Introduction
At present, there is a lack of effective clinical
treatments for long-term facial nerve lesions. The predominant
available therapies are facial nerve decompression surgery and
facial nerve transplantation (1).
However, these treatments have their own limitations. Although
facial nerve decompression is a suitable therapeutic option for
facial nerve damage due to trauma or other causes of facial nerve
compression, it requires that the anatomy of the facial nerve must
be kept intact. There is also a certain time limit (within one
month) in which it should be performed, otherwise the likelihood of
successful treatment is reduced (2).
Facial nerve transplantation, which involves connecting the two
broken ends of the facial nerve via suture, or neural
transplantation (connecting the facial nerve with a nerve taken
from another area of the body), is a common method used for
reconstructing the facial nerve (3).
However, the neural transplantation only serves as a bridge. If the
break is from a superior anatomical position, it will take a long
time (six months) for the facial nerve to reach the muscles
(4). During this period, the motor
end plate (nerve and muscle joint) may shrink, which leads to
disuse atrophy. This is the main cause of permanent facial
paralysis (2).
The present study explored a novel treatment
strategy, which aimed to restore the facial nerve reflex arc via an
artificial facial nerve system (AFNS). The AFNS contains a
signal-collecting microelectrode, processing chip and stimulating
microelectrode, as well as a system control program on a computer.
The processing chip and system control program communicate with
each other to process signals and control the current stimulus
(5). The signal-collecting
microelectrode is implanted into normal facial muscles, and
receives electrical signal from normal facial muscles. This
information is transported to the artificial facial nerve system
processing chip. The chip analyzes this information, determines the
movement patterns of the normal side, then sends a signal to the
stimulating microelectrode implanted in the paralyzed muscle,
maintaining a synchronous movement with the normal side.
In previous research by the current authors, the
reflex arc was restored using an AFNS (5). In this previous research, the
processing chip was outside of the body and was connected to a
computer, in which there was a system controlling program (System
Controller). This program helped us to set the working parameters
and confirm the function of the AFNS.
The purpose of the present study was to implant the
processing chip, together with the microelectrodes, into the
paralyzed rabbits to make the system fully implantable. This was
named the implantable AFNS (IAFNS). The communication system
between the chip and the System Controller was changed from wired
to wireless. The processing chips were improved to adapt to the
wireless communication. The IAFNS was implanted into animal models
in order to restore orbicularis oculi muscle function. The
effectiveness, stability and security of the wireless communication
between the processing chip and the System Controller was assessed
in animal experiments in the current study.
Materials and methods
Animals and equipment
A total of 12 healthy male New Zealand rabbits
(weight, 2–2.5 kg; age, 6±0.41 months) were used in the present
study [permission number: SCXK (SH) 2012–0007 and SYXK (SH)
2009–0086]. The temperature and humidity of the lab were 24–26°C
and 60%, respectively. Rabbit food (pellet feed as required) and
water (100 ml/kg) were provided every day. The present study was
approved by the Ethics Committee of Shanghai General Hospital,
Shanghai Jiao Tong University School of Medicine (Shanghai, China).
An SMB100A signal generator (Rohde & Schwarz UK, Ltd.,
Hampshire, UK) and an E4419B EPM series power meter (Agilent
Technologies, Inc., Santa Clara, CA, USA) were used for low
frequency interference experiments. High frequency interference
experiments were conducted using an N5181A 100 KHz-6 GHz MXG analog
signal generator and an N1912A P-series power meter (Agilent
Technologies, Inc. Santa Clara, CA, USA). The radiation field
strength test was performed using an EMI test receiver 20 Hz-40 GHz
(ESU40; Rohde & Schwarz UK, Ltd.).
Processing chip
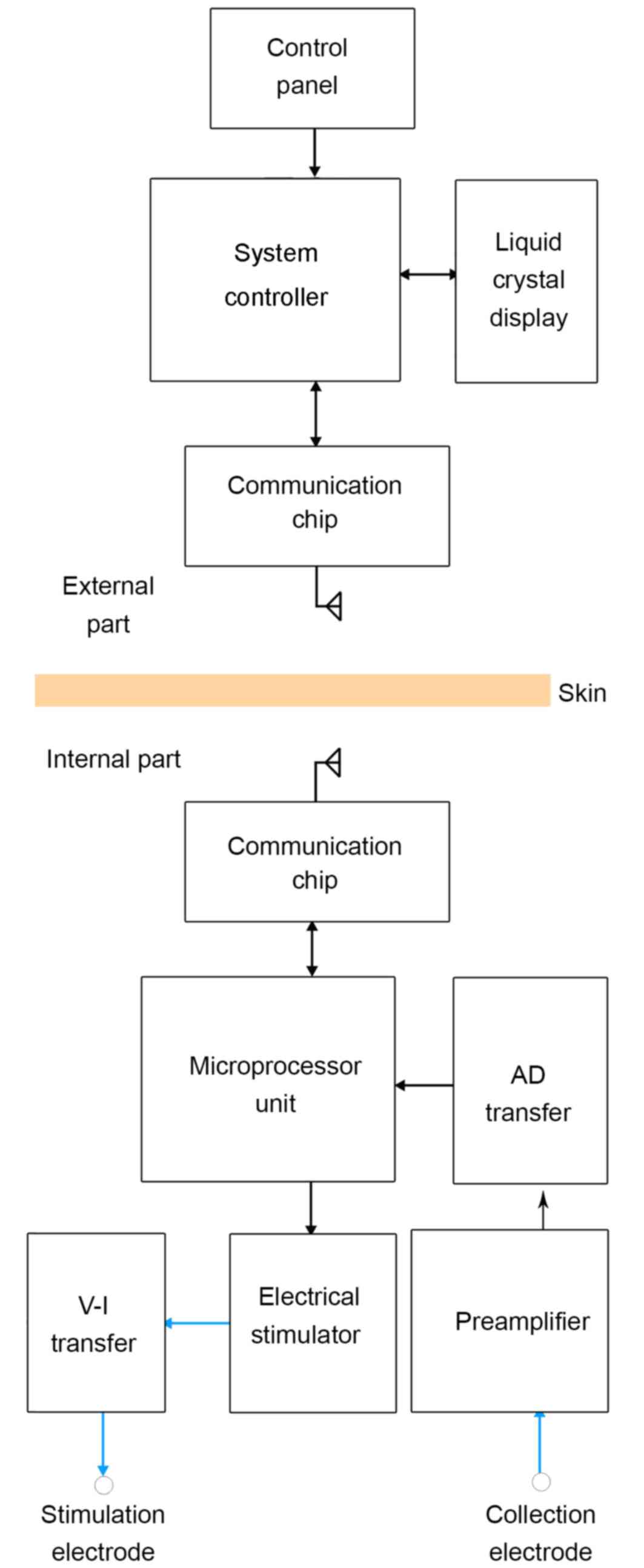
The IAFNS consisted of two parts: An external part
and an internal part. The external part contained the System
Controller and its wireless communication chip module. The internal
part contained the processing chip and microelectrodes. The
communication between each part was via the wireless communication
chip module. The signal from the uninjured side of the face was
collected and analyzed by the processing chip. Then, a stimulating
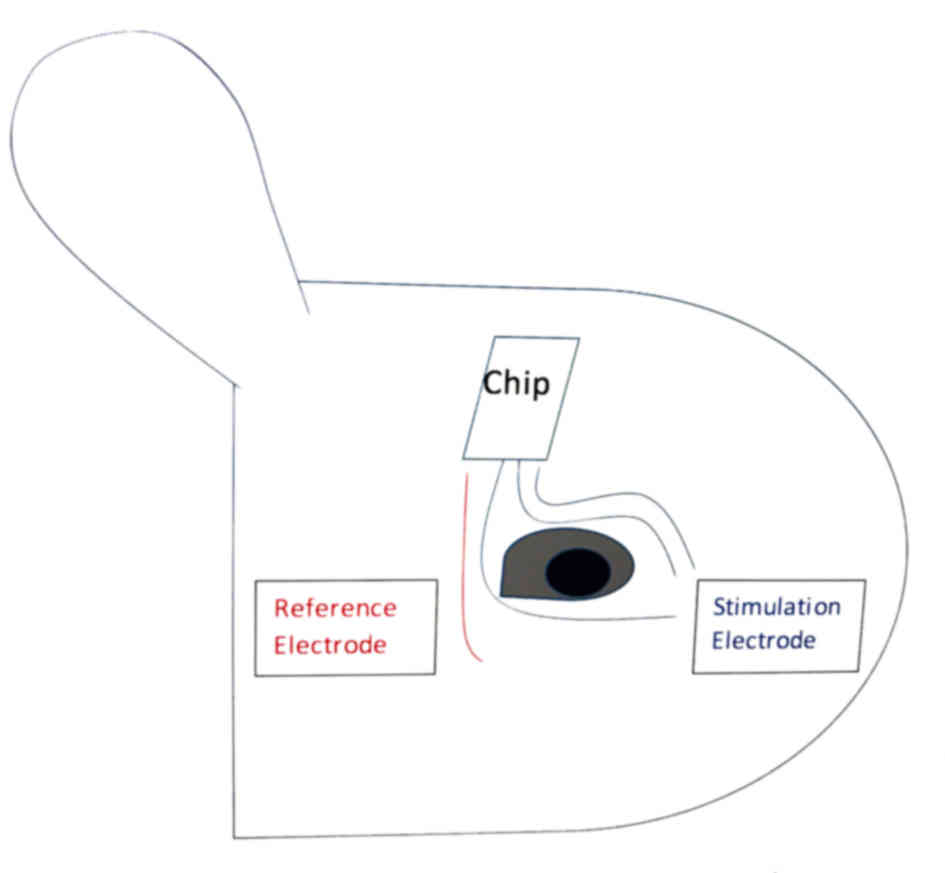
signal was sent out to stimulate the injured side (Fig. 1).
Based on previous research by the current authors
(5), the processing chip consisted
of three parts: The signal collection module, the stimulating
module and the wired communication module. The signal collection
module of the chip was composed of three microelectrodes, including
two collection electrodes and one reference electrode. The
stimulating module included a three-channel stimulation electrode
and one reference electrode. These electrodes were made of nano
platinum black, which was demonstrated to be safe and effective in
previous research (6). In the
current research the processing chip was implanted into the
forehead of the facial nerve paralyzed animal model. The
communication between the processing chip and the System Controller
was changed from wired to wireless. A new communication module was
added into the processing chip. Two-way data communication was
established by radio frequency communication. The communication
module used a CC1110 low power consumption microprocessor unit
(MPU) chip (produced by Texas Instruments, Inc., Dallas, TX, USA),
which contains a standard enhanced 8051 MCU and a wireless
transceiver chip CC1100, with 433 MHz frequency-shift keying
communication unit connecting with the MPU through the Serial
Peripheral Interface (Fig. 2). The
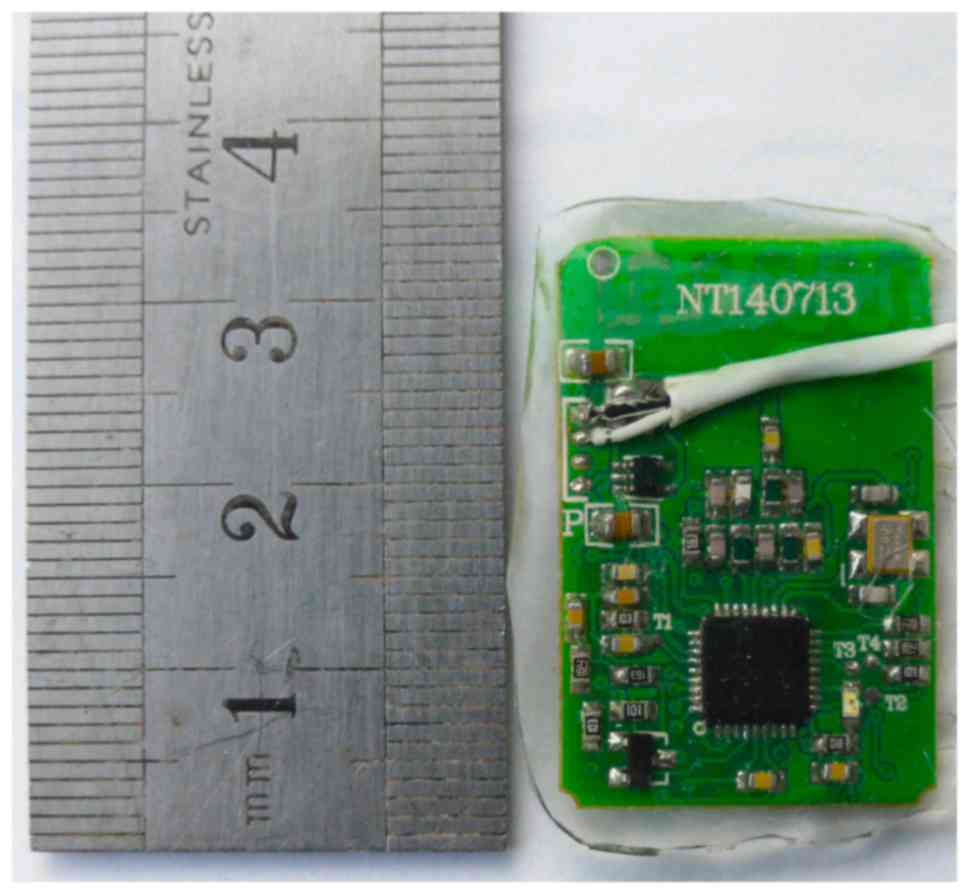
chip was 3×2 cm, and was implanted into the forehead of the rabbit
(Fig. 3).
System Controller
The System Controller on the computer outside the
body was designed to indicate the working status and control the
implanted processing chip via two-way wireless data communication.
This program obtained the contralateral facial excitation mode by
analyzing the wavelength, voltage and frequency of the input
signal, as well as the MPU output of the corresponding stimulus
mode through an electrical stimulator. This program could display
the normal side wavelength as well as adjust the electric detection
algorithm and threshold and stimulation parameters (stimulation
intensity and frequency, range of wave and stimulation time) of the
implanted part, to ensure the best working state was
maintained.
Electromagnetism yield in radiation
assessment and anti-interference tests
Electromagnetism yield in radiation assessment and
anti-interference tests of the chip were performed in Shanghai
Testing & Inspection Institute for Electrical Equipment
(Shanghai, China). The tests were performed in an electromagnetic
darkroom (26°C), 21.7×13.0×13.0 m, produced by Albatross Projects
GmbH (Nattheim, Germany). Three rabbits and three chips were used
in the test.
Anti-interference test of the
chip
The purpose of this test was to assess the stability
of the the processing chip under the interference of
electromagnetic radiation. For low anti-interference, an SMB100A
signal generator (Rohde & Schwarz UK, Ltd., Fleet, UK) and an
E4419B EPM series power meter (Agilent Technologies, Inc.) were
used. An N5181A 100 KHz-6 GHz MXG analog signal generator (Agilent
Technologies, Inc.) and an N1912A P-series power meter (Agilent
Technologies, Inc.) were employed for high frequency interference.
The chip was placed on a 0.8 m high insulation test panel. The
signal generator antenna was placed 3 m from the test chip.
Pyramid-shaped absorbing material was placed between the chip and
antenna. Low and high frequency tests were performed at 80 MHz-1
GHz and 1–2.5 GHz, respectively; the test field intensity was 3
V/m, the test frequency increased by 1% every time (according to
the national standard YY 0505-2012). For each frequency, the
scanning of amplitude modulation carrier reflected the residence
time in the chip movement and response time (~0.5 sec). The test
chip was assessed under two polarized states of the transmitting
antenna: One antenna in the perpendicular polarization position,
another in the horizontal polarization position. The
anti-interference test results had four ranks: Level A, the
electric device performed normally in the test; level B, the
function was lost or the performance temporarily reduced, but
readily restored with no need of operator intervention; level C,
the function was lost or the performance temporarily reduced, but
could return to normal following operator intervention; and level
D, due to hardware or software damage, or missing data, the lost
function failed to return to normal.
Electromagnetism yield in radiation
assessment
The electromagnetic radiation energy level produced
by the chip was assessed using the ESU EMI Test Receiver 20 Hz-40
GHz (ESU40; Rohde & Schwarz UK, Ltd.). The chip in active
status launched and received signals; every cycle had a firing time
of 10 msec and receiving time of ~40 msec.
The chip functioned in three states: State 1, in
vitro environment condition (three chips); state 2, implanted
within the rabbit with the skin not sutured (three rabbits); and
state 3, implanted within the rabbit with sutured skin (three
rabbits). Test distances investigated were 0, 1, 3 and 10 m
(according to the national standard YY 0505-2012).
Establishment of facial paralysis
model
A total of 12 adult male New Zealand rabbits were
used in facial nerve system rebuilding experiments. Following
administration of 3 ml/kg 1% pentobarbital sodium via the marginal
ear vein, the junction of the right auricle cartilage and bone
border was identified. Skin on the masseter muscle surface and
subcutaneous tissues was cut to expose the facial nerve branches. A
facial nerve monitoring probe (NTS-2000; NCC Medical Co., Ltd.,
Shanghai, China) was used to stimulate the nerve branch and
masseter muscle and jaw twitches were observed. These nerve
branches extend towards the proximal end and join the posterior and
superior nerves to form the stylomastoid foramen. When the probe
stimulated the stylomastoid foramen, twitches in the neck, cheek,
eyelid and ear muscle were detected. A partial facial nerve stem
segment (2–3 mm) at the stylomastoid foramen was excised and in
order to prevent recanalization, the distal and proximal ends were
rotated and the severed distal and proximal ends of the nerve were
stimulated by the facial nerve monitoring probe 10 min later. When
no twitching was observed in the neck, ears and eyes, disconnection
was confirmed. All incisions were sutured and stitches were removed
after 7 days to allow for facial expression observation in rabbits.
Electromyography (EMG; NTS-2000) was performed to examine
orbicularis oculi muscle activity on the surgical (right) side to
confirm denervation.
Implantation of the IAFNS
One week after the facial paralysis model was
established, all 12 rabbits were subsequently anesthetized with 3
ml/kg 1% pentobarbital sodium via the marginal ear vein. The skin
was incised on the parietal transverse surface to expose the skull
periosteum and the subcutaneous tissue was separated to make a 4×5
cm space for insertion of the processing chip. Once the chip was
inserted, the subcutaneous tissue was sutured.
The signal collection module of the chip was
composed of three electrodes, including two collection electrodes
and one reference electrode. Two collection electrodes were
embedded into the superior and inferior orbicularis oculi
respectively. The reference electrode was embedded into the outer
canthus. At implantation the upper and lower eyelid skin was
sterilized. A 2-ml guided needle was used to penetrate from the
lateral limbus of the eye epidermis to the inside, near the inner
canthus. The electrode wire was inserted and positioned in the
orbicularis oculi muscle via the guide needle, which was
subsequently carefully removed. The reference electrode was
embedded into the lateral canthus skin using the same method.
The stimulation module included three-channel
stimulation and one reference electrode. Two stimulating electrodes
were embedded into the superior orbicularis oculi and the other
into the inferior orbicularis oculi. The reference electrode was
embedded into the outer canthus as indicated in Fig. 1.
In vivo and in vitro validity and
stability of the IAFNS
The IAFNS was run for 5 h daily for 30 consecutive
days. Communication between the processing chip and the System
Controller was established every 3 days to control and observe the
working state of the processing chip. The stimulation frequencies
were 30 and 50 Hz (7). Every 3 days,
the smallest stimulus intensity that could induce the movement of
the paralyzed muscle was recorded. At 0, 10, 20 and 30 days after
the surgery, the uninjured cornea was stimulated 20 times, using a
cotton bud to irritate the uninjured eyelid to close. The eyelid
movement of the injured side was recorded at the same time to
evaluate the synchronization of both sides (8).
Statistical analysis
SAS 9.4 (SAS Institute, Cary, NC, USA) was used for
statistical processing (t-tests). Data are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Experimental animals
A total of 12 male rabbits were evaluated. One
rabbit (no. 11) was sacrificed by anesthetic injection via the
marginal ear vein (150 mg/kg, 1% pentobarbital sodium) as no signal
was received from the chip 25 days after implantation. Another
rabbit (no. 1) succumbed to mortality 4 days following facial
paralysis surgery as a result of infection. The 10 remaining
rabbits were sacrificed by anesthetic injection (150 mg/kg, 1%
pentobarbital sodium) via the marginal ear vein after research.
Anti-interference test and
electromagnetism yield in radiation assessment of the processing
chip
Chips ran stably according to the anti-interference
test, and signal emission performance did not decrease following
the test, indicating results of level A. The results of
electromagnetism yield in radiation test (Tables I and II) indicated that the processing chip
implanted in rabbits with the skin not sutured had the highest
radiation field intensity, compared with the in vitro state
and implanted state in sutured rabbits. The lowest intensity of the
three states was exhibited when the chip was implanted and sutured
in the skin. In each state, radiation field intensity decreased
with increasing distance and the minimum intensity observed was at
10 m.
 | Table I.Electromagnetic radiation energy level
(Horizontal level) under three states. |
Table I.
Electromagnetic radiation energy level
(Horizontal level) under three states.
|
| Electromagnetic
radiation energy level (dBµV/M) |
|---|
|
|
|
|---|
| Horizon level
(m) | State 1 | State 2 | State 3 |
|---|
| 0 |
63.97±0.06 |
63.97±3.98 |
58.7±1.82 |
| 1 |
64.3±0.17 |
62.73±2.00 |
55.47±6.62 |
| 3 |
64.13±0.12 |
51.97±2.06 |
48.07±0.81 |
| 10 |
52.07±1.95 |
44.97±0.21 |
46.3±2.17 |
 | Table II.Electromagnetic radiation energy level
(vertical level) under three states. |
Table II.
Electromagnetic radiation energy level
(vertical level) under three states.
|
| Electromagnetic
radiation energy level (dBµV/M) |
|---|
|
|
|
|---|
| Vertical level
(m) | State 1 | State 2 | State 3 |
|---|
| 0 |
57.5±0.00 |
57.37±3.50 |
51.4±1.47 |
| 1 |
63.93±0.06 |
56.2±4.94 |
56.4±0.20 |
| 3 |
63.47±0.49 |
51.77±1.40 |
51.67±1.10 |
| 10 |
41.4±1.57 |
40.87±0.75 |
42.17±2.35 |
Implantation of the IAFNS
All rabbits were healthy and underwent facial
neurosurgery to induce facial paralysis on the right side of the
face. During surgery, the denervated distal stump was stimulated
with a nerve monitoring system probe. Tics in the ocular region and
mouth corner were accompanied by an electromyography monitoring
sound, which appeared at the same time the facial nerve was
transected. After 10 min, the denervated proximal stump was
stimulated with the nerve monitoring system probe, no tics and
monitoring sound were detected in the left external ear, ocular
region, mouth corner or cervical muscle. One day after the surgery,
rabbits exhibited an inability to close the right eyelid and the
mouth corner was deviated. Seven days after the surgery, EMG was
performed, which revealed denervated muscle on the injured side in
all experimental rabbits (sensitivity, 100 µV/D; scanning speed, 50
msec/D), indicating successful establishment of unilateral
peripheral facial paralysis. The processing chips were implanted
into the animal model and communication with the System Controller
was established.
In vivo and in vitro validity and
stability of the IAFNS
The stimulating threshold, which was the minimum
stimulus intensity that induced closure of the eye on the paralyzed
side, was defined as 50 µV, according to a previous study (9). Throughout the 30-days experimental
period, the threshold of each rabbit was measured every 3 days at
two different frequencies (30 and 50 Hz). Positive and negative
square waves were used at a current intensity of 1–6 mA. The
stimulation frequencies used were 30 and 50 Hz and the mean
intensity was 3.45±0.24 mA and 2.23±0.14 mA, respectively. Results
demonstrated no significant difference in the current intensity
threshold of the stimulating electrode between 0 and 30 days
post-surgery in rabbits with the skin sutured (Tables III and IV).
 | Table III.Signal intensities (mA) at a
stimulating threshold frequency of 30 Hz on chips implanted in
rabbits (n=10) for 30 days. |
Table III.
Signal intensities (mA) at a
stimulating threshold frequency of 30 Hz on chips implanted in
rabbits (n=10) for 30 days.
|
|
Days after
surgery |
|---|
|
|
|
|---|
| Rabbit no. | 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 |
|---|
| 2 | 3.5 | 3.6 | 3.7 | 3.7 | 3.8 | 3.8 | 3.8 | 3.7 | 3.7 | 3.6 | 3.6 |
| 3 | 3.5 | 3.6 | 3.6 | 3.6 | 3.7 | 3.7 | 3.7 | 3.7 | 3.7 | 3.6 | 3.6 |
| 4 | 3.5 | 3.5 | 3.5 | 3.6 | 3.6 | 3.6 | 3.6 | 3.7 | 3.7 | 3.7 | 3.6 |
| 5 | 3.4 | 3.4 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.6 |
| 6 | 3.5 | 3.3 | 3.3 | 3.4 | 3.4 | 3.5 | 3.5 | 3.5 | 3.4 | 3.5 | 3.4 |
| 7 | 3.6 | 3.3 | 3.3 | 3.5 | 3.5 | 3.3 | 3.5 | 3.3 | 3.4 | 3.5 | 3.6 |
| 8 | 3.0 | 2.8 | 3.0 | 3.2 | 3.2 | 3.1 | 3.3 | 2.9 | 2.9 | 3.0 | 3.0 |
| 9 | 3.2 | 2.9 | 3.0 | 3.2 | 3.3 | 3.5 | 3.2 | 3.0 | 3.0 | 2.9 | 3.0 |
| 10 | 3.4 | 3.2 | 3.3 | 3.5 | 3.4 | 3.5 | 3.1 | 3.4 | 3.4 | 3.4 | 3.4 |
| 12 | 3.7 | 3.7 | 3.7 | 3.8 | 3.8 | 3.8 | 3.7 | 3.7 | 3.6 | 3.6 | 3.7 |
| Mean ± SD | 3.43±0.20 | 3.33±0.30 | 3.39±0.26 | 3.50±0.19 | 3.52±0.20 | 3.53±0.22 | 3.49±0.23 | 3.44±0.30 | 3.43±0.28 | 3.43±0.27 | 3.45±0.25 |
 | Table IV.Signal intensities (mA) at a
stimulating threshold frequency of 50 Hz on chips implanted in
rabbits (n=10) for 30 days. |
Table IV.
Signal intensities (mA) at a
stimulating threshold frequency of 50 Hz on chips implanted in
rabbits (n=10) for 30 days.
|
|
Days after
surgery |
|---|
|
|
|
|---|
| Rabbit no. | 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 |
|---|
| 2 | 2.3 | 2.2 | 2.4 | 2.4 | 2.3 | 2.3 | 2.3 | 2.3 | 2.2 | 2.2 | 2.2 |
| 3 | 2.4 | 2.3 | 2.2 | 2.3 | 2.5 | 2.3 | 2.3 | 2.3 | 2.4 | 2.3 | 2.3 |
| 4 | 2.4 | 2.3 | 2.3 | 2.5 | 2.3 | 2.2 | 2.4 | 2.3 | 2.3 | 2.4 | 2.3 |
| 5 | 2.0 | 1.9 | 1.9 | 2.1 | 2.1 | 2.0 | 1.9 | 2.1 | 2.2 | 2.1 | 2 |
| 6 | 2.0 | 2.1 | 2.2 | 2.3 | 2.2 | 2.2 | 2.2 | 2.3 | 2.1 | 2.2 | 2.1 |
| 7 | 2.2 | 2.3 | 2.3 | 2.1 | 2.2 | 2.4 | 2.3 | 2.3 | 2.2 | 2.4 | 2.3 |
| 8 | 2.3 | 2.2 | 2.3 | 2.4 | 2.3 | 2.5 | 2.4 | 2.3 | 2.3 | 2.3 | 2.4 |
| 9 | 2.2 | 2.1 | 2.3 | 2.4 | 2.3 | 2.4 | 2.1 | 2.3 | 2.4 | 2.2 | 2.3 |
| 10 | 2.3 | 2.0 | 2.0 | 2.2 | 2.3 | 2.4 | 2.2 | 2.3 | 2.3 | 2.2 | 2.4 |
| 12 | 2.1 | 2.0 | 2.1 | 2.0 | 2.2 | 2.1 | 2.0 | 1.9 | 1.9 | 2.0 | 2 |
| Mean ± SD | 2.22±0.15 | 2.14±0.14 | 2.2±0.16 | 2.27±0.16 | 2.27±0.11 | 2.28±0.15 | 2.21±0.17 | 2.24±0.13 | 2.23±0.15 | 2.23±0.13 | 2.23±0.15 |
At 0, 10, 20 and 30 days after the surgery, the
uninjured cornea was stimulated 20 times in facial nerve paralyzed
rabbits using a cotton bud to irritate the uninjured eyelid to
close. The electrical stimulator, triggered by the computer,
emitted the corresponding electrical stimulation to the injured
orbicularis oculi muscle. The number of contractions from the
injured orbicularis oculi muscle triggered by the uninjured side is
indicated in Table V. Almost all
cases of stimulation induced muscle movement in the injured side.
No significant differences were found between the two groups at any
time point.
 | Table V.Number of times orbicularis oculi
muscle contraction was exhibited when the uninjured eyes of rabbits
(n=10) were stimulated to close 20 times. |
Table V.
Number of times orbicularis oculi
muscle contraction was exhibited when the uninjured eyes of rabbits
(n=10) were stimulated to close 20 times.
|
| Days after
surgery |
|---|
|
|
|
|---|
| Rabbit no. | 0 | 10 | 20 | 30 |
|---|
| 2 | 20 | 20 | 19 | 19 |
| 3 | 20 | 19 | 19 | 20 |
| 4 | 20 | 20 | 19 | 20 |
| 5 | 19 | 19 | 20 | 20 |
| 6 | 20 | 20 | 19 | 19 |
| 7 | 19 | 20 | 20 | 19 |
| 8 | 20 | 19 | 20 | 19 |
| 9 | 20 | 19 | 20 | 20 |
| 10 | 20 | 20 | 20 | 19 |
| 12 | 18 | 19 | 19 | 18 |
Discussion
At present, all treatments for facial nerve
decompression are directed to the facial nerve, with the primary
purpose of restoring its anatomical and functional integrity
(10). However, the structure and
function of the facial nerve in some patients does not fully
recover (11–13). Sönmez et al (14) implanted a weight in the upper eyelid
to close the eyelid with gravity and prevent blindness
complications, but this results in poor quality of life.
A previous study focused on restoration of the
paralyzed muscle instead of nerve function (15). Muscle movement information was
extracted by multi-channel electroencephalogram large matrix. An
electronic processing chip was able to process the movement
information and control the electrical stimulator to release
current, which directly induced skeletal muscle movement. However,
the signal was weak and the data flow processing was time
consuming. Therefore, this method may be applicable to slow action
movements alone and may not be suitable for rapid movements, such
as the facial muscles.
With appropriate currents directly stimulating the
muscle, safety and effectiveness, the use of implantable electronic
devices has been accepted by the majority of scholars (16). Implantable electronic devices have
been widely applied in the biomedical field, including cochlear
implants and artificial prosthesis control system devices (15,17). The
movement of a prosthesis may be controlled by gathering and
analyzing muscle electromyography signals (18).
The IAFNS demonstrated in the present study may be
used clinically in three ways. Firstly, the artificial nerve system
may be used to replace the facial nerve center and facial nerve, to
stimulate the facial muscles directly, to restore expression and
maintain face symmetry. This may be used to treat patients with
facial paralysis and restore facial muscle contraction function.
Secondly, the artificial nerve system may be used to temporarily
maintain the facial muscle tension for a patient being prepared for
facial nerve transplantation in order to prevent facial muscle
disuse atrophy and may be stopped once facial nerve function has
recovered. Thirdly, the artificial nerve system may be used as a
supplement for facial nerve transplantation in the instance that
neural transplantation is invalid or is ineffective. In this case,
the system may be able to stimulate facial muscles to provide the
extra contraction strength, rendering symmetrical movement in the
paralyzed and contralateral areas.
In a previous study by the current authors (9), the time domain and peak absolute
potentials of EMG signals during the natural continuous
eye-opening, natural continuous eye-closing, natural blinking and
evoked eye-closing states were compared. Absolute potentials of
initial myoelectric signals, which triggered the orbicularis oculi
muscle to potently contract during natural blinking and evoked
eye-closing states, were 55.39±6.25 and 52.16±3.69 µV,
respectively; therefore, the chip stimulating threshold was
determined as 50 µV.
The positions of the stimulating electrode were
determined according to previous research (19). The optimal position is able to
improve the utilization efficiency of the stimulus current and
therefore the minimum current may be used to achieve the largest
muscles contraction.
In the present study, stimulation frequencies were
determined predominantly by the eyelid movement stimulated in the
facial nerve paralyzed rabbits. At first, stimulation frequencies
of 20, 22, 25, 30, 50 Hz were assessed: 20, 22 and 25 Hz could
induce orbicularis oculi muscle movement, but these movements were
too weak to close the eyelids completely. At frequencies of 30 and
50 Hz, the paralyzed orbicularis oculi muscle could be stimulated
to provoke the complete closure of eyes in the facial nerve
paralyzed rabbits, with 30 and 50 Hz yielding mean intensities of
3.45 and 2.23 mA, respectively. There were no significant
differences in the stimulation intensities between 0 and 30 days
after implantation. The results indicated that the artificial
facial nerve system reconstructed the nerve reflex arc that was
damaged by induced facial nerve injury.
In the present study, 30 days after implantation in
the animal model, the processing chip worked stably. The
synchronicity of the movement on both sides was assessed on 0, 10,
20 and 30 days after implantation. Results indicated that high
synchronicity was obtained and length of time the implant was
embedded within the rabbits did not affect the results.
At the Shanghai Testing & Inspection Institute
for Electrical Equipment, the anti-interference ability of the
implantable artificial facial nerve system chip was assessed and
results confirmed that the system was not affected by high
intensity radiation during operation. In addition, the present
study also evaluated the radiation field intensity of the
artificial facial nerve chip under states 1, 2 and 3. Test
distances analyzed were 0, 1, 3 and 10 m. Results indicated that
most of the radiation field intensity was <60 dB at three
states, except 0 m in state 3. In further research, the processing
chip will be coated with insulating material; the chip was in a
bare-metal state in this research, which led to a high radiation
field intensity.
In conclusion, the present study indicated that the
artificial facial nerve system applied an appropriate muscle
stimulation signals to produce the corresponding muscle
contractions to perform specific actions. Implantation of the
artificial facial nerve system into rabbits restored the function
of induced facial paralysis on the paralyzed side and provoked eye
closing and blinking. Furthermore, the system functioned well while
implanted in the body. From the findings of the present study, it
is suggested that the wireless communication between the processing
chip and the system control program is reliable, which could
displayed the contralateral electromyography activity and the
working state of the implanted chip, adjust the appropriate
parameters to reconstruct the paralyzed muscle.
Acknowledgements
The authors are thankful for financial support
received from the Science and Technology Commission of Shanghai Key
Research Projects (grant no. 11441900102).
References
|
1
|
Lu Xg, Cai Zg, Yu Gy and Peng X: Surgical
treatment of transected peripheral facial nerve injury. Beijing Da
Xue Xue Bao. 43:106–111. 2011.(In Chinese). PubMed/NCBI
|
|
2
|
Sardesai MG and Moe K: Recent progress in
facial paralysis: Advances and obstacles. Curr Opin Otolaryngol
Head Neck Surg. 18:266–271. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Volk GF, Pantel M and Guntinas-Lichius O:
Orlando Guntinas-Lichius: Concepts in facial nerve reconstruction.
Head Face Med. 6:252010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Langhals NB, Urbanchek MG, Ray A and
Brenner MJ: Update in facial nerve paralysis: Tissue engineering
and new technologies. Curr Opin Otolaryngol Head Neck Surg.
22:291–299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang YJ, Li KY, Liu JQ, Xu DY, Rui YF and
Yan CS: Artificial facial nerve reflex restores eyelid closure
following orbicularis oculi muscle denervation. Neural Regen Res.
5:1750–1755. 2010.
|
|
6
|
Zhang Y, Li KY, Jin C, Wang YT, Geng L,
Sun YJ, Tian HC, Liu JQ and Jin XJ: Comparative studies on the
implantation of nanoplatinum black and pure platinum electrodes in
the rabbit orbicularis oculi muscle. J Laryngol Otol. 128:679–689.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jie T, Zhiqiang G, Guodong F, Yubin X,
Xiuyong D, Tingting C and Yang Z: The effective stimulating pulse
for restoration of blink function in unilateral facial nerve
paralysis rabbits, verified by a simple FES system. Eur Arch
Otorhinolaryngol. 273:2959–2964. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yi X, Jia J, Deng S, Shen SG, Xie Q and
Wang G: A blink restoration system with contralateral EMG triggered
stimulation and real-time artifact blanking. IEEE Trans Biomed
Circuits Syst. 7:140–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dongyue X, Keyong L, Jingquan L, Yujuan W
and Yuefeng R: Quantitative features of myoelectric signals in the
orbicularis oculi muscle during different motion states. Neural
Regen Res. 5:1895–9. 2010.
|
|
10
|
Hadlock T: Facial paralysis: Research and
future directions. Facial Plast Surg. 24:260–267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi Y, Zhou L, Tian J and Wang Y:
Transplantation of neural stem cells overexpressing glia-derived
neurotrophic factor promotes facial nerve regeneration. Acta
Otolaryngol. 129:906–914. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bozkurt G, Mothe AJ, Zahir T, Kim H,
Shoichet MS and Tator CH: Chitosan channels containing spinal
cord-derived stem/progenitor cells for repair of subacute spinal
cord injury in the rat. Neurosurgery. 67:1733–1744. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kabatas S and Teng YD: Potential roles of
the neural stem cell in the restoration of the injured spinal cord:
Review of the literature. Turk Neurosurg. 20:103–110.
2010.PubMed/NCBI
|
|
14
|
Sönmez A, Oztürk N, Durmuş N, Bayramiçli M
and Numanoğlu A: Patients' perspectives on the ocular symptoms of
facial paralysis after gold weight implantation. J Plast Reconstr
Aesthet Surg. 61:1065–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pohlmeyer EA, Oby ER, Perreault EJ, Solla
SA, Kilgore KL, Kirsch RF and Miller LE: Toward the restoration of
hand use to a paralyzed monkey: Brain-controlled functional
electrical stimulation of forearm muscles. PLoS One. 4:e59242009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Laizou PC: Signal-processing techniques
for cochlear implants. IEEE Eng Med Biol Mag. 18:34–46. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hochberg LR, Serruya MD, Friehs GM, Mukand
JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD and Donoghue JP:
Neuronal ensemble control of prosthetic devices by a human with
tetraplegia. Nature. 442:164–171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wessberg J, Stambaugh CR, Kralik JD, Beck
PD, Laubach M, Chapin JK, Kim J, Biggs SJ, Srinivasan MA and
Nicolelis MA: Real-time prediction of hand trajectory by ensembles
of cortical neurons in primates. Nature. 408:361–365. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Li K, Jin C, Wang Y, Geng L, Sun
YJ and Tian H: Electrical stimulation characteristics of denervated
orbicularis oculi muscle. Neurol Sci. 36:1379–1386. 2015.
View Article : Google Scholar : PubMed/NCBI
|