Introduction
Cervical cancer is the second most commonly
diagnosed cancer and the third leading cause of cancer-associated
mortality among females in less developed countries (1). There are 527,600 new cervical cancer
cases each year and 265,700 mortalities due to cervical cancer,
which is the highest mortality rate of all gynecological
malignancies (1,2). Cervical cancer is more prevalent in
developing countries, where >85% of cases occur (3). China has the highest incidence of
cervical cancer worldwide with ~140,000 new cervical cancer cases
each year, accounting for ~38% of the global incidence (4).
microRNAs (miRNAs or miRs) are a type of short
length, highly conservative, non-coding RNA, which are able to
affect gene expression by binding to the 3′-untranslated region of
target genes (5). miRNAs may act as
either oncogenic factors or tumor suppressors in various types of
cancer (6). In human cervical
cancer, the miR-27b cluster was previously found to serve an
oncogenic role in cervical cancer by promoting proliferation and
was upregulated by papillomavirus 16 E7 (7). Conversely, several miRs, including
miR-646, miR-141 and miR-205, were observed to be downregulated in
cervical cancer (8–10). Various miRs act as tumor suppressors,
including the miR-10 family, which suppresses cancer cell
proliferation and promotes cancer cell apoptosis (11,12).
However, there are limited studies focusing on the relationship
between miR-10-5p, another member of the miR-10 family, and
cervical carcinoma.
Brain-derived neurotrophic factor (BDNF) is a
transcription factor that serves a key function in the process of
neural differentiation (13).
Previous studies have suggested that BDNF is also a key regulator
of cancer (14–16). However, little is known about the
molecular pathways of BDNF in cervical cancer.
The current study aimed to investigate the
association between miR-10-5p and the biological characteristics of
cervical cancer cell lines. The results indicated that upregulation
of miR-10-5p has inhibitory effects on cervical cancer. In
addition, it was demonstrated that the pro-oncogenic gene BDNF was
suppressed by upregulation of miR-10-5p in cervical cancer
cells.
Materials and methods
Cervical cancer cell culture
Five cervical cancer cell lines were studied: SiHa,
HeLa, CaSki, C4-I and C-33a. These cell lines were all obtained
from the American Type Culture Collection (Manassas, VA, USA).
Normal cervical epithelial cells from the human uterus were
purchased from ScienCell Research Laboratories (San Diego, CA,
USA). All cells were cultured in Dulbecco's modified Eagle's medium
(DMEM), high-glucose, supplemented with 10% fetal calf serum (both
from Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
in a tissue culture incubator at 37°C with 5% CO2 until
90% confluence was reached. All specimens were immediately
snap-frozen in liquid nitrogen and stored at −80°C for subsequent
experimentation.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA (2 µg) from cervical cell lines was
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). A SuperScript™ one-step RT-PCR kit (Invitrogen;
Thermo Fisher Scientific, Inc.) was used to perform RT-qPCR. For
reverse transcription, the first strand was synthesized using oligo
(dT) primers, DEPC-treated water, reverse transcriptase buffer,
dNTPS mixture, RNase inhibitor and reverse transcriptase. The
reaction steps were set by incubating at 42°C for 1 h followed by a
10 min incubation at 92°C to denature the reverse transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.) and separate
complementary strands. cDNA was subsequently used to perform PCR
using β-actin as an internal control. The sequences of the primers
used were as follows: miR-10-5p, 5′-UACCCUGUAGAUCCGAAUUUGUG-3′;
BDNF forward, 5′-CTACGAGACCAAGTGCAATCC-3′ and reverse,
5′-AATCGCCAGCCAATTCTCTTT-3′; and β-actin forward,
5′-CTCCATCCAGGCGCTGT-3′ and reverse, 5′-GCTGTCACCTTCACCGTTCC-3′.
qPCR was performed in a total final 20 µl volume consisting of 0.5
µl cDNA, 1 µl Taq polymerase, 1.1 µl TaqMan probe, 0.4 µl, 10 nM
dNTPs, 2 µl 10× PCR buffer and 15 µl DEPC-treated water. The
thermocycling conditions of PCR were performed as follows: 95°C for
10 min; 65°C for 45 sec; 72°C for 1 min, for 35 cycles. All
reactions including control groups were performed in triplicate.
The results were analyzed by 2−ΔΔCq method (17).
Lentivirus production
In the present study, human miR-10-5p was
transfected into lentivirus (lentimiR10a-5p) and empty lentivirus
lenti-miRNA control (mi-RC) was used as a negative control. All
products were purchased from SunBio Biotech, Co., Ltd. (Beijing,
China). Lenti-miR-10a-5p or lenti-miRC [100 nM; GenScript (Nanjing)
Co., Ltd., Nanjing, China] were then transduced into HeLa and SiHa
cells using Lipofectamine 2000 (Biomics Biotechnologies Co., Ltd.,
Nantong, China) following a previously published protocol (18). HeLa and SiHa cells were selected from
the five cell lines studied to determine miR-10-5p expression
levels. HeLa and C4-1 cells were HPV-18 infected cervical carcinoma
cell lines and SiHa and CaSki were HPV-16 infected cells, while
C-33a was a HPV negative cell line. Additionally, HeLa and SiHa
were from the local cervical cancer tissues, while C4-1 and CaSki
were from metastatic tissues. CaSki and C4-1 cells were difficult
to culture, therefore SiHa and HeLa cells were selected to conduct
the following experiments. All cells were cultured in a 6-well
plate (3×105) using DMEM with 5 µg/ml puromycin at 37°C
with 5% CO2 for 48 h prior to subsequent
experimentation.
Cervical cancer cell viability
assay
The viability of HeLa and SiHa cells was evaluated
using an MTT assay (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. In a 96-well plate (5,000
cells/well), lentivirus-transduced HeLa and SiHa cells were
maintained for 5 days at 20°C. All cervical cancer cell lines were
incubated with a volume fraction of 10% FBS DMEM culture liquid at
37°C and 5% CO2. Cells were collected from the plates
using trypsin and washed with PBS. Cell viability was measured
using an MTT assay kit (Beyotime Institute of Biotechnology,
Haimen, China) and the optical density at a wavelength of 490 nm
was detected using a microplate reader (Multiskan MK3; Thermo
Fisher Scientific, Inc.).
Cervical cancer cell cycle
analysis
The HeLa (3×104) or SiHa cells
(3×104) lentiviral transfected with miR-10-5p or the
mi-RC controls were placed in a 10 ml centrifuge tube with 100 µl
PBS suspension and 1 ml 75% ethanol. Cells were then fixed
overnight at 4°C. Cells were separated by centrifugation at 8,000 ×
g for 5 min at 4°C. The supernatant was removed by aspirating and
the pellet was washed with PBS, then subjected to centrifugation
8,000 × g for 5 min at 4°C. Cells were digested for 15 min with
RnaseA (cat no. R4875; Yushen Biotechnology Co., Ltd., Taichung,
Taiwan), then stained for 10 min with propidium iodide. The cell
cycle was evaluated using FACSCalibur flow cytometry (BD
Biosciences, Franklin Lakes, NJ, USA), and the results were
analyzed using Multicycle AV 1.0 software (Phoenix Flow Systems,
San Diego, CA, USA).
Western blot analysis
For western blot analysis, cells from the control
and miR-10-5p groups were lysed with lysis buffer (Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol. Proteins were isolated by centrifugation at 13,000 × g at
5°C for 5 min. A BCA kit (Shanghai Qcbio Science and Technologies
Co., Ltd., Shanghai, China) was utilized for protein quantification
according to the manufacturer's protocol. A total of 50 ng protein
was then loaded on to each lane for SDS-PAGE. The concentrations of
stacking and resolving gel were 5 and 15% respectively. Following
SDS-PAGE, proteins were transferred to PVDF membranes [cat no.
L03014; GenScript (Nanjing) Co., Ltd.]. Blocking was performed with
5% dry milk for 1 h at room temperature. Rabbit anti-BDNF antibody
(1:200, cat no. sc-20981; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was used as the primary antibody and was incubated at 4°C
overnight. Goat anti-rabbit horseradish-peroxidase conjugated
antibody [1:1,000 dilution, cat no. A00098; GenScript (Nanjing)
Co., Ltd.] was used as the secondary antibody. The internal control
was β-actin (1:1,000 dilution, cat no. sc-7210; Sigma-Aldrich;
Merck KGaA). Incubation with the secondary antibody was 45 min at
room temperature. The membranes were washed with 10X TBST (cat no.
P0231; Beyotime Institute of Biotechnology) three times and
visualized using an enhanced chemiluminescence film system
according to the manufacture's protocol (GE Healthcare Life
Sciences, Little Chalfont, UK).
Statistical analysis
Data are presented as the mean ± standard deviation.
Comparisons between two groups were evaluated using the two-tailed
Student's t-test with SPSS 19.0 software (SPSS Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-10-5p expression in cervical
cancer cells and normal cervical cells
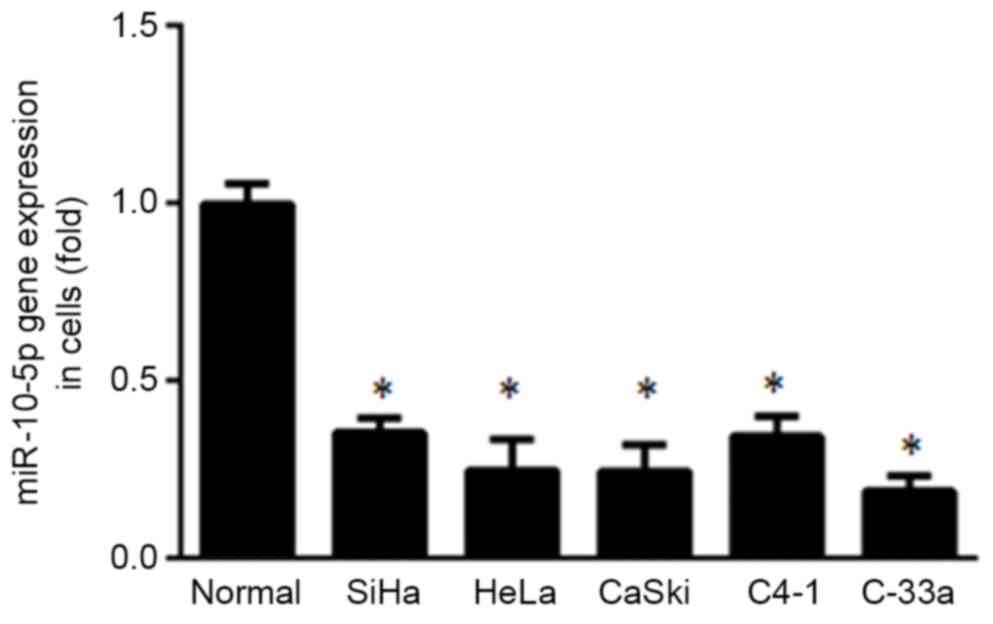
miR-10-5p expression was evaluated in five cervical
cancer cell lines (SiHa, HeLa, CaSki, C4-1 and C-33a) and normal
cervical cells using RT-qPCR. It was demonstrated that miR-10-5p
expression was significantly lower in all cervical cancer cell
lines compared with normal cervical cells (P<0.05; Fig. 1).
Effect of miR-10-5p on the viability
and cell cycle of cervical cancer cells
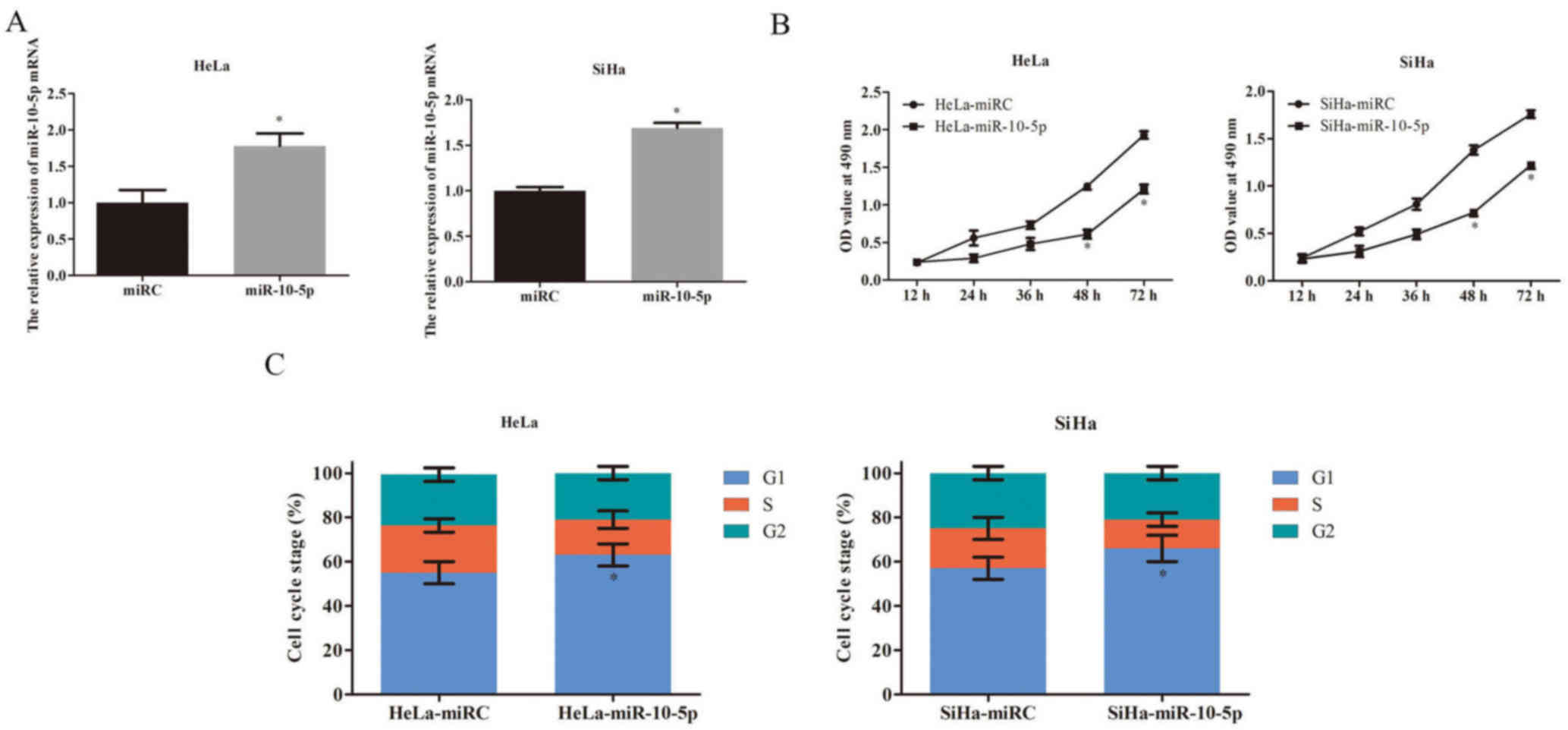
As miR-10-5p was significantly downregulated in
cervical cancer cells compared with normal cervical cells, it was
hypothesized that overexpression of miR-10-5p may have an
inhibitory effect on the viability of cervical cancer cells.
Therefore, miR-10-5p was upregulated in HeLa and SiHa cells using
lentiviral miR-10-5p. Compared with lentiviral miRC, lentiviral
miR-10-5p caused a significant increase in the expression of
miR-10-5p in both HeLa and SiHa cells (P<0.05; Fig. 2A).
An MTT assay indicated that overexpression of
miR-10-5p was able to significantly reduce cell viability at day 4
and 5 compared with the control in HeLa and SiHa cells (P<0.05;
Fig. 2B). To better understand its
inhibitory mechanism, the effect of miR-10-5p overexpression on
cervical cancer cell cycle regulation was evaluated by flow
cytometry. The results indicated that miR-10-5p overexpression
suppressed cervical cancer cell cycle progression, as significantly
more HeLa and SiHa cells were arrested at G1 stage in
miR-10-5p-upregulated cells compared with the control (P<0.05;
Fig. 2C).
BDNF mRNA and protein expression in
cells
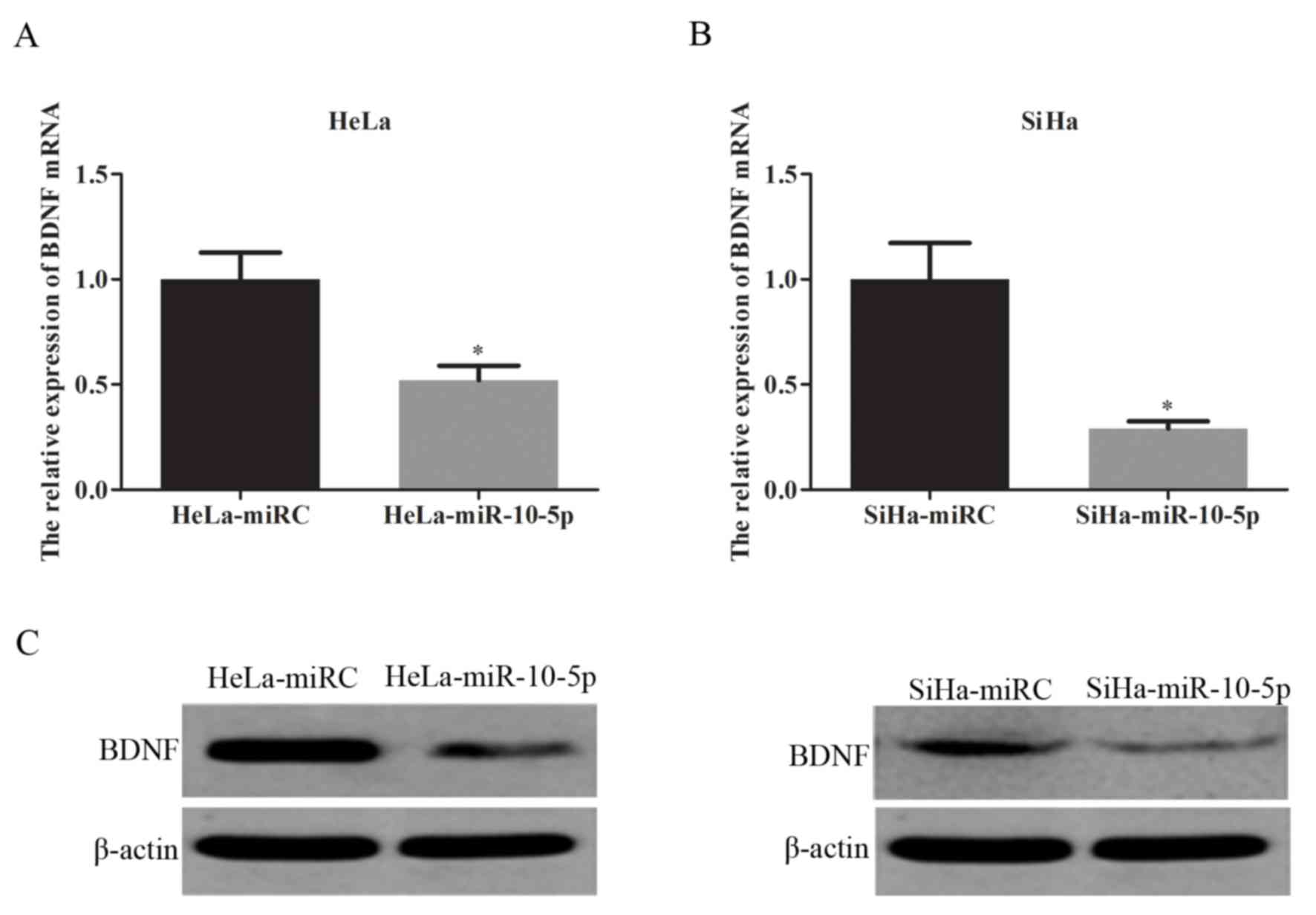
BDNF is a target gene of miR-10-5p (19). The current study aimed to investigate
the association between BDNF and miR-10-5p in two different
cervical cancer cell lines (HeLa and SiHa). BDNF mRNA expression
was evaluated using RT-qPCR and BDNF protein expression was
assessed by western blot analysis. The results indicated that
overexpression of miR-10-5p significantly inhibited BDNF mRNA
expression in HeLa (P<0.05; Fig.
3A) and SiHa cells (P<0.05; Fig.
3B) compared with the control. miR-10-5p overexpression also
suppressed BDNF protein expression in HeLa and SiHa cells compared
with the control (Fig. 3C).
Discussion
miRNA has previously been identified as an important
factor in cell proliferation, division, metastasis or apoptosis in
cervical cancer (20). In the
present study, it was demonstrated that miR-10-5p was significantly
downregulated in cervical cancer compared with normal cervical
cells. A previous study reported that miR-10-5p was downregulated
in chronic laryngeal epithelial premalignant lesions (21).
The current study indicated that the overexpression
of mir-10a-5p reduced cell viability and delayed cell cycle
progression in cervical cancer cells. In laryngeal epithelial
premalignant lesions, miR-10-5p induced cancer cell apoptosis
(21). The current findings are
consistent with the aberrant downregulation of miR-10-5p in cancer
and support the proposal that miR-10-5p has a tumor suppressor role
in various types of cancer.
In the present study, it was identified that BDNF
was a target gene of miR-10-5p in cervical cancer cells. In
previous studies, it was proposed that miR-10a/10b targeted cell
apoptosis or cell division related genes, such as MIB1 LPO, to
induce apoptosis and cell cycle arrest (22,23).
Therefore, the current study aimed to explore the relationship
between miR-10a-5p and BDNF in the development of cancer. In some
types of cancer, BDNF may act as an oncogene. Suppressing BDNF
expression has been reported to inhibit cancer proliferation
(14,24).
The current results indicated that upregulation of
miR-10-5p expression decreased viability and inhibited cell cycle
progression in cervical cancer cell lines. It was also indicated
that BDNF was the target gene of miR-10-5p, and therefore miR-10-5p
may be a key cervical cancer regulator. These data may help to
identify novel molecular pathways to provide targeted gene therapy
for cervical cancer patients.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nam EJ, Kim JW, Hong JW, Jang HS, Lee SY,
Jang SY, Lee DW, Kim SW, Kim JH, Kim YT, et al: Expression of the
p16INK-4a and Ki-67 in relation to the grade of cervical
intraepithelial neoplasia and high-risk human papillomavirus
infection. J Gynecol Oncol. 19:162–168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oluwole OP, Okoyomo OO, Onile TG, Alabi OO
and Gbejegbe JO: Cervical carcinoma, still a burden:
Histopathological analysis and review on the literature. Adv Lab
Med Int. 6:1–6. 2016.
|
|
4
|
Laukkanen P, Läärä E, Koskela P, Pukkala
E, Virkkunen H and Lehtinen M: Population fraction of cervical
neoplasia attributable to high-risk human papillomaviruses. Future
Oncol. 6:709–716. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nilsen TW: Mechanisms of microRNA-mediated
gene regulation in animal cells. Trends Genet. 23:243–249. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang B, Pan X, Cobb GP and Anderson TA:
MicroRNAs as oncogenes and tumor suppression. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu F, Zhang S, Zhao Z, Mao X, Huang J, Wu
Z, Zheng L and Wang Q: MicroRNA-27b up-regulated by human
papillomavirus 16 E7 promotes proliferation and suppresses
apoptosis by targeting polo-like kinase2 in cervical cancer.
Oncogarget. 7:19666–19679. 2016. View Article : Google Scholar
|
|
8
|
Wang WT, Zhao YN, Yan JX, Weng MY, Wang Y,
Chen YQ and Hong SJ: Differentially expressed microRNAs in the
serum of cervical squamous cell carcinoma patients before and after
surgery. J Hematol Oncol. 7:62014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma Q, Wan G, Wang S, Yang W, Zhang J and
Yao X: Serum of microRNA-205 as a novel biomarker for cervical
cancer patients. Cancer Cell Int. 14:812014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mi Y, Wang L, Zong L, Pei M, Lu Q and
Huang P: Genetic variants in microRNA target sites of 37 selected
cancer-related genes and the risk of cervical cancer. PLoS One.
9:e860612014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Safan A, Seifolesiami M, Yahaghi E,
Sedaghati F and Khameneie MK: Retracted Article: Upregulation of
miR-20a and miR-10a expression levels act as potential biomarkers
of aggressive progression and poor prognosis in cervical cancer.
Tumour Biol. Oct 1–2015.(Epub ahead of print).
|
|
12
|
Zhang L, Sun J, Wang B, Ren JC, Su W and
Zhang T: MicroRNA-10b triggers the epithelial-mesenchymal
transition (EMT) of laryngeal carcinoma Hep-2 cells by directly
targeting the E-cadherin. Appl Biochem Biotechnol. 176:33–44. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McAllister AK: Neurotrophins and neuronal
differentiation in the central nervous system. Cell Mol Life Sci.
58:1054–1060. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao L, Liu X, Lin EJ, Wang C, Choi EY,
Riban V, Lin B and During MJ: Environmental and genetic activation
of a brain-adipocyte BDNF/leptin axis causes cancer remission and
inhibition. Cell. 142:52–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaplan DR, Matsumoto K, Lucarelli E and
Thiele CJ: Induction of TrkB by retinoic acid mediates biologic
responsiveness to BDNF and differentiation of human neuroblastoma
cells. Eukaryotic Signal Transduction Group. Neuron. 11:321–331.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu Z, Shen WJ, Kraemer FB and Azhar S:
MicroRNAs 125a and 455 repress lipoprotein-supported
steroidogenesis by targeting scavenger receptor class B type I in
steroidogenic cells. Mol Cell Biol. 32:5035–5045. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo D, Hou X, Zhang H, Sun W, Zhu L, Liang
J and Jiang X: More expressions of BDNF and TrkB in multiple
hepatocellular carcinoma and anti-BDNF or K252a induced apoptosis,
supressed invasion of HepG2 and HCCLM3 cells. J Exp Clin Cancer
Res. 30:972011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Müller S: In silico analysis of regulatory
networks underlined the role of miR-10b-5p and its target BDNF in
huntington's disease. Transl Neurodegener. 3:172014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du X, Lin LI, Zhang L and Jiang J:
microRNA-195 inhibits the proliferation, migration and invasion of
cervical cancer cells via the inhibition of CCND2 and MYB
expression. Oncol Lett. 10:2639–2643. 2015.PubMed/NCBI
|
|
21
|
Hu Y and Liu H: MicroRNA-10a-5p and
microRNA-34c-5p in laryngeal epithelial premalignant lesions:
Differential expression and clinicopathological correlation. Eur
Arch Otorhinolaryngol. 272:391–399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Ling CC, Li L, Qin Y, Qi J, Liu X,
You B, Shi Y, Zhang J, Jiang Q, et al: MicroRNA-10a/10b represses a
novel target gene mib1 to regulate angiogenesis. Carrdiovasc Res.
110:140–150. 2016. View Article : Google Scholar
|
|
23
|
Stadthagen G, Tehler D, Høyland-Kroghsbo
NM, Wen J, Krogh A, Jensen KT, Santoni-Rugiu E, Engelholm LH and
Lund AH: Loss of miR-10a activates lpo and collaborates with
activated Wnt signaling in inducing intestinal neoplasia in female
mice. PLoS Genet. 9:e10039132013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, McMruphy T, Xiao R, Slater A, Huang
W and Cao L: Hypothalamic gene transfer of BDNF inhibits breast
cancer progression and metastasis in middle age obese mice. Mol
Ther. 22:1275–1284. 2014. View Article : Google Scholar : PubMed/NCBI
|