Introduction
Anti-neutrophil cytoplasmic antibody
(ANCA)-associated vasculitis (AAV) is a common autoimmune disease
with rapid progression, and is one of the causes of secondary
kidney disease. The clinical symptoms of AAV patients are mainly
fatigue, fever and emaciation (1,2). AAV
often leads to multiple organ involvement, especially affecting the
kidneys, altering glomerular capillaries and small arteries,
leading to renal injury and causing focal necrotic
glomerulonephritis, accompanied with crescent formation and renal
insufficiency. Most importantly, the progression of AAV is rapid
and the 5-year survival rate of patients is <60% in the absence
of timely and effective renal replacement and immunosuppressive
therapy (3,4). Creatinine (SCr) found in the serum is
the product of muscle metabolism in the human body; it can be
filtered by glomeruli and excreted in the urine. SCr is often used
as a major clinical indicator of renal function (5). The erythrocyte sedimentation rate
(ESR), or blood sedimentation for short, refers to the
sedimentation rate of erythrocytes under certain laboratory
conditions. In blood samples from a variety of pathological
conditions, ESR is significantly increased, reflecting the activity
of the disease to a certain extent (6). In this study, Scr levels and the ESR
were measured in patients with renal injury caused by AAV, and the
relationship between the values obtained and the pathology and
prognosis of the patients was analyzed, so as to provide reference
markers for prevention and treatment of AAV disease.
Patients and methods
General data
A total of 86 patients with AAV treated in the
Affiliated Hospital of Qingdao University from December 2010 to
November 2011 were enrolled in the study. Patients who met the
following conditions were included: patients diagnosed with AAV via
renal biopsy and laboratory examination; patients with positive
serum ANCA, accompanied by renal injury, and who had not received
renal replacement therapy; and patients/gardians who signed the
informed consent. Patients were excluded if they had an immune
deficiency or concurrent infection; if they had used hormones in
the past 3 months; or if they were suffering from cardiac
insufficiency or malignant tumor. According to their age, these
patients were divided into an elderly group (n=45) and a
non-elderly group (n=41). Other general data differing between the
patients in the two groups had no statistical significance
(P>0.05) (Table I). The study was
approved by the Ethics Committee of The Affiliated Hospital of
Qingdao University.
 | Table I.General data of objects in the
study. |
Table I.
General data of objects in the
study.
| Item | Elderly group
(n=45) | Non-elderly group
(n=41) |
t-value/χ2 | P-value |
|---|
| Sex (M/F) | 21/17 | 19/22 | 0.322 | 0.571 |
| BMI
(kg/m2) | 20.35±2.36 | 20.63±2.43 | 0.542 | 0.589 |
| MAP (mmHg) | 96.87±12.37 | 97.35±11.58 | 0.185 | 0.854 |
| WBC
(109/l) | 16.06±5.93 | 15.84±5.74 | 0.174 | 0.862 |
| Course of disease
(months) | 4.03±5.83 | 4.14±5.35 | 0.091 | 0.928 |
Pathological examination
All patients underwent renal biopsies and took
vitamin K orally 2 days before the procedure. On the day of
operation, patients were put on a light liquid diet. Patients
assumed a prone position, and a 10 cm-thick pillow was put under
their abdomen to better expose the lower back. The patients held
their breath and cooperated with the doctor during the biopsy.
After the operation, the patients relaxed and lied on their back
for 24 h. The tissue samples were fixed with 4% formaldehyde, and
then turned into conventional paraffin-embedded sections for
hematoxylin and eosin (H&E), periodic acid Schiff reaction
(PAS), periodic acid-silver methenamine (PASM) and Masson trichrome
stainings. An experienced pathologist observed the prepared slides
under a light microscope (Leica Microsystems, Inc., Buffalo. Grove,
IL, USA).
Laboratory examination
After 8 h of fasting, 5 ml venous blood samples were
collected from patients. The blood samples were placed in special
glass tubes with anticoagulant, and then ESRs were detected using
the ESR-30 fully automatic dynamic ESR analyzer (Shanghai Xunda
Medical Instrument Co., Ltd., Shanghai, China). Additionally, other
3–5 ml venous blood samples were collected from patients in the
morning, and placed at room temperature for 1 h, before
centrifugation at 2,053 × g for 20 min at 4°C. Next, the
supernatants were taken and stored at −80°C. The Scr level was
detected using the sarcosine oxidase method, with the relevant kits
provided by Guangzhou Wondfo Biotech, and using a 7170A fully
automatic biochemical analyzer (Hitachi, Japan). Relevant
parameters of the assays included a temperature of 37.0°C, with the
samples placed in the test plate and detected using the two-point
termination method (measuring point between 14–34, dominant
wavelength of 700 nm, and sub-wavelength of 505 nm). After
completing the measurements, the results were obtained via a
printer automatically.
Follow-up
The patients in the two groups were followed up for
5 years, and the clinical features annotated including the
end-stage renal disease characteristics (the estimated value of
glomerular filtration rate <15 ml/min or dialysis) and death
rates were recorded.
Evaluation criteria
The morphologic grading of ANCA-associated nephritis
of Berden (7) was used to classify
the pathological type of renal injury into one of four types: 1)
Focal type for ≥50% normal glomeruli. 2) Crescentic type for ≥50%
crescentic glomeruli. 3) Mixed type for <50% crescentic
glomeruli, <50% normal glomeruli and <50% global sclerotic
glomeruli. 4) sclerotic type for ≥50% global sclerotic
glomeruli.
As mentioned above, the Scr level was detected using
the sarcosine oxidase method, and the ESR level was detected using
the full-automatic ESR analyzer. If the levels of Scr and ESR were
higher than normal, they were designated as high. The distribution
of high levels of Scr and ESR in patients with different
pathological types was statistically analyzed.
Statistical analysis
The SPSS version 19.0 (SPSS Inc., Chicago, IL, USA)
software was used for data processing. Measurement data were
presented as mean ± standard deviation, and the t-test was used for
analysis. Enumeration data were presented as rate, and analyzed via
Chi-square test. The Kaplan-Meier analysis was used for survival
analysis, and receiver operator characteristic (ROC) curve analysis
was used for prognosis prediction. Logistic regression analysis was
used for influencing factor of prognosis. A P<0.05 indicates
that a given difference is statistically significant.
Results
Seric Scr levels and ESRs of the
patients in the two groups
The mean levels of seric Scr and the ESR in the 86
patients were 406.87±12.37 µmol/l and 83.83±7.64 mm/1 h,
respectively; with the levels of Scr and ESR in the elderly group
being significantly higher than those in the non-elderly group
(p<0.05) (see Table II for
details).
 | Table II.Comparisons of Scr and ESR levels of
patients in the two groups. |
Table II.
Comparisons of Scr and ESR levels of
patients in the two groups.
| Group | No. of cases | Scr (µmol/l) | ESR (mm/1 h) |
|---|
| Elderly group | 45 | 481.78±13.48 | 87.93±6.23 |
| Non-elderly
group | 41 | 398.04±13.98 | 75.05±5.32 |
| t-value |
| 28.692 | 10.260 |
| P-value |
| <0.001 | <0.001 |
Pathological analysis of patients and
relationship between different pathological types and Scr levels
and ESR
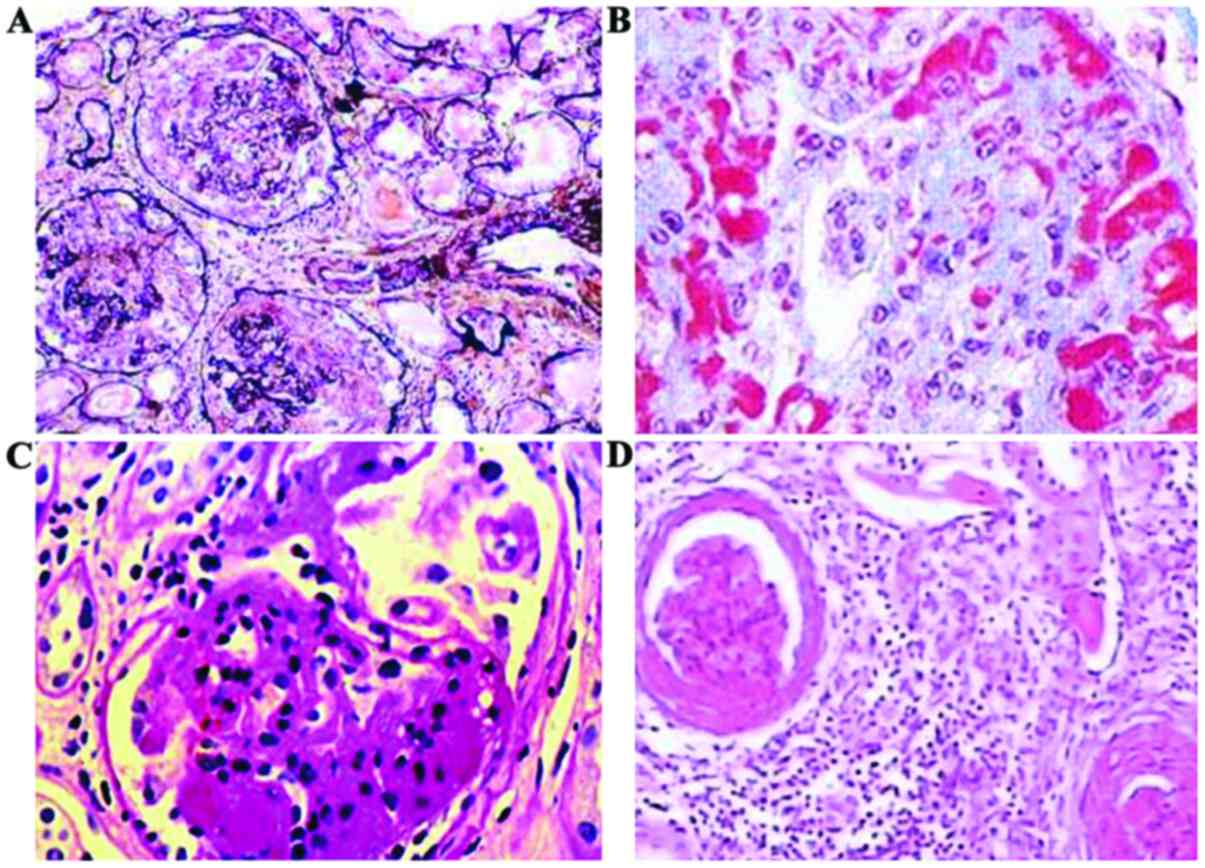
Pathology results showed 30 cases of crescentic, 24
of sclerotic, 18 of mixed and 14 of focal types (Fig. 1). The patients with high levels of
Scr and high ESR presented mainly the crescentic and sclerotic
types, the patients in this group with focal and mixed types were
significantly less (P<0.05) (Table
III).
 | Table III.High Scr and ESR levels in patients
with different pathological types (n, %). |
Table III.
High Scr and ESR levels in patients
with different pathological types (n, %).
| Pathological
type | No. of cases | High Scr level | High ESR level |
|---|
| Crescentic type | 30 | 29 (96.67) | 28 (93.33) |
| Sclerotic type | 24 | 21 (87.50) | 20 (83.33) |
| Mixed type | 18 | 8
(44.44) | 7
(38.89) |
| Focal type | 14 | 5
(35.71) | 4
(28.57) |
| χ2 |
| 28.567 | 28.731 |
| P-value |
| <0.001 | <0.001 |
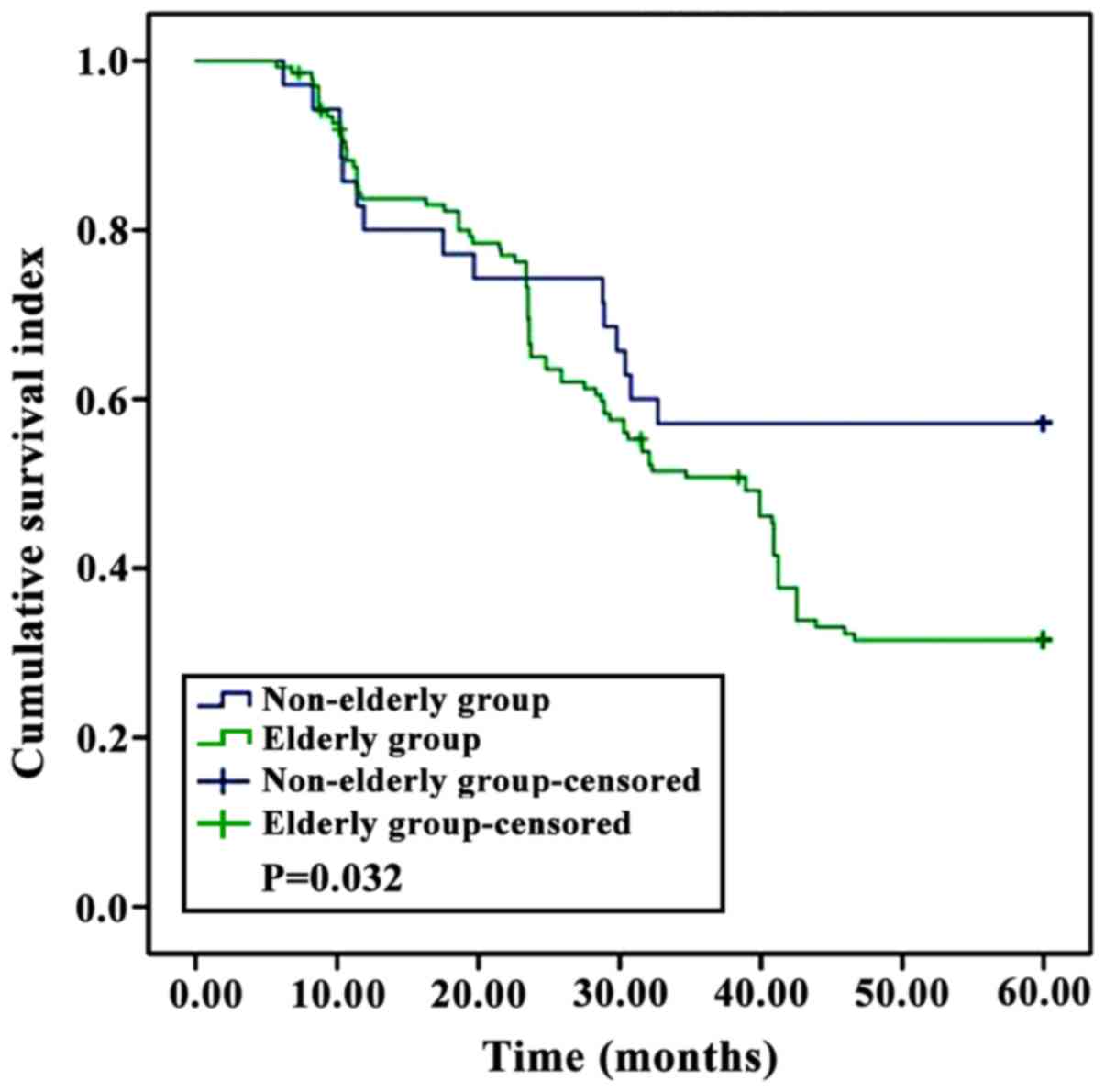
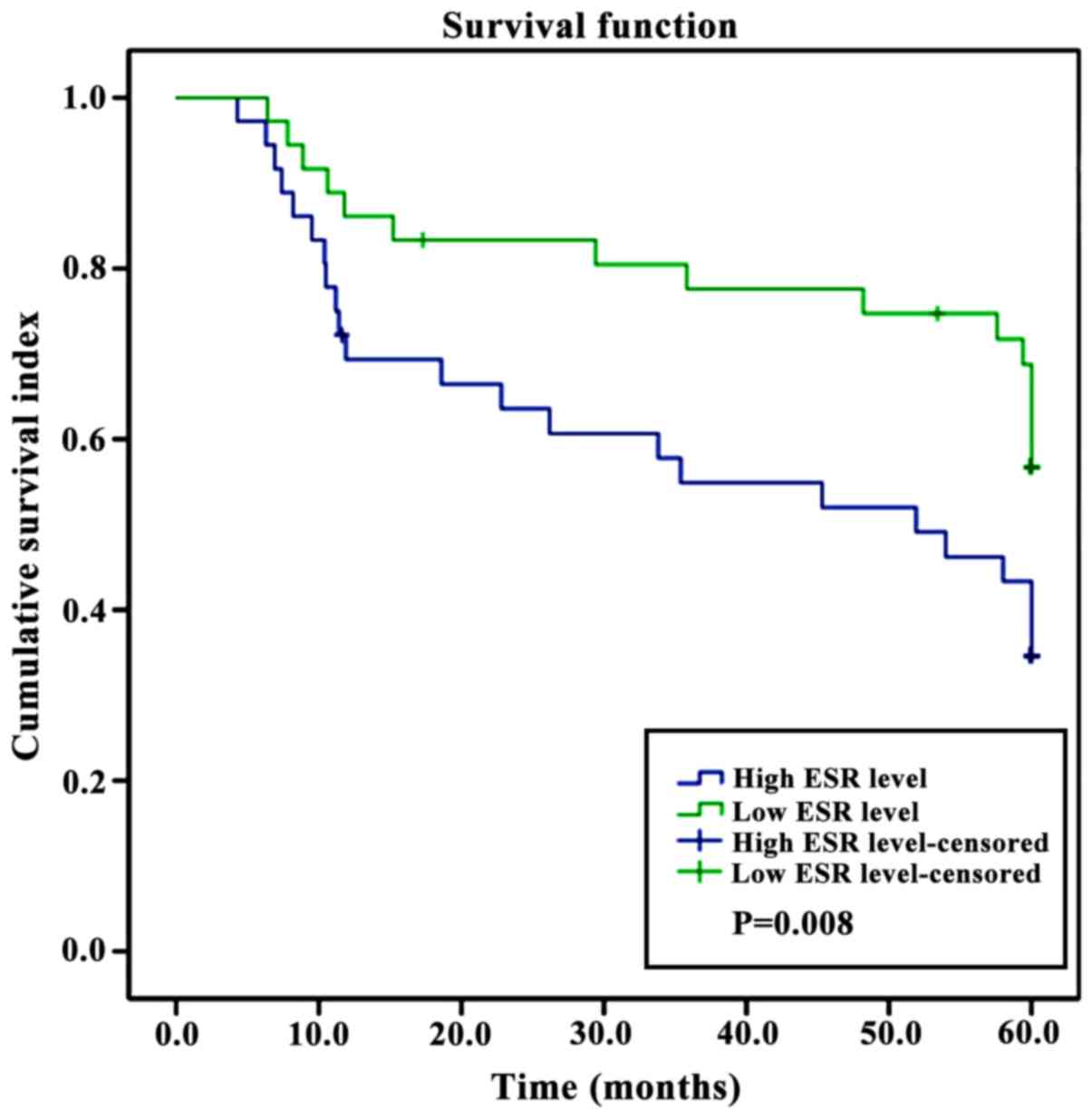
Survival rate of patients
Kaplan-Meier analysis showed that the survival rate
of patients in the elderly group was significantly lower than that
of patients in the non-elderly group, and the survival rate of
patients with high levels of Scr and ESR was significantly lower
than that of patients with low levels of Scr and ESR (P<0.05)
(Figs. 2–4).
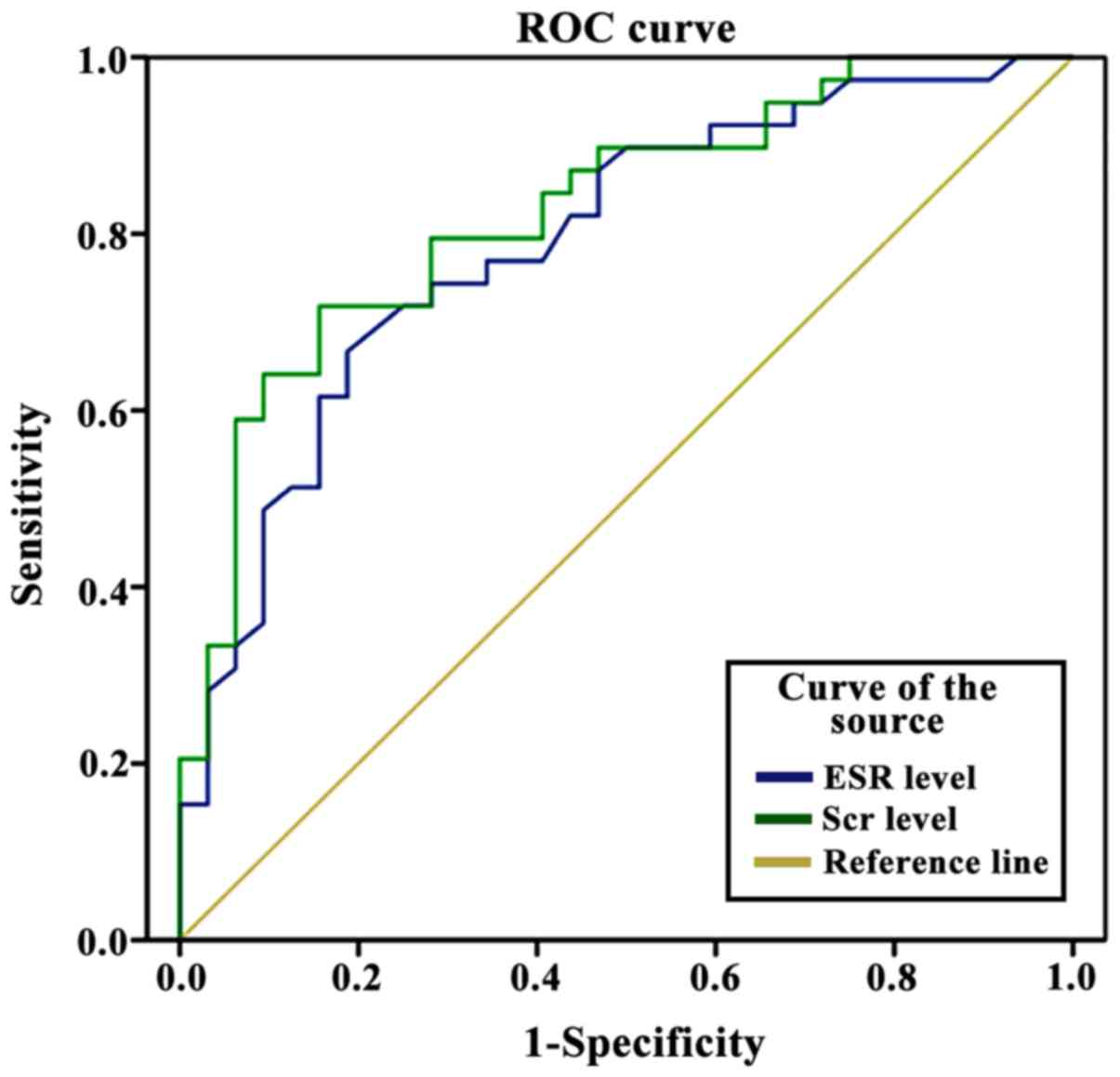
ROC analysis
The ROC analyses using the Scr and ESR values showed
an area under the curve (AUC) of Scr level of 0.901, with 90.2%
sensitivity, 89.5% specificity, and a cut-off value of 392.5
µmol/l; while the AUC of the ESR value was 0.864, with a
sensitivity of 89.2%, a specificity of 88.5% and a cut-off value of
72.8 mm/1 h (Fig. 5).
Analysis of prognosis influencing
factors
With poor prognosis as the dependent variable and
age, sex, educational level, course of disease, Scr and ESR values
as independent variables, the logistic regression analysis
performed showed that Scr (OR=2.315, P=0.004) and ESR (OR=1.847,
P=0.003) were independent risk factors affecting the poor prognosis
of patients (P<0.05) (Table
IV).
 | Table IV.Logistic regression analysis of
prognosis influencing factors. |
Table IV.
Logistic regression analysis of
prognosis influencing factors.
| Factor | β | S.E | Wald | OR | 95% CI | P-value |
|---|
| Age | 0.337 | 0.502 | 3.713 | 0.738 | 0.375–0.972 | 0.213 |
| Sex | 0.437 | 0.517 | 4.072 | 0.236 | 0.114–0.779 | 0.319 |
| Educational
level | −0.417 | 0.613 | 5.327 | 0.173 | 0.456–0.856 | 0.173 |
| Course of
disease | −0.615 | 0.824 | 6.405 | 0.237 | 0.196–0.512 | 0.218 |
| Scr | 1.426 | 0.749 | 7.757 | 2.315 | 1.475–5.252 | 0.004 |
| ESR | 1.433 | 0.517 | 8.524 | 1.847 | 1.113–4.347 | 0.003 |
Discussion
ANCA-associated vasculitis (AAV) is a kind of
autoimmune disease occurring commonly in the elderly; and its
pathological characteristics include necrotic inflammation in
vascular walls and abnormal death of neutrophils. AAV belongs to
the small-vessel vasculitis group of systemic vasculitis, with
positive ANCA antibodies in serum and a lack of immune complex
deposition in vascular walls (8,9). ANCA is
an autoantibody with monocyte lysosomal components and PMN
cytoplasmic particles as special antigens (immunoglobulin-like)
(10). At present, the detection
methods for ANCA include mainly the enzyme-linked immunosorbent
assay (ELISA) and the indirect immunofluorescence methods. ANCA
detection was incorporated into the standardized detection system
in the 1990s in China, so the detection rate of AAV has been
increasing ever since. The pathogenesis of AAV remains unclear.
However, many factors have been implicated in the development of
the disease. Genetic factors include the CD226-encoded antigen, the
HLA gene (HLA-DR4 and HLA-DR13), the PTPN22 protein and the IL-I0
gene, which can participate in the immune mechanisms of AAV.
Moreover, environmental factors such as
hydrocarbons, silica dust, microbial (Escherichia coli,
Staphylococcus aureus and Klebsiella pneumoniae)
infections and drugs (hydralazine, propylthiouracil, minocyline and
penicillamine) have been associated with AAV (11). A general route for the development of
AAV would theoretically begin with environmental factors
stimulating inflammatory mediators (TNF-α, IL-10 and IL-8); which
would, in turn, activate PMN cells to express antigen components
(PR3 and MPO) onto their cell surface, and get combined with ANCAs,
leading to the full activation of PMN. During the process of PMN
activation, the expression of adhesion molecules is increased, so
the PMN and endothelial cells contact each other closely; which
results in various inflammatory mediators, lysosomal enzymes and
toxic oxygen free radicals play a direct cytotoxic effect, causing
vascular endothelial injury in multiple organ sites, especially the
in the kidneys (12). Clinical
symptoms of patients with renal injury caused by AAV include
oliguria, anuria, decreased renal function or acute oliguric renal
failure. If there is no timely treatment, AAV develops rapidly and
ultimately leads to irreversible end-stage renal failure (13).
A clinical treatment method for patients with
AAV-related renal injury consists often of a glucocorticoid
(methylprednisolone) combined with cytotoxic drugs (14). Clinically, renal biopsy is often used
to diagnose AAV-related renal injury and evaluate the prognosis.
But high-sensitivity and high-specificity markers for early
diagnosis and prognosis evaluation would be preferable due to the
invasive nature of the renal biopsy procedure (15,16).
Scr is the final metabolite of phosphocreatine via a
non-enzymatic dehydration reaction. Scr is excreted by glomeruli
but not reabsorbed by renal tubules. The normal seric level of Scr
ranges from 44 to 133 µmol/l, and high levels can reflect the
severity of glomerular injury (17,18).
ESR, on the other hand, is a non-specific marker
that reflects well the status of the AAV disease. The acceleration
of the ESR is often associated with a variety of diseases, such as
acute bacterial inflammation, active tuberculosis, nephritis,
myocarditis, pneumonia, rheumatoid arthritis, systemic lupus
erythematosus, and others; and tissue damage and necrosis also
accelerate the ESR (19,20). The results of this study showed that
the mean level of Scr and the ESR in 86 patients was, respectively,
406.87±12.37 µmol/l and 83.83±7.64 mm/h. Additionally, the levels
of Scr and the ESR of patients in the elderly group were
significantly higher than those of patient in the non-elderly group
(P<0.05). This is because renal injury leads to decreased
glomerular filtration function and changes in the glomerular
filtration rate; so the concentration of Scr is increased, and with
the worsening of the disease, the ESR is significantly
accelerated.
The pathology results in this study showed 30 cases
of crescentic type, 24 cases of sclerotic type, 18 cases of mixed
type and 14 cases of focal type. The number of patients with high
Scr and ESR values was significantly higher in those patients with
crescentic and sclerotic types than in patients with focal and
mixed types. This may be due to the fact that patients of focal
type have a more stable renal function, and the patients with the
mixed types generally have a more benign renal disease; while the
patients with sclerotic type cannot have their renal function
restored and those with crescentic type have also a more serious
alteration of their kidneys.
Different renal outcomes are closely related to the
survival and life quality of patients affected with AAV. Treatment
is aimed at improving the long-term survival and life quality of
patients. Kaplan-Meier analysis showed that the survival rate of
patients in the elderly group was significantly lower than that of
patients in the non-elderly group. The survival rate of patients
with high Scr and ESR values was significantly lower than that of
patients with low Scr and ESR values. Furthermore, the ROC curve
test showed Scr levels and the ESR should be very useful in
diagnosis and prognosis of AAV, and logistic regression analysis
showed that high Scr levels and an accelerated ESR were independent
risk factors each leading to poor prognosis. These findings suggest
that the levels of Scr and the ESR values can reflect the severity
of renal injury to a certain extent and help in understand the
activity, remission or relapse condition of the kidney disease. The
changes in the two markers should be monitored during clinical
treatment and follow-up, so as to take effective intervention
measures to improve the prognosis of patients with AAV. However,
this study was limited by a small sample size, so long-term
large-sample studies are still needed before issuing precise
recommendation.
In conclusion, monitoring the serum level of Scr and
the ESR value in patients with renal injury by AAV can help
practitioners prescribe interventions that may alter the course of
the disease and improve the prognosis for the patients.
References
|
1
|
Holle JU, Wieczorek S and Gross WL: The
future of ANCA-associated vasculitis. Rheum Dis Clin North Am.
36:609–621. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang S, Zhang Y, Yin SW, Gao XJ, Shi WW,
Wang Y, Huang X, Wang L, Zou LY, Zhao JH, et al: Neutrophil
extracellular trap formation is associated with autophagy-related
signalling in ANCA-associated vasculitis. Clin Exp Immunol.
180:408–418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jones RB, Furuta S, Tervaert JWC, Hauser
T, Luqmani R, Morgan MD, Peh CA, Savage CO, Segelmark M, Tesar V,
et al: European Vasculitis Society (EUVAS): Rituximab versus
cyclophosphamide in ANCA-associated renal vasculitis: 2-year
results of a randomised trial. Ann Rheum Dis. 74:1178–1182. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Turner-Stokes T, Sandhu E, Pepper RJ,
Stolagiewicz NE, Ashley C, Dinneen D, Howie AJ, Salama AD, Burns A
and Little MA: Induction treatment of ANCA-associated vasculitis
with a single dose of rituximab. Rheumatology (Oxford).
53:1395–1403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Massenhove J, Lameire N, Dhondt A,
Vanholder R and Van Biesen W: Prognostic robustness of serum
creatinine based AKI definitions in patients with sepsis: A
prospective cohort study. BMC Nephrol. 16:1122015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sung HH, Jeon HG, Jeong BC, Seo SI, Jeon
SS, Choi HY and Lee HM: Clinical significance of prognosis using
the neutrophil-lymphocyte ratio and erythrocyte sedimentation rate
in patients undergoing radical nephroureterectomy for upper urinary
tract urothelial carcinoma. BJU Int. 115:587–594. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rahmattulla C, Berden AE and Bajema IM:
L21. Kidneys in ANCA-associated vasculitis: What to learn from
biopsies? Presse Med. 42:563–565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilde B, Van Paassen P, Van Breda Vriesman
P, Cohen Tervaert JW and Hilhorst M: Estimating renal survival
using the ANCA-associated glomerulonephritis classification: A
validation study. Presse Med. 42:747–748. 2013. View Article : Google Scholar
|
|
9
|
Quintana LF, Peréz NS, De Sousa E, Rodas
LM, Griffiths MH, Solé M and Jayne D: ANCA serotype and
histopathological classification for the prediction of renal
outcome in ANCA-associated glomerulonephritis. Nephrol Dial
Transplant. 29:1764–1769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Konstantinov KN, Ulff-Møller CJ and
Tzamaloukas AH: Infections and antineutrophil cytoplasmic
antibodies: Triggering mechanisms. Autoimmun Rev. 14:201–203. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Syed R, Rehman A, Valecha G and El-Sayegh
S: Pauci-immune crescentic glomerulonephritis: An ANCA-associated
vasculitis. BioMed Res Int. 2015:4028262015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanna A and Pusey CD: The
histopathological classification of ANCA-associated
glomerulonephritis comes of age. J Rheumatol. 44:265–267. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Naidu GS, Sharma A, Nada R, Kohli HS, Jha
V, Gupta KL, Sakhuja V and Rathi M: Histopathological
classification of pauci-immune glomerulonephritis and its impact on
outcome. Rheumatol Int. 34:1721–1727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Walsh M, Casian A, Flossmann O, Westman K,
Höglund P, Pusey C and Jayne DR: European Vasculitis Study Group
(EUVAS): Long-term follow-up of patients with severe
ANCA-associated vasculitis comparing plasma exchange to intravenous
methylprednisolone treatment is unclear. Kidney Int. 84:397–402.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abe Y, Shima T, Izumi Y, Kitamura M,
Yamashita H, Tsuji Y, Sasaki O, Maeda C, Kawahara C, Torisu A, et
al: Successful management of lupus nephritis with high titers of
myeloperoxidase anti-neutrophil cytoplasmic antibodies using
tacrolimus. Intern Med. 54:2929–2933. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Zhang W, Yu HJ, Pan XX, Shen PY, Ren
H, Wang WM and Chen N: Clinical and pathological study on patients
with primary antineutrophil cytoplasmic autoantibody-associated
vasculitis with renal immune complex deposition. J Clin Rheumatol.
21:3–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boyer TD, Sanyal AJ, Pappas SC, Wong F and
Jamil K: P0198: Percentage change in serum creatinine (SCr) is a
sensitive indicator of therapeutic response to terlipressin in
hepatorenal syndrome type 1 (HRS-1). J Hepatol. 62:S379–S380. 2015.
View Article : Google Scholar
|
|
18
|
Wei DZ, Ge M, Wang CX, Lin QY, Li MJ and
Li P: Geographical distribution of the Serum creatinine reference
values of healthy adults. Nan Fang Yi Ke Da Xue Xue Bao.
36:1555–1560. 2016.(In Chinese). PubMed/NCBI
|
|
19
|
Kantor ED, Udumyan R, Signorello LB,
Giovannucci EL, Montgomery S and Fall K: Adolescent body mass index
and erythrocyte sedimentation rate in relation to colorectal cancer
risk. Gut. 65:1289–1295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McArthur BA, Abdel MP, Taunton MJ, Osmon
DR and Hanssen AD: Seronegative infections in hip and knee
arthroplasty: Periprosthetic infections with normal erythrocyte
sedimentation rate and C-reactive protein level. Bone Joint J.
97-B:939–944. 2015. View Article : Google Scholar : PubMed/NCBI
|