Introduction
In western countries, endometrial cancer is well
known as one of the most common gynecological cancers and in Asian
countries, the morbidity has been increasing (1). The incidence of endometrial cancer has
had annual increases of 1.9% among women ≥50 years and 1.3% among
women <50 years of age. The mortality has been multiplying by
1.1% per year and for 2017, ~10,585 deaths are anticipated
(2). At present, the primary
standard treatment for endometrial cancer is surgery. Chemotherapy,
radiation therapy or their combination is chosen for patients with
a medium or high recurrence risk. Most endometrial cancer patients
were diagnosed in the early stage and treated with surgery alone or
with additional radiation therapy (3). However, a significant proportion of
endometrial cancer patients with metastasis or in the advanced
stage still require systematic chemotherapy (4,5).
Taxanes, platinum and adriamycin alone or in combination are
currently used for recurrent or progressing endometrial cancer
patients. The response rate of adriamycin and cisplatin combination
in advanced endometrial carcinoma patients is up to 42% (6). However, the search for effective agents
against either recurrent or advanced endometrial cancer remains
disappointing (7). Accordingly, the
development of novel compounds against endometrial cancer has
attracted great attention and continuous research efforts are being
made towards this goal. However, only a small number of studies on
novel compounds with activity against endometrial cancer are
currently available and the mechanisms remain largely elusive.
Novel antitumor agents derived and identified from
natural sources have attracted exponential interest. The compounds
represent a key alternative for the improvement of existing
standard cancer therapies (8,9).
Paeoniflorin (PAE) is a principal bioactive component of the root
of Paeonia lactiflora Pall., a medicinal herb species found
in Korea, Japan and China (10). In
Traditional Chinese Medicine, PAE has been widely used (11). It has been reported that PAE exerts
numerous pharmacological effects, such as anti-inflammatory
(12,13), anti-allergy (14,15),
immunoregulatory (16,17), neuroprotective (18) and hepatoprotective effects (19). Recent studies have suggested that PAE
may be a novel potential antitumor agent. PAE inhibits the
proliferation of a variety of human cancer cell types, including
gastric carcinoma (10,11), lung cancer (19) and hepatocellular carcinoma cells
(20). PAE was reported to induce
cell cycle arrest via activating p53/14-3-3 zeta in HT29 colorectal
cancer cells (21) and to promote
apoptosis by downregulating matrix metalloproteinase-9 and
upregulating microRNA-16 in human glioma cells (22). However, the anti-cancer effects of
PAE on endometrial cancer have remained indeterminate.
In the present study, RL95-2 human endometrial
cancer cells were selected to investigate the potential effect of
PAE on the inhibition of cell proliferation. In addition, changes
of protein expression in the mitogen-activated protein kinases
(MAPKs) and nuclear factor-κB (NF-κB) signaling pathways as
underlying mechanisms of PAE-mediated antitumor activity were
studied. According to these attributes, PAE may be a promising
novel candidate compound for the treatment of endometrial
cancer.
Materials and methods
Reagents
Paeoniflorin (purity, ≥98%) and 5-fluorouracil
(5-FU) were obtained from Aladdin Biochem Technology Co., Ltd.
(Shanghai, China). Cell Signaling Technology, Inc. (Beverly, MA,
USA) supplied the inhibitors SB203580, PD98059 and MG-132. Wuxi
Jinpu Bio-Technology Co., Ltd. (Jiangsu, China) provided the
inhibitor BI-78D3. The Cell Counting Kit-8 (CCK-8) was obtained
from Dojindo Laboratories (Kumamoto, Japan).
Cell culture
The Cell Bank of the Chinese Academy of Sciences
(Shanghai, China) supplied the RL95-2 human endometrial carcinoma
cell line, and the HECCL-1 human endometrial carcinoma cell line
was purchased from BeNa Culture Collection (Suzhou, China). Cells
were cultured in Dulbecco's modified Eagle's medium/F12 (HyClone;
GE Healthcare, Little Chalfont, UK) with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100
µg/ml streptomycin and 100 U/ml penicillin (Beyotime Institute of
Biotechnology, Inc., Haimen, China) at 37°C in a humidified
atmosphere containing 5% CO2. A total of 2 h prior to
the penicillin process, 50 µM SB203580, PD98059, BI-78D3 and MG-132
(Sigma-Alrich; Merck KGaA; Darmstadt, Germany) were pre-incubated
with RL95-2 for 1 h at room temperature.
Cell proliferation assay
RL95-2 cells were seeded into 96-well plates at
1×104 cells/well and treated for the assigned times with
either vehicle (0.1% dimethyl sulfoxide) or PAE at the indicated
concentrations. The CCK-8 assay was performed to assess cell
proliferation according to the manufacturer's instructions. The
numerical absorption values obtained with an ELISA plate reader
(Thermo Fisher Scientific, Inc.) at a wavelength of 450 nm were
used to evaluate the cell viability.
Western blot analysis
RL95-2 cells were treated and the lysate was
prepared as described previously (23). The protein (20 µg) was separated by
10% SDS-PAGE and transferred onto polyvinylidene difluoride
membranes (EMD Millipore; Billerica, MA, USA). Immunoblots were
exposed to primary antibodies at room temperature for 1 h.
Antibodies used were against p38 (9212; 1:2,000 dilution),
phosphorylated (p)-p38 (4511l 1:1,000 dilution), NF-κB p65 (8242l
1:1,500 dilution), p-p65 (3033l 1:2,000 dilution), extracellular
signal-regulated kinase (ERK; 4695; 1:1,000 dilution), p-ERK (4370l
1:1,500 dilution) (all from Cell Signaling Technology, Inc.), c-Jun
N-terminal kinase (JNK; ab179461; 1:1,000 dilution) or p-JNK
(ab76572; 1:1,500 dilution) (all from Abcam, Cambridge, MA, USA).
Subsequently, blots were incubated at room temperature for 40 min
with horseradish peroxiase-conjugated secondary antibodies (BA1054;
1:20,000 dilution; Boster Biological Technology, Wuhan, China). The
bands with immunoreactive proteins on the membrane were assessed by
the ECL Plus Western Blotting Detection system (EMD Millipore).
GAPDH was selected as the reference protein (kC-5G5; 1:10,000
dilution; Kangchen Biotech Co., Ltd., Shanghai, China).
Statistical analysis
Data analysis was performed using SPSS software
(version 22.0; IBM Corp., Armonk, NY, USA). All values are
expressed as the mean ± standard deviation. Student's t-test was
applied to assess differences between two samples. Multiple
comparison tests were performed via one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
PAE inhibits human endometrial cancer
cell proliferation
The effect of PAE on human endometrial cancer cell
proliferation was initially assessed in RL95-2 cells. As depicted
in Fig. 1A, PAE treatment at 100,
200, 400 and 800 µg/ml for 24 h resulted in a marked inhibition of
proliferation in a dose-dependent manner. 400 µg/ml PAE exerted
significant anti-proliferative activity (P<0.001) and was chosen
to assess the role of time in PAE-induced proliferative inhibition.
As displayed in Fig. 1B, treatment
with 400 µg/ml PAE for 24, 48 and 72 h inhibited cell proliferation
in a time-dependent manner. Similar results were obtained in anther
endometrial cancer cell line, HECCL-1 (Fig. 1C).
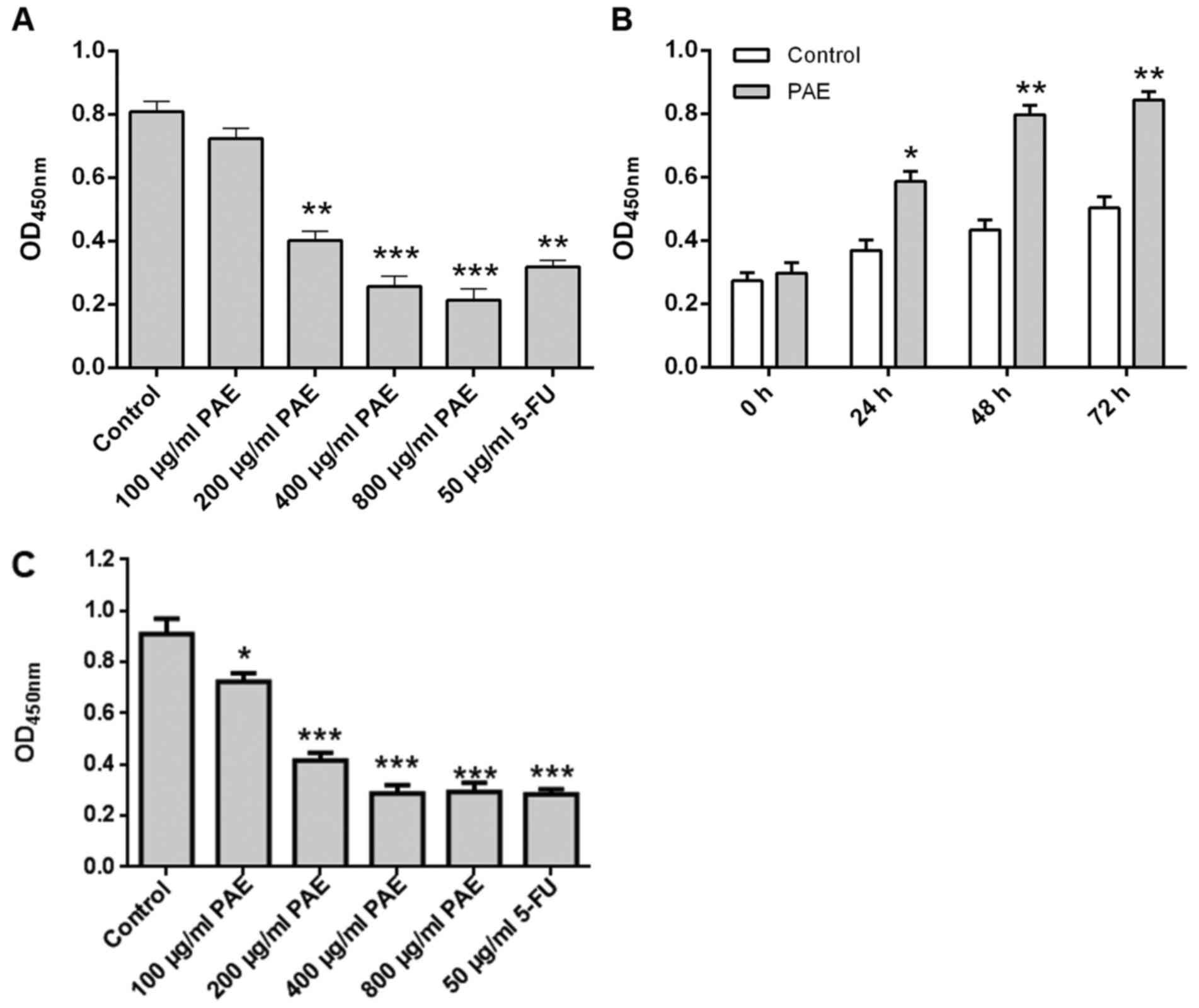 | Figure 1.Effect of PAE on the proliferation of
RL95-2 cells. (A) RL95-2 cells were incubated overnight, exposed to
PAE (100, 200, 400 and 800 µg/ml) or 50 µg/ml 5-FU and then
cultured for 24 h. (B) Cells were incubated overnight, exposed to
400 µg/ml PAE and then cultured for 24, 48 and 72 h. (C) HECCL-1
cells were incubated overnight, exposed to PAE (100, 200, 400 and
800 µg/ml) or 50 µg/ml 5-FU and then cultured for 24 h. Inhibition
of proliferation was measured via the Cell Counting Kit-8 assay.
Values are expressed as the mean ± standard deviation (n=3).
***P<0.001, **P<0.01 and *P<0.05 vs. control. PAE,
paeoniflorin; 5-FU, 5-fluorouracil; OD450, optical density at 450
nm. |
Roles of MAPK signaling pathways in
PAE-induced proliferative inhibition
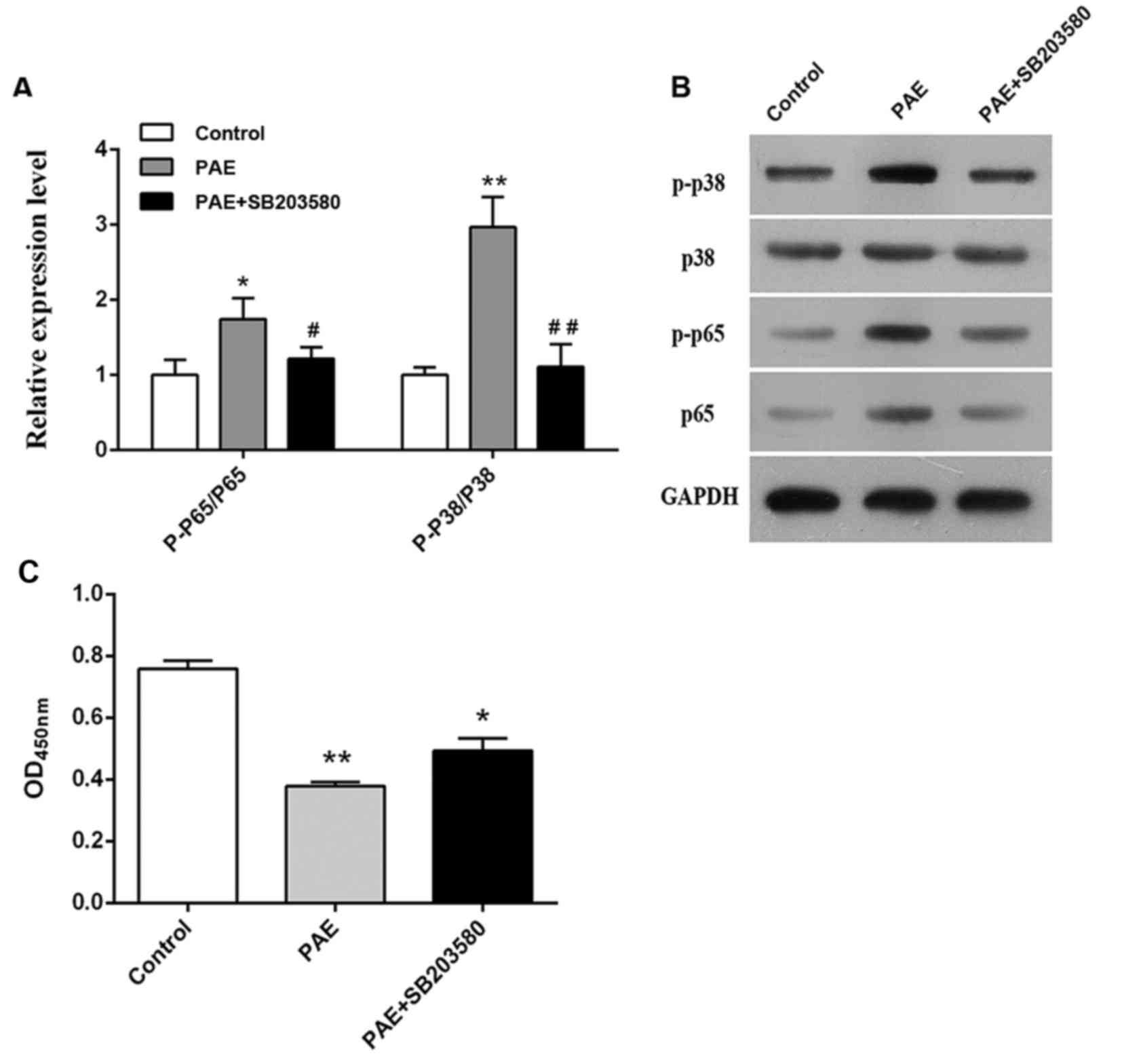
Role of p38 MAPK in PAE-induced proliferative
inhibition
Following vehicle or PAE treatment of RL95-2 cells
for 24 h, the levels of p38, p-p38, p65 and p-p65 were detected by
western blot analysis. PAE significantly upregulated p-p65 and
p-p38 ratio (P<0.05, P<0.01; Fig.
2A and B), suggesting that PAE activated the p38 MAPK signaling
pathway in RL95-2 cells. To clarify the role p38 MAPK activation in
PAE-mediated proliferative inhibition, the p38 MAPK inhibitor
SB203580 was added. Addition of SB203580 abolished the increase in
p-p65 and p-p38 and prevented the proliferative inhibition induced
by PAE (P<0.01; Fig. 2C). These
findings suggested that the p38 MAPK signaling pathway may have an
important role in the anti-proliferative effect of PAE on RL95-2
cells.
Role of ERK in PAE-induced proliferative
inhibition
Next, the effect of ERK in the proliferative
inhibition induced by PAE was evaluated. As depicted in Fig. 3A and B, PAE treatment caused a
significant increase of p-ERK and p-p65 levels, which was
significantly inhibited by ERK inhibitor PD98059. However, in the
proliferation assay, pre-treatment with PD98059 did not affect the
PAE-induced proliferative inhibition (P>0.05; Fig. 3C).
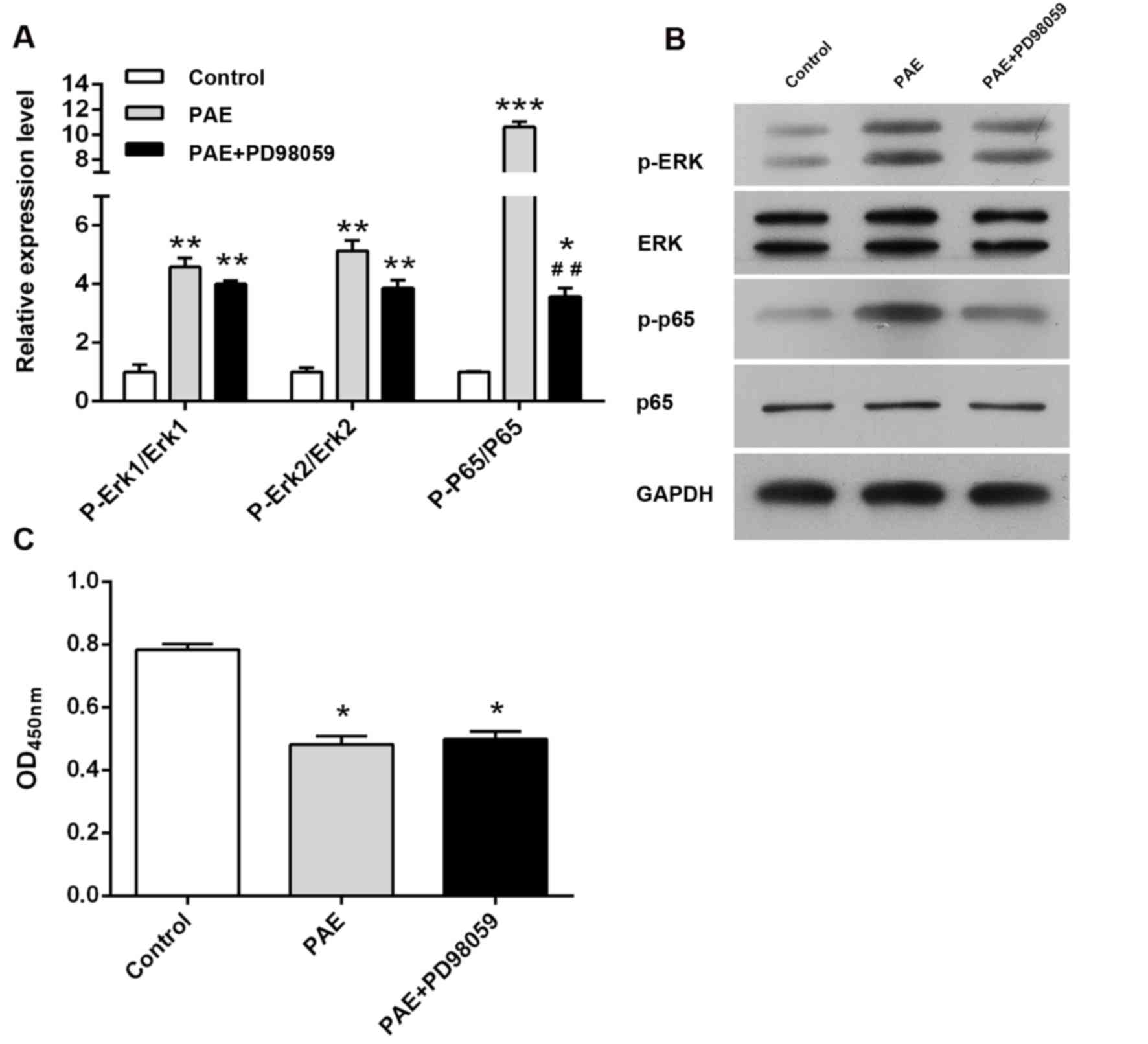 | Figure 3.Role of ERK in PAE-induced
proliferative inhibition. (A and B) RL95-2 cells were treated with
vehicle, 400 µg/ml PAE or 400 µg/ml PAE + PD98059 for 24 h, and
ERK, p-ERK, p65 and p-p65 levels were detected by western blot
analysis. (C) Cells were exposed to vehicle, 400 µg/ml PAE or 400
µg/ml PAE + PD98059 for 24 h and cell viability was measured via
the Cell Counting Kit-8 assay. Values are expressed as the mean ±
standard deviation (n=3). ***P<0.001, **P<0.01 and *P<0.05
vs. control; ##P<0.01 vs. PAE group. PAE,
paeoniflorin; p-p65, phosphorylated p65; OD450, optical density at
450 nm; ERK, extracellular signal-regulated kinase. |
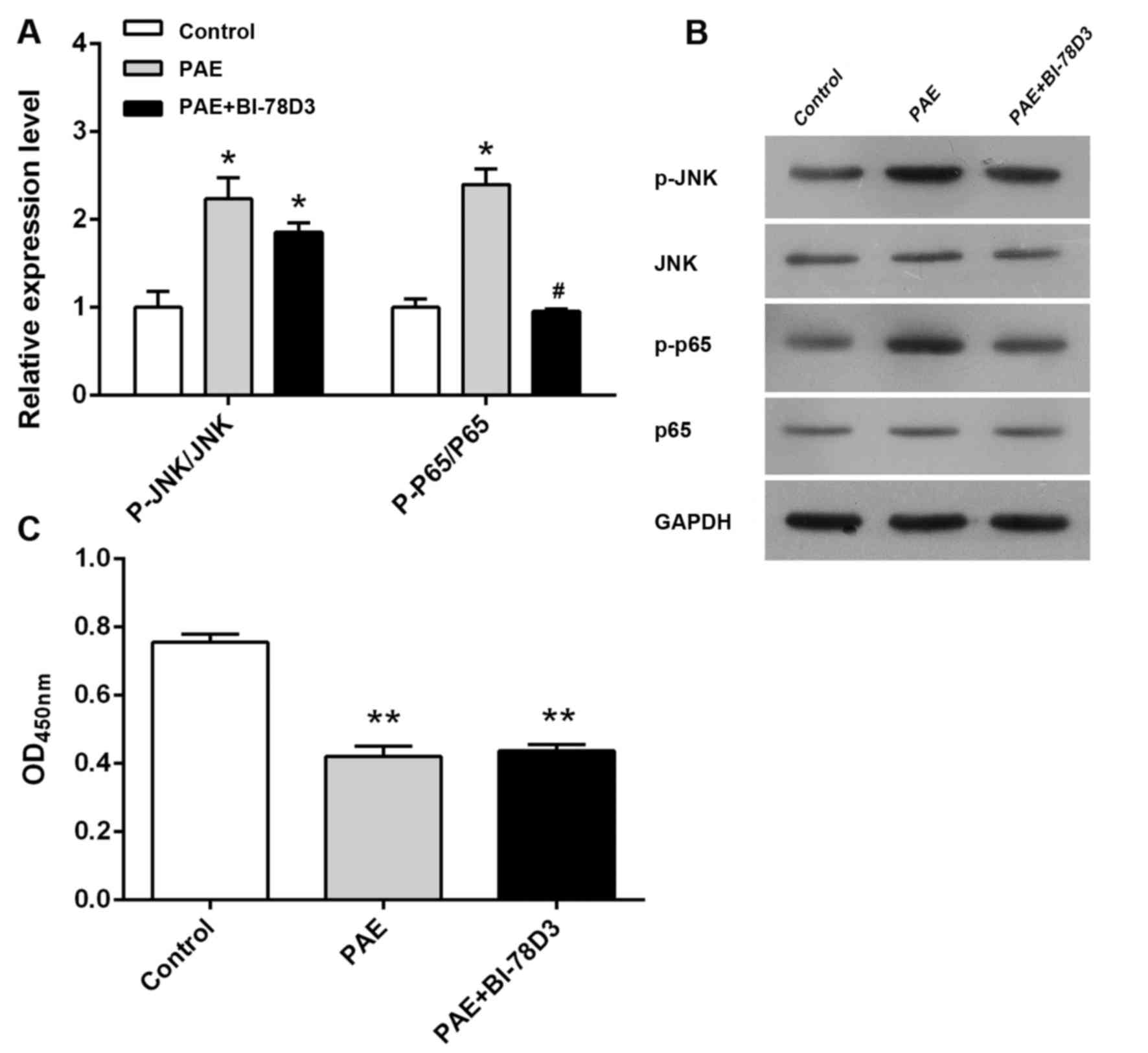
Role of JNK in PAE-induced proliferative
inhibition
As illustrated in Fig.
4, PAE treatment caused a marked increase of p-JNK levels
compared with those in the control group. JNK inhibitor BI-78D3
significantly abolished the PAE-induced increase in p-JNK
expression. However, BI-78D3 pre-treatment did not significantly
affect the anti-proliferative effect of PAE, suggesting that JNK
inhibition did not interfere with the ability of PAE to inhibit
cell proliferation (P>0.05, Fig.
4).
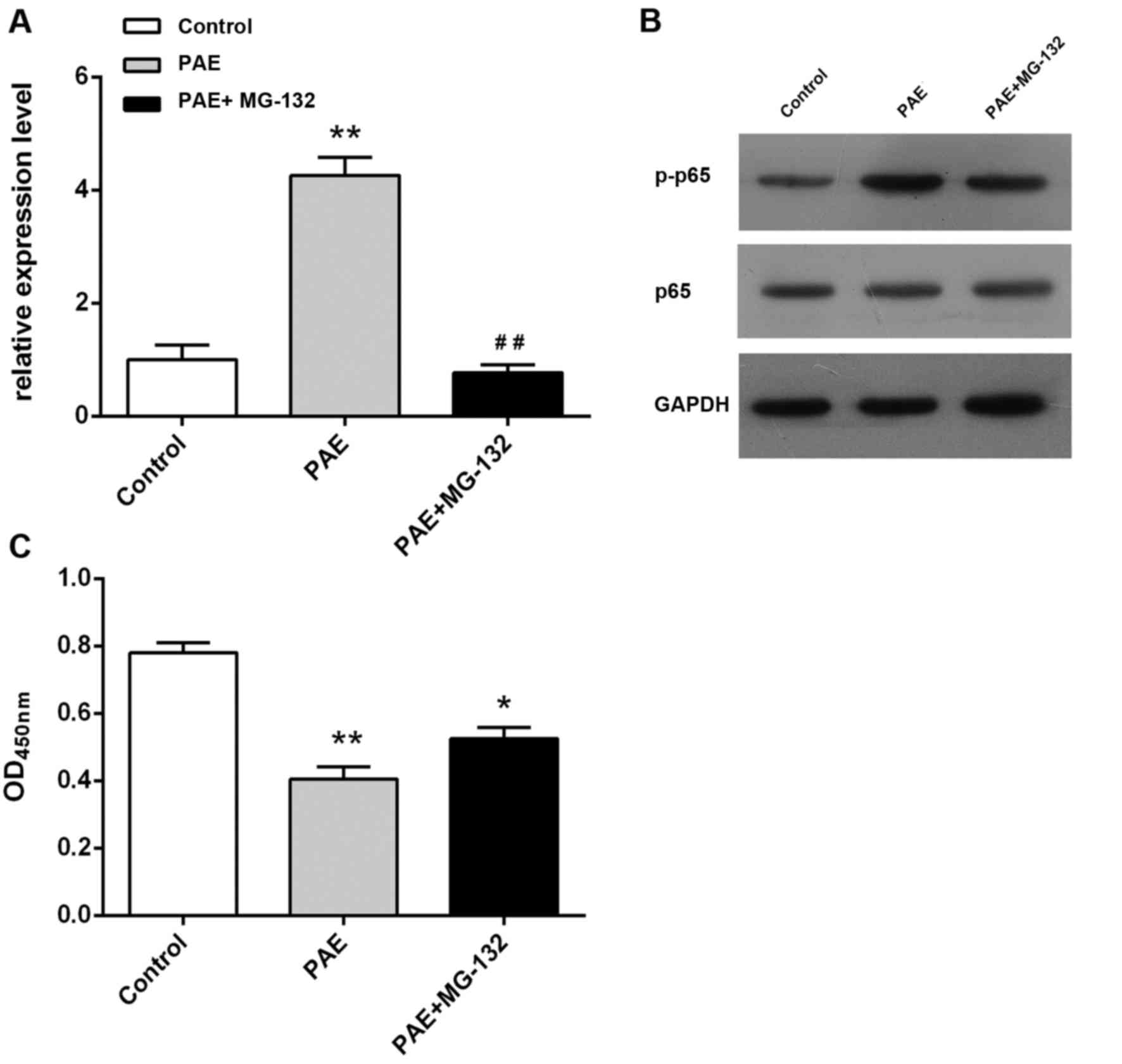
Role of NF-κB signaling pathway in PAE-induced
proliferative inhibition
The present study further examined the effect of PAE
on NF-κB p65 and p-p65 levels in order to explore the role of NF-κB
in the PAE-mediated proliferative inhibition. PAE treatment of
RL95-2 cells resulted in a marked upregulation of p-p65 levels
(P<0.05, P<0.01; Fig. 5A and
B), suggesting that PAE activated the NF-κB signaling pathway.
It was then assessed whether NF-κB activation had a role in
PAE-induced proliferative inhibition. Pre-treatment with the NF-κB
inhibitor MG-132 attenuated the increase in p-p65 expression. In
addition, MG-132 pre-treatment yielded a significant suppression of
PAE-induced proliferative inhibition (P<0.05; Fig. 5C). These observations indicated that
the anti-proliferative activity of PAE against RL95-2 cells is
mediated via the activation of the NF-κB signaling pathway.
Discussion
The present study aimed to investigate the
anti-cancer activity of PAE in human endometrial cancer cells and
the potential underlying mechanisms. The results revealed that PAE
induced a significant dose- and time-dependent inhibition of
endometrial cancer cell growth. As the MAPK and NF-κB signaling
pathways are crucial in cancer cell proliferation, the roles of
associated signaling proteins in PAE-induced antitumor activity
were then explored.
The MAPK family is involved in the regulation of
gene expression in response to the extracellular stimulation
signals and activation of various cell responses, such as
apoptosis, differentiation and cell proliferation (13). MAPKs include three key groups: p38
MAPK, JNK and ERK (24). In the
present study, PAE increased the levels of phosphorylated p38 MAPK,
ERK and JNK, suggesting that the p38 MAPK, ERK and JNK signaling
pathways were activated during the treatment of RL95-2 cells with
PAE. Furthermore, the function of MAPK signaling pathways in the
anti-proliferative effects of PAE was assessed by applying p38
MAPK, ERK and JNK kinase inhibitors. Pre-treatment with p38 MAPK
inhibitor effectively prevented PAE-induced proliferative
inhibition, whereas ERK and JNK kinase inhibitors did not. This
result suggested that PAE inhibits the proliferation of RL95-2
cells through the p38 MAPK signaling pathway, but not through the
ERK and JNK pathways.
p38 MAPK has been reported to have a critical role
in various cancer types, including prostate (25), breast (26) and liver cancer (27). MAPK inhibitors may be promising drugs
against solid tumors of lung, liver, bladder, breast and prostate
(28). It has been suggested that
PAE may act as an antitumor agent against other solid tumor types
via the p38 MAPK signaling pathway. In fact, PAE exerts antitumor
activities against various solid tumor types, including gastric
carcinoma (10,11), lung cancer (19), liver cancer (20), while the involvement of the p38 MAPK
pathway in its antitumor activities requires further
investigation.
NF-κB, a heterodimeric complex composed of proteins
p65 and p50, is an important transcriptional regulatory factor. It
inhibits cell metastasis, invasion, cell cycle, apoptosis and cell
proliferation by regulating a variety of target genes (29). In the present study, PAE was found to
increase the levels of p-p65, suggesting that PAE activates the
NF-κB signaling pathway. Furthermore, pre-treatment with NF-κB
inhibitor partially inhibited PAE-induced proliferative inhibition
in RL95-2 cells. As NF-κB is one of the substrates of the MAPKs
signaling pathways and is therefore correlated with them, the
present study also examined the effect of MAPK inhibitors on the
levels of p-p65. The results demonstrated that all MAPK inhibitors
decreased the levels of p-p65, but only p38 MAPK inhibitor
prevented PAE-induced proliferative inhibition. These findings
suggested that PAE inhibits RL95-2 cell proliferation partially via
the NF-κB signaling pathway.
NF-κB has a key role in inflammatory and immune
regulation and is found in a diversity of cells (30). Zhang et al (12) reported that PAE revokes colitis
induced by dextran sulfate sodium through the Toll-like receptor
4-dependent pathway. In rats with adjuvant arthritis, Wu et
al (16) found that PAE caused
immune tolerance by enhancing the desensitization of β2-adrenergic
receptor. These studies demonstrated that PAE displays
anti-inflammatory or immuoregulatory activities, whereas the
involvement of NF-κB in these processes requires further study.
Of note, emerging evidence has indicated a positive
correlation between NF-κB activation and cancer progression in a
variety of tumor types (31,32). In human gastric cancer, Wu et
al (8) found that PAE restrains
NF-κB activation and promotes the apoptosis induced by 5-FU.
Furthermore, Fang et al (10)
reported that PAE modulates multidrug resistance of
SGC7901/vincristine by effectively inhibiting the activation of
NF-κB. Furthermore, PAE inhibited NF-κB activation by significantly
decreasing p65 expression and inhibiting intra-nuclear p65
transcription activation (10).
These studies appear to contradict the findings of the present
study. However, the dual effects of PAE on NF-κB occurred under
different circumstances, indicating that PAE may be a potential
drug candidate for treating cancer, inflammation and immunological
diseases via bidirectional modulation of the NF-κB signaling
pathway.
The findings of the present study therefore
confirmed that PAE inhibits endometrial cancer cell proliferation
via activating the MAPK and NF-κB signaling pathways, which may
also be associated with the anti-inflammatory and immunoregulatory
activities of PAE. For the treatment of endometrial cancer,
targeting these signaling pathways mat provide an efficient
alternative approach. The present study is expected to provide a
foundation for future study on endometrial cancer therapy and more
extensive applications of PAE.
In conclusion, the present study demonstrated that
PAE, a principal bioactive component of Paeonia lactiflora
Pall., significantly inhibited the proliferation of endometrial
cancer cells, thereby providing a potential novel approach for
clinical endometrial cancer treatment. Furthermore, the antitumor
effect of PAE was found to be mediated via the activation of p38
MAPK and NF-κB signaling pathways, which is likely linked to the
anti-inflammatory and immunoregulatory activities of PAE.
Acknowledgements
The present study was supported by the
Administration of Traditional Chinese Medicine of Shandong
Province, China (grant nos. 2005040 and 2013052).
References
|
1
|
Fatima I, Chandra V, Saxena R, Sanghani Y,
Hajela K, Negi MP, Sankhwar PL, Jain SK and Dwivedi A:
2,3-diaryl-2H-1-benzopyran derivatives interfere with classical and
non-classical estrogen receptor signaling pathways, inhibit akt
activation and induce apoptosis in human endometrial cancer cells.
Mol Cell Endocrinol. 348:198–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alteri R, Bertaut T, Brooks D, et al:
Cancer Facts and Figures 2016. Atlanta, GA: American Cancer
Society; 2016
|
|
3
|
Horan TC, Zompa MA, Seto CT, Kim KK, Moore
RG and Lange TS: Description of the cytotoxic effect of a novel
drug abietyl-isothiocyanate on endometrial cancer cell lines.
Invest New Drugs. 30:1460–1470. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fleming GF: Systemic chemotherapy for
uterine carcinoma: Metastatic and adjuvant. J Clin Oncol.
25:2983–2990. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singapore Cancer Network (SCAN)
Gynaecological Cancers Systemic Therapy Workgroup: Singapore cancer
network (SCAN) guidelines for the systemic therapy of endometrial
(uterine) cancer. Ann Acad Med Singapore. 44:434–439.
2015.PubMed/NCBI
|
|
6
|
Thigpen JT, Brady MF, Homesley HD,
Malfetano J, DuBeshter B, Burger RA and Liao S: Phase III trial of
doxorubicin with or without cisplatin in advanced endometrial
carcinoma: A gynecologic oncology group study. J Clin Oncol.
22:3902–3908. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H and Narahara H: 15-Deoxy-Δ(12,
14)-prostaglandin j (2) induces growth inhibition, cell cycle
arrest and apoptosis in human endometrial cancer cell lines. Int J
Mol Med. 31:778–788. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu H, Li W, Wang T, Shu Y and Liu P:
Paeoniflorin suppress NF-κB activation through modulation of I
kappaB alpha and enhances 5-fluorouracil-induced apoptosis in human
gastric carcinoma cells. Biomed Pharmacother. 62:659–666. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Duan X, Yang G, Zhang X, Deng L,
Zheng H, Deng C, Wen J, Wang N, Peng C, et al: Honokiol crosses BBB
and BCSFB, and inhibits brain tumor growth in rat 9L intracerebral
gliosarcoma model and human U251 xenograft glioma model. PLoS One.
6:e184902011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang S, Zhu W, Zhang Y, Shu Y and Liu P:
Paeoniflorin modulates multidrug resistance of a human gastric
cancer cell line via the inhibition of NF-κB activation. Mol Med
Rep. 5:351–356. 2012.PubMed/NCBI
|
|
11
|
Nie XH, Ou-yang J, Xing Y, Li DY, Dong XY,
Liu RE and Xu RX: Paeoniflorin inhibits human glioma cells via
STAT3 degradation by the ubiquitin-proteasome pathway. Drug Des
Devel Ther. 9:5611–5622. 2015.PubMed/NCBI
|
|
12
|
Zhang J, Dou W, Zhang E, Sun A, Ding L,
Wei X, Chou G, Mani S and Wang Z: Paeoniflorin abrogates
DSS-induced colitis via a TLR4-dependent pathway. Am J Physiol
Gastrointest Liver Physiol. 306:G27–G36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gan Y, Cui X, Ma T, Liu Y, Li A and Huang
M: Paeoniflorin upregulates β-defensin-2 expression in human
bronchial epithelial cell through the p38 MAPK, ERK, and NF-κB
signaling pathways. Inflammation. 37:1468–1475. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee B, Shin YW, Bae EA, Han SJ, Kim JS,
Kang SS and Kim DH: Antiallergic effect of the root of Paeonia
lactiflora and its constituents paeoniflorin and paeonol. Arch
Pharm Res. 31:445–450. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen T, Guo ZP, Wang L, Qin S, Cao N, Li
MM, Jia RZ and Wang TT: Paeoniflorin suppresses vascular damage and
the expression of E-selectin and ICAM-1 in a mouse model of
cutaneous arthus reaction. Exp Dermatol. 22:453–457. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu H, Wei W, Song L, Zhang L, Chen Y and
Hu X: Paeoniflorin induced immune tolerance of mesenteric lymph
node lymphocytes via enhancing beta 2-adrenergic receptor
desensitization in rats with adjuvant arthritis. Int
Immunopharmacol. 7:662–673. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He DY and Dai SM: Anti-inflammatory and
immunomodulatory effects of Paeonia lactiflora pall., a
traditional chinese herbal medicine. Front Pharmacol. 2:102011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong H, Li R, Yu C, Xu T, Zhang X and Dong
M: Paeoniflorin inhibition of 6-hydroxydopamine-induced apoptosis
in PC12 cells via suppressing reactive oxygen species-mediated
pkcdelta/NF-κB pathway. Neuroscience. 285:70–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hung JY, Yang CJ, Tsai YM, Huang HW and
Huang MS: Antiproliferative activity of paeoniflorin is through
cell cycle arrest and the FAS/FAS ligand-mediated apoptotic pathway
in human non-small cell lung cancer A549 cells. Clin Exp Pharmacol
Physiol. 35:141–147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu JT, He W, Song SS and Wei W:
Paeoniflorin inhibited the tumor invasion and metastasis in human
hepatocellular carcinoma cells. Bratisl Lek Listy. 115:427–433.
2014.PubMed/NCBI
|
|
21
|
Wang H, Zhou H, Wang CX, Li YS, Xie HY,
Luo JD and Zhou Y: Paeoniflorin inhibits growth of human colorectal
carcinoma HT 29 cells in vitro and in vivo. Food Chem Toxicol.
50:1560–1567. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li W, Qi Z, Wei Z, Liu S, Wang P, Chen Y
and Zhao Y: Paeoniflorin inhibits proliferation and induces
apoptosis of human glioma cells via microRNA-16 upregulation and
matrix metalloproteinase-9 downregulation. Mol Med Rep.
12:2735–2740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim JY, Lee SG, Chung JY, Kim YJ, Park JE,
Oh S, Lee SY, Choi HJ, Yoo YH and Kim JM: 7,
12-Dimethylbenzanthracene induces apoptosis in RL95-2 human
endometrial cancer cells: Ligand-selective activation of cytochrome
P450 1B1. Toxicol Appl Pharmacol. 260:124–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li W, Fan M, Chen Y, Zhao Q, Song C, Yan
Y, Jin Y, Huang Z, Lin C and Wu J: Melatonin induces cell apoptosis
in AGS cells through the activation of JNK and P38 MAPK and the
suppression of nuclear factor-Kappa B: A novel therapeutic
implication for gastric cancer. Cell Physiol Biochem. 37:2323–2338.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khandrika L, Lieberman R, Koul S, Kumar B,
Maroni P, Chandhoke R, Meacham RB and Koul HK: Hypoxia-associated
p38 mitogen-activated protein kinase-mediated androgen receptor
activation and increased HIF-1alpha levels contribute to emergence
of an aggressive phenotype in prostate cancer. Oncogene.
28:1248–1260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsai PW, Shiah SG, Lin MT, Wu CW and Kuo
ML: Up-regulation of vascular endothelial growth factor C in breast
cancer cells by heregulin-beta 1. A critical role of p38/nuclear
factor-kappa B signaling pathway. J Biol Chem. 278:5750–5759. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iyoda K, Sasaki Y, Horimoto M, Toyama T,
Yakushijin T, Sakakibara M, Takehara T, Fujimoto J, Hori M, Wands
JR and Hayashi N: Involvement of the p38 mitogen-activated protein
kinase cascade in hepatocellular carcinoma. Cancer. 97:3017–3026.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koul HK, Pal M and Koul S: Role of p38 MAP
kinase signal transduction in solid tumors. Genes Cancer.
4:342–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Greten FR and Karin M: The IKK/NF-kappaB
activation pathway-a target for prevention and treatment of cancer.
Cancer Lett. 206:193–199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang X, Wu M, Jiang H, Hao J, Zhang Q,
Zhu Q, Saren G, Zhang Y, Meng X and Yue X: Angiotensin II
upregulates endothelial lipase expression via the NF-kappa B and
MAPK signaling pathways. PLoS One. 9:e1076342014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Van Waes C: Nuclear factor-kappa B in
development, prevention and therapy of cancer. Clin Cancer Res.
13:1076–1082. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li J, Jia H, Xie L, Wang X, Wang X, He H,
Lin Y and Hu L: Association of constitutive nuclear factor-KappaB
activation with aggressive aspects and poor prognosis in cervical
cancer. Int J Gynecol Cancer. 19:1421–1426. 2009. View Article : Google Scholar : PubMed/NCBI
|