Introduction
The contribution of hypertension to mortality and
morbidity in people's health is preventable, for its etiologic
influence and its growing influence in stroke, kidney failure and
heart attack (1). Based on the
reports from World Health Organization (WHO), high levels of blood
pressure, even when just suboptimal, have responsibility for 49%
ischaemic heart disease and 62% cerebrovascular disease (2). The rise of BP within a patient with
hypertension results from control mechanisms for blood pressure,
such as vascular resistance of periphery, volume of circulating
blood and cardiac output. It is a crucial task to choose the
appropriate therapy for every patient (3).
During the past years, invasive and non-invasive
techniques have brought improved vascular changes within
experimental animals and hypertensive patients (4). In both animal and human models, there
is an association between hypertension and aortic remodeling
(5), which is featured by structural
vascular alterations and destroyed endothelium-dependent
vasodilation (6). Therefore,
endothelium is essential to the vascular structure and tone
(7). A declined aortic diameter
within hypertensive subject in middle age may also make sense to
increase pulse pressure via strengthening particular impedance,
which contradicts the traditional phenotype of hypertensive aortic
featured by degenerated and calcific vascular wall and increased
aortic diameter (8).
To account for various mechanisms of blood pressure,
scientists have developed the therapy of targeted
anti-hypertension. Even though anti-hypertensive drugs, like
calcium-channel blockers, receptor blockers of angiotensin II (Ang
II) and inhibitors of angiotensin-converting enzyme (ACE), have
extreme application in clinical treatment, there is no resolution
for vascular changes induced by hypertension (9). Thus, it is necessary to develop new
therapeutic tactics and drugs for vascular remodeling related with
hypertension. With the characteristics of ‘multi-target’,
composition of many compatible herbs and multiple compounds in one
prescription, conventional Chinese herbs have achieved a good
acceptance in China that attempts to decrease side effects and
promote efficacy (10). Cyathula
officinalis (C. officinalis), with family of Amaranthaceae
family, belongs to an herbaceous plant perennially with wide
distribution in tropical regions of Africa and Asia, and especially
in Korea, Vietnam and China. C. officinalis Kuan's roots,
C. officinalis Kuan, in Chinese called ‘Chuan Niu Xi’, have
functions to remove blood stasis and restore menstrual flow, ease
joint movement, as well as induce diuresis for treatment of
stranguria (11). It is often
applied as emmenagogue, atonic, antiarthritic, anti-fertility agent
and diuretic to nourish kidneys and liver, fortify muscles and
bones, and activate circulation (12). C. officinalis Kuan has been
extracted with diverse active compounds in biology, such as
palmitic acids, hyterocyclic compounds and phytoecdysteroids
(13,14), whose biological attributes have been
featured. Nevertheless, the anti-hypertensive attributes of C.
officinalis Kuan have attracted little attention.
In the present study, we made efforts to evaluate
the impacts of C. officinalis Kuan on the arterial
remodeling in spontaneously hypertensive rats (SHRs). The results
indicated that C. officinalis Kuan could improve the
arterial remodeling by decreasing endothelin-1 (ET-1) and
increasing endothelial nitric oxide synthase (eNOS) and ATIR
expression.
Materials and methods
Animal treatments
Male rats, 12-weeks-old with spontaneous
hypertension (SHR) (245–285 g) were obtained from the Shaanxi Jiahe
Phytochem Co., Ltd. (Xian, China). SHR were separated into 5 groups
randomly with 8 rats in each group: SHR treated by 0.9% saline were
considered to be a model of hypertension (SHR); SHR in the other 4
groups were administered with 3, 6 and 12 g/kg C.
officinalis Kuan or 2.5 mg/kg enalapril. The rats had a
dark/light cycle of 12/12 h at fixed temperature of 22–23°C with
available water and food freely. The administration was once a day
for eight weeks. Monitoring of blood pressure was once a week using
a tail BP Series Automatic non-invasive blood pressure measuring
system (BP-300A; Chengdu Techman Software Co., Ltd., Chengdu,
China) during the experimental period. Animal Care and Use
Committee of Shanghai Putuo People's Hospital approved this study
according to the guidelines on Ethical Care for Experimental
Animals.
Histological assessment
At the end of the experiments, the rats were
euthanized with an overdose of chloral hydrate. The aorta of the
rats was harvested, and fixed with 10% formalin, dehydrated and
embedded into paraffin. Next, sections with thickness of 4 µm were
cut, and then stained with hematoxylin and eosin (H&E). The
Olympus BX51 microscope with the camera of Olympus DP71 CCD from
Olympus (Tokyo, Japan) was used to capture digital images
(magnification, ×200). A blinded manner was used to perform
analysis on all images.
Measurement of serum NO level
The serum concentration of nitric oxide (NO) was
measured by Nitric Oxide assay kit (Nitrate reductase method, A012;
Nanjing Jiancheng Bioengineering Institute, Nanjing, China) in
accordance with instructions of the manufacturer.
RNA extraction and analysis on
quantitive reverse transcription-polymerase chain reaction
(qRT-PCR)
Whole RNA was extracted from aorta by snap-freezing
and samples of carotid by RNAiso Plus and PrimeScript reagent kit
of reverse reaction (DRR037A) (both from Takara, Dalian, China) was
used to carry out reverse transcription reaction on RNA in
accordance with manufacturer's instructions. Quantitative analysis
on the change of expression level was conducted by SYBR Premix Ex
Taq (DRR041A; Takara) in ABI 7500 (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The primer sequences of PCR were: ET-1 forward,
5′-TGTTCCCTAACCTGTCTTC-3′ and reverse, 5′-ACACTCCCTAAGGACTTTC-3′;
eNOS forward, 5′-CTTTCGGAAGGCGTTTGAC-3′ and reverse,
5′-AACTCTTGTGCTGCTCAGG-3′; Ang II receptor type 1 (AT1R) forward,
5′-CTCTGTTCTACGGCTTTC-3′ and reverse, 5′-CTTCTGTCAGGGCATTAC-3′;
GAPDH forward, 5′-GTCGGTGTGAACGGATTTG-3′ and reverse,
5′-TCCCATTCTCAGCCTTGAC-3′. The change in expression of mRNA within
rats treated by saline, C. officinalis Kuan or enalapril was
assessed by the 2−ΔΔCq method.
Western blotting
Whole protein was isolated out of snap-frozen aorta
samples using radioimmunoprecipitation buffer, supplemented with
protease inhibitor (Beyotime Institute of Biotechnology, Shanghai,
China). The concentration of protein was estimated employing the
assay kit of bicinchoninic acid (Thermo Fisher Scientific, Inc.).
Equivalently quantitive protein (30 µg) was divided subsequently on
12% SDS-PAGE gels, and then was moved onto membranes of
nitrocellulose (EMD Millipore, Billerica, MA, USA). Following
blocking, these membranes were immunoblotted overnight in 4°C with
first antibodies: Anti-ET-1, anti-eNOS, anti-ATIR and anti-GAPDH.
Horseradish peroxidase-conjugated second antibodies were used to
incubate membranes after they were washed (1:1,000; Beyotime
Institute of Biotechnology) for 1 h at 37°C. Tris-buffered saline
including Tween-20 of 20% was used to wash these membranes
(Amresco, LLC, Solon, OH, USA). Detection for signals employed an
improved system of chemiluminescence (Pierce, Rockford, IL, USA)
and their determination employed software of ImageJ version 1.46
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The quantitive values are in mean ± SD. GraphPad
Prism software, version 5.0 (GraphPad Software, Inc., San Diego,
CA, USA) was used to analyze nonlinear regression of each curve for
dose-response. Calculation of data used one-way analysis on
variance (ANOVA) and analysis on statistical calculations used SPSS
18.0 statistical software (SPSS Inc., Chicago, IL, USA). Comparison
among data from various groups used one-way ANOVA. P<0.05 was
considered to indicate a statistically significant difference.
Results
C. officinalis Kuan therapy reduces
blood pressure in SHR
Monitoring of blood pressure was conducted
fortnightly at indicative time. In week 0, different doses of C.
officinalis Kuan (3, 6 and 12 g/kg) or 2.5 mg/kg enalapril
therapy did not show any impact on blood pressure in comparison to
group of SHR (Table I). After 2
week, the blood pressure was 161.34±4.38, 181.50±3.15, 181.08±5.43
and 176.46±4.11 mmHg for enalapril, 3, 6 and 12 g/kg C.
officinalis Kuan treatment, respectively, compared with SHR
with the blood pressure of 187.91±4.89 mmHg. At the 8th week, there
were no differences in the blood pressure between 12 g/kg C.
officinalis Kuan and enalapril treatment. These results suggest
that C. officinalis Kuan treatment significantly reduced the
blood pressure of SHR.
 | Table I.The blood pressure in SHR with
enalapril or C. officinalis Kuan treatment. |
Table I.
The blood pressure in SHR with
enalapril or C. officinalis Kuan treatment.
| Groups | 0 week | 2 weeks | 4 weeks | 6 weeks | 8 weeks |
|---|
| SHR | 185.67±6.31 | 187.91±4.89 | 191.48±6.51 | 193.88±3.31 | 195.51±4.88 |
| SHR+enalapril | 189.64±5.83 |
161.34±4.38b |
154.84±4.17b |
155.31±4.38b |
154.29±4.38b |
| SHR+3 g/kg | 182.85±4.95 |
181.50±3.15a |
181.74±3.21b |
178.28±3.17b |
177.63±5.49b |
| SHR+6 g/kg | 186.81±3.74 |
181.08±5.43a |
175.38±4.29b |
172.00±4.11b |
167.21±6.11b |
| SHR+12 g/kg | 181.34±5.84 |
176.46±4.11b |
170.93±7.17b |
162.15±4.46b |
158.50±3.94b |
C. officinalis Kuan treatment inhibits
arterial remodeling in SHR
It has been suggested previously that an evaluation
of arterial alterations may offer valuable information on
hypertensive damage of organs in people. In the present study, it
evaluated the prevention of C. officinalis Kuan from
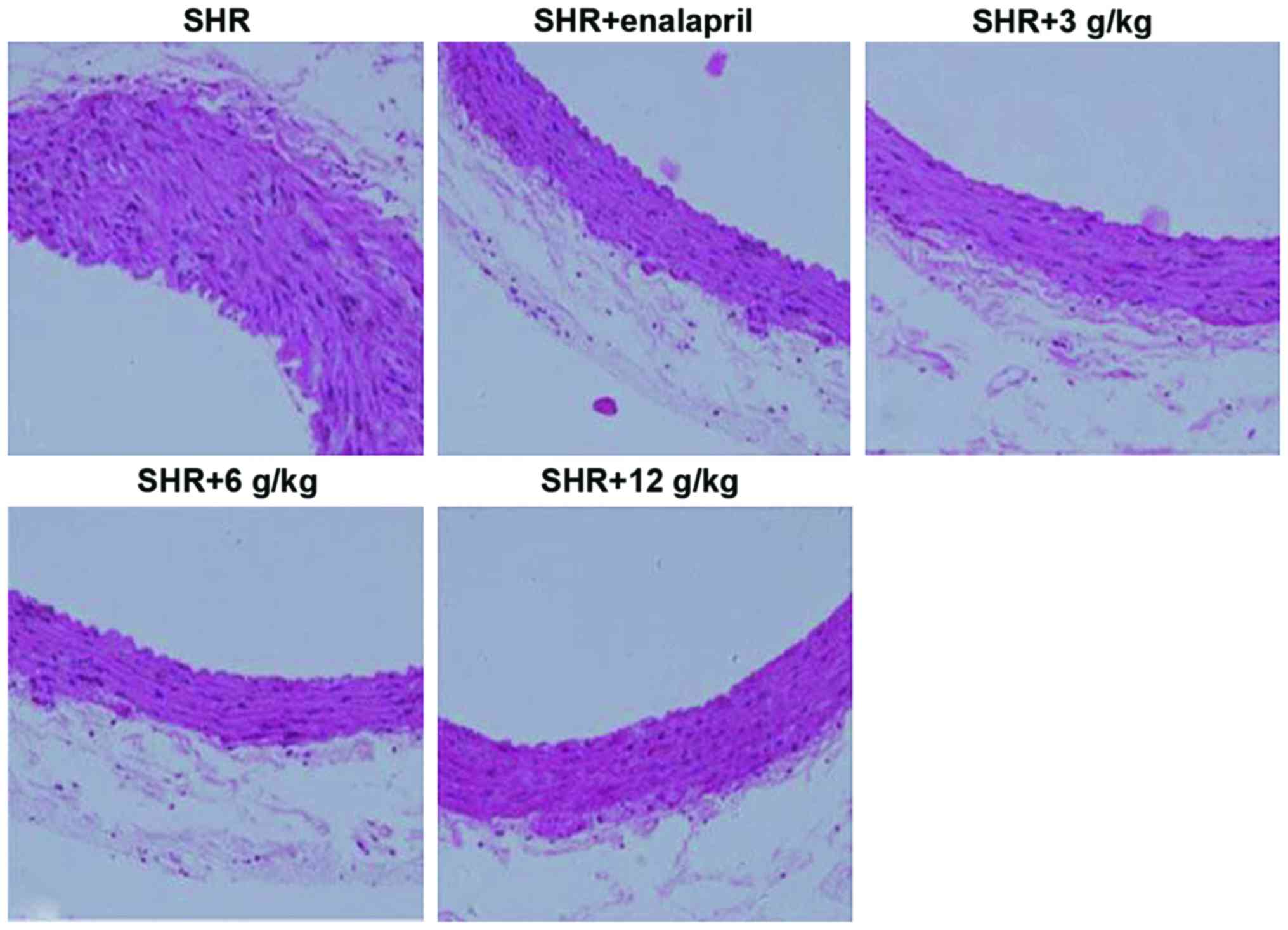
arterial remodeling employing staining with H&E. As Fig. 1 shows the aorta's medial thickness
within the SHR+enalapril, as SHR+3 g/kg, SHR+6 g/kg and SHR+12
g/kg, C. officinalis Kuan group was significantly low
compared with that of SHR group, with obvious decrease in SHR+6
g/kg C. officinalis Kuan group.
C. officinalis Kuan treatment
increases NO and eNOS expression and decreases ET-1 and AT1R
expression in SHR
As shown in Fig. 2A,
the serum level of NO in enalapril and different doses of C.
officinalis Kuan treatment was significantly increased in
comparison to SHR. Measurement of expression shown by ET-1, eNOS
and AT1R within aorta used qRT-PCR and western blotting. Different
doses of C. officinalis Kuan or enalapril treatment
significantly decreased the transcriptional level of ET-1 and AT1R,
while increased the transcriptional level of eNOS in the aorta of
SHR (Fig. 2B and C). Whereas
different doses of C. officinalis Kuan treatment
significantly reduced the protein level of ET-1 as well as raised
eNOS in the aorta of SHR significantly, but had no effect on the
protein expression of ATIR (Fig.
2D). Moreover, the transcriptional level of ET-1 and AT1R was
significantly decreased in the carotid of SHR with enalapril or
different doses of C. officinalis Kuan treatment, which
showed increased transcriptional level of eNOS (Fig. 2E).
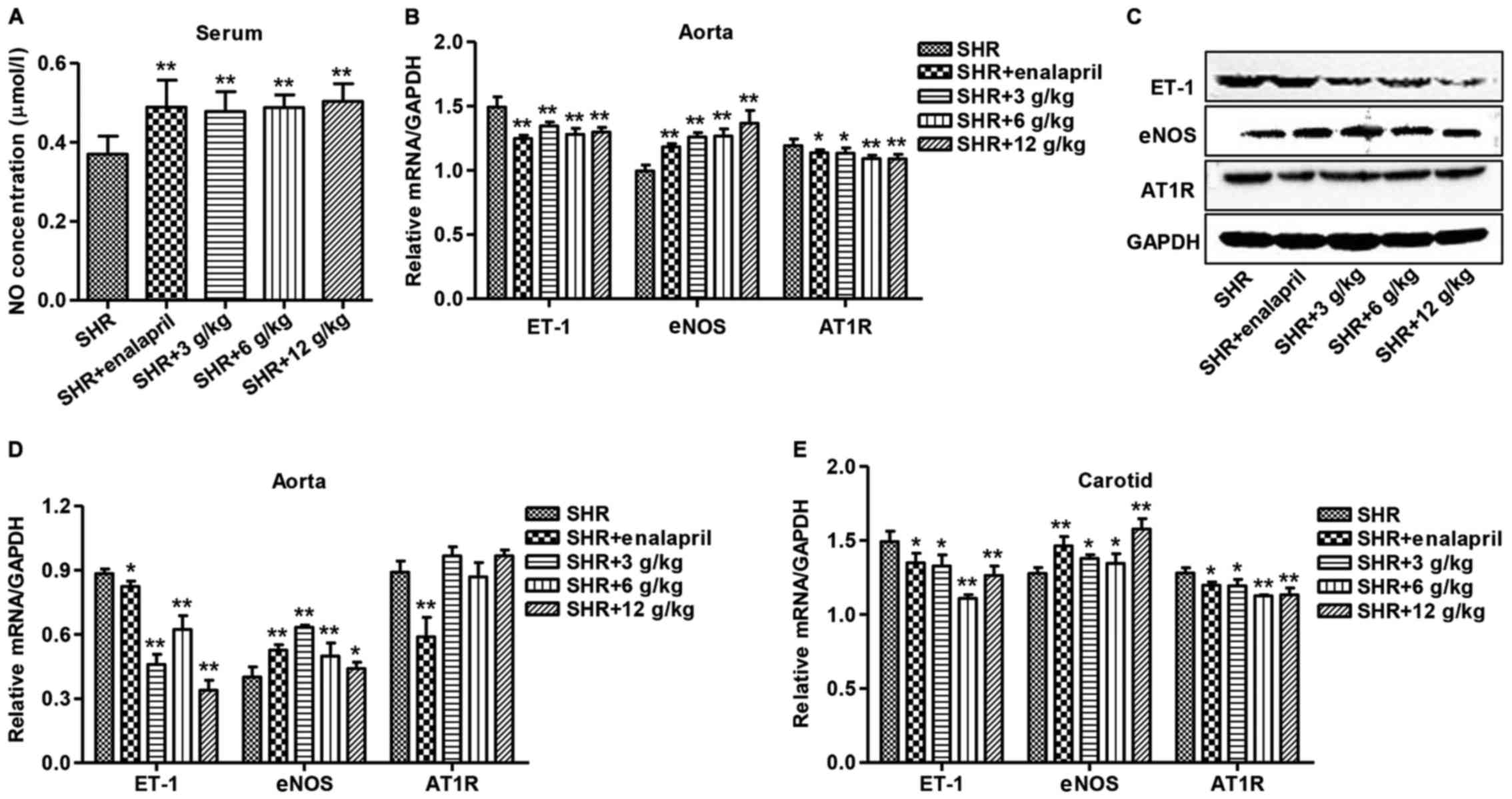 | Figure 2.Effect of C. officinalis Kuan
on serum NO and expression of ET-1, eNOS and AT1R in SHR. After
treatment of SHR with enalapril or different doses of C.
officinalis Kuan (3, 6 or 12 g/kg). (A) The serum NO
concentration was measured by NO assay kit (nitrate reductase
method), (B) the protein and mRNA expression, (C) ET-1, AT1R and
eNOS in aorta employed qRT-PCR, and (D) western blotting for
measurement and protein in ET-1, eNOS and AT1R in carotid was
measured by western blotting. (E) Western blotting for measurement
and protein in ET-1, eNOS and AT1R in carotid was measured by
western blotting. C. officinalis, Cyathula
officinalis; NO, nitric oxide; ET-1, endothelin-1; eNOS,
endothelial nitric oxide synthase; AT1R, angiotensin II receptor
type 1; SHR, spontaneously hypertensive rat. *P<0.05 and
**P<0.01. |
Discussion
Although it was demonstrated in contemporary
pharmacological studies that diverse pharmacological activities
were possessed by C. officinalis Kuan, containing
immunostimulant, antitumor, analgesic, anti-inflammatory,
eliminating blood stasis, anti-aging, inducing diuresis to treat
stranguria, recovering menstrual flow (15,16), it
is still unknown whether C. officinalis Kuan would affect
arterial change. Within this study, we evaluated the impact of
C. officinalis Kuan in the procedure of arterial change
induced from hypertension and demonstrated that C.
officinalis Kuan inhibited the blood pressure and arterial ET-1
and AT1R expression as well as increased serum NO level and
arterial eNOS expression in SHR. This report is the first to show
administration of C. officinalis Kuan improves the arterial
remodeling, by decreasing blood pressure, ET-1 and AT1R expression
and increasing the NO and eNOS expression.
Hypertension, a major public health problem,
affecting up to one billion people worldwide (17) and exhibiting aortic remodeling
including aortic hypertrophy, collagen accumulation and impaired
endothelium dependent vasorelaxation (18), among which the main adaptive
mechanisms are rearranged formations of extracellular matrix and
vascular remodeling to increase blood pressure chronically and
growing mortality and morbidity (19,20),
characterized in part by the proliferation and hypertrophy of
vascular smooth muscle cells (21).
Hypertensive vascular remodeling is contributed by the increased
vascular cell, inflammation, fibrosis and hypertrophy (22). After C. officinalis Kuan
administration for 2 weeks, SHR group had a significant decline in
aorta's medial thickness and blood pressure, which was in line with
the effect of enalapril in SHR. Enalapril is an orally
anti-hypertensive agent with efficacy, affecting risk factors on
cardiovascular and preventing decrease within renal function as
well as other organ injury positively (23). C. officinalis Kuan presented
significant prevention of both vascular function and structure from
remodeling, indicating the relation between beneficial impact of
C. officinalis Kuan and the influence to blood pressure.
In the present study, it was revealed by us that
hypertension's pathogenesis is related to NO activity, whereas
C. officinalis Kuan may have a hypertensive function that
was progressed via elevating the NO level in serum, as well as
preventing endothelial impacts, which is in accord with our study
that anti-hypertension role is to improve NO production (24). NO belongs to a crucial vasodilator,
which is indispensable for maintaining regular blood pressure.
Besides, activity of impaired NO takes responsibility for
hypertension pathophysiology (25).
Hypertension has a pathological feature as the dysfunctional
relaxation dependent on endothelium (26). Endothelial vascular cells in the
sub-type of M are activated by ACh, releasing NO, and finally
inducing vascular vasodilator (27),
resulting in decreased average arterial pressure, sympathetic
activity and heart rate within rats through activated adenosine A2A
receptors as well as reduced M1 receptor and ACh levels (28). Accumulating evidence suggests that
dysfunctional eNOS, enhanced activity of xanthine oxidase,
increased NADPH oxidase activity, and decreased antioxidant defense
during the aging process are linked to dysfunction of the
endothelium and consequent development of hypertension (29). In the present study, it was
discovered that C. officinalis Kuan could significantly
increase the expression of eNOS in SHR, mimicing the effect of
enalapril.
ET-1 affects hypertension. In addition to the impact
on people by raising blood pressure, myocardial hypertrophies and
vascular are induced by ET-1, as independent risk elements for
cardiovascular mortality and morbidity (30). It has been shown that over-activated
ET-1 can exacerbate both aortic and cardiac remodeling that could
be corrected by ET antagonists (31,32). In
the present study, it was discovered that C. officinalis
Kuan could significantly decrease the expression of ET-1 and AT1R
in SHR, and mimic the effect of enalapril. Enalapril has the
ability to decrease plasma levels in Ang II through blocking its
last step of activation and offering anti-hypertensive actions
(23). AT1R stimulation regulated
ACE2 and Ang-(1–7) expression in aorta of SHR (33). Less activated AT1R as well as their
endocellular signaling reduce plasma levels of Ang II (4) and alters the balance of
ACE2/Ang-(1–7)/Mas axis with ACE/Ang II/AT1R axis to
improve vascular remodeling (34).
This study was the first to demonstrate that C.
officinalis Kuan significantly improved arterial remodeling in
SHR through decreasing ET-1 and AT1R expression and increasing eNOS
and NO expression.
Acknowledgements
This study was funded by Independent Innovation
Research Funding for Putuo District Health System (KW1305),
‘Xinglin New Star’ Project of Shanghai (ZY3-RCPY-2-2071) and
Shanghai Grassroots Senior Experts in Traditional Chinese Medicine
Heritage Research Studio Construction Projects (JCZYGZS-020).
References
|
1
|
James PA, Oparil S, Carter BL, Cushman WC,
Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML,
MacKenzie TD, Ogedegbe O, et al: 2014 Evidence-based guideline for
the management of high blood pressure in adults: Report from the
panel members appointed to the Eighth Joint National Committee (JNC
8). JAMA. 311:507–520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Derosa G, Bonaventura A, Romano D, Bianchi
L, Fogari E, D'Angelo A and Maffioli P: Effects of
enalapril/lercanidipine combination on some emerging biomarkers in
cardiovascular risk stratification in hypertensive patients. J Clin
Pharm Ther. 39:277–285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Antza C, Stabouli S and Kotsis V:
Combination therapy with lercanidipine and enalapril in the
management of the hypertensive patient: An update of the evidence.
Vasc Health Risk Manag. 12:443–451. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schiffrin EL: The vascular phenotypes in
hypertension: Relation with the natural history of hypertension. J
Am Soc Hypertens. 1:56–67. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lujan HL, Janbaih H and DiCarlo SE:
Structural remodeling of the heart and its premotor
cardioinhibitory vagal neurons following T(5) spinal cord
transection. J Appl Physiol (1985). 116:1148–1155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guerrero EI, Ardanaz N, Sevilla MA,
Arévalo MA and Montero MJ: Cardiovascular effects of nebivolol in
spontaneously hypertensive rats persist after treatment withdrawal.
J Hypertens. 24:151–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gómez-Roso M, Montero MJ, Carrón R and
Sevilla MA: Cardiovascular changes in spontaneously hypertensive
rats are improved by chronic treatment with zofenopril. Br J
Pharmacol. 158:1911–1921. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schiffrin EL: Vascular remodeling in
hypertension: Mechanisms and treatment. Hypertension. 59:367–374.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Higashi Y, Sasaki S, Nakagawa K, Ueda T,
Yoshimizu A, Kurisu S, Matsuura H, Kajiyama G and Oshima T: A
comparison of angiotensin-converting enzyme inhibitors, calcium
antagonists, beta-blockers and diuretic agents on reactive
hyperemia in patients with essential hypertension: A multicenter
study. J Am Coll Cardiol. 35:284–291. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schmidt BM, Ribnicky DM, Lipsky PE and
Raskin I: Revisiting the ancient concept of botanical therapeutics.
Nat Chem Biol. 3:360–366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou R, Li BG and Zhang GL: Chemical study
on Cyathula officinalis Kuan. J Asian Nat Prod Res.
7:245–252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng H, Du X, Liu J, Han X, Cao X and Zeng
X: Novel polysaccharide from Radix Cyathulae officinalis
Kuan can improve immune response to ovalbumin in mice. Int J Biol
Macromol. 65:121–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park HY, Lim H, Kim HP and Kwon YS:
Downregulation of matrix metalloproteinase-13 by the root extract
of Cyathula officinalis Kuan and its constituents in
IL-1β-treated chondrocytes. Planta Med. 77:1528–1530. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Xu J, Zhao XJ, Gao WY, Zhang SZ and
Guo YQ: A new heterocyclic compound from Cyathula
officinalis Kuan. Chin Chem Lett. 21:70–72. 2010. View Article : Google Scholar
|
|
15
|
Ye P, Peng J and Liu J: The research
development of Cyathula officinalis kuan. Zhongguo Yaowu
Huaxue Zazhi. 35:51–53. 2007.
|
|
16
|
Han X, Shen S, Liu T, Du X, Cao X, Feng H
and Zeng X: Characterization and antioxidant activities of the
polysaccharides from Radix Cyathulae officinalis Kuan. Int J
Biol Macromol. 72:544–552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kearney PM, Whelton M, Reynolds K, Muntner
P, Whelton PK and He J: Global burden of hypertension: Analysis of
worldwide data. Lancet. 365:217–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu TT, Guo K, Chen HC, Lan CZ, Wang J,
Huang LL, Wang XH, Zhang Z and Gao S: Effects of traditional
Chinese medicine Xin-Ji-Er-Kang formula on 2K1C hypertensive rats:
Role of oxidative stress and endothelial dysfunction. BMC
Complement Altern Med. 13:1732013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghiadoni L, Taddei S and Virdis A:
Hypertension and endothelial dysfunction: Therapeutic approach.
Curr Vasc Pharmacol. 10:42–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shantsila A and Shantsila E: Arterial
stiffening in hypertension: Beyond blood pressure levels. J Hum
Hypertens. 24:303–305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feihl F, Liaudet L, Levy BI and Waeber B:
Hypertension and microvascular remodelling. Cardiovasc Res.
78:274–285. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao P, Xu TT, Lu J, Li L, Xu J, Hao DL,
Chen HZ and Liu DP: Overexpression of SIRT1 in vascular smooth
muscle cells attenuates angiotensin II-induced vascular remodeling
and hypertension in mice. J Mol Med (Berl). 92:347–357. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barrios V, Escobar C and Echarri R: Fixed
combinations in the management of hypertension: Perspectives on
lercanidipine-enalapril. Vasc Health Risk Manag. 4:847–853. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang H, Shen Z, Chu Y, Li Y, Li J, Wang
X, Yang W, Zhang X, Ju J, Xu J, et al: Serum metabolomics research
of the anti-hypertensive effects of Tengfu Jiangya tablet on
spontaneously hypertensive rats. J Chromatogr B Analyt Technol
Biomed Life Sci. 1002:210–217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gordish KL, Kassem KM, Ortiz PA and
Beierwaltes WH: Moderate (20%) fructose-enriched diet stimulates
salt-sensitive hypertension with increased salt retention and
decreased renal nitric oxide. Physiol Rep. 5:52017. View Article : Google Scholar
|
|
26
|
Zhai YL, Zhu L, Shi SF, Liu LJ, Lv JC and
Zhang H: Elevated soluble VEGF receptor sFlt-1 correlates with
endothelial injury in IgA nephropathy. PLoS One. 9:e1017792014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wassmann S, Laufs U, Stamenkovic D, Linz
W, Stasch JP, Ahlbory K, Rösen R, Böhm M and Nickenig G: Raloxifene
improves endothelial dysfunction in hypertension by reduced
oxidative stress and enhanced nitric oxide production. Circulation.
105:2083–2091. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang MY, Chen J, Wang J, Xiao F, Zhang
HH, Zhang CR, Du DS, Cao YX, Shen LL and Zhu DN: Nitric oxide
modulates cardiovascular function in the rat by activating
adenosine A2A receptors and inhibiting acetylcholine release in the
rostral ventrolateral medulla. Clin Exp Pharmacol Physiol.
38:380–386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang YM, Huang A, Kaley G and Sun D: eNOS
uncoupling and endothelial dysfunction in aged vessels. Am J
Physiol Heart Circ Physiol. 297:H1829–H1836. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang X, Wang X, Hu F, Zhou B, Chen HB,
Zha D, Liu Y, Guo Y, Zheng L and Xiu J: A novel hydrodynamic
approach of drag-reducing polymers to improve left ventricular
hypertrophy and aortic remodeling in spontaneously hypertensive
rats. Int J Nanomed. 11:6743–6751. 2016. View Article : Google Scholar
|
|
31
|
Lee TM, Lin MS, Chou TF, Tsai CH and Chang
NC: Effect of pravastatin on development of left ventricular
hypertrophy in spontaneously hypertensive rats. Am J Physiol Heart
Circ Physiol. 289:H220–H227. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amiri F, Virdis A, Neves MF, Iglarz M,
Seidah NG, Touyz RM, Reudelhuber TL and Schiffrin EL:
Endothelium-restricted overexpression of human endothelin-1 causes
vascular remodeling and endothelial dysfunction. Circulation.
110:2233–2240. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Igase M, Strawn WB, Gallagher PE, Geary RL
and Ferrario CM: Angiotensin II AT1 receptors regulate ACE2 and
angiotensin-(1–7) expression in the aorta of spontaneously
hypertensive rats. Am J Physiol Heart Circ Physiol.
289:H1013–H1019. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iwai M, Nakaoka H, Senba I, Kanno H,
Moritani T and Horiuchi M: Possible involvement of
angiotensin-converting enzyme 2 and Mas activation in inhibitory
effects of angiotensin II type 1 receptor blockade on vascular
remodeling. Hypertension. 60:137–144. 2012. View Article : Google Scholar : PubMed/NCBI
|