Introduction
Chronic periodontitis (CP) is an infectious disease
that affects the periodontium and gradually destroys periodontal
tissues (1). Bacterial plaque is a
well-known cause of CP, which stimulates a local inflammatory
response and activation of the innate immune system (1,2). This
eventually results in the characteristic pathology of periodontal
disease, the main clinical features of which are advancing gingival
inflammation, irreversible alveolar bone loss, and the loosening
and/or loss of teeth (3). Numerous
studies (4,5) have highlighted the role of T lymphocyte
cells in periodontitis; in particular, T lymphocyte phenotype and
function are important in the susceptibility, onset and severity of
periodontitis (6).
Regulatory T (Treg) cells are a critical
sub-population of CD4+ T cells that are essential for
maintaining self-tolerance and preventing autoimmunity, for
limiting chronic inflammatory diseases, and for regulating
homeostatic lymphocyte expansion (7–11). A
recent study by Wang et al (12) demonstrated that the imbalance between
Treg cells and T helper 17 (Th17) cells plays an essential role in
the progression of periodontitis.
Interleukin (IL)-35, as a Forkhead box P3
(Foxp3)+ Treg cell immunosuppressive/anti-inflammatory
cytokine, is required for the maximum regulatory activity of Treg
cells (13). IL-35 is a heterodimer
formed by an IL-12p35 subunit and an IL-27β chain, the latter of
which is encoded by the Epstein-Barr virus-induced 3 (EBi3) gene
(14). The known functions of IL-35
include: Maintenance of the peripheral immune system; regulation of
the proliferation of T effector cells; inhibition of Th17 cell
differentiation and IL-17 synthesis (15). Thus, it has a close association with
immunological and infectious diseases (16,17).
Although studies on IL-35 are relatively few, and the signal
transduction mechanisms involved in its actions are not yet
elucidated, IL-35 therapy shows promising potential for the
treatment of immunological and infectious diseases (15,18).
In the present study, the expression of Foxp3,
IL-12p35 and EBi3 mRNA in peripheral blood mononuclear cells
(PBMCs) and periodontal tissue, and the concentration of IL-35
protein in serum and gingival crevicular fluid (GCF), were compared
between patients with CP and healthy individuals. Elucidating the
potential signaling mechanisms of IL-35 in CP may provide a basis
for improvements in the future clinical treatments of
periodontitis.
Materials and methods
Study population
The study included 20 patients with CP (the CP
group) and 20 healthy individuals (the control group) at Shengjing
Hospital of China Medical University (Shenyang, China).
Participants were recruited from February to December 2013, and
their ages ranged from 18 to 55 years. Subjects were included
according to the following three criteria: i) A diagnosis of
moderate to severe chronic periodontitis [moderate: 4 mm<pocket
depth (PD) ≤6 mm and clinical attachment loss (CAL) 3–5 mm, or
radiographic bone loss between one-third and one-half root length;
severe: PD>6 mm and CAL>5 mm, or radiographic bone loss ≥
one-half root length (19)]; ii)
retention of ≥20 teeth; iii) being generally healthy and without
systemic disease. Exclusion criteria included: i) Recent intake of
any pharmaceutical that had the potential to influence the outcome
of the study or inflammatory clinical indices, e.g. antibiotics;
ii) use of systemic antibiotics or local antimicrobial agents in
the previous 3 months prior to the start of the trial; iii)
pregnancy or lactation in female subjects; iv) received periodontal
supportive treatment within 6 months prior to the start of the
trial. The study protocol was approved by the Ethics Committee of
Shengjing Hospital of China Medical University. All trial
participants provided informed consent.
Clinical examination
To diagnose and document periodontal disease, trial
participants were assessed for probing depth (PD) and clinical
attachment level (CAL) by a single examiner using a Florida Probe
system (Florida Probe Corporation, Gainesville, FL, USA). Testing
was conducted for six sites per tooth for all teeth.
GCF and periodontal tissue
collection
A tooth site without untreated caries, overhang
fillings, food impaction or any inflammation with the exception of
CP in each quadrant was selected. In the majority of cases the
mesiobuccal site of the first molar was selected. If the first
molar was not available, a second molar or a premolar in the same
quadrant was selected. Plaque and chunks of calculus were removed
and the tooth was then dried with dry, sterile cotton and an air
gun. After waiting for 1 min, a Whatman paper strip was inserted,
until mild resistance was encountered, for GCF collection from a
periodontal pocket. Each paper strip was left in its position for
30 sec. Paper strips contaminated with blood were discarded. Four
paper strips were collected from each participant (a total of 80
sites from the CP or control groups) and inserted into an Eppendorf
tube, 200 µl PBS added and the tubes were then stored at −80°C.
Patients received local anesthesia and periodontal
tissue biopsies were obtained by surgical excision from the
labial/buccal surface of the gingival margin/papilla of multirooted
teeth. Tissue biopsies from patients with periodontitis were
collected with flap surgery. Healthy biopsies were also collected
from patients following surgery for impacted teeth. Each tissue was
repeatedly washed with 0.9% saline until blood was no longer seen,
then 1 ml of RNAiso Reagent (Takara Biotechnology Co., Ltd.,
Dalian, China) was added to each sample and the sample was stored
in an RNase-free Eppendorf tube at −80°C.
Blood collection
Peripheral venous blood was drawn from each
individual and collected in heparin tubes. PBMCs were isolated from
2 ml blood by density gradient centrifugation using the separation
medium Ficoll according to the manufacturer's instructions (Haoyang
Biotechnology Co., Ltd., Tianjin, China). Peripheral blood taken at
rest was centrifuged (20°C, 400 × g for 20 min) and serum aspirated
into new tubes. PBMCs and sera were separately stored at −80°C
until use.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
PBMCs or 1-mm3 periodontal tissue samples
were processed for total RNA extraction in 1 ml RNAiso Reagent at
4°C. RNA quality was determined using a bioanalyzer and total RNA
was quantified using a spectrophotometer (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
A260nm/A280nm between 1.8 and 2.0. RT was
performed using a PrimeScript RT system (Takara Biotechnology Co.,
Ltd.) following the manufacturer's recommendations. The expression
levels of I Foxp3, IL-12p35, EBi3 and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) transcripts were quantified using RT-qPCR
with SYBR Premix Ex Taq II (TaKaRa Biotechnology Co. Ltd.) and a
LightCycler system (Roche Molecular Biochemicals, Mannheim,
Germany) in accordance with the manufacturer's protocol. Primers
for Foxp3, IL-12p35, EBi3 and GAPDH are listed in Table I. Cycling conditions used were: 95°C
for 30 sec, 95°C for 5 sec and 60°C for 34 sec for 40 cycles, then
95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec. In the qPCR
process, 2 µl cDNA was added per well, using three wells per
sample. Relative target gene quantification was obtained according
to the 2−ΔΔCq method (12). Target gene mRNA expression was
normalized to GAPDH mRNA expression, and the adjusted expression
for healthy individuals was used as a reference (fold change, 1).
In the mRNA analysis, 20 patients per group were included.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Target | Direction | Sequence (5′-3′) |
|---|
| Foxp3 | Forward |
CTGGCAAATGGTGTCTGCAAGT |
|
| Reverse |
CTGCCCTTCTCATCCAGAAGATG |
| IL-12p35 | Forward |
AGGAATGTTCCCATGCCTTCA |
|
| Reverse |
CCAATGGTAAACAGGCCTCCAC |
| EBi3 | Forward |
GACCTCACAGACTACGGGGAAC |
|
| Reverse |
CGGGAAGCCCTTGCTACTT |
| GAPDH | Forward |
TGGTGAAGACGCCAGTGGA |
|
| Reverse |
GCACCGTCAAGGCTGAGAAC |
Enzyme-linked immunosorbent assay
(ELISA)
Sera and GCFs were tested using an IL-35 ELISA kit
(USCN, Co., Ltd., Wuhan, China) in strict accordance with the
manufacturer's instructions. The optical density of each sample was
determined at 450 nm. A standard curve was generated, and data were
expressed in units of pg/ml IL-35 per liter of serum or GCF.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean for each group. An unpaired Student's t-test was used to
analyze differences between groups using SPSS version 19.0 software
(IBM Corp., Armonk, NY, USA). The correlation analysis between
clinical indicators and cytokines was analyzed by the Pearson rank
correlation test. P<0.05 was considered to indicate a
statistically significant result.
Results
Patient information and clinical
parameters
A total of 40 participants were included in this
study, with an age range from 18 to 55 years. Table II outlines the basic characteristics
and clinical parameters of each group. The mean age of the CP group
was 37.12±2.55 years and that of the healthy group was 35.23±2.36
years. The CP group consisted of 7 men and 13 women while the
healthy group was composed of 6 men and 14 women. Differences in
age and sex were not statistically significant between the two
groups (P>0.05 for both). The PD of the healthy controls was
1.60±0.05 mm while for the CP group the PD was 4.72±0.26 mm, a
statistically significant increase (P<0.05). The mean PD and CAL
for the CP group were significantly higher than those of the
healthy group (P<0.05 for both).
 | Table II.Patient information and clinical
parameters. |
Table II.
Patient information and clinical
parameters.
| Group | Sample size | Age (years) | Sex (M/F) | PD (mm) | CAL (mm) |
|---|
| Control | 20 | 35.23±2.36 | 6/14 | 1.60±0.05 | 0.12±0.02 |
| CP | 20 | 37.12±2.55 | 7/13 |
4.72±0.26a |
2.18±0.30a |
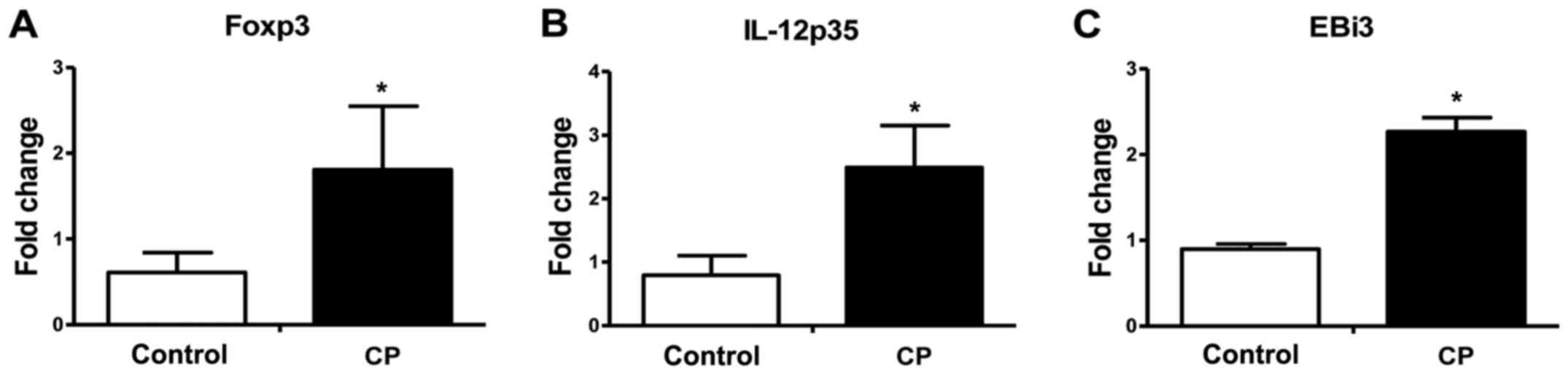
Foxp3, IL-12p35 and EBi3 mRNAs in
periodontal tissues
The mRNA expression levels of Foxp3, and of the
IL-35 subunits, IL-12p35 and EBi3, in periodontal tissue were
analyzed using RT-qPCR. As shown in Fig.
1, significantly increased Foxp3, IL-12p35 and EBi3 mRNA
expression in periodontal tissue (Foxp3, 2.21-fold; IL-12p35,
2.49-fold; EBi3, 2.27-fold; P<0.05 for all) was observed for the
CP group in comparison with the healthy control group.
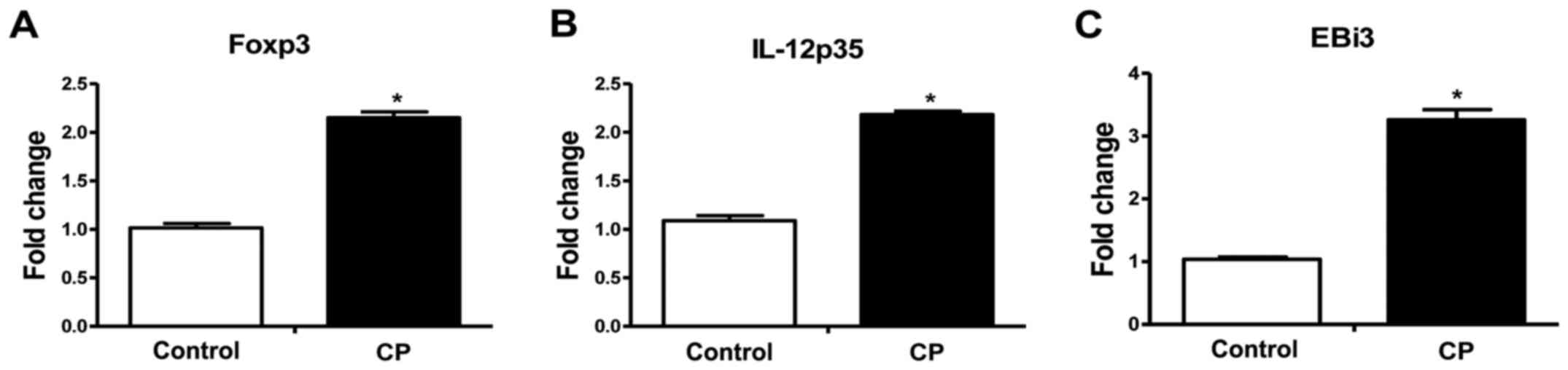
Foxp3, IL-12p35 and EBi3 mRNA in
PBMCs
The mRNA expression levels of Foxp3, IL-12p35 and
EBi3 were also examined in PBMCs using RT-qPCR. As shown in
Fig. 2, significantly increased
Foxp3, IL-12p35 and EBi3 mRNA expression in PBMCs (Foxp3,
2.15-fold; IL-12p35, 2.17-fold; EBi3, 3.06-fold; P<0.05 for all)
was observed for the CP group in comparison with the healthy
control group.
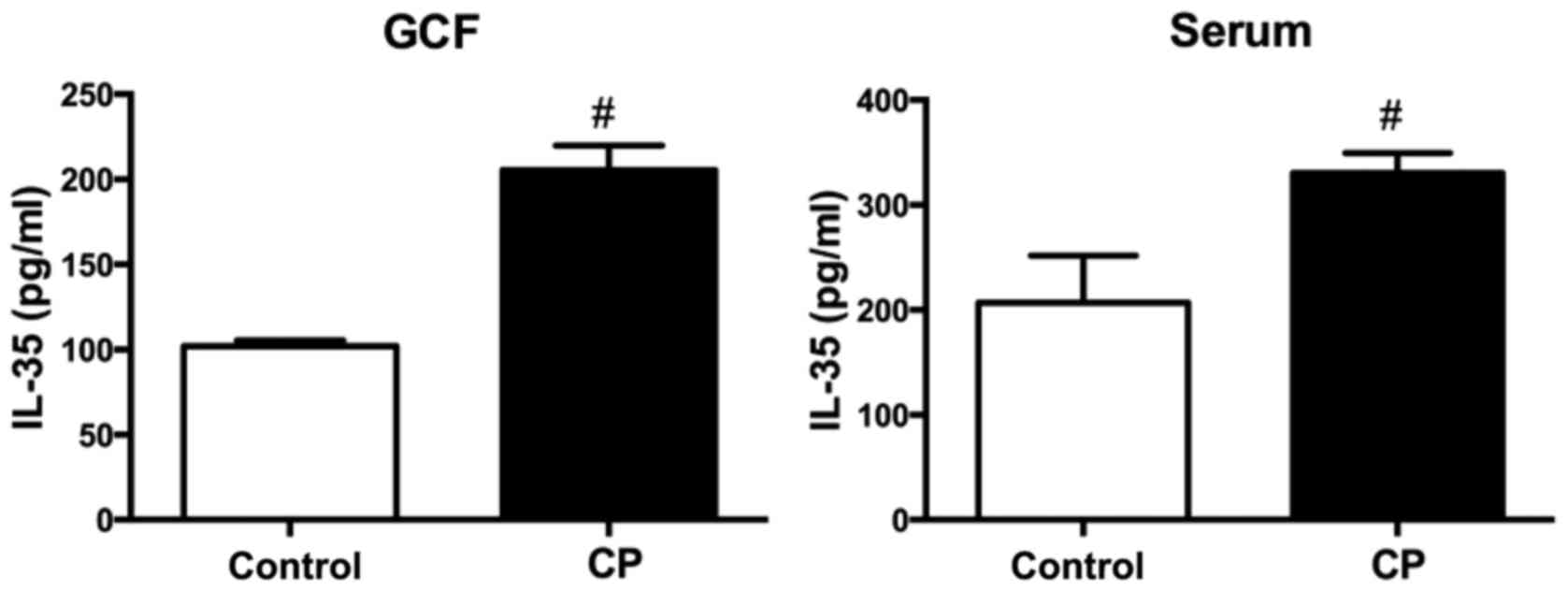
IL-35 protein in GCF and serum
Table III and
Fig. 3 show the mean levels of IL-35
protein in GCF and serum samples from the CP and healthy control
groups. The mean concentration of IL-35 protein was 205.56±1.61
ng/ml in GCF and 330.42±4.30 ng/ml in serum from the CP group,
while for the healthy control group it was 101.88±0.37 ng/m in GCF
and 206.89±10.06 ng/ml in serum. The mean concentration of IL-35
protein in the GCF and serum was significantly higher for the CP
group compared with the healthy group (P<0.001 for both).
 | Table III.Concentration of IL-35 protein in GCF
and serum. |
Table III.
Concentration of IL-35 protein in GCF
and serum.
| Group | Sample size | GCF (ng/ml) | Serum (ng/ml) |
|---|
| Control | 20 | 101.88±0.37 | 206.89±10.06 |
| CP | 20 |
205.56±1.61a |
330.42±4.30a |
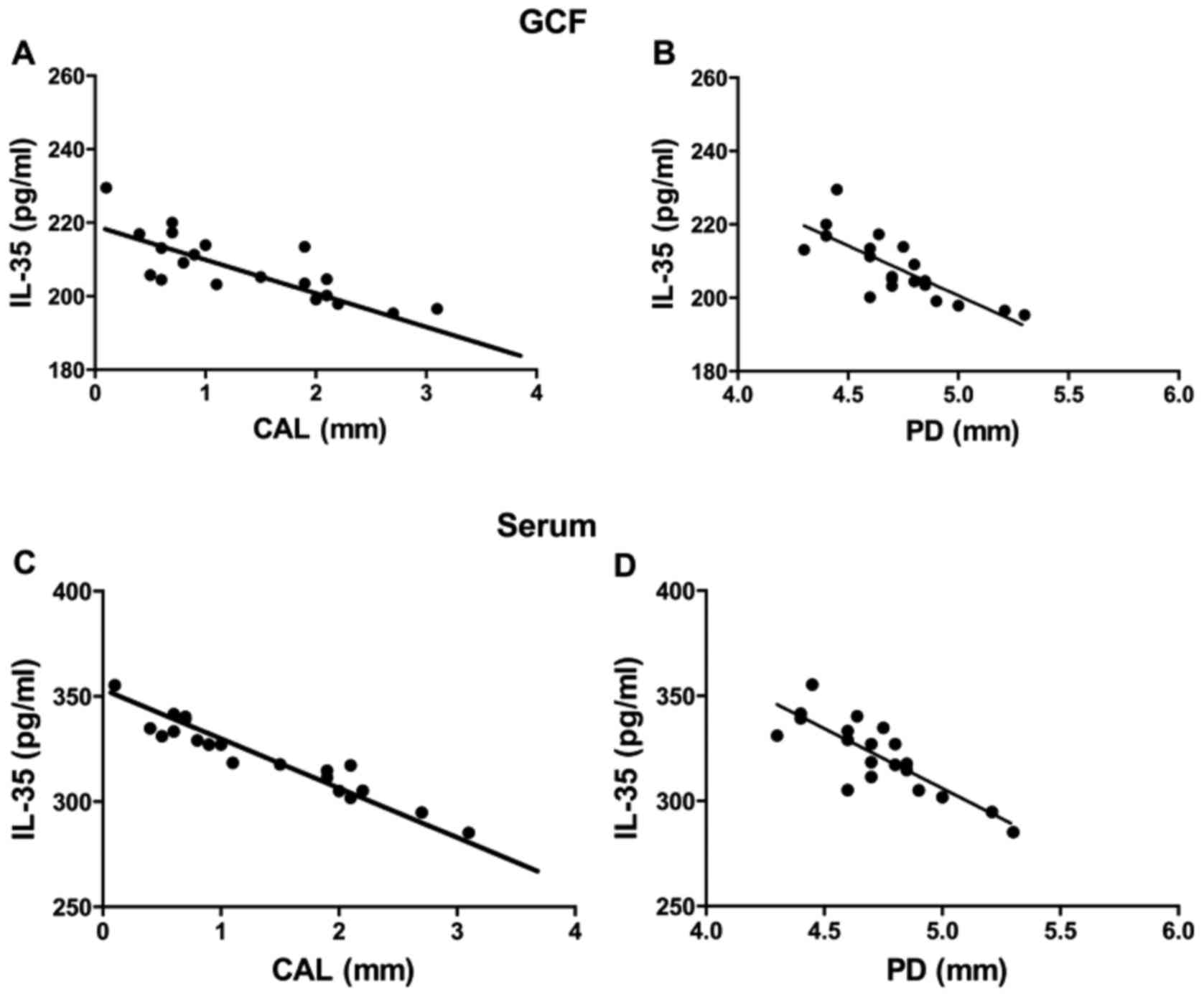
Correlation analysis
Using the Pearson rank correlation test, the
concentration of IL-35 in the GCF with the CAL at detection sites
of the CP group exhibited a negative correlation (P<0.001,
R2=0.6101; Fig. 4A), and
the concentrations of IL-35 and PD at detection sites were also
significantly negative correlation (P<0.001,
R2=0.6173; Fig. 4B).
Similarly, the concentration of IL-35 in the serum of the CP group
with CAL at detection sites exhibited a negative correlation
(P<0.001, R2=0.9119; Fig.
4C), and PD at detection sites was also negatively correlated
with the concentration of serum IL-35 (P<0.001,
R2=0.6812; Fig. 4D).
Discussion
Treg cells are necessary in the maintenance of
immune homeostasis and the prevention of autoimmune disease. There
is evidence (20,21) to suggest that anti-inflammatory Treg
cells also play an important role in the development of periodontal
disease and are involved in the subsequent inflammation and bone
resorption. The infiltration of Treg cells into periodontal tissue
reflects their ability to inhibit tissue damage (22). Foxp3 plays an integral role in
regulating the differentiation of Treg cells (23). In the present study, Foxp3 was
detected in periodontal tissues and PBMCs using RT-qPCR and its
expression was found to be significantly higher in the CP group
compared with the healthy control group.
IL-35 is an immunosuppressive/anti-inflammatory
cytokine, expressed by Foxp3+ Treg cells, that belongs
to the IL-12 family of cytokines (24). IL-35 is a dimeric protein comprised
of an α chain (p35) and a β chain (EBi3) (14). Unlike other cytokines of the IL-12
family, IL-35 acts as an inhibitory factor for chronic
inflammation, autoimmune disease and other immune disorders
(7), and the expression of IL-35 in
Treg cells is associated with their immune inhibitory ability
(25). It has also been suggested
that IL-35, as an inhibitory cytokine, plays a central role in
infection and immune regulation (26), which includes inhibiting the
proliferation of T cells and their effects. In the present study,
it was observed that IL-35 was strongly detected in periodontitis
tissues and the expression of IL-35 protein was increased in
tissues from the CP group compared with those of healthy
controls.
As long-living, non-Foxp3-dependent cells within the
body, IL-35-producing inducible Treg cells secrete IL-35, which may
inhibit the spread of inflammation, increase the number of Treg
cell subsets and enhance immune regulation (27). Niedbala et al (15) found that an EBi3-p35-Fc fusion
protein promoted the proliferation of
CD4+CD25+ Treg cells and inhibited
CD4+CD25− T cells in vitro. These
observations may explain why as increased expression of IL-35
protein in tissues from the CP group compared with those of healthy
controls was detected in the present study.
There is evidence indicating that a loss of IL-35 is
associated with the progression of various diseases, including
numerous inflammatory diseases (28,29).
IL-35 is required for effective Treg cells; animals lacking
functional IL-35 exhibit an enhanced inflammatory immune response
and progressive deterioration from disease (30,31). The
present study found that the concentration of IL-35 in the GCF of
the CP group showed a negative correlation with CAL or PD in
detection sites, and the concentration of IL-35 in the serum of the
CP group correlated with CAL and PD in a similar manner. These data
suggest that IL-35 in autoimmune or infectious diseases may
regulate the local microenvironment and peripheral immune response,
maintaining its homeostasis.
The analysis of IL-12p35 and EBi3 (IL-35) mRNAs
using RT-qPCR in PBMCs and periodontal tissues from patients with
CP in the present study revealed significantly higher expression in
the CP group compared with the healthy control group. This is a
similar result to that of Kalburgi et al, which to the best
of our knowledge is the only other study to address the role of
IL-35 in periodontal disease, albeit using semi-quantitative RT-PCR
(32). The present study also found
that the mean concentration of IL-35 protein in GCFs and sera from
the CP group was significantly higher than for the healthy group,
paralleling gene expression. These results suggest that IL-35 is an
essential factor for the immune response of Treg cells, and may be
useful in the prognosis of CP. Similarly, Nakajima et al
(33) reported that the proportion
of CD4+CD25+ T cells and Foxp3 expression in
periodontal disease tissues was increased compared with those of
gingivitis controls. Since EBi3 is a downstream target of Foxp3
(7), the high expression of Foxp3
mRNA, as detected in the present study, would promote EBi3 subunit
formation and therefore explain the high IL-35 protein expression
that was observed.
The results of the present study indicate that IL-35
expression increases with CP development, which may help to
attenuate the process of chronic periodontitis. In addition,
although patients included in the study did not exhibit systemic
disease, periodontitis may cause, or aggravate, chronic
inflammation in systemic disease. More specifically, gram-negative
anaerobic bacteria at the bottom of periodontal pockets may invade
epithelial cells and hide in host cells, aggravating the
destruction of periodontal tissue; they can potentially also invade
endothelial cells and access the blood circulation to stimulate a
host immune response and cause systemic inflammation (34–36).
IL-35, as a negative regulator of immune factors, may slow or
inhibit the development of periodontal disease and thus,
indirectly, also slow systemic disease. IL-35 is a relatively
recently identified cytokine that has not been studied in many
disease models. Since the parameters investigated in the present
study are few, it is unclear whether IL-35 enhances or antagonizes
the effects of other cytokines or immune cells in CP. In future, it
will be necessary to increase sample sizes and study a greater
number of parameters to more fully understand the role of IL-35 in
CP.
IL-35, as a Treg-specific suppressor of inflammatory
cytokines, may maintain the balance between bacterial infection in
chronic periodontal patients and effector cells by regulating the
immune system, in order to avoid periodontal tissue damage caused
by an overstimulated immune system (24,32). The
detection of higher levels of IL-35 protein and subunit mRNA in
diseased tissue compared with healthy tissue indicate that it may
play an important role in the development of CP. As GCF is easily
sampled, and simple, sensitive and reliable detection methods for
IL-35 are available, IL-35 is potentially an important diagnostic
tool for clinical use. However, as an appropriate treatment
strategy for periodontitis, further studies on IL-35 are required
to understand the precise functional role of this cytokine in
periodontal disease and within immune and inflammatory regulatory
networks.
Acknowledgements
This study was supported by the Natural Science
Foundation of China (grant no. 81570988).
References
|
1
|
Di Benedetto A, Gigante I, Colucci S and
Grano M: Periodontal disease: Linking the primary inflammation to
bone loss. Clin Dev Immunol. 2013:5037542013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mendes L, Azevedo NF, Felino A and Pinto
MG: Relationship between invasion of the periodontium by
periodontal pathogens and periodontal disease: A systematic review.
Virulence. 6:208–215. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yucel-Lindberg T and Båge T: Inflammatory
mediators in the pathogenesis of periodontitis. Expert Rev Mol Med.
15:e72013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gonzales JR: T- and B-cell subsets in
periodontitis. Periodontol 2000. 69:181–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campbell L, Millhouse E, Malcolm J and
Culshaw S: T cells, teeth and tissue destruction - what do T cells
do in periodontal disease? Mol Oral Microbiol. 31:445–456. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hernández M, Dutzan N, García-Sesnich J,
Abusleme L, Dezerega A, Silva N, González FE, Vernal R, Sorsa T and
Gamonal J: Host-pathogen interactions in progressive chronic
periodontitis. J Dent Res. 90:1164–1170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Collison LW, Workman CJ, Kuo TT, Boyd K,
Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS and Vignali DA:
The inhibitory cytokine IL-35 contributes to regulatory T-cell
function. Nature. 450:566–569. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shevach EM, DiPaolo RA, Andersson J, Zhao
DM, Stephens GL and Thornton AM: The lifestyle of naturally
occurring CD4+ CD25+ Foxp3+
regulatory T cells. Immunol Rev. 212:60–73. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xystrakis E, Boswell SE and Hawrylowicz
CM: T regulatory cells and the control of allergic disease. Expert
Opin Biol Ther. 6:121–133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coombes JL, Robinson NJ, Maloy KJ, Uhlig
HH and Powrie F: Regulatory T cells and intestinal homeostasis.
Immunol Rev. 204:184–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Annacker O, Pimenta-Araujo R,
Burlen-Defranoux O and Bandeira A: On the ontogeny and physiology
of regulatory T cells. Immunol Rev. 182:5–17. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Wang J, Jin Y, Gao H and Lin X:
Oral administration of all-trans retinoic acid suppresses
experimental periodontitis by modulating the Th17/Treg imbalance. J
Periodontol. 85:740–750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Collison LW and Vignali DA:
Interleukin-35: Odd one out or part of the family? Immunol Rev.
226:248–262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi J, Leung PS, Bowlus C and Gershwin
ME: IL-35 and Autoimmunity: A comprehensive perspective. Clin Rev
Allergy Immunol. 49:327–332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niedbala W, Wei XQ, Cai B, Hueber AJ,
Leung BP, McInnes IB and Liew FY: IL-35 is a novel cytokine with
therapeutic effects against collagen-induced arthritis through the
expansion of regulatory T cells and suppression of Th17 cells. Eur
J Immunol. 37:3021–3029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu Y, Dong C, Yue Y and Xiong S: In vivo
delivery of interleukin-35 relieves coxsackievirus-B3-induced viral
myocarditis by inhibiting Th17 cells. Arch Virol. 159:2411–2419.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Terayama H, Yoshimoto T, Hirai S, Naito M,
Qu N, Hatayama N, Hayashi S, Mitobe K, Furusawa J, Mizoguchi I, et
al: Contribution of IL-12/IL-35 common subunit p35 to maintaining
the testicular immune privilege. PLoS One. 9:e961202014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guan SY, Leng RX, Khan MI, Qureshi H, Li
XP, Ye DQ and Pan HF: Interleukin-35: A potential therapeutic agent
for autoimmune diseases. Inflammation. 40:303–310. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu X, Offenbacher S, Lόpez NJ, Chen D,
Wang HY, Rogus J, Zhou J, Beck J, Jiang S, Bao X, et al:
Association of interleukin-1 gene variations with moderate to
severe chronic periodontitis in multiple ethnicities. J Periodontal
Res. 50:52–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dutzan N, Gamonal J, Silva A, Sanz M and
Vernal R: Over-expression of forkhead box P3 and its association
with receptor activator of nuclear factor-kappa B ligand,
interleukin (IL)-17, IL-10 and transforming growth factor-beta
during the progression of chronic periodontitis. J Clin
Periodontol. 36:396–403. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Joosten SA and Ottenhoff TH: Human CD4 and
CD8 regulatory T cells in infectious diseases and vaccination. Hum
Immunol. 69:760–770. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohlrich EJ, Cullinan MP and Seymour GJ:
The immunopathogenesis of periodontal disease. Aust Dent J. 54
Suppl 1:S2–S10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alroqi FJ and Chatila TA: T regulatory
cell biology in health and disease. Curr Allergy Asthma Rep.
16:272016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Collison LW, Chaturvedi V, Henderson AL,
Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ,
Brown SA, et al: IL-35-mediated induction of a potent regulatory T
cell population. Nat Immunol. 11:1093–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Collison LW, Pillai MR, Chaturvedi V and
Vignali DA: Regulatory T cell suppression is potentiated by target
T cells in a cell contact, IL-35- and IL-10-dependent manner. J
Immunol. 182:6121–6128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Clavel G, Thiolat A and Boissier MC:
Interleukin newcomers creating new numbers in rheumatology: IL-34
to IL-38. Joint Bone Spine. 80:449–453. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma J and Xie LZ: Eukaryotic expression and
biological activity of human interleukin-35. Zhongguo Yi Xue Ke Xue
Yuan Xue Bao. 35:618–622. 2013.(In Chinese). PubMed/NCBI
|
|
28
|
Zhang YL, Zhou XY, Guo XY and Tu JW:
Association between serum interleukin-35 levels and severity of
acute pancreatitis. Int J Clin Exp Med. 8:7430–7434.
2015.PubMed/NCBI
|
|
29
|
Egwuagu CE, Yu CR, Sun L and Wang R:
Interleukin 35: Critical regulator of immunity and
lymphocyte-mediated diseases. Cytokine Growth Factor Rev.
26:587–593. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu JQ, Liu Z, Zhang X, Shi Y, Talebian F,
Carl JW Jr, Yu C, Shi FD, Whitacre CC, Trgovcich J and Bai XF:
Increased Th17 and regulatory T cell responses in EBV-induced gene
3-deficient mice lead to marginally enhanced development of
autoimmune encephalomyelitis. J Immunol. 188:3099–3106. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tirotta E, Duncker P, Oak J, Klaus S,
Tsukamoto MR, Gov L and Lane TE: Epstein-Barr virus-induced gene 3
negatively regulates neuroinflammation and T cell activation
following coronavirus-induced encephalomyelitis. J Neuroimmunol.
254:110–116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kalburgi NB, Muley A, Shivaprasad BM and
Koregol AC: Expression profile of IL-35 mRNA in gingiva of chronic
periodontitis and aggressive periodontitis patients: A
semiquantitative RT-PCR study. Dis Markers. 35:819–823. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakajima T, Ueki-Maruyama K, Oda T, Ohsawa
Y, Ito H, Seymour GJ and Yamazaki K: Regulatory T-cells infiltrate
periodontal disease tissues. J Dent Res. 84:639–643. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Olsen I: From the Acta Prize Lecture 2014:
The periodontal-systemic connection seen from a microbiological
standpoint. Acta Odontol Scand. 73:563–568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Linden GJ and Herzberg MC: Working group 4
of the joint EFP/AAP workshop: Periodontitis and systemic diseases:
A record of discussions of working group 4 of the joint EFP/AAP
workshop on periodontitis and systemic diseases. J Periodontol. 84
4 Suppl:S20–S23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paquette DW: The periodontal
infection-systemic disease link: A review of the truth or myth. J
Int Acad Periodontol. 4:101–109. 2002.PubMed/NCBI
|