Introduction
The prime objective of restorative dentistry is to
maintain pulpal vitality and function. Occasionally, pulp tissue
may be exposed accidentally during a clinical procedure, such as
deep caries removal or restorative procedures. In cases where the
pulp tissue is healthy or diagnosed with reversible pulpitis,
direct pulp capping is regarded as an effective approach to
preserve the pulp vitality and function by forming a dentinal
bridge against pulp exposure (1,2). Calcium
ions (Ca2+) released from calcium hydroxide or mineral
trioxide aggregate have been certified as classic direct pulp
capping materials, and are key factors promoting the healing
process (3–5). Besides, as a ubiquitous second
messenger, Ca2+ is involved in abundant physiological
functions, including proliferation and differentiation. The role of
calcium signaling in osteogenic differentiation is of prime
interest for both normal bone homeostasis and bone regeneration
(6). However, the mechanism
underlying the effect of Ca2+ on the odontoblastic
differentiation of dental pulp cells has yet to be fully
elucidated.
The elevation of cytosolic calcium concentration can
be achieved by two major mechanisms: Discharge of intracellular
Ca2+ stores, and extracellular Ca2+ influx.
Ca2+ current is mediated mostly by calcium channels
located in membranes. The intracellular stores are regulated by
inositol triphosphate (IP3) receptors, while
extracellular Ca2+ influx is mainly regulated by the
canonical transient receptor potential (TRPC) channels and the ORAI
channels (7). ORAI channels and
certain TRPC members are regulated by depletion of intracellular
stores, which refers to the store-operated Ca2+ entry
(SOCE) (8). SOCE is a broadly
existent mechanism in non-excitable cells, responding to the
cytosolic Ca2+ change and refilling Ca2+
stores. A recent study revealed that ORAI1 is involved in the
odontogenic differentiation of human dental pulp cells (HDPCs),
which implied that Ca2+ mobilization is vital for the
odontogenic differentiation (9).
Furthermore, several TRPC members are activated by diacylglycerol
in the store-independent manner, which refers to the
receptor-operated Ca2+ entry (ROCE) (8). Another previous study demonstrated that
rat odontoblasts expressed RNA transcripts for TRPC1 and TRPC6
(10).
TRPC6, the Ca2+-permeable non-selective
cation channel, has been suggested as a ROCE channel. Activation of
TRPC6 by diacylglycerol (DAG) analogues, such as
1-oleoyl-2-acetyl-sn-glycerol (OAG) (11), resulted in elevated intracellular
Ca2+, which then influenced the calcium entry or calcium
signaling. However, limited information is available on the
importance of TRPC channels in dental pulp cells. Consequently, the
present study hypothesized that TRPC6 is expressed in the human
dental pulp and participates in the odontogenic differentiation of
dental pulp cells.
Materials and methods
Tooth preparation
In this study, all teeth were obtained from the
Department of Oral and Maxillofacial Surgery, Guanghua School and
Hospital of Stomatology, Sun Yat-sen University, (Guangzhou, China)
between December 2014 and February 2015. A total of 22 caries-free
human wisdom teeth were extracted from 22 different patients aged
18–25 undertaking orthodontic treatment. All patients provided
informed consent prior to treatment. The experimental protocols
were approved by the Ethics Committee of the Guanghua School of
Stomatology.
Tissue sample preparation
Four healthy teeth were randomly sectioned to reveal
the healthy pulp inside, fixed with 4% paraformaldehyde overnight
and then decalcified in 10% EDTA for 3 months. Subsequently,
decalcified tissues were embedded in paraffin and placed onto
slides. Following deparaffinization, five random sections with
integral structure in each tooth were selected for
immunohistochemical staining. Four pulp tissue samples from another
four teeth were prepared to detect TRPC6 for western blotting, as
described below.
Cell culture and odontoblastic
differentiation
HDPCs were established from healthy third molars by
growing the minced explants as described previously (12). In brief, teeth were split and the
dental pulp tissue was collected into a 35-mm Petri dish. The
tissues were minced into pieces and cultured antiseptically during
the whole process. Cells were cultured in Dulbecco's modified
Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) at 37°C in
the presence of 95% air and 5% CO2. To induce odontogenic
differentiation, cells at passage 3 were grown in 6-well dishes at
a density of 1×105 cells per well with a DMEM containing
10% FBS, 0.2 mmol/l ascorbic acid, 10 mmol/l β-glycerophosphate and
100 nmol/l dexamethasone. The medium were refreshed every 2 days
for 14 days. Subsequently, Alizarin red S staining was performed to
confirm the mineralization. HDPCs from four healthy third molars
extracted from patients (two female and two male) were used to
detect TRPC6 expression level by western blotting analysis. In
addition, HDPCs from another 10 teeth were prepared for the
subsequent experiments, such as the experiment to induce
odontoblast differentiation of HDPCs, Ca2+ concentration
detection and the knockdown experiments.
The human embryonic kidney 293T cell line was
purchased from American Type Culture Collection (Manassas, VA,
USA). 293T cells were grown in 100 mm2 dishes at a
density of 1.2×106 cells per well with DMEM containing
10% FBS.
Short hairpin RNA (shRNA) and
lentivirus constructs
Lentivirus-based shRNA knockdown pLKO.1 puro vector
(Addgene plasmid #8453; Addgene, Cambridge, MA, USA) was used to
stably knockdown the expression of TRPC6 (Genbank accession no.
NM_004621.5), and shRNA constructs were generated as described
previously (13). Target sequences
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) were selected
through software provided on the manufacturer's website. Oligo
sequences were annealed, subcloned into the pLKO.1 vector by T4 DNA
ligase between the AgeI and EcoRI sites, according to
the manufacturer's protocols. The selected TRPC6 hairpin oligo
sequence for the study was as follows:
5′-GCGACAGAGCATCATTGACGCAAATCTGTGAAGCCACAGATGGGATTTGCGTCAATGATGCTCTGGTGCTTTTTT-3′.
In addition, a non-target scrambled shRNA (with a sequence of
5′-GCGCGTAGTAATGACAATCCGCGCTCTGTGAAGCCACAGATGGGAGCGCGGATTGTCATTACTACTTGCTTTTTT-3′)
served as the negative control. The shRNA-expressing vectors were
transfected into 293T cells together with the lentiviral helper
plasmids to generate respective lentiviruses. After 48 h,
lentiviruses were collected from the culture medium and used to
infect target cells. HDPCs were transfected with the lentiviruses
three times a day, and the medium was replaced with growth medium
supplemented with 10% FBS. Next, medium containing 2 µg/ml
puromycin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used
to isolate cells stably transduced with shRNA. The successful
knockdown of TRPC6 was verified by western blot analysis.
Western blot analysis
Total protein was extracted from the HDPCs or pulp
tissue samples using RIPA medium containing phenylmethanesulfonyl
fluoride (Beyotime Institute of Biotechnology, Haimen, China).
Protein concentrations were determined using a BCA Protein Assay
kit (P0012; Beyotime Institute of Biotechnology). Equivalent
amounts of diluted protein samples were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
onto a polyvinylidene difluoride membrane. After blocking for 1 h
at room temperature in 5% skim milk solution, the membranes were
subsequently incubated with anti-TRPC6 antibodies (1:500; ab62461;
Abcam, Cambridge, UK), anti-dentin sialophosphoprotein (anti-DSPP)
antibodies (1:500; sc73632; Santa Cruz Biotechnologies, Inc.,
Dallas, TX, USA), anti-dentin matrix protein-1 (anti-DMP-1)
antibodies (1:500; sc73633; Santa Cruz Biotechnology, Inc.)
overnight at 4°C. Secondary antibody anti-mouse IgG/anti-rabbit
IgG, HRP-linked antibody (7076S/7074S; Cell Signaling Technology,
Inc., Danvers, MA, USA) was then added at a dilution of 1:5,000 for
1 h at room temperature after the membranes were washed with
Tris-buffered saline with Tween-20. Relative band intensities were
detected by densitometry using Quantity One 1-D analysis software
(version 4.6.2; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Immunohistochemical staining
Immunohistochemical staining was performed mainly in
the humidity chamber as described in a previous study (12). Briefly, each section was pretreated
with 3% hydrogen peroxide in ice-cold methanol for 20 min in order
to block endogenous peroxidase activity. Next, slide-loaded
sections were bathed in 0.01 M sodium citrate buffer (pH 6.0) at
95–100°C in a microwave oven for antigen retrieval. After blocking
with normal goat serum for 20 min at room temperature, slides were
incubated with primary anti-TRPC6 antibody (1:100; ab62461; Abcam)
overnight at 4°C. Slides were subsequently incubated with the
secondary antibodies, goat anti-rabbit IgG (1:200; ab205718, Abcam)
and stained with 3,3′-diaminobenzidine (Boster Systems, Inc.,
Pleasanton, CA, USA) and hematoxylin according to the
manufacturer's instructions. Finally, the slides were observed
using a light microscope.
Measurement of cytosolic
Ca2+ concentration([Ca2+]c)
HDPCs at passage 3 were plated into 96-well assay
plates at a density of 1×105 cells per well and loaded
with 5 mM Fura 2/AM for 30 min at 37°C. [Ca2+]c in cells
was determined by a spectrofluorometer at excitation wavelengths of
340 and 380 nm, and an emission wavelength of 510 nm. Background
fluorescence intensities were subtracted from each data point. The
calcium concentration can be calculated according to the following
formula: [Ca2+]c = Kd × β × (R-Rmin) / (Rmax-R), where
Kd represents the dissociation constant and β is a constant. Rmax
and Rmin were obtained while the cells were perfused and exposed to
high and zero calcium settings, respectively (14). Next, 100 µM OAG (Sigma-Aldrich; Merck
KGaA) was used as an analogue of DAG to activate TRPC6. The absence
of extracellular Ca2+ was determined by incubation of
HDPCs in Ca2+-free Hank's balanced salt solution (Thermo
Fisher Scientific, Inc.) with 3 mM EGTA. Supplement of
extracellular Ca2+ was achieved by addition of 2 mmol/l
CaCl2.
Statistical analysis
The values are demonstrated as the mean ± standard
deviation. Statistical analyses were performed using one-way
analysis of variance, followed by the Bonferroni
multiple-comparison test, with a statistically significant
difference detected when P<0.05. Statistical analyses were
performed with SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Expression of TRPC6 in human dental
pulp tissue and HDPCs
Pulp tissue harbors various cell types. To determine
which type of cells in the pulp tissue express TRPC6, the TRPC6
antibody was used to perform immunohistochemical staining in the
pulp tissue samples. The results indicated that TRPC6 was expressed
in the dental pulp tissue particularly in the odontoblast layer
(Fig. 1A). In the negative control
group, no positive staining was detected in the healthy dental pulp
(Fig. 1A). In addition, the protein
expression of TRPC6 in pulp tissue, as well as in HDPCs, was
further detected by western blotting. TRPC6 protein expression in
dental pulp tissues was significantly higher compared with that in
HDPCs (P<0.05; Fig. 1B and C).
Thus, these findings suggest that both human dental pulp tissues
and HDPCs expressed TRPC6.
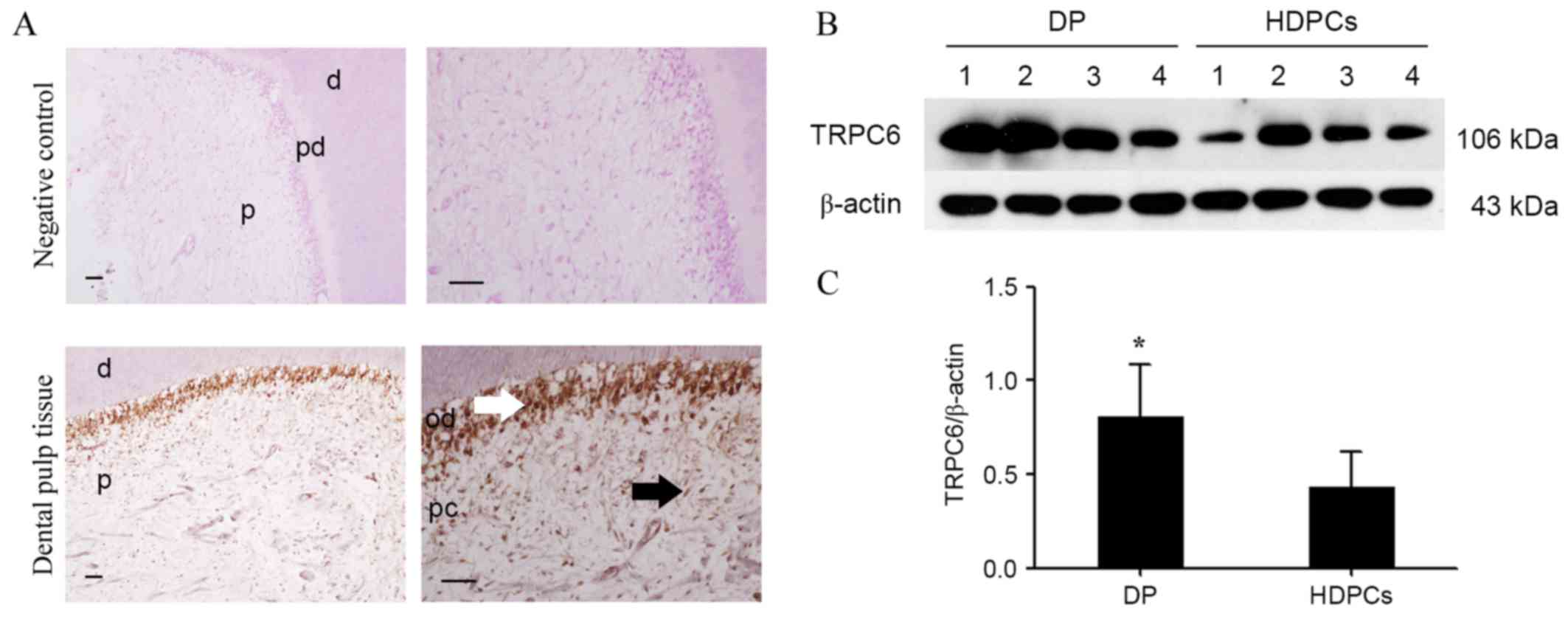 | Figure 1.Expression of TRPC6 in human dental
pulp tissue and HDPCs. (A) Positive anti-TRPC6 staining was
detected in the cytoplasm of odontoblasts (white arrow) situated in
the outermost layer of healthy dental pulp. Positive anti-TRPC6
staining was not evident in the pulp fibrobasts (black arrow) and
the negative control of healthy pulp tissues. Scale bar, 100 µm.
Upper images, magnification ×200. Lower images, magnification ×400.
(B) Expression of TRPC6 protein (106 kDa) was detected by western
blotting in the dental pulp tissue (n=4) and HDPCs (n=4). β-actin
was used as a loading control. (C) Densitometric values for each
group are shown in the bar graph as the mean ± standard deviation.
All data are representative of three separate experiments.
*P<0.05 vs. HDPCs. HDPCs, human dental pulp cells; TRPC6,
transient receptor potential channel 6; DP, dental pulp; d, dentin;
pd, predentin; p, pulp; od, odontoblast; pc, human dental pulp
cell. |
TRPC6 is markedly upregulated during
odontoblastic differentiation of HDPCs
Subsequent to confirming that TRPC6 is expressed in
HDPCs by immunohistochemical staining, the expression levels of
TRPC6, as well as that of the odontoblastic
differentiation-associated proteins DSPP and DMP-1, were further
analyzed during cell differentiation. In the differentiated cells,
the expression level of TRPC6 was enhanced in a time-dependent
manner compared with that in undifferentiated cells, with
significantly increased levels observed at 7 and 14 days
(P<0.05; Fig. 2A and B). A
similar trend was observed for the DSPP and DMP-1 expression levels
(P<0.05; Fig. 2A, C and D).
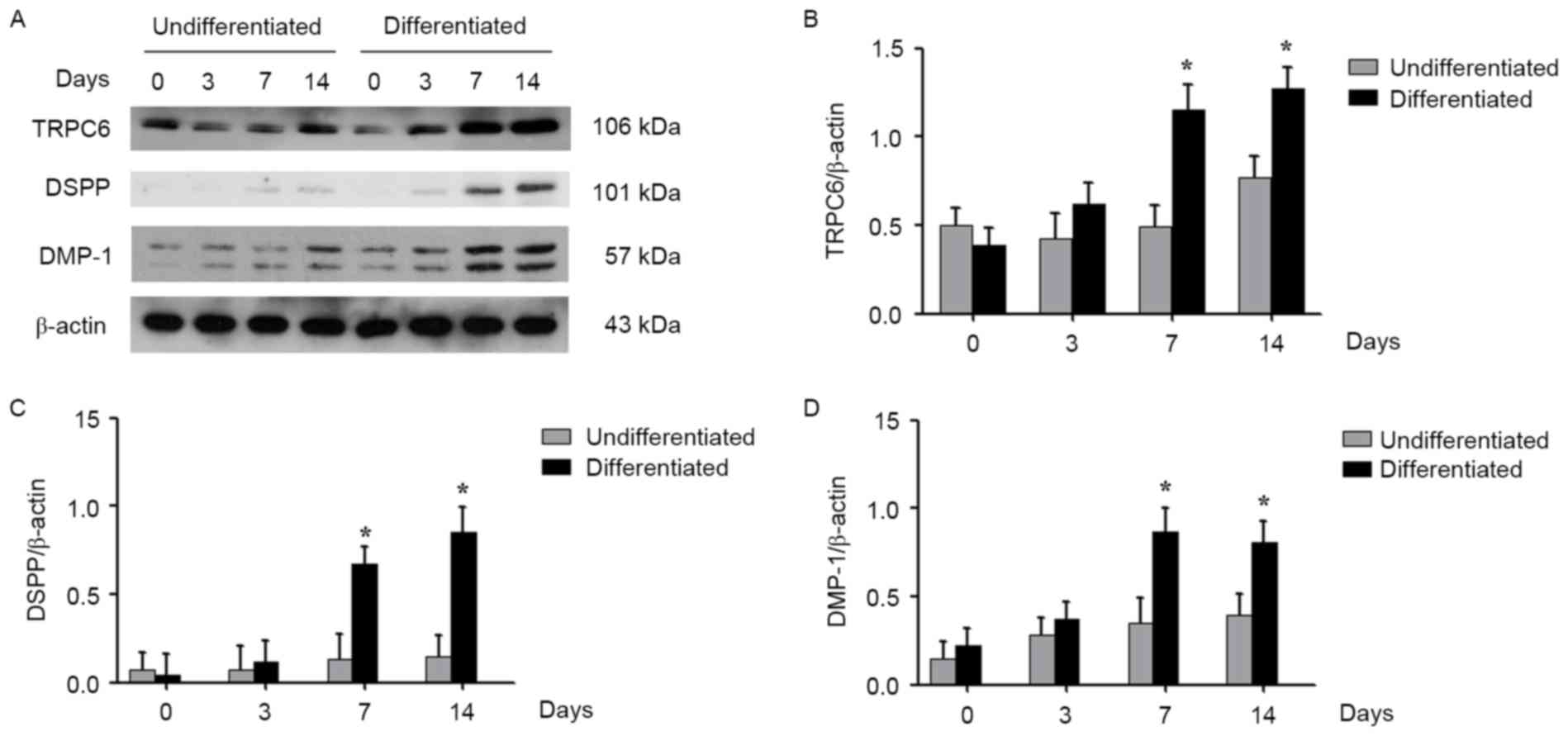 | Figure 2.Expression of TRPC6 during
odontoblastic differentiation of HDPCs. (A) Western blotting of
TRPC6, DSPP, and DMP-1 in HDPCs undergoing odontogenic
differentiation, with total proteins collected at 0, 3, 7 and 14
days. Quantitative analysis of (B) TRPC6, (C) DSPP and (D) DMP-1 in
HDPCs is shown. The results are expressed as the mean ± standard
deviation of three different experiments. *P<0.05 vs. cells on
day 0. HDPCs, human dental pulp cells; TRPC6, transient receptor
potential channel 6; DSPP, dentin sialophosphoprotein; DMP-1,
dentin matrix protein 1. |
Knockdown of TRPC6 inhibits the
odontoblastic differentiation of HDPCs
To verify whether the upregulation of TRPC6 was
indispensable during odontoblastic differentiation, lentiviruses
contained TRPC6 shRNA were used to knock down TRPC6 expression in
HDPCs. The results demonstrated that the lentiviruses expressing
shRNA were able to knock down the endogenous TRPC6 by up to 75%
(Fig. 3A). In addition, TRPC6
knockdown functionally inhibited Ca2+ influx, indicating
that ROCE was impaired (Fig. 3B). In
the TRPC6-shRNA group, a significant decline was detected in the
calcium nodule formation (Fig. 3C).
Accordingly, the protein levels of DSPP and DMP-1 were all also
reduced in the TRPC6 knockdown groups on day 14 after odontoblastic
induction (Fig. 3D and E), which
indicated that knockdown of TRPC6 inhibited the odontoblastic
differentiation of HDPCs.
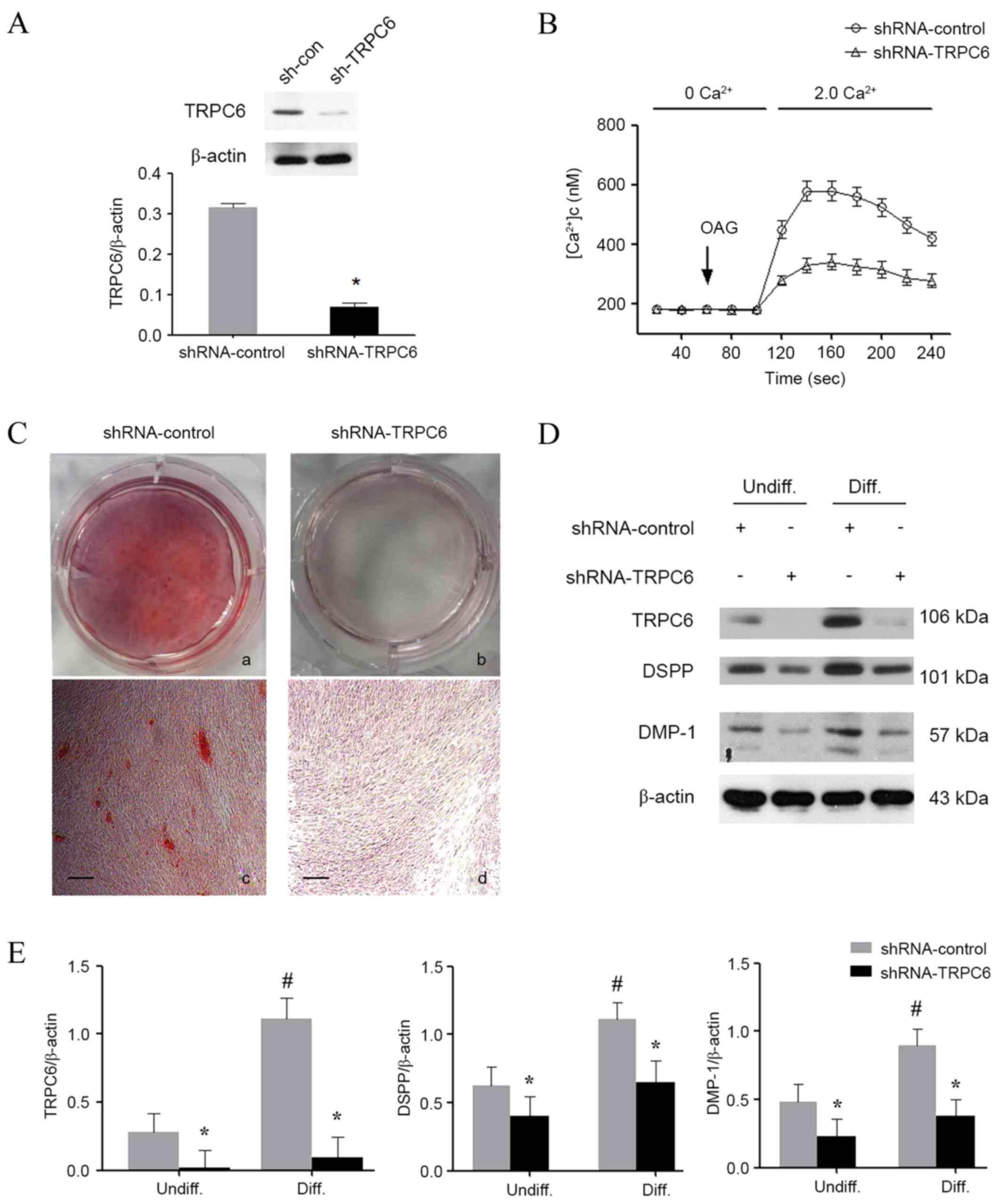 | Figure 3.Effect of TRPC6 shRNA on
odontoblastic differentiation of HDPCs. (A) Western blotting was
performed to confirm the successful TRPC6 knockdown. (B)
Intracellular Ca2+ imaging assay was performed to
confirm the TRPC6 function following shRNA transfection. (C)
Alizarin red S staining in HDPCs on day 14 after odontogenic
differentiation in the absence or presence of TRPC6 shRNA. Scale
bar, 200 µm. Magnification, ×50. (D) Western blotting was performed
to examine the expression levels of TRPC6, DSPP and DMP-1 in all
groups. (E) Quantitative analysis of TRPC6, DSPP and DMP-1 protein
expression levels in HDPCs. The results are expressed as the mean ±
standard deviation of three different experiments. *P<0.05 vs.
control group; #P<0.05 vs. undifferentiated control.
HDPCs, human dental pulp cells; TRPC6, transient receptor potential
channel 6; DSPP, dentin sialophosphoprotein; DMP-1, dentin matrix
protein 1; OAG, 1-oleoyl-2-acetyl-sn-glycerol; Undiff,
undifferentiated; Diff, differentiated. |
Discussion
TRPC6 is a receptor-operated Ca2+ channel
that serves an important role in regulating Ca2+ influx
in the majority of non-excitable cells (15–17). A
recent study reported that TRPC6 is expressed in the brain, kidney,
smooth muscle tissues, as well as in immune and blood cells
(18). In the present study, it was
demonstrated that TRPC6 was expressed in dental pulp tissue, and
was required for the odontogenic differentiation of HDPCs. To the
best of our knowledge, this is the first study reporting an
essential role of TRPC6 in the odontoblastic differentiation of
HDPCs.
Although a recent study demonstrated that TRPC6 mRNA
was detected in rat odontoblasts by single-cell reverse
transcription-polymerase chain reaction (10), there is limited knowledge about the
role of TRPC6 in dental pulp cells. The results of the present
immunohistochemistry experiments revealed that TRPC6 was expressed
in human dental pulp tissue and mainly in the odontoblast layer.
Odontoblasts are considered to be the terminally differentiated
cells, which are responsible for dentin formation. During dentin
formation, Ca2+ is conveyed to the extracellular
mineralization front against concentration gradients by
odontoblasts (19). In contrast to
odontoblasts, HDPCs can be easily obtained from extracted teeth.
Besides, HDPCs as a heterogeneous population consist of the
multipotent stem/progenitor cells that can differentiate into
odontoblast-like cells during the formation of the reparative
dentin due to the similar phenotypical properties they share with
odontoblasts (20,21). Since the endogenous expression of
TRPC6 in HDPCs was detected by western blotting in the present
study, it is presumed that TRPC6 was involved in the odontoblastic
differentiation of HDPCs.
In vitro experiments in the present study
identified that TRPC6 expression was significantly enhanced in a
time-dependent manner when odontoblastic differentiation was
induced in HDPCs, particularly on days 7 and 14. This finding leads
to the question of whether TRPC6 was indispensable during
odontoblastic differentiation. Thus, the function of TRPC6 in the
odontoblastic differentiation of HDPCs was explored. The results
demonstrated that downregulation of TRPC6 inhibited the
odontoblastic differentiation and mineralization, as indicated by
the reduction in the deposition of mineralized matrix and by the
downregulation of DSPP and DMP-1 levels. DMP-1 and DSPP are known
as mineralization markers in the odontoblasts-like differentiation
of HDPCs. DMP-1 is essential to the formation and mineralization of
dentine (22), while DSPP is the
predominant non-collagen protein in dentin formation (23,24).
These results demonstrated a tight coupling between TRPC6 and
odontoblastic differentiation, which may be mediated by calcium
signaling.
A recent study brought to light that the ORAI1
protein, a calcium channel operated by calcium store, served an
important role in odontogenic differentiation (9). Knockdown of ORAI1 expression suppressed
odontogenic differentiation and mineralization in vivo and
in vitro (9). Meanwhile, it
has been confirmed that mutation of ORAI1 may lead to the
deficiency of dental enamel calcification (25). TRPC6 is believed to be activated by
DAG and it is not sensitive to the change of calcium concentration
in the endoplasmic reticulum, which defined TRPC6 as a
receptor-operated channel rather than a store-operated channel
(11,26). However, several lines of evidence
indicated that TRPC6 may also affect the SOCE (27,28).
Notably, TRPC6 was also found to react with ORAI1 (29). Although the detailed mechanism
remains debated, functional studies in different cell types implied
a direct link between TRPC6, Ca2+ signaling and cellular
responses. Collectively, these factors affected various cellular
functions and maintained the cellular homeostasis (18,30–32).
Ca2+ was recognized to be a critical
cellular cation that regulates physiological and pathologic
processes, including proliferation, transcription and contraction.
In clinical practice, Ca2+ released from pulp-capping
materials was proven to participate in forming calcium carbonate,
which affected the proliferation and differentiation of HDPCs and
then promoted the mineralization (33). Furthermore, an in vitro study
observed that addition of extracellular Ca2+ increased
the expression of bone-associated genes, including BMP-2, and
promoted odontoblastic differentiation of dental pulp cells
(34). Therefore, further
investigations focusing on the association between TRPC6 and
calcium signaling, as well as the possible underlying pathway, are
required.
In conclusion, the present study demonstrated that
TRPC6 was expressed in dental pulp tissue and was involved in the
odontogenic differentiation process of HDPCs. This raised the
possibility that TRPC6 may be a useful therapeutic target in
promoting reparative dentin formation.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81600862,
81670984 and 81560184) and the Guangdong Natural Science Foundation
(no. 2016A030310227).
References
|
1
|
Aguilar P and Linsuwanont P: Vital pulp
therapy in vital permanent teeth with cariously exposed pulp: A
systematic review. J Endod. 37:581–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cho SY, Seo DG, Lee SJ, Lee J, Lee SJ and
Jung IY: Prognostic factors for clinical outcomes according to time
after direct pulp capping. J Endod. 39:327–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mizuno M and Banzai Y: Calcium ion release
from calcium hydroxide stimulated fibronectin gene expression in
dental pulp cells and the differentiation of dental pulp cells to
mineralized tissue forming cells by fibronectin. Int Endod J.
41:933–938. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Natale LC, Rodrigues MC, Xavier TA, Simões
A, de Souza DN and Braga RR: Ion release and mechanical properties
of calcium silicate and calcium hydroxide materials used for pulp
capping. Int Endod J. 48:89–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sangwan P, Sangwan A, Duhan J and Rohilla
A: Tertiary dentinogenesis with calcium hydroxide: A review of
proposed mechanisms. Int Endod J. 46:3–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barradas AM, Fernandes HA, Groen N, Chai
YC, Schrooten J, van de Peppel J, van Leeuwen JP, van Blitterswijk
CA and de Boer J: A calcium-induced signaling cascade leading to
osteogenic differentiation of human bone marrow-derived mesenchymal
stromal cells. Biomaterials. 33:3205–3215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berridge MJ, Bootman MD and Roderick HL:
Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev
Mol Cell Biol. 4:517–529. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng KT, Ong HL, Liu X and Ambudkar IS:
Contribution and regulation of TRPC channels in store-operated Ca2+
entry. Curr Top Membr. 71:149–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sohn S, Park Y, Srikanth S, Arai A, Song
M, Yu B, Shin KH, Kang MK, Wang C, Gwack Y, et al: The Role of
ORAI1 in the odontogenic differentiation of human dental pulp stem
cells. J Dent Res. 94:1560–1567. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kwon M, Baek SH, Park CK, Chung G and Oh
SB: Single-cell RT-PCR and immunocytochemical detection of
mechanosensitive transient receptor potential channels in acutely
isolated rat odontoblasts. Arch Oral Biol. 59:1266–1271. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hofmann T, Obukhov AG, Schaefer M,
Harteneck C, Gudermann T and Schultz G: Direct activation of human
TRPC6 and TRPC3 channels by diacylglycerol. Nature. 397:259–263.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin Z, Song Z, Qin W, Li J, Li WJ, Zhu HY
and Zhang L: Expression of nucleotide-binding oligomerization
domain 2 in normal human dental pulp cells and dental pulp tissues.
J Endod. 35:838–842. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qin W, Lin ZM, Deng R, Li DD, Song Z, Tian
YG, Wang RF, Ling JQ and Zhu XF: p38a MAPK is involved in
BMP-2-induced odontoblastic differentiation of human dental pulp
cells. Int Endod J. 45:224–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grynkiewicz G, Poenie M and Tsien RY: A
new generation of Ca2+ indicators with greatly improved
fluorescence properties. J Biol Chem. 260:3440–3450.
1985.PubMed/NCBI
|
|
15
|
Monet M, Francoeur N and Boulay G:
Involvement of phosphoinositide 3-kinase and PTEN protein in
mechanism of activation of TRPC6 protein in vascular smooth muscle
cells. J Biol Chem. 287:17672–17681. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen S, He FF, Wang H, Fang Z, Shao N,
Tian XJ, Liu JS, Zhu ZH, Wang YM, Wang S, et al: Calcium entry via
TRPC6 mediates albumin overload-induced endoplasmic reticulum
stress and apoptosis in podocytes. Cell Calcium. 50:523–529. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Foster RR, Zadeh MA, Welsh GI, Satchell
SC, Ye Y, Mathieson PW, Bates DO and Saleem MA: Flufenamic acid is
a tool for investigating TRPC6-mediated calcium signalling in human
conditionally immortalised podocytes and HEK293 cells. Cell
Calcium. 45:384–390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Albarran L, Berna-Erro A, Dionisio N,
Redondo PC, Lopez E, Lopez JJ, Salido GM, Sabate JM Brull and
Rosado JA: TRPC6 participates in the regulation of cytosolic basal
calcium concentration in murine resting platelets. Biochim Biophys
Acta. 1843:789–796. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Linde A and Lundgren T: From serum to the
mineral phase. The role of the odontoblast in calcium transport and
mineral formation. Int J Dev Biol. 39:213–222. 1995.PubMed/NCBI
|
|
20
|
Hilkens P, Gervois P, Fanton Y,
Vanormelingen J, Martens W, Struys T, Politis C, Lambrichts I and
Bronckaers A: Effect of isolation methodology on stem cell
properties and multilineage differentiation potential of human
dental pulp stem cells. Cell Tissue Res. 353:65–78. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kawashima N: Characterisation of dental
pulp stem cells: A new horizon for tissue regeneration? Arch Oral
Biol. 57:1439–1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Butler WT and Ritchie H: The nature and
functional significance of dentin extracellular matrix proteins.
Int J Dev Biol. 39:169–179. 1995.PubMed/NCBI
|
|
23
|
Lee SY, Kim SY, Park SH, Kim JJ, Jang JH
and Kim EC: Effects of recombinant dentin sialoprotein in dental
pulp cells. J Dent Res. 91:407–412. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee YH, Kim GE, Cho HJ, Yu MK, Bhattarai
G, Lee NH and Yi HK: Aging of in vitro pulp illustrates change of
inflammation and dentinogenesis. J Endod. 39:340–345. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McCarl C, Picard C, Khalil S, Kawasaki T,
Röther J, Papolos A, Kutok J, Hivroz C, Ledeist F, Plogmann K, et
al: ORAI1 deficiency and lack of store-operated Ca2+ entry cause
immunodeficiency, myopathy, and ectodermal dysplasia. J Allergy
Clin Immunol. 124:1311–1318. e7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parekh AB and Putney JW Jr.:
Store-operated calcium channels. Physiol Rev. 85:757–810. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ichikawa J and Inoue R: TRPC6 regulates
cell cycle progression by modulating membrane potential in bone
marrow stromal cells. Br J Pharmacol. 171:5280–5294. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
El Boustany C, Bidaux G, Enfissi A,
Delcourt P, Prevarskaya N and Capiod T: Capacitative calcium entry
and transient receptor potential canonical 6 expression control
human hepatoma cell proliferation. Hepatology. 47:2068–2077. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jardin I, Gómez LJ, Salido GM and Rosado
JA: Dynamic interaction of hTRPC6 with the Orai1-STIM1 complex or
hTRPC3 mediates its role in capacitative or non-capacitative Ca(2+)
entry pathways. Biochem J. 420:267–276. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Winn MP, Conlon PJ, Lynn KL, Farrington
MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S,
Burchette JL, et al: A mutation in the TRPC6 cation channel causes
familial focal segmental glomerulosclerosis. Science.
308:1801–1804. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding Y, Winters A, Ding M, Graham S,
Akopova I, Muallem S, Wang Y, Hong JH, Gryczynski Z, Yang SH, et
al: Reactive oxygen species-mediated trpc6 protein activation in
vascular myocytes, a mechanism for vasoconstrictor-regulated
vascular tone. J Biol Chem. 286:31799–31809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tauseef M, Knezevic N, Chava KR, Smith M,
Sukriti S, Gianaris N, Obukhov AG, Vogel SM, Schraufnagel DE,
Dietrich A, et al: TLR4 activation of TRPC6-dependent calcium
signaling mediates endotoxin-induced lung vascular permeability and
inflammation. J Exp Med. 209:1953–1968. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
An S, Gao Y, Ling J, Wei X and Xiao Y:
Calcium ions promote osteogenic differentiation and mineralization
of human dental pulp cells: Implications for pulp capping
materials. J Mater Sci Mater Med. 23:789–795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tada H, Nemoto E, Kanaya S, Hamaji N, Sato
H and Shimauchi H: Elevated extracellular calcium increases
expression of bone morphogenetic protein-2 gene via a calcium
channel and ERK pathway in human dental pulp cells. Biochem Biophys
Res Commun. 394:1093–1097. 2010. View Article : Google Scholar : PubMed/NCBI
|

















