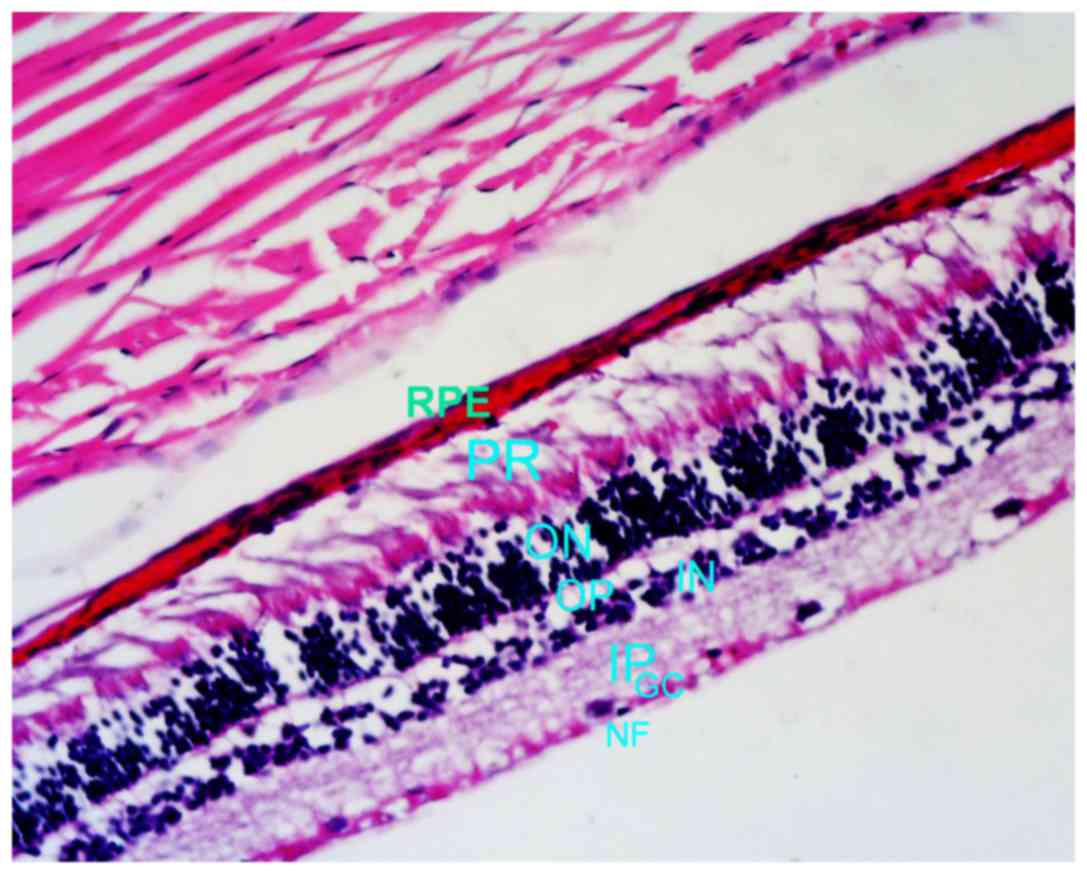
In the control eyes, the cells of the nerve fiber, ganglion cell,
inner plexiform, inner nuclear, outer plexiform, outer nuclear,
photoreceptor cell layers, and retinal pigment epithelium were
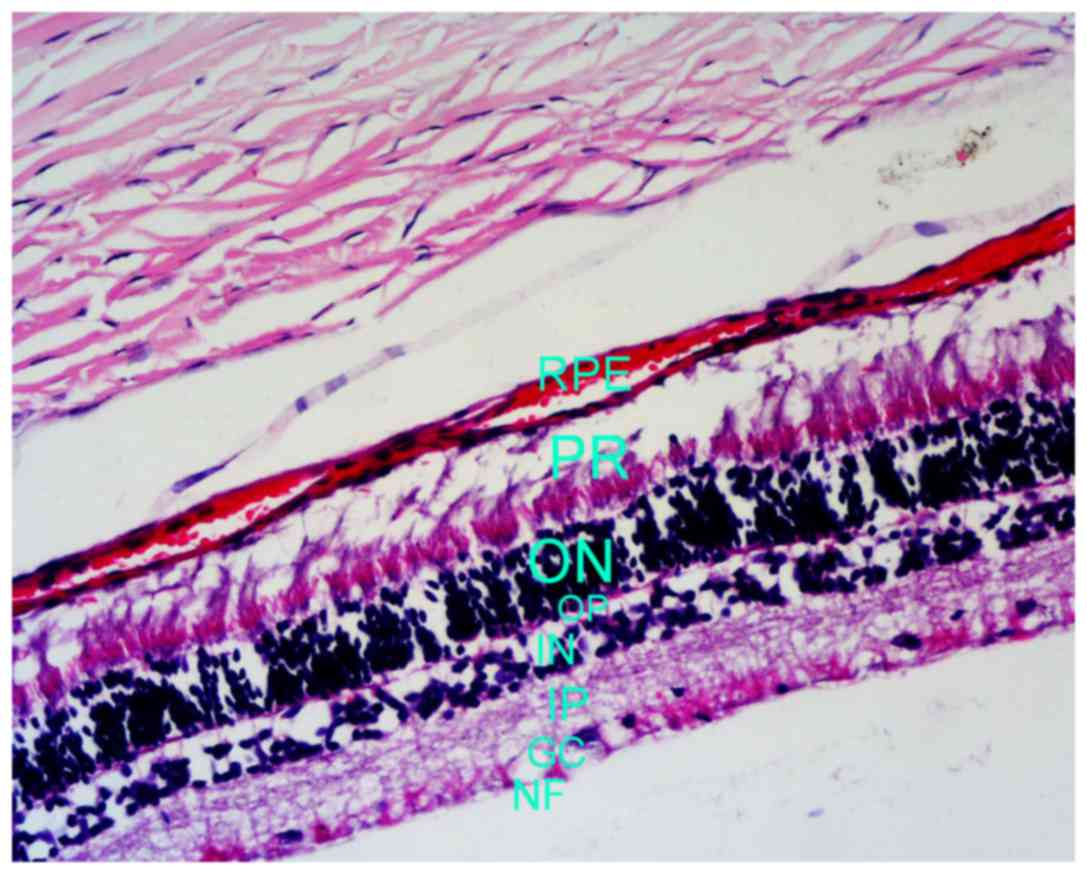
morphologically intact and well organized (Fig. 1). Similarly, the cells in the
corresponding layers of treatment eyes were morphologically intact
and well organized, and exhibited no bleeding, exudation or retinal
detachment (Fig. 2).
 | Figure 1.Control eye (hematoxylin and eosin
staining; magnification, ×100). RPE, retinal pigment epithelium;
PR, photoreceptor cell layer, ON, outer plexiform layer; OP, outer
plexiform layer; IN, inner nuclear layer; IP, inner plexiform
layer; GC, ganglion cell layer; NF, nerve fiber layer. |
Discussion
In the present study, we tested the suitability of
Suncon medical adhesive for 23G minimally invasive vitrectomy, with
a specific focus to potential toxicity to retina. In addition to
ophthalmologic evaluation, we recorded b-wave, which reflects the
electrical activity in bipolar cells and Müller cells, and
represents the functional status of retina (6). We demonstrate that intravitreal
injection of 0.05 ml of Suncon medical adhesive causes no damage to
the retinal function. Light-microscopy examination found that the
cells in each layer of the retina in the treatment group were
morphologically normal. Furthermore, no obvious inflammatory
reaction and no adverse effects on function and morphology of
retinal cells were observed.
In conclusion, Suncon medical adhesive injected at
doses of 0.05 ml is well-tolerated by the retina. Therefore, the
Suncon medical adhesive is a suitable alternative to fibrin glue.
This may be especially relevant for patients with thinner suture in
the scleral incision, to prevent incision leakage and incomplete
closure.
Acknowledgements
This study was supported by the Major Research
Project of Jiangsu Provincial Health Department (grant no.
H201054).
References
|
1
|
Batman C, Ozdamar Y, Mutevelli S, Sonmez
K, Zilelioglu G and Karakaya J: A comparative study of tissue glue
and vicryl suture for conjunctival and scleral closure in
conventional 20-gauge vitrectomy. Eye (Lond). 23:1382–1387. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Batman C, Ozdamar Y, Aslan O, Sonmez K,
Mutevelli S and Zilelioglu G: Tissue glue in sutureless
vitreoretinal surgery for the treatment of wound leakage.
Ophthalmic Surg Lasers Imaging. 39:100–106. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang M and Ye Y: Medical adhesive in the
treatment of eyelid injury. J Ocular Trauma Occup Eye Dis.
25:8502003.(In Chinese).
|
|
4
|
Setlik DE, Seldomridge DL, Adelman RA,
Semchyshyn TM and Afshari NA: The effectiveness of isobutyl
cyanoacrylate tissue adhesive for the treatment of corneal
perforations. Am J Ophthalmol. 140:920–921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meskin SW, Ritterband DC, Shapiro DE,
Kusmierczyk J, Schneider SS, Seedor JA and Koplin RS: Liquid
bandage (2-octyl cyanoacrylate) as a temporary wound barrier in
clear corneal cataract surgery. Ophthalmology. 112:2015–2021. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu L and Wu D: Clinical visual
electrophysiology. Science Press; Beijing: pp. 17–20. 1999
|