Introduction
Carnosine is a dipeptide composed of β-alanine and
histidine amino acids, and is widely abundant in the brain tissues
and muscle. It was first identified by a Russian scientist
(1) and later in a number of other
countries (2–8). Carnosine has been demonstrated to
possess antioxidant properties. Reactive oxygen species (ROS) and
aldehydes are produced by fatty acid oxidation and it has been
indicated that carnosine is able to scavenge these molecules.
Carnosine is a zwitterion with a negative and positive end, and is
a a well-known compound that reduces advanced glycation end
products. End products of advanced glycation may have a critical
role in the pathogenesis of a number of diseases, including
diabetes mellitus, renal failure, atherosclerosis and
neurodegenerative disease (9).
Carnosine has also been demonstrated to reduce the development of
atherosclerotic plaque (10).
Chronic glycolysis has been reported to accelerate
the aging process and the production of carnosine, a crucial
therpeutic candidate for neurodegeneration (11). Carnosine is abundant in cerebrospinal
fluid, innervated tissues and lenses. Carnosine possesses
physiological buffering, wound healing, antioxidant and
radioprotectant properties. In addition, it has metal ion
chelating, free-radical scavenging, anti-tumour compound,
immunomodulator (12) and
anti-ageing properties (13,14). However, its proper function remains
unknown. The present study investigated the protective effect of
carnosine against salsolinol-induced cellular damage.
Free oxygen radicals support the formation of some
species that are detrimental to biological molecules. ROS are
involved in the aging process (15)
and have been indicated to participate in the pathogenesis of joint
disease, diabetes, atherosclerosis and Parkinson's disease
(16,17). Lipid peroxidation (LPO) generates
malondialdehyde (MDA), which may potentially damage the proteins by
producing cross-links (18).
Previous studies have indicated that carnosine may react with
aldehydes to prevent proteins from advanced glycation. This
suggests that the high levels of carnosine may be enough to protect
against salsolinol induced neurotoxicity.
Salsolinol is a well-known compound that is widely
used as a pesticide, piscicide and insecticide. Salsolinol's
toxicity has been extensively studied in various in vitro
(19–21) and in vivo (22) systems. Synergistic neurotoxicity may
also occur when a small dose of different exogenous factors are
applied together. Combination of salsolinol and lipopolysaccharide
may result in synergistic toxicity (23). A previous study reported that
carnosine may be useful against neurotoxicity (9). The present study analyzes the
suppressive effect of carnosine against salsolinol-induced
Parkinson's disease in rats and rat brain endothelial cells.
Materials and methods
Materials
Dulbecco's modified Eagle medium (DMEM), dimethyl
sulphoxide (DMSO), sulforhodamine B (SRB), fetal bovine serum
(FBS), antibiotics (penicillin-streptomycin) and EDTA were
purchased from Sigma-Aldrich (Merck KGaA; Darmstadt, Germany).
2,7-Dichlorodihydrofluorescein diacetate (DCFH-DA) was obtained
from Santa Cruz Biotechnology, Inc., (Dallas, TX, USA). Rat brain
endothelial cells (bc3h1) were purchased from the American Type
Culture Collection (Manassas, VA, USA).
Animals
A total of 24 healthy, male albino rats were
purchased from the Shanghai Animal House (Shangai Medical College,
Shanghai, China), weighing 180–200 g, and were selected for the
present study. Rats were maintained in polypropylene cages, under
standard condition (relative humidity 62±5% and temperature
25±0.5°C) with a 12-h light dark cycle with access to food and
water ad libitum. Experimental animal groups were designated as
follows: Groups I, II, III and IV (all n=4). All animal experiments
were carried out in agreement with the ethical standards of China
Medical University, which conforms to the Guide for the Care and
Use of Laboratory Animals published by the U.S. National Institutes
of Health (NIH Publication no. 85–23, revised 1996).
Treatments
Group I received normal saline, group II 100 µg
salsolinol, group III 50 µg salsolinol + 50 µg carnosine, and group
IV 100 µg salsolinol + 100 µg carnosine. Following 72 h, the
animals were sacrificed, and brain tissues were surgically removed.
Brain tissue homogenate was prepared and used for the subsequent
investigations.
In vitro studies
Cell culture
Rat brain endothelial cells were cultured in DMEM
growth medium containing FBS and 1% penicillin-streptomycin. Cells
were maintained under standard conditions in a CO2
incubator at 37°C with an atmosphere containing 5%
CO2.
Fluorescence microscopy
Rat brain endothelial cells were cultured in a dish.
Cells were treated with 100 µg/ml salsolinol, 50 µg/ml salsolinol +
50 µg/ml carnosine or 100 µg salsolinol + 100 µg carnosine,
respectively. Following treatment, cells were centrifuged at 500 ×
g for 5 min at 4°C, and cell volume was adjusted to
104-105 cells/ml. Cells were incubated with
acridine orange (AO) and ethidium bromide (EB) dye for 30 min at
room temperature. Cells were viewed under a fluorescence microscope
(Olympus Corp., Tokyo, Japan), as previously described by
Muthuraman et al (24).
Determination of ROS production
Rat brain endothelial cells were cultured at
2.5×105 cells/well in a 6-well plate under 37°C and 5%
CO2. Cells were treated with 100 µg/ml salsolinol, 50
µg/ml salsolinol + 50 µg/ml carnosine or 100 µg salsolinol + 100 µg
carnosine, respectively. Following treatment, the cells were
treated with DCFH-DA for 30 min at 37°C in an atmosphere containing
5% CO2. Cells were viewed for fluorescence under a
fluorescence microscope (Olympus Corp.), as previously described by
Muthuraman et al (24).
Determination of lipid peroxidation
Cells were seeded in a dish at 2.5×105
cells/well in a 6-well plate. Cells were treated with 100 µg/ml
salsolinol, 50 µg/ml salsolinol + 50 µg/ml carnosine or 100 µg
salsolinol + 100 µg carnosine, respectively. At the end of all
treatment, LPO levels were determined using a kit according to
method outlined by Muthuraman et al (25). MDA content was measured with a
spectrophotometer at 534 nm (Cary 100 UV–Vis; Agilent Technologies,
Inc., Santa Clara, CA, USA).
Determination of reduced glutathione
Cells were cultured and grown at 2.5×105
cells per well in a 6-well plate. Cells were treated with 100 µg/ml
salsolinol, 50 µg/ml salsolinol + 50 µg/ml carnosine or 100 µg
salsolinol + 100 µg carnosine, respectively. GSH levels were
measured using a kit according to the method outlined by Muthuraman
et al (25). The resultant
yellow product was measured at 405 nm using a Cary 100 UV-Vis
spectrophotometer.
Determination of superoxide dismutase (SOD) and
catalase enzyme activities
Cells were seeded in a dish at 2.5×105
cells/well in a 6-well plate. Cells were treated with 100 µg/ml
salsolinol, 50 µg/ml salsolinol + 50 µg/ml carnosine or 100 µg
salsolinol + 100 µg carnosine, respectively. SOD and catalase
enzyme activities were measured using a kit (SOD assay kit,
19160-1KT-F; catalase assay kit, CAT100-1KT; both Sigma-Aldrich;
Merck KGaA) according to the method oulined by Muthuraman et
al (25).
In vivo studies
Determination of lipid peroxidation
Lipid peroxidation was determined using a kit
according to the spectrophotometric method of Muthuraman et
al (25). MDA content was
measured by determining the thiobarbituric acid reactive species
(TBARS). The resultant product was determined at 534 nm using a
Cary 100 UV-Vis spectrophotometer.
Determination of reduced glutathione (GSH)
The level of GSH was measured using a kit according
to the spectrophotometric method of Muthuraman et al
(25). The yellow product color was
measured using a according to the spectrophotometer at 405 nm.
Determination of SOD and catalase enzyme
activities
SOD and catalase enzyme activities were measured
using a kit according to the method of Muthuraman et al
(25).
Histopathological examination
A total of 24 rats were anesthetized with diethyl
ether (Sigma-Aldrich; Merck KGaA) and sacrificed by decapitation.
Brain tissues were removed and kept in 4% paraformaldehyde at 4°C
for 60 min. Hippocampus sections (4-µm thick) were prepared with
use of microtome and stained with hematoxylin and eosin. Sections
were qualitatively analyzed by light microscopy as previously
described (26).
Statistical analysis
All experimental data are expressed as the mean ±
standard error of the mean. The treated and control groups were
compared using Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
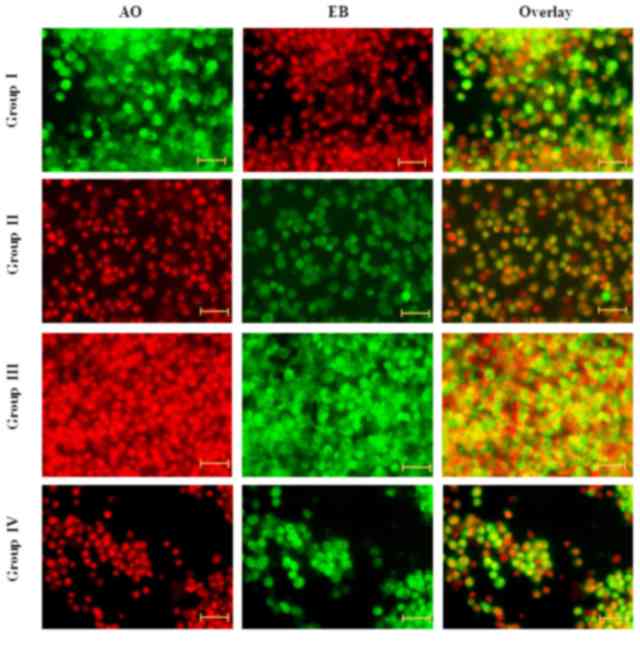
Effect of carnosine on apoptosis
Fluorescence microscopy examination was performed to
assess whether the neuroprotective effect of carnosine was
associated with the morphological aspect of cell death and
apoptosis and the morphological features of cell death. DNA-binding
AO and EB dyes were used to differentiate between viable and
non-viable cells, as chromatin condensation in the stained nucleus
is useful to identify viable, apoptotic and necrotic cells. The
neuroprotective effect of carnosine against salsolinol in the rat
brain endothelial cells was presented (Fig. 1). Fluorescence analysis indicated
normal cell size and morphology in control cells (group I); whereas
salsolinol-induced rat brain endothelial cells exhibited altered
cell morphology, including apoptosis and necrosis (group II).
Administration of 50 µg/ml carnosine and 50 µg/ml salsolinol (group
III) markedly reduced the apoptosis and necrosis of rat brain
endothelial cells. Administration of 100 µg/ml carnosine and 100
µg/ml rotenone (group IV) markedly reduced the occurrence of
apoptosis and necrosis in the endothelial cells towards normal
levels (Fig. 1).
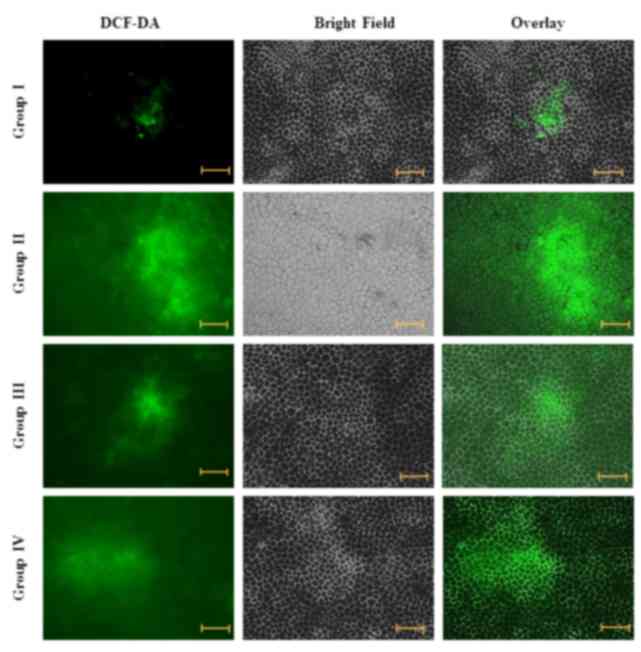
Effect of carnosine on intracellular ROS
level
ROS are able to facilitate signal transduction
processes in the cellular region. Fluorescence studies demonstrated
that there was little green fluorescence in the control cells
(group I), whereas green fluorescence was markedly increased in
salsolinol-treated cells (group II). Administration of 50 µg/ml
carnosine and 50 µg/ml salsolinol (group III) markedly reduced the
level of ROS in rat brain endothelial cells. Administration of 100
µg/ml carnosine and 100 µg/ml salsolinol (group IV) markedly
reduced the level of ROS in the rat brain endothelial cells,
towards normal levels (Fig. 2).
Effect of carnosine on MDA and GSH content in rat
brain endothelial cells
The neuroprotective effect of carnosine against
salsolinol-induced toxicity in rat brain endothelial cells is
presented in Table I. MDA content in
control cells was 21.15±1.20 nmol/g, whereas it significantly
increased to 36.15±1.0 nmol/g in salsolinol-treated rat brain
endothelial cells (group II; P<0.05; Table I). Administration of 50 µg/ml
carnosine and 50 µg/ml salsolinol (group III) significantly reduced
(31.36±1.2 nmol/g) MDA content in the rat brain endothelial cells,
compared with group II (P<0.05; Table
I). Administration of 100 µg/ml carnosine and 100 µg/ml
salsolinol (group IV) significantly reduced MDA content (23.7±1.1
nmol/g) in the rat brain endothelial cells, compared with group II
(P<0.05; Table I).
 | Table I.Effect of carnosine against
salsolinol induced LPO, GSH, SOD and catalase levels in the rat
brain endothelial cells. |
Table I.
Effect of carnosine against
salsolinol induced LPO, GSH, SOD and catalase levels in the rat
brain endothelial cells.
| Parameter | Group I | Group II | Group III | Group IV |
|---|
| MDA, nmol/g | 21.15±1.12 |
36.15±1.0a |
31.36±1.2b |
23.70±1.1b |
| GSH, mg/g | 73.23±2.2 |
34.14±1.2a |
47.45±1.2b |
64.80±2.2b |
| SOD, U/mg | 2.80±0.03 |
1.80±0.02a |
1.92±0.02b |
2.50±0.03b |
| Catalase, U/g | 6.70±0.07 |
3.33±0.05a |
3.80±0.02b |
5.75±0.05b |
GSH content in control cells was 73.23±2.2 mg/g
(group I), whereas it was significantly reduced to 34.14±1.2 mg/g
in salsolinol-treated rat brain endothelial cells (group II;
P<0.05; Table I). Administration
of 50 µg/ml carnosine and 50 µg/ml salsolinol (group III)
significantly increased GSH content to 47.45±1.2 m/g in the rat
brain endothelial cells, compared with group II (P<0.05;
Table I). Administration of 100
µg/ml carnosine and 100 µg/ml salsolinol (group IV) significantly
increased GSH content to 64.8±2.2 mg/g in the rat brain endothelial
cells, compared with group II (P<0.05; Table I).
Effect of carnosine on antioxidant enzymes in rat
brain endothelial cells
The neuroprotective effect of carnosine against the
salsolinol-induced toxicity of rat brain endothelial cells is
presented in Table I. SOD activity
was identified to be 2.8±0.03 U/mg in the control rat brain
endothelial cells (group I), whereas it was significantly reduced
to 1.8±0.02 U/g in the salsolinol-induced rat brain endothelial
cells (group II; P<0.05; Table
I). Administration of 50 µg/ml carnosine and 50 µg/ml
salsolinol (group III) significantly increased SOD activity to
1.92±0.02 U/g in the rat brain endothelial cells, as compared with
group II (P<0.05; Table I).
Administration of 100 µg/ml carnosine and 100 µg/ml alsolinol
(group IV) has significantly increased SOD activity to 2.5±0.03 U/g
compared with group II (P<0.05; Table
I).
Catalase activity was identified to be 6.7±0.07 U/g
in the control rat brain endothelial cells (group I), whereas it
was significantly reduced to 3.33±0.05 U/g in the
salsolinol-induced rat brain endothelial cells (group II;
P<0.05; Table I). Administration
of 50 µg/ml carnosine and 50 µg/ml salsolinol (group III)
significantly increased catalase activity to 3.8±0.02 U/g in the
rat brain endothelial cells, as compared with group II (P<0.05;
Table I). Administration of 100
µg/ml carnosine with 100 µg/ml salsolinol (group IV) significantly
increased catalase activity to 5.75±0.05 U/g compared with group II
(P<0.05; Table I).
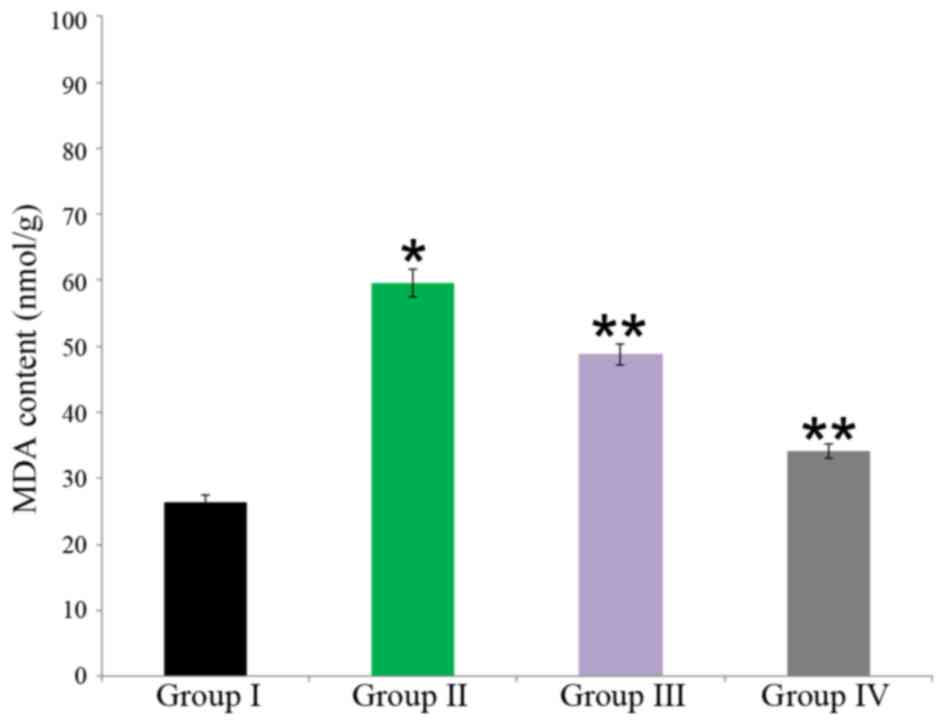
Effect of carnosine on MDA content in rat brain
tissue
The neuroprotective effect of carnosine against
salsolinol in male albino rats is demonstrated in Fig. 1. MDA content in the control was
26.40±1.1 nmol/g, whereas it significantly increased to 59.55±2.1
nmol/g in salsolinol treated rat brain (group II; P<0.05).
Administration of 50 µg/ml carnosine and 50 µg/ml salsolinol (group
III) significantly reduced compared with group II (48.76±1.6
nmol/g) MDA content in the rat brain (P<0.05. Administration of
100 µg/ml carnosine and 100 µg/ml salsolinol (group IV)
significantly reduced MDA content (34.11±1.1 nmol/g) in the rat
brain (P<0.05; Fig. 3).
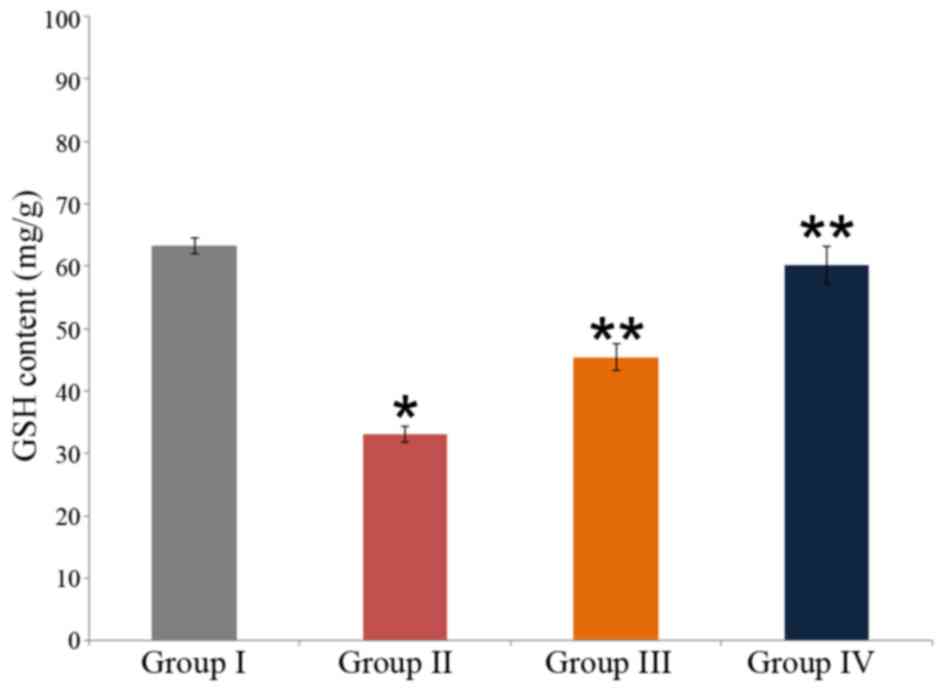
Effect of carnosine on GSH content in rat brain
tissue
The neuroprotective effect of carnosine against
salsolinol in male albino rats is indicated in Fig. 4. The GSH content in the control was
63.3±1.2 mg/g (group I), whereas it was significantly reduced to
33.10±1.2 mg/g in salsolinol treated rat brain (group II;
P<0.05). Administration of 50 µg/ml carnosine and 50 µg/ml
salsolinol (group III) significantly increased GSH content to
45.45±2.1 m/g in the rat brain, as compared with the content in
group II. Administration of 100 µg/ml carnosine and 100 µg/ml
salsolinol (group IV) significantly increased GSH content to
60.21±3.0 mg/g in the rat brain, as compared with group II
(P<0.05; Fig. 4).
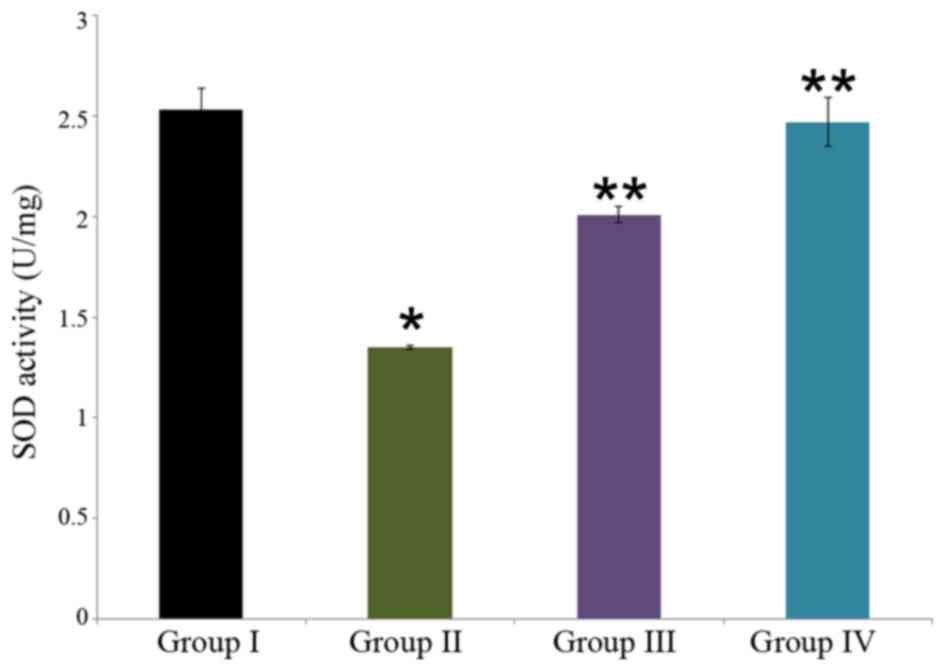
Effect of carnosine on SOD activity in rat brain
tissue
The neuroprotective effect of carnosine against
salsolinol in male albino rats is presented in Fig. 5. SOD activity was identified to be
2.53±0.11 U/mg in the control rat brain (group I), whereas it was
significantly reduced to 1.35±0.01 U/mg in the salsolinol treated
rat brain (group II; P<0.05). Administration of 50 µg/ml
carnosine and 50 µg/ml salsolinol (group III) significantly
increased SOD activity to 2.01±0.04 U/mg in the rat brain compared
with group II (P<0.05). Administration of 100 µg/ml carnosine
and 100 µg/ml salsolinol (group IV) significantly increased SOD
activity to 2.47±0.12 U/mg in the rat brain, as compared with group
II (P<0.05; Fig. 5).
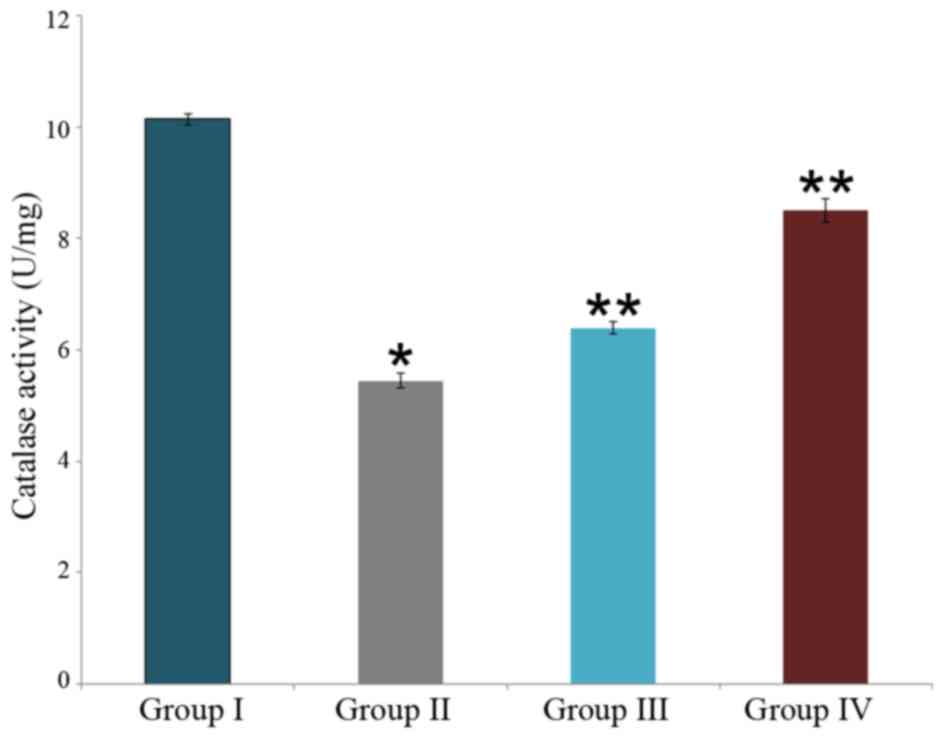
Effect of carnosine on catalase activity in rat
brain tissue
The neuroprotective effect of carnosine against
salsolinol in male albino rats is presented in Fig. 6. Catalase activity was identified to
be 10.14±0.1 U/g in the control rat brain (group I), this was
significantly reduced to 5.45±0.13 U/g in the salsolinol treated
rat brain (P<0.05; group II). Administration of 50 µg/ml
carnosine and 50 µg/ml salsolinol (group III) significantly
increased catalase activity to 6.4±0.11 U/g in the rat brain, as
compared with group II (P<0.05). Administration of 100 µg/ml
carnosine and 100 µg/ml salsolinol (group IV) significantly
increased catalase activity to 8.5±0.21 U/g in the rat brain, as
compared with group II (P<0.05; Fig.
6).
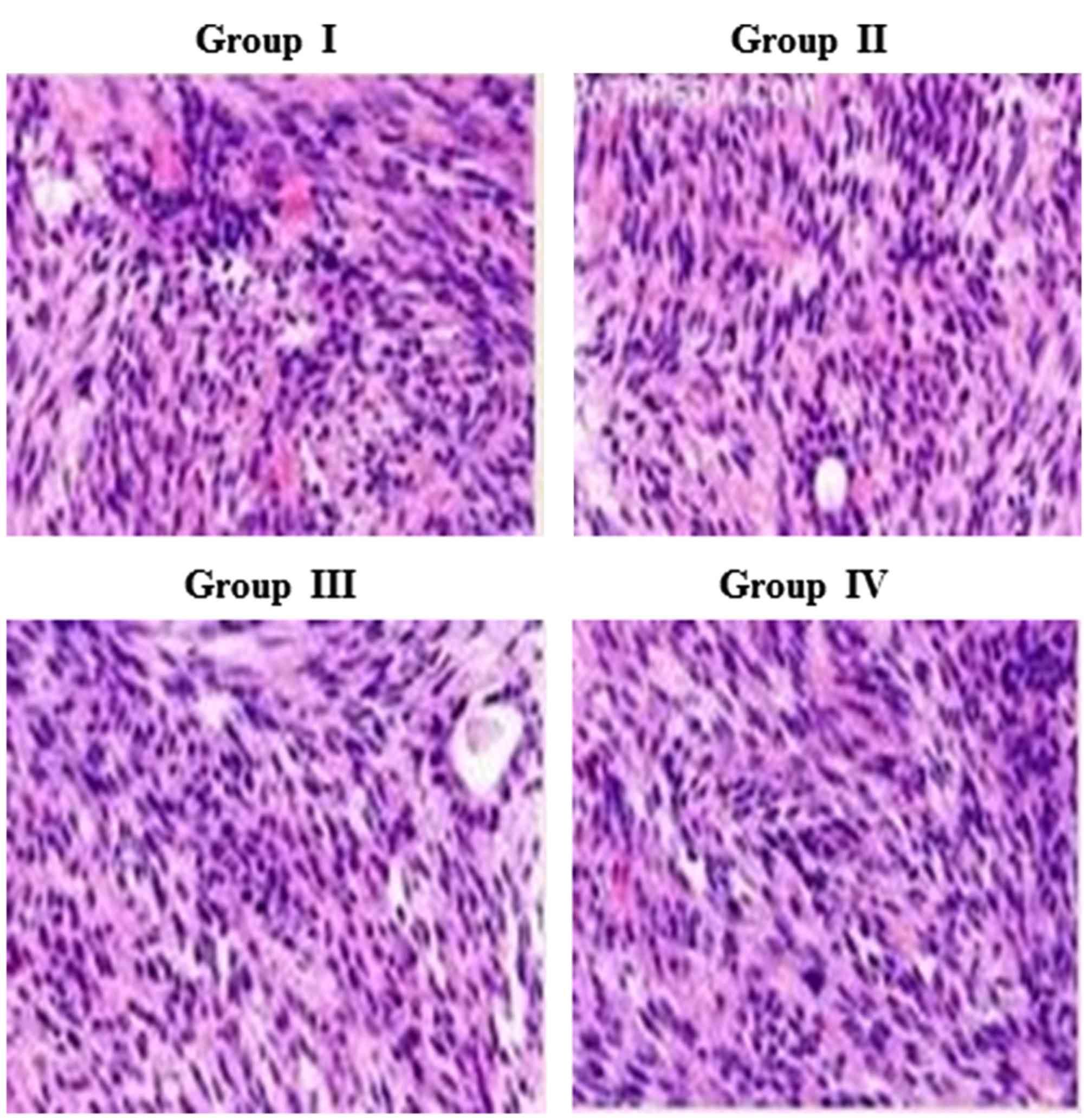
Effect of carnosine on rat brain
histopathology
The neuroprotective effect of carnosine against
salsolinol in male albino rats is presented in Fig. 7. Histopathological analysis
demonstrated a normal cellular architecture in control rats (group
I), whereas salsolinol-induced rat brain exhibited altered cellular
structure, including apoptosis and necrosis of cells (group II).
Administration of 50 µg/ml carnosine and 50 µg/ml salsolinol (group
III) markedly improved the rat brain cells. Administration of 100
µg/ml carnosine and 100 µg/ml salsolinol (group IV) markedly
improved the rat brain cell architecture, returning to normal
(Fig. 7).
Discussion
The experimental results in the present study
demonstrated that carnosine had neuroprotective effects on the male
albino rats, rat brain endothelial cells and appear to exhibit a
clear dose-dependence when exposing cells to higher concentrations.
Induction of tumor cell apoptosis is an essential property of
anti-cancer therapeutics (27).
Apoptosis is defined as a morphological and biochemical alteration
of cells and therefore, a morphological study is vital for
apoptosis investigations. In the current study, carnosine treatment
exerted increasing suppressive effects on the male albino rats and
rat brain endothelial cells with increasing concentrations.
Morphological studies are important to understand
the cytotoxic impact of the carnosine with apoptosis. Carnosine has
been demonstrated to protect rat brain endothelial cells against
the toxic effects of amyloid peptides. Carnosine protects cells by
scavenging MDA and 4-hydroxynonenal, which usual react with
macromolecules (28). Therefore, ROS
production and involvement are one of the potential mechanisms.
Interference using stimulating peptide catabolism, scavenging
superoxide radicals and effects on second messenger processes are
considered to be further explanations. The results of the current
study indicate that carnosine treatment reduced lipid peroxidation
in the rat brain and endothelial cells. Carnosine also
significantly increased GSH and antioxidant enzyme activities,
which further confirms the protective effects of carnosine.
Carnosine significantly normalized cell morphology
at an in vivo and in vitro level. It has been
demonstrated that accumulation of glycated and damaged proteins
occurs during normal aging (29) and
in larger quantities during Alzheimer's disease (30,31).
Carnosine protects against age-related macromolecular damage via
the production of ROS (1). Carnosine
is an anti-glycating compound (13)
and previous studies have indicated that carnosine may delay
senescence (14) and inhibit DNA
oxidation in human fibroblasts (32). The present study demonstrated that
carnosine acts against lipid peroxidation and antioxidant markers
by altering its toxicity and inhibiting protein damage. Brain,
muscle and lens tissues in animals contain high amount of carnosine
(33).
The results of the current study indicated that
salsolinol may induce neurotoxicity in the male albino rat brain
and rat brain endothelial cells (34,35).
Treatment with carnosine exhibited a significant improvement in
neurotoxicity. The lower concentration (50 µg/ml) of carnosine used
in the present study was able to significantly protect against
neurotoxicity induced by salsolinol in the rat brain and
endothelial cells. Salsolinol is known to cause neurotoxicity via
the inhibition of mitochondrial complex II and by initiating
apoptosis through the increased production of free radicals
(36). Carnosine has been
demonstrated to possess neuroprotective effects through the
inhibition of apoptosis with a consequent reduction in the
production of ROS. Although, even at a high concentration,
carnosine may partially recover or inhibit the toxicity induced by
salsolinol in the rat brain and endothelial cells. Investigation of
carnosine in combination with other drugs for their synergistic
action would be worthwhile and notable as it may be helpful in
determining the therapeutic effect of the carnosine as a
monotherapy and in combination with other drugs.
In conclusion, salsolinol exerted neurotoxicity in
the male albino rat brain tissues and rat brain endothelial cells.
This cytotoxicity was reversed following treatment with carnosine,
as demonstrated by the results of the current study. The in
vivo levels of MDA, GSH, SOD and catalase were renormalized
following carnosine treatment, and the cellular architecture of the
rat brain also began to return to normal. Morphological and
apoptotic changes were evaluated by fluorescence microscopy, which
confirmed that there was a reduction of apoptosis following
carnosine treatment. The level of ROS was reduced in the current
study due to a decrease in the oxidative stress. MDA, GSH, SOD and
catalase levels were also measured in vitro, and these
findings matched the in vivo measurements. The experimental
results of the present study may conclude that salsolinol exerts
neurotoxicity, and treatment with carnosine may significantly
reverse this toxicity.
References
|
1
|
Gulewitsch Wl and Amiradžibi S: Ueber das
carnosin, eine neue organische base des Fleischextractes. Berichte
Der Deutschen Chemischen Gesellschaft. 33:1902–1903. 1900.
View Article : Google Scholar
|
|
2
|
Aruoma OI, Laughton MJ and Halliwell B:
Carnosine, homocarnosine and anserine: Could they act as
antioxidants in vivo? Biochem J. 264:863–869. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi SY, Kwon HY, Kwon OB and Kang JH:
Hydrogen peroxide-mediated Cu, Zn-superoxide dismutase
fragmentation: Protection by carnosine, homocarnosine and anserine.
Biochim Biophys Acta. 1472:651–657. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klebanov GI, Teselkin YuO, Babenkova IV,
Lyubitsky OB, Rebrova OYu, Boldyrev AA and Vladimirov YuA: Effect
of carnosine and its components on free-radical reactions. Membr
Cell Biol. 12:89–99. 1998.PubMed/NCBI
|
|
5
|
Babizhayev MA, Seguin MC, Gueyne J,
Evstigneeva RP, Ageyeva EA and Zheltukhina GA: L-carnosine
(beta-alanyl-L-histidine) and carcinine (beta-alanylhistamine) act
as natural antioxidants with hydroxyl-radical-scavenging and
lipid-peroxidase activities. Biochem J. 304:509–516. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karton A, O'Reilly RJ, Pattison DI, Davies
MJ and Radom L: Computational design of effective, bioinspired HOCl
antioxidants: The role of intramolecular Cl+ and H+ shifts. J Am
Chem Soc. 134:19240–19245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan KM and Decker EA: Endogenous skeletal
muscle antioxidants. Crit Rev Food Sci Nutr. 34:403–426. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kohen R, Yamamoto Y, Cundy KC and Ames BN:
Antioxidant activity of carnosine, homocarnosine, and anserine
present in muscle and brain. Proc Natl Acad Sci USA. 85:pp.
3175–3179. 1988; View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vistoli G, De Maddis D, Cipak A, Zarkovic
N, Carini M and Aldini G: Advanced glycoxidation and lipoxidation
end products (AGEs and ALEs): An overview of their mechanisms of
formation. Free Radic Res. 47 Suppl 1:S3–S27. 2013. View Article : Google Scholar
|
|
10
|
Reddy VP, Garrett MR, Perry G and Smith
MA: Carnosine: A versatile antioxidant and antiglycating agent. Sci
Aging Knowledge Environ. 2005:pe122005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hipkiss AR: Does chronic glycolysis
accelerate aging? could this explain how dietary restriction works?
Ann N Y Acad Sci. 1067:361–368. 2006.PubMed/NCBI
|
|
12
|
Boldyrev AA, Formazyuls VE and Sergienko
VI: Biological significance of histidine-containing dipeptides with
special reference to carnosine: Chemistry, distribution, metabolism
and medical application. Sov Sci Rev D Physicochem Biol. 13:1–60.
1994.
|
|
13
|
Hipkiss AR, Holliday R, McFarland G and
Michaelis J: Carnosine and senescence. Lifespan. 4:1–3. 1993.
|
|
14
|
McFarland GA and Holliday R: Retardation
of the senescence of cultured human fibroblasts by carnosine. Exp
Cell Res. 212:167–175. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harman D: Aging: A Theory Based on Free
Radical and Radiation Chemistry. UCRL Publication no. 3078.
Univesrity of California; Berkrley, CA: 1955
|
|
16
|
Halliwell B and Gutteridge JMC: Free
Radicals in Biology and Medicine. Clarendon Press; Oxford: 1989,
View Article : Google Scholar
|
|
17
|
Schubert D, Behl C, Lesley R, Brack A,
Dargusch R, Sagara Y and Kimua H: Amyloid peptides are toxic via a
common oxidative mechanism. Proc Natl Acad Sci USA. 92:pp.
1989–1993. 1995; View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Libondi T, Ragone R, Vincenli D, Stiuso P,
Auricchio G and Collona G: In vitro cross-linking of calf lens
alpha-crystallin by malondialdehyde. Int J Peptide Protein Res.
44:342–347. 1994. View Article : Google Scholar
|
|
19
|
Hartley A, Stone JM, Heron C, Cooper JM
and Schapira AH: Complex I inhibitors induce dose-dependent
apoptosis in PC12 cells: Relevance to Parkinson's disease. J
Neurochem. 63:1987–1990. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao HM, Hong JS, Zhang W and Liu B:
Distinct role for microglia in rotenone-induced degeneration of
dopaminergic neurons. J Neurosci. 22:782–790. 2002.PubMed/NCBI
|
|
21
|
Freestone PS, Chung KK, Guatteo E, Mercuri
NB, Nicholson LF and Lipski J: Acute action of rotenone on nigral
dopaminergic neurons-involvement of reactive oxygen species and
disruption of Ca2+ homeostasis. Eur J Neurosci. 30:1849–1859. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Caboni P, Sherer TB, Zhang N, Taylor G, Na
HM, Greenamyre JT and Casida JE: Rotenone, deguelin, their
metabolites, and the rat model of Parkinson's disease. Chem Res
Toxicol. 17:1540–1548. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao HM, Hong JS, Zhang W and Liu B:
Synergistic dopaminergic neurotoxicity of the pesticide rotenone
and inflammogen lipopolysaccharide: Relevance to the etiology of
Parkinson's disease. J Neurosci. 23:1228–1236. 2003.PubMed/NCBI
|
|
24
|
Muthuraman P, Kim DH, Muthuviveganandavel
V, Vikramathithan J and Ravikumar S: Differential bio-potential of
ZnS nanoparticles to normal MDCK cells and cervical carcinoma HeLa
cells. J Nanoscience Nanotechnol. 16:8279–8286. 2016. View Article : Google Scholar
|
|
25
|
Muthuraman P, Muthuviveganandavel V and
Kim DH: Cytotoxicity of zinc oxide nanoparticles on antioxidant
enzyme activities and mRNA expression in the cocultured C2C12 and
3T3-L1 cells. Appl Biochem Biotechnol. 175:1270–1280. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Muthuviveganandavel V, Muthuraman P, Muthu
S and Srikumar K: A study of low dose cypermethrin induced
histopathology, lipid peroxidation and marker enzyme changes in
male rats. Pesticide Biochemistry Physiol. 91:12–16. 2008.
View Article : Google Scholar
|
|
27
|
Frankfurt OS and Krishan A:
Apoptosis-based drug screening and detection of selective toxicity
to cancer cells. Anticancer Drugs. 14:555–561. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Markesbery WR: Oxidative stress hypothesis
in Alzheimer's disease. Free Radical Biol Med. 23:134–147. 1997.
View Article : Google Scholar
|
|
29
|
Stadtman ER: Protein oxidation and aging.
Science. 257:1220–1224. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smith MA, Perry G, Richey PL, Sayre LM,
Anderson VE, Beal MF and Kowall N: Oxidative damage in Alzheimer's.
Nature. 382:120–121. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Smith MA, Rudnicka-Nawrot M, Richey PL,
Praprotnik D, Mulvihill P, Miller CA, Sayre LM and Perry G:
Carbonyl-related post-translational modification of neurofilament
protein in neurofibrillary pathology in Alzheimer's disease. J
Neurochem. 64:2660–2666. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kantha SS, Wada S, Tanaka H, Takeuchi M,
Watabe S and Ochi H: Carnosine sustains the retention of cell
morphology in continuous fibroblast culture subjected to
nutritional insult. Biochem Biophys Res Commun. 223:278–282. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Perry TL, Hansen S, Stedman D and Love D:
Homocarnosine in human cerebrospinal fluid: An age-dependent
phenomenon. J Neurochem. 15:1203–1206. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Das JR and Tizabi Y: Additive protective
effects of donepezil and nicotine against salsolinol-induced
cytotoxicity in SH-SY5Y cells. Neurotox Res. 16:194–204. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song JX, Shaw PC, Wong NS, Sze CW, Yao XS,
Tang CW, Tong Y and Zhang YB: Chrysotoxine, a novel bibenzyl
compound selectively antagonizes MPP+, but not rotenone,
neurotoxicity in dopaminergic SH-SY5Y cells. Neurosci Lett.
521:76–81. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Storch A, Kaftan A, Burkhardt K and
Schwarz J: 1-Methyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline
(salsolinol) is toxic to dopaminergic neuroblastoma SH-SY5Y cells
via impairment of cellular energy metabolism. Brain Res. 855:67–75.
2000. View Article : Google Scholar : PubMed/NCBI
|