Introduction
Traditional Chinese medicine (TCM) has been used for
a long time and has a large market value as a medicinal product.
Saussureae involucratae Flos, also known as the blood lotus
or thumb flower, is a well-known traditional Chinese medicine used
to treat a number of different conditions, including rheumatoid
arthritis, lower abdominal pain in women, retained placenta and
amenorrhea (1–3). Saussureae involucratae Flos
grows in gravel near the snow line of mountains at 4800–5800 m
above sea level, in areas that include Sinkiang, Qinghai, Tibet and
Gansu (4,5). It is a typical alpine plant and a rare
Chinese medicinal herb with resistance to the cold, which is hard
to obtain. Saussureae involucratae Flos contains flavonoids,
alkaloids and sesquiterpenoids (6),
and has been demonstrated to dispel cold and remove dampness,
stimulate menstrual flow and promote blood circulation (6). The primary component of jiangu is
Saussureae involucratae Flos, however it also consists of 12
types of natural medicine, listed in Table I. These natural components include
Punica granatum L. Semen (7),
Meconopsis horridula (8),
Gentianae Macrophyllae Radix Flos (9), Tinospora sinensis (10), Caryophylli Flos (11), Amomi Fructus Rotundus and
Cassiae Semenv (12) that are
known to be potent in promoting blood circulation to remove blood
stasis and preventing osteoarthritis. In addition, Cinnamomi
Cortex (13), Zingiberis
Rhizoma, Carthami Flos (14) and Polygonati Rhizoma (15) are components of jiangu with
anti-inflammatory and analgesic effects. The jiangu capsule acts to
promote blood circulation, eliminate cold and dampness from the
body and reduce inflammation. Therefore, it has been proposed as a
novel treatment of rheumatoid arthritis and different types of
osteoarthritis (16).
 | Table I.Composition of the jiangu capsule. |
Table I.
Composition of the jiangu capsule.
| Chinese name | Latin
name | Scientific name | Supplier details | Ratio (%) |
|---|
| Xue lian hua | Saussureae
involucratae Flos | Saussurea medusa
Maxim | Tibet Autonomous
Region Medicine Company, Lhasa, China | 20.41 |
| Qin jiao hua | Gentianae
Macrophyllae Radix Flos | Gentiana Straminea
Maxim | Tibet Autonomous
Region Drug Mill, Lhasa, China | 13.62 |
| Shi liu zhi | Punica granatum L.
Semen | Punica granatum
L | Tibet Autonomous
Region Medicine Company, Lhasa, China | 20.41 |
| Rou gui | Cinnamomi
Cortex | Cinnamomum cassia
Presl | Xi'an Chinese
medicine yinpian factory co., LTD, Xi'an, China | 4.08 |
| Gan jiang | Zingiberis
Rhizoma | Zingiber
officinale Rosc | Xi'an Chinese
medicine yinpian factory co., LTD, Xi'an, China | 6.80 |
| Ding xiang | Caryophylli
Flos | Eugenia
caryophyllata Thunb | Xi'an Chinese
medicine yinpian factory co., LTD, Xi'an, China | 3.40 |
| Hong hua | Carthami
Flos | Carthamus
tinctorius L | Xi'an Chinese
medicine yinpian factory co., LTD, Xi'an, China | 3.40 |
| Kuan jin teng | Tinospora
sinensis | Tinospora sinensis
(Lour) Merr. | Tibet Autonomous
Region Medicine Company, Lhasa, China | 6.80 |
| Dou kou | Amomi Fructus
Rotundus. | Amonum kravanh
Pierre ex Gugnep | Xi'an Chinese
medicine yinpian factory co., LTD, Xi'an, China | 4.08 |
| Jue ming zi | Cassiae
Semen | Cassia
obtusifolia L | Xi'an Chinese
medicine yinpian factory co., LTD, Xi'an, China |
6.80 |
| Huang jing | Polygonati
Rhizoma coll.et Hemsl | Polygonatum
kingianum | Xi'an Chinese
medicine yinpian factory co., LTD, Xi'an, China |
6.80 |
| Duo ci lv rong
gao | Meconopsis
horridula | Meconopsis
horridula Hook.f. et Thoms | Tibet Sunbara
shenshui Drug Mill, Lhasa, China |
3.40 |
Limited information exists on the safety of
treatment with jiangu; therefore the present study assessed the
toxicity of acute and chronic oral doses of jiangu. The potential
toxicity following 14 days or 6 months of repeated oral
administration was assessed in mice or rats, respectively, in order
to assess the safety of the jiangu capsule.
Materials and methods
Jiangu capsule preparation
The jiangu capsule was prepared using 12 herbs,
which are listed in Table I with the
purchasing details. Cinnamomi Cortex, Zingiberis Rhizoma,
Caryophylli Flos, Amomi Fructus Rotundus, respectively, were
smashed through an 80 mesh sieve and then mixed with extracts I and
II. The compounds Saussureae involucratae Flos, Gentianae
Macrophyllae Radix Flos, Punica granatum L. Semen,
Tinospora sinensis, Cassiae Semen, Carthami
Flos and Meconopsis horridula underwent reflux
extraction in ten parts 65% ethanol to one part powder for 1 h at
60°C. This was repeated twice under low pressure using the filtrate
from the previous extraction to produce extract I at a relative
density of 1.30–1.33. Polygonati Rhizoma and the dregs from
the previous extraction were mixed and decocted twice in 10× water.
To produce extract II, the filtrate was combined without the dregs
under low pressure at 60°C, to produce a relative density of
1.30–1.33. Extract I and II were collected and dried at low
pressure at a temperature of 50–60°C prior to mixing with
Cinnamomi Cortex, Zingiberis Rhizoma, Caryophylli
Flos and Amomi Fructus Rotundus. The granule was
produced following the use of a 14-mesh sieve with 80% ethanol,
used to soften the material and the 16-mesh sieve was used to
prepare the granule. This compound was enclosed into a size 0
capsule (0.30 g per capsule). A patent for the herbal formula has
been applied for from the State Intellectual Property Office of the
P.R. China (Beijing, China) under the ID: 201510149957.7.
Experimental animals and housing
conditions
A total of 30 ICR mice (15 females and 15 males, 4
weeks old), weighing 18–22 g, were used for the acute toxicity
study. The chronic toxicity test was conducted with a total of 120
4-week old Sprauge-dawley (SD) rats at a gender ratio of 50:50
weighing 80–100 g. All animals were obtained from the Experimental
Animal Center of Xi'an Jiaotong University (Xi'an, China). Animals
were kept in colony cages; 10 rats or 5 mice per cage, under the
following laboratory conditions in a ventilated room: 18–25°C,
35–50% humidity and a 12 h light dark cycle. Animals were fed a
standard commercial diet and tap water. The experimental protocol
was undertaken in accordance with the Guidelines of Care and Use of
Laboratory Animals issued by the Chinese Council on Animal Research
and the Guidelines of Animal Care (17). The present study was approved by the
Animal Ethical Committee of Xi'an Jiaotong University (Xi'an,
China).
Acute toxicity study
The highest dose method was adopted for the acute
toxicity test (18). Doses were
calculated by evaluating the dissolving capacity of the jiangu
capsule in distilled water. ICR mice were randomly assigned to one
of two groups: A control group of 10 mice and a medicated group of
20 mice (5 per cage, segregated by gender). The medicated group
received a 40% concentration of Jiangu capsule, 0.4 ml/10 g/day (16
g/kg/day) by oral gavage (treatment was administered twice in 24 h
with an interval of 6 h between doses). The control group was
administered distilled water (0.4 ml/10 g/day). Mice had ad
libitum access to food and water. The mice were observed for
general behavior changes, toxicity and mortality continuously for 4
h after dosing, intermittently during a 24 h period and then kept
for a further 14 days.
Chronic toxicity study
The chronic toxicity study was conducted over 6.5
months. The 120 SD rats were housed in groups of 10 in plastic
cages, segregated by gender. The animals were randomly grouped into
one of four groups: A control group and high, medium and low dose
groups. Each group consisted of 15 females and 15 males. The weight
of each animal was recorded. The jiangu capsule was administered
orally for 6 months (6 times per week) to the high, medium and low
dose groups in the following concentrations: 8, 4 and 2 g/kg/day,
respectively. During a period of 14 days post treatment, rats were
fed with standard commercial diet and tap water and had ad
libitum access to feed and drinking water. The control group
received distilled water (20 ml/kg/day). Following 6 months of
treatment, 3 months post-treatment and 14 days of withdrawal
without any drug treatment, 10 rats from each group (5 females and
5 males) were sacrificed by cervical dislocation under anesthesia
with 20% urethane (ethyl carbamate; i.p., 1,000 mg/kg; Shanghai
Hengyuan Biological Technology Co., Ltd., Shanghai, China).
Sacrificed rats were used to assess relevant parameters of
hematological and biochemical analysis, urinalysis, systematic
anatomy, organ weights and histopathological examination (18).
General condition, body weight
changes, and food and water consumption
The general condition of different groups was
observed for 1 h following treatment, then intermittently for 4 h
and thereafter over a period of 24 h. The following assessments
were carried out daily using a previously published scale: Pelage
was determined by assessing luster; behavior and activity were
determined by assessing agility; respiration was determined by
assessing if the rats had even breathing; rats were examined for
the presence of any abnormal secretions from the eyes, nose and
genitals; and the colour of the rats' urine was assessed (18,19).
Body weight and food consumption were recorded weekly (20).
Hematological parameters
Blood samples of rats in the four groups were
collected following month 3 and 6, and following 14 days of
withdrawal and analyzed using a BC-5500 Auto Hematological
auto-analyzer (Mindray Medical International, Ltd., Shenzhen,
China). The hematological parameters assessed included red blood
cell count, mean corpuscular hemoglobin concentration, hematocrit,
mean corpuscular hemoglobin, white blood cell (WBC) count, platelet
count, haemoglobin and WBC differential count (21).
Biochemical parameters
At the end of 3 and 6 months treatment, and 14 days
of withdrawal, urine was collected using a syringe whilst rats were
anesthetized with 20% urethane (Ethyl carbamate; 0.5 ml/100 g;
Shanghai Hengyuan Biological Technology Co., Ltd). The detection of
blood, nitrite, pH, urobilinogen, bilirubin, protein, glucose and
ketone bodies in urine was performed using visual eight league test
strips (Guangzhou Huadu Gao'erbao Biological Technology Co., Ltd
Guangzhou, China). Blood samples were centrifuged at 3,000 × g for
10 min at 4°C. Serum was then analyzed using the CL-8000 automatic
biochemistry analyzer (Shimadzu Corporation, Kyoto, Japan) for
biochemical parameters. These parameters included blood urea
nitrogen, creatinine, cholesterol, total bilirubin, total protein,
albumin, glucose, alanine aminotransferase, aminotransferase,
alkaline phosphatase, triglyceride and creatine kinase (22).
Systematic anatomy
Immediately following blood collection, all animals
were sacrificed by cervical dislocation under 20% urethane
anesthesia (1,000 mg/kg, Shanghai Hengyuan Biological Technology
Co., Ltd) and organs including heart, liver, spleen, lungs,
kidneys, adrenal glands, thymus, uterus, ovary, testis, prostate,
thyroid, stomach, bladder, pancreas, thoracic cavity and abdominal
cavity were removed and observed for gross lesions.
Organ weights
Following organ collection, blood was removed using
filter paper and the connective tissue around each organ was cut.
Upon sacrifice, the organ weights of all animals were measured
using an electronic balance. These organs included heart, liver,
spleen, lung, kidney, adrenal gland, thymus, prostate, testis,
ovary, brain, uterus and epididymis (23).
Histopathological examination
Careful pathological examinations were performed on
the following organs: Heart, liver, spleen, lung, kidney, adrenal
gland, thymus, stomach, small intestine, large intestine, thyroid,
parathyroid, brain, cerebellum, brainstem, pancreas, uterus, ovary,
breast, testis, epididymis, prostate, bone marrow, spinal cord,
lymph nodes, esophagus, trachea, pituitary gland, bladder,
arteries, sciatic nerve and optic nerve. The aforementioned organ
samples were fixed in 10% formalin for 36 h at 25°C, embedded in
paraffin, sliced into sections 5-µm thick, stained with
haematoxylin for 5–10 min and then stained with eosin for 1–3 min
at 25°C. Samples were observed using an optical microscope
(24,25).
Statistical analysis
Data were expressed as the mean ± standard error of
the mean and analyzed using one-way analysis of variance followed
by least significant difference correction for multiple comparisons
tests. All figures were created using GraphPad Prism version 5.01
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Acute oral toxicity of jiangu capsule
treatment in mice
The highest dose (16 g/kg) of orally administered
jiangu capsule did not induce any signs of acute toxicity or
mortality in mice. Mice in the control and treatment groups had
glossy fur, moved with ease and demonstrated normal food intake,
urination and defecation during the acute toxicity assessment. The
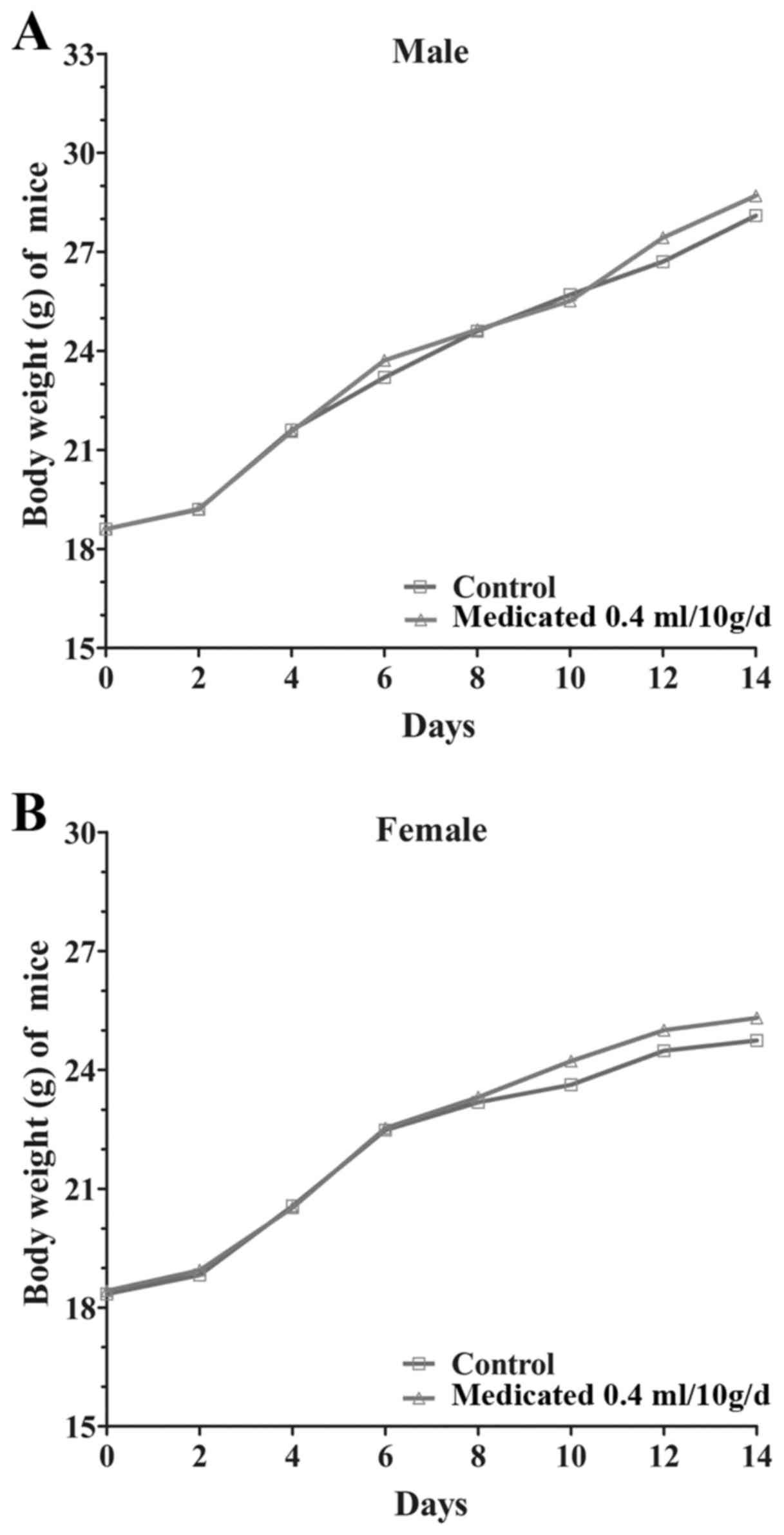
body weight gain of the treated groups was similar to the control
group during the study (Fig. 1).
Following the experiment, all mice were sacrificed and
morphological observations demonstrated that the size and exterior
colors of internal organs (including the heart, liver, spleen, lung
and kidney) of the treated groups were the same as the control
group. The results indicated that the maximum dose of jiangu
capsule in mice is ~16 g/kg.
Chronic oral toxicity study of jiangu
capsule treatment in SD rats
The general condition, body weight changes, food and
water consumption were assessed. No rats succumbed during the 6
months of oral administration. There was no change in appearance,
activity or excrement of the treated group when compared with the
control group during the chronic toxicity test. There was no
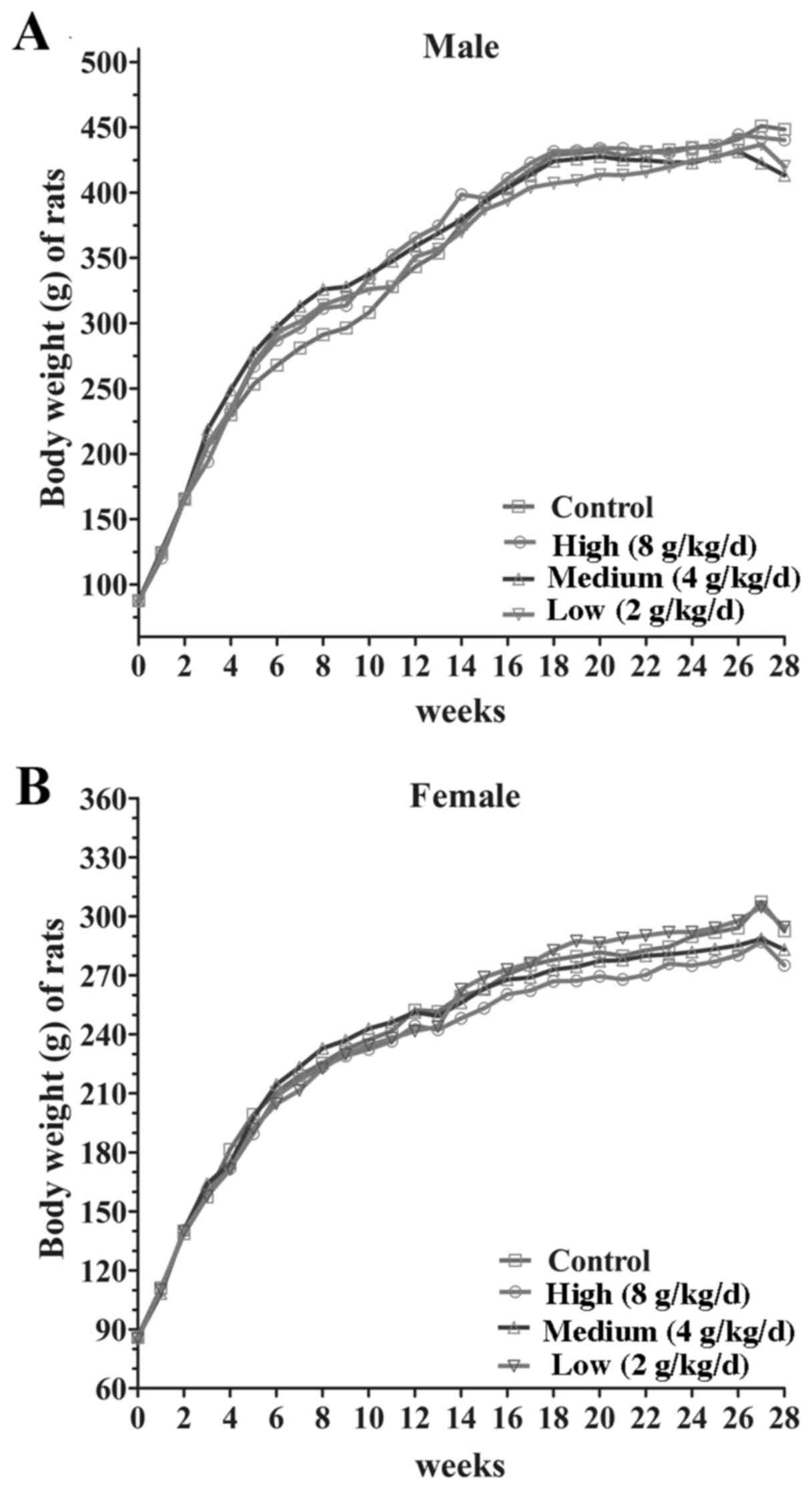
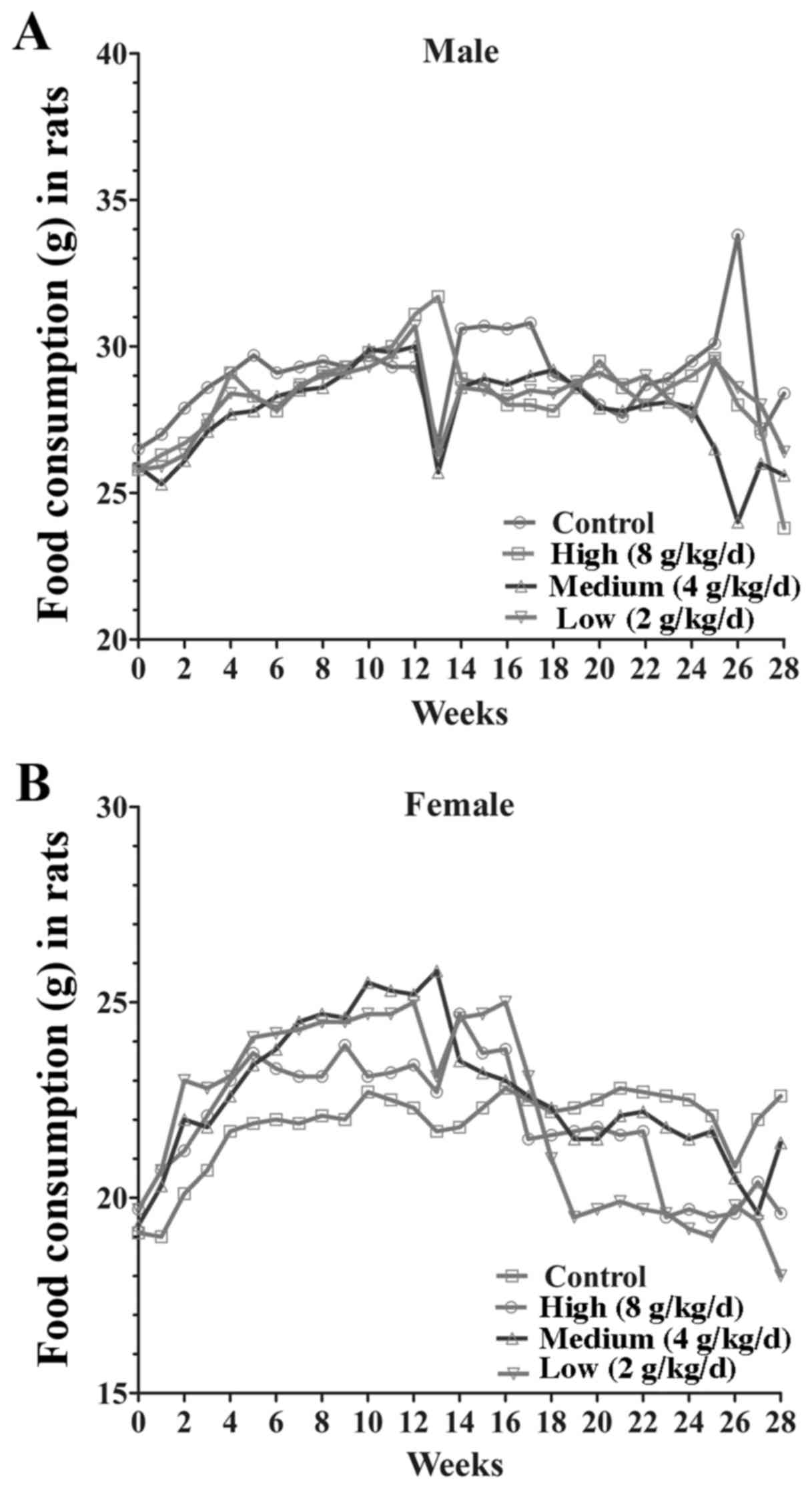
significant difference between the body weight gain (P>0.05) and
food consumption of male and female rats at each dose (8, 4, 2
g/kg/day; Fig. 2). Following 14 days
of withdrawal, there were no significant differences among the
groups in body weight gain and food intake (Fig. 3). These results demonstrated that the
jiangu capsule exhibits no marked effects on body weight or food
consumption in rats.
Hematological parameters
The hematological parameters of groups treated with
the jiangu capsule were within the normal physiological ranges (red
blood cell count: 7.2–9.6×1012/l, hemoglobin: 120–170
g/l, hematocrit: 35–50%, mean corpuscular hemoglobin: 15–20 pg,
mean corpuscular hemoglobin concentration: 30–40 g/l, platelet
count: 50–100×109/l, white blood cell count:
6.0–15.0×109/l, lymphocyte: 65–84%, neutrophil: 9–34%,
monocyte: 0–5%) and the medicated groups exhibited no significant
difference when compared with the control group (Table II; P>0.05). The data suggested
that jiangu capsule treatment exerted no discernable effect on
hematological parameters.
 | Table II.Effect of chronic oral administration
on the hematological parameters of rats following treatment with
jiangu capsule. |
Table II.
Effect of chronic oral administration
on the hematological parameters of rats following treatment with
jiangu capsule.
|
| Treatment
period |
|---|
|
|
|
|---|
| Parameter | Normal
physiological ranges | 3 months | 6 months | 14 days of
withdrawal |
|---|
| Control |
|
|
|
|
| RBC,
×1012/l | 7.2–9.6 |
8.22±0.43 |
8.31±0.42 |
8.30±0.44 |
| HGB,
g/l | 120–170 |
166.32±5.26 |
165.25±6.10 |
167.71±4.75 |
| HCT,
% | 35–50 |
45.82±2.56 |
46.28±3.33 |
45.94±2.54 |
| MCH,
pg | 15–20 |
20.26±0.67 |
19.92±0.61 |
20.25±0.62 |
| MCHC,
g/l | 30–40 |
363.89±21.34 |
358.98±32.03 |
366.07±22.77 |
| PLT,
×109/l |
50–100 |
723.10±89.69 |
713.70±85.83 |
702.00±97.23 |
| WBC,
×109/l |
6.0–15.0 |
14.13±3.70 |
14.71±3.52 |
14.26±3.33 |
|
Lymphocyte, % | 65–84 |
71.50±5.34 |
68.70±4.32 |
68.50±3.31 |
|
Neutrophil, % |
9–34 |
21.70±6.55 |
24.10±3.31 |
23.50±3.44 |
|
Monocyte, % | 0–5 |
6.80±1.87 |
7.20±2.66 |
8.00±2.40 |
| High dose (8
g/kg) |
|
|
|
|
| RBC,
×1012/l | 7.2–9.6 |
8.25±0.36 |
8.18±0.45 |
8.30±0.44 |
| HGB,
g/l | 120–170 |
166.28±6.78 |
167.78±6.63 |
167.71±4.75 |
| HCT,
% | 35–50 |
44.78±2.71 |
45.83±2.88 |
45.94±2.54 |
| MCH,
pg | 15–20 |
20.16±0.25 |
20.53±0.53 |
20.25±0.62 |
| MCHC,
g/l | 30–40 |
372.62±27.79 |
367.69±31.70 |
366.07±22.77 |
| PLT,
×109/l |
50–100 |
695.30±125.38 |
762.00±105.99 |
750.10±73.88 |
| WBC,
×109/l |
6.0–15.0 |
13.64±4.19 |
14.70±3.59 |
14.40±3.22 |
|
Lymphocyte, % | 65–84 |
70.50±6.08 |
67.70±5.96 |
67.80±4.71 |
|
Neutrophil, % |
9–34 |
21.60±7.15 |
24.40±2.80 |
23.40±2.22 |
|
Monocyte, % | 0–5 |
7.90±2.64 |
762.00±105.99 |
8.80±4.29 |
| Medium dose (4
g/kg) |
|
|
|
|
| RBC,
×1012/l | 7.2–9.6 |
8.07±0.36 |
8.21±0.46 |
8.33±0.41 |
| HGB,
g/l | 120–170 |
162.46±5.27 |
165.27±7.37 |
164.73±6.27 |
| HCT,
% | 35–50 |
45.38±2.06 |
45.88±2.98 |
46.34±2.99 |
| MCH,
pg | 15–20 |
20.14±0.56 |
20.13±0.33 |
19.79±0.56 |
| MCHC,
g/l | 30–40 |
358.55±17.20 |
361.49±27.19 |
356.90±27.85 |
| PLT,
×109/l |
50–100 |
711.10±84.18 |
776.80±87.30 |
716.80±91.34 |
| WBC,
×109/l |
6.0–15.0 |
14.02±3.40 |
13.92±3.76 |
14.63±3.11 |
|
Lymphocyte, % | 65–84 |
66.40±4.97 |
67.60±4.40 |
68.90±3.63 |
|
Neutrophil, % |
9–34 |
25.10±3.25 |
24.60±3.69 |
21.90±4.07 |
|
Monocyte, % | 0–5 |
8.50±3.75 |
776.80±87.30 |
9.20±2.44 |
| Low dose (2
g/kg) |
|
|
|
|
| RBC,
×1012/l | 7.2–9.6 |
8.14±0.29 |
8.25±0.38 |
8.37±0.39 |
| HGB,
g/l | 120–170 |
165.47±6.17 |
166.45±5.95 |
166.95±4.59 |
| HCT,
% | 35–50 |
45.94±2.47 |
45.99±2.71 |
46.08±2.79 |
| MCH,
pg | 15–20 |
20.36±0.94 |
20.18±0.36 |
19.96±0.46 |
| MCHC,
g/l | 30–40 |
361.26±26.19 |
363.33±29.13 |
363.68±26.71 |
| PLT,
×109/l |
50–100 |
764.20±101.53 |
711.10±86.98 |
716.00±87.36 |
| WBC,
×109/l |
6.0–15.0 |
14.28±3.16 |
14.75±3.76 |
13.95±3.47 |
|
Lymphocyte, % | 65–84 |
70.60±2.72 |
71.10±5.86 |
68.20±4.16 |
|
Neutrophil, % |
9–34 |
23.80±3.94 |
21.20±4.39 |
24.60±2.84 |
|
Monocyte, % | 0–5 |
5.60±2.22 |
711.10±86.98 |
7.20±3.16 |
Biochemical parameters
The biochemical values of rats in the jiangu
capsule-treated and control groups are presented in Table III. The parameter index of the
treated group was within the normal physiological range. There were
no significant differences in biochemical parameters between the
control and medicated groups (P>0.05; Table III). Urine from all the animals was
a transparent liquid with a light yellow color, and no abnormal
changes in the urinalysis results were observed in the treatment
group compared with the control group. The results suggest that
jiangu capsule treatment has no significant impact on biochemical
parameters or urine composition.
 | Table III.Effect of chronic oral administration
of jiangu capsule on the biochemical parameters of rats |
Table III.
Effect of chronic oral administration
of jiangu capsule on the biochemical parameters of rats
|
| Treatment
period |
|---|
|
|
|
|---|
| Biochemical
parameter | 3 months | 6 months | 14 days
withdrawal |
|---|
| Control |
|
|
|
| Total
proteins, g/l |
70.43±3.09 |
70.07±3.32 |
69.78±4.58 |
|
Albumin, g/l |
33.82±3.76 |
33.21±3.32 |
32.08±3.56 |
| ALT,
U/l |
41.01±4.86 |
42.87±4.31 |
41.50±5.12 |
| AST,
U/l |
56.62±4.29 |
63.03±5.03 |
57.88±3.37 |
| ALP,
U/l |
84.49±9.34 |
83.93±8.79 |
76.85±13.92 |
|
Glucose, mmol/l |
7.01±0.79 |
6.72±0.86 |
7.22±0.64 |
| Total
bilirubin, µmol/l |
4.86±1.29 |
5.03±1.44 |
6.30±1.30 |
| Urea,
mmol/l |
7.65±0.76 |
8.41±0.73 |
8.12±0.91 |
|
Creatinine, µmol/l |
69.43±8.94 |
72.90±9.71 |
71.87±9.99 |
| TC,
mmol/l |
1.77±0.30 |
1.59±0.25 |
1.62±0.22 |
| TG,
mmol/l |
1.04±0.17 |
0.95±0.21 |
0.96±0.16 |
| CK,
U/l |
579.00±81.23 |
679.90±74.04 |
663.60±66.30 |
| High dose (8
g/kg) |
|
|
|
| Total
proteins, g/l |
69.48±3.79 |
69.89±4.05 |
70.06±3.62 |
|
Albumin, g/l |
31.91±4.06 |
31.05±4.50 |
70.06±3.62 |
| ALT,
U/l |
44.96±4.30 |
43.62±5.26 |
70.06±3.62 |
| AST,
U/l |
56.39±5.48 |
57.98±5.50 |
70.06±3.62 |
| ALP,
U/l |
84.43±9.88 |
4.10±1.12 |
84.14±11.59 |
|
Glucose, mmol/l |
7.27±0.80 |
4.10±1.12 |
7.10±0.98 |
| Total
bilirubin, µmol/l |
4.97±1.44 |
4.10±1.12 |
7.06±1.57 |
| Urea,
mmol/l |
8.02±0.78 |
8.46±0.74 |
8.48±0.96 |
|
Creatinine, µmol/l |
74.10±9.32 |
71.25±8.62 |
70.70±9.98 |
| TC,
mmol/l |
1.63±0.30 |
1.63±0.26 |
1.64±0.28 |
| TG,
mmol/l |
1.05±0.23 |
0.98±0.22 |
0.99±0.21 |
| CK,
U/l |
650.70±75.81 |
695.10±77.53 |
655.10±64.84 |
| Medium dose (4
g/kg) |
|
|
|
| Total
proteins, g/l |
69.18±2.29 |
68.37±3.40 |
69.76±3.56 |
|
Albumin, g/l |
31.59±3.14 |
30.83±3.31 |
32.17±3.99 |
| ALT,
U/l |
42.85±4.96 |
43.28±4.98 |
45.84±6.52 |
| AST,
U/l |
57.36±2.94 |
58.62±4.90 |
59.02±4.43 |
| ALP,
U/l |
75.25±12.78 |
81.69±8.37 |
83.04±11.02 |
|
Glucose, mmol/l |
7.24±0.59 |
6.96±1.01 |
6.92±0.89 |
| Total
bilirubin, µmol/l |
4.49±1.19 |
6.77±1.41 |
6.46±1.74 |
| Urea,
mmol/l |
7.96±0.75 |
8.48±0.96 |
8.54±0.85 |
|
Creatinine, µmol/l |
69.39±5.43 |
74.23±9.82 |
73.39±9.34 |
| TC,
mmol/l |
1.60±0.27 |
1.64±0.24 |
1.65±0.24 |
| TG,
mmol/l |
1.00±0.19 |
1.03±0.15 |
1.04±0.15 |
| CK,
U/l |
654.70±77.43 |
703.90±75.05 |
662.50±58.18 |
| Low dose (2
g/kg) |
|
|
|
| Total
proteins, g/l |
69.56±3.75 |
69.80±2.67 |
69.19±3.20 |
|
Albumin, g/l |
31.29±3.49 |
31.94±3.85 |
31.68±4.00 |
| ALT,
U/l |
42.76±4.55 |
42.10±5.54 |
42.94±5.44 |
| AST,
U/l |
55.68±4.60 |
57.56±4.87 |
57.21±2.41 |
| GGT,
U/l |
4.50±1.23 |
3.96±1.27 |
4.89±1.32 |
| ALP,
U/l |
83.19±8.67 |
81.79±10.59 |
77.75±8.15 |
|
Glucose, mmol/l |
7.18±0.71 |
7.32±0.77 |
6.93±0.81 |
| Total
bilirubin, µmol/l |
4.09±1.45 |
5.91±1.25 |
5.40±1.16 |
| Urea,
mmol/l |
8.29±0.46 |
8.47±0.76 |
8.46±0.77 |
|
Creatinine, µmol/l |
75.66±5.53 |
71.50±8.40 |
73.25±8.71 |
| TC,
mmol/l |
1.66±0.27 |
1.52±0.25 |
1.65±0.22 |
| TG,
mmol/l |
0.98±0.18 |
0.96±0.19 |
1.06±0.16 |
| CK,
U/l |
663.70±73.11 |
673.10±69.67 |
684.40±65.24 |
Systematic anatomy
Internal organs were observed by the naked eye. No
effusion was observed in the pleural or peritoneal cavities and no
abnormal colors or morphological changes were identified in the
organs of any rat.
Organ weights
The organ weights of the rats medicated with the
jiangu capsule and the control group is presented in Table IV. There were no significant
differences when compared with the control group during the study
(P>0.05). The results demonstrate that jiangu capsule treatment
does not influence organ weight.
 | Table IV.Effect of chronic oral administration
of jiangu capsule on organ weights of rats. |
Table IV.
Effect of chronic oral administration
of jiangu capsule on organ weights of rats.
|
| Treatment
period |
|---|
|
|
|
|---|
| Parameter | 3 months | 6 months | 14 days of
withdrawal |
|---|
| Control |
|
|
|
|
Heart |
0.33±0.03 |
0.36±0.05 |
0.36±0.06 |
|
Liver |
2.93±0.24 |
2.64±0.29 |
2.39±0.24 |
|
Spleen |
0.21±0.03 |
0.21±0.05 |
0.16±0.03 |
|
Lung |
0.78±0.21 |
0.52±0.14 |
0.65±0.10 |
|
Kidney |
0.68±0.05 |
0.63±0.08 |
0.61±0.06 |
|
Brain |
0.65±0.11 |
0.59±0.14 |
0.56±0.12 |
|
Uterusa |
0.21±0.04 |
0.27±0.06 |
0.23±0.04 |
|
Testiclesa |
0.82±0.08 |
0.73±0.03 |
0.74±0.06 |
|
Epididymisa |
0.45±0.08 |
0.37±0.03 |
0.42±0.05 |
|
Prostatea |
0.09±0.01 |
0.16±0.02 |
0.16±0.05 |
| Adrenal
glands |
22.45±6.98 |
24.12±10.04 |
20.91±7.88 |
|
Thymus |
115.83±25.27 |
86.41±20.02 |
93.67±20.78 |
|
Ovariesa |
49.91±8.47 |
45.20±8.12 |
52.81±11.42 |
| High dose (8
g/kg) |
|
|
|
|
Heart |
0.34±0.04 |
0.36±0.04 |
0.36±0.05 |
|
Liver |
2.76±0.33 |
2.88±0.41 |
2.43±0.24 |
|
Spleen |
0.20±0.04 |
0.22±0.06 |
0.17±0.06 |
|
Lung |
0.65±0.21 |
0.53±0.06 |
0.64±0.12 |
|
Kidney |
0.69±0.06 |
0.64±0.05 |
0.61±0.06 |
|
Brain |
0.67±0.15 |
0.57±0.13 |
0.56±0.12 |
|
Uterusa |
0.27±0.10 |
0.25±0.02 |
0.23±0.03 |
|
Testiclesa |
0.77±0.04 |
0.72±0.03 |
0.73±0.13 |
|
Epididymisa |
0.40±0.08 |
0.36±0.03 |
0.43±0.05 |
|
Prostatea |
0.10±0.01 |
0.17±0.02 |
0.17±0.03 |
| Adrenal
glands |
19.52±7.63 |
28.18±9.19 |
26.44±10.69 |
|
Thymus |
104.95±34.19 |
77.25±25.16 |
79.86±24.57 |
|
Ovariesa |
41.63±1.46 |
46.78±24.26 |
48.20±11.34 |
| Medium dose (4
g/kg) |
|
|
|
|
Heart |
0.34±0.04 |
0.36±0.05 |
0.36±0.04 |
|
Liver |
2.85±0.26 |
2.82±0.66 |
2.52±0.31 |
|
Spleen |
0.24±0.06 |
0.21±0.06 |
0.18±0.04 |
|
Lung |
0.82±0.25 |
0.53±0.07 |
0.62±0.07 |
|
Kidney |
0.69±0.08 |
0.62±0.05 |
0.61±0.09 |
|
Brain |
0.68±0.15 |
0.58±0.13 |
0.58±0.12 |
|
Uterusa |
0.25±0.07 |
0.23±0.03 |
0.23±0.05 |
|
Testiclesa |
0.77±0.07 |
0.74±0.10 |
0.74±0.17 |
|
Epididymisa |
0.39±0.07 |
0.39±0.04 |
0.43±0.09 |
|
Prostatea |
0.08±0.02 |
0.17±0.04 |
0.16±0.02 |
| Adrenal
glands |
23.41±9.52 |
21.53±13.22 |
24.37±9.77 |
|
Thymus |
103.43±19.46 |
74.74±20.55 |
87.54±24.60 |
|
Ovariesa |
48.14±9.50 |
47.83±11.14 |
52.70±6.07 |
| Low dose (2
g/kg) |
|
|
|
|
Heart |
0.34±0.06 |
0.37±0.04 |
0.36±0.06 |
|
Liver |
2.76±0.28 |
2.67±0.47 |
2.53±0.22 |
| Low dose (2
g/kg) |
|
|
|
|
Spleen |
0.23±0.05 |
0.21±0.04 |
0.17±0.03 |
|
Lung |
0.71±0.18 |
0.52±0.07 |
0.66±0.05 |
|
Kidney |
0.70±0.06 |
0.63±0.04 |
0.62±0.05 |
|
Brain |
0.68±0.18 |
0.57±0.11 |
0.57±0.11 |
|
Uterusa |
0.23±0.09 |
0.24±0.04 |
0.24±0.06 |
|
Testiclesa |
0.84±0.10 |
0.73±0.10 |
0.75±0.05 |
|
Epididymisa |
0.42±0.11 |
0.39±0.03 |
0.44±0.04 |
|
Prostatea |
0.10±0.03 |
0.17±0.02 |
0.16±0.03 |
| Adrenal
glands |
25.32±9.61 |
27.24±6.18 |
21.96±6.31 |
|
Thymus |
111.38±45.44 |
81.62±25.89 |
92.41±18.23 |
|
Ovariesa |
46.04±8.46 |
51.38±7.02 |
49.98±6.68 |
Histopathological examination
No histopathological changes were observed in the
organs of all rats that were treated with the jiangu capsule. The
aforementioned results indicate that the jiangu capsule did not
exert an effect on the body weight, food intake, hematological
parameters, urine composition, biochemical parameters, systematic
anatomy, histopathological parameters or the weight of internal
organs of rats that underwent the chronic study.
Discussion
Traditional herbal medicine serves an important role
in complementary and alternative medicine. Saussureae
involucratae Flos has been widely applied as a clinical
treatment for thousands of years (26). The jiangu capsule is composed of 12
types of natural medicine and has been used to treat a number of
conditions, including arthralgia, cold and sore muscles, stiff
limbs, inconvenient flexion and activity limitation caused by
osteoarthritis (27,28). However, there is a little information
regarding jiangu capsule treatment in relation to toxicity.
Therefore, the present study aimed to assess the toxicology and
identify the safe dosage of the jiangu capsule prior to clinical
trials.
Hematological and serum biochemical parameters are
important markers of the physiological and pathological state of
the blood. Thus, variations in these parameters may indicate
toxicity associated with the compound being assessed. In the
chronic study, oral administration for 6 months did not lead to any
significant differences in the hematological and biochemical
parameters of the treatment groups when compared with controls.
These results suggest that the jiangu capsule has no significant
toxicological effects on the hemopoietic system.
Urinalysis is known to be the first sign of kidney
or urinary tract diseases and may offer important clues regarding
the nature of pathological processes (29). The current study did not detect any
adverse changes in urine routines when comparing the treatment and
control groups. This indicates that the jiangu capsule does not
induce toxic effects on the kidney or urinary systems. Assessment
of the internal organ weights and histopathological examination of
the treatment groups identified no abnormalities and the results
were similar to those obtained from the control group. Differences
in the organ weights of the two groups were not significant and
were not considered to be associated with jiangu treatment. The
results obtained therefore indicate that the jiangu capsule is
relatively non-toxic.
In the acute toxicity test, the highest oral dose of
the jiangu capsule (concentration, 40%) administered was 16
g/kg/day (yield of extract of the raw material, 47.04 g/kg), which
is equal to a 356-fold increase of the recommended clinical dosage
for a 60 kg human. The mice treated with the jiangu capsule
exhibited unlimited movement, normal food consumption and normal
excretion throughout the experiment. The acute study demonstrated
that there were no adverse changes or mortality in mice following
jiangu administration.
In the chronic toxicity study, there were no visible
signs of morbidity or mortality in rats that received the highest
dose of 8 g/kg/day (yield of extract of the raw material 47.04
g/kg). The highest dose is equivalent to 178× of the proposed
clinical dosage for a 60 kg human. There were no changes in the
general condition, hematological and biochemical values,
urinalysis, systematic anatomy, organ weights or histopathological
examination in the treatment groups compared with the control
group. The results of the chronic study demonstrated that the
jiangu capsule does not produce long-term or delayed toxicity in
rats following administration.
In conclusion, the present study demonstrated that
high doses of jiangu capsule are relatively safe in rats and mice,
due to no fatalities occurring or abnormal results in acute and
chronic toxicity detected following assessments. Therefore, the
jiangu capsule may be used for oral administration without toxic
effects. The jiangu capsule may possess the potential to treat
diseases including arthralgia, cold and sore muscles, stiff limbs,
inconvenient flexion and activity limitation caused by different
types of osteoarthritis.
Acknowledgements
The present study was supported by funds from the
National Natural Science Foundation of China (grant no. 81170176),
the Scientific Research Foundation for the Returned Overseas
Chinese Scholars, the State Education Ministry (grant no. 2012-08),
Shaanxi Province Science and Technology Plan Project (grant no.
2014KTCL03-10), and the Ministry of Science and Technology and
Technological Special Project for ‘Significant New Drugs
Development’ (grant no. 2011ZX09401-308-32).
References
|
1
|
Song LR: China Herbal. 1st edition.
Shanghai Science and Technology Publisher; Shanghai: 1999, (In
Chinese).
|
|
2
|
Jung JH, Kim Y, Lee CO, Kang SS, Park JH
and Im KS: Cytotoxic constituents of Saussurea lappa. Arch Pharm
Res. 21:153–156. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng RL, Liu GS, Xing GX, Jia ZJ, Du M
and Tan LQ: Free radical scavenging and antifatigue activities of
Saussurea involucrate polysaccharides. Zhongguo Yao Li Xue
Bao. 14 Suppl:S47–S49. 1993.(In Chinese). PubMed/NCBI
|
|
4
|
Su KY, Yu CY, Chen YP, Hua KF and Chen YL:
3,4-Dihydroxytoluene, a metabolite of rutin, inhibits inflammatory
responses in lipopolysaccharide-activated macrophages by reducing
the activation of NF-κB signaling. BMC Complement Altern Med.
14:212014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma HP, Fan PC, Jing LL, Yao J, He XR, Yang
Y, Chen KM and Jia ZP: Anti-hypoxic activity at simulated high
altitude was isolated in petroleum ether extract of Saussurea
involucrate. J Ethnopharmacol. 137:1510–1515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Li and Wang XL: Research on chemical
composition of pharmacology and its clinic application of
Saussurea Involucrata. J Southwest Univ Nationalities.
29:424–428. 2003.(In Chinese).
|
|
7
|
Singh RP, Murthy Chidambara KN and
Jayapakasha GK: Studies on the antioxidant activity of pomegranate
(Punica granatum) peel and seed extracts using in vitro
models. J Agric Food Chem. 50:81–86. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu HF, Ding LS, Wang H and Zhang XF:
Advances in the research of phytochemistry and pharmacology of
meconopsis vig. Nat Prod Res Dev. 23:163–168. 2011.(In
Chinese).
|
|
9
|
Yu F, Yu F, Li R and Wang R: Inhibitory
effects of the Gentiana macrophylla (Gentianaceae)
extract on rheumatoid arthritis of rats. J Ethnopharmacol.
95:77–81. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jain S, Sherlekar B and Barik R:
Evaluation of antioxidant potential of Tianspora Cordifolia
and Tinospaora Sinensis. Int J Clin Pharmacol Res.
1:122–128. 2010.
|
|
11
|
Rodrigues TG, Fernandes A Jr, Sousa JP,
Bastos JK and Sforcin JM: In vitro and in vivo effects of clove on
proinflammatory cytokines production by macrophages. Nat Prod Res.
23:319–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu B, Gong HX, Xiao XF and Ye GF:
Advances in studies on chemical constituents of Cassia Semen
and their pharmacological activities. Drug Eval Res. 33:312–315.
2010.(In Chinese).
|
|
13
|
Lee SH, Lee SY, Son DJ, Lee H, Yoo HS,
Song S, Oh KW, Han DC, Kwon BM and Hong JT: Inhibitory effect of
2′-hydroxyeinnamadehyde on nitric oxide production through
inhibition of NF-kappa B activation in RAW 264.7 cells. Biochem
Pharmacol. 69:791–799. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu RY and Tong SH: The research progress
of the chemical components and pharmacological effects of
safflower. Chin Pharmaceuticals. 19:86–87. 2010.(In Chinese).
|
|
15
|
Kim GY, Lee JY, Lee JO, Ryu CH, Choi BT,
Jeong YK, Lee KW, Jeong SC and Choi YH: Partial characterization
and immunostimulatory effect of a novel polysaccharide-protein
complex extracted from Phellinus linteus. Biosci Biotechnol
Biochem. 70:1218–1226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yin H: Studies on chemical constituents,
pharmacological activities and clinical application of
Saussureae Involucratae Herba. J Qiqihar Univ Med.
34:1010–1012. 2013.(In Chinese).
|
|
17
|
He ZM, Li GP, Zhu DS and Lu SM: Guidelines
of Management and Use of Laboratory Animals. Science press;
Beijing: 2016, (In Chinese).
|
|
18
|
Qi C: The Methodology of Traditional
Chinese Medicine Pharmacology Research. 2nd edition. People's
Medical Publishing House; Beijing: 2006, (In Chinese).
|
|
19
|
Wei W, Wu XM and Li YJ: Experimental
Methodology of Pharmacology. 4th edition. People's Medical
Publishing House; Beijing: 2010, (In Chinese). View Article : Google Scholar
|
|
20
|
Mu LH, Huang ZX, Liu P, Hu Y and Gao Y:
Acute and subchronic oral toxicity assessment of the herbal formula
Kai-Xin-San. J Ethnopharmacol. 138:351–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chiranthanut N, Teekachunhatean S,
Panthong A, Khonsung P, Kanjanapothi D and Lertprasertsuk N:
Toxicity evaluation of standardized extract of Gynostemma
pentaphyllum Makino. J Ethnopharmacol. 149:228–234. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ha H, Lee JK, Lee HY, Seo CS, Kim JH, Lee
MY, Koh WS and Shin HK: Evaluation of safety of the herbal formula
Ojeok-san: Acute and sub-chronic toxicity studies in rats. J
Ethnopharmacol. 131:410–416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ezeja MI, Anaga AO and Asuzu IU: Acute and
sub-chronic toxicity profile of methanol leaf extract of Gouania
longipetala in rats. J Ethnopharmacol. 151:1155–1164. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bancroft John D and Gamble Marilyn: Theory
and practice of histological techniques, sixth edition Churchill
Livingstone. USA: 2007
|
|
25
|
Tahraoui A, Israili ZH and Lyoussi B:
Acute and sub-chronic toxicity of a lyophilized aqueous extract of
Centaurium erythraea in rodents. J Ethnopharmacol.
132:48–55. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chik WI, Zhu L, Fan LL, Yi T, Zhu GY, Gou
XJ, Tang YN, Xu J, Yeung WP, Zhao ZZ, et al: Saussurea
involucrata: A review of the botany, phytochemistry and
ethnopharmacology of a rare traditional herbal medicine. J
Ethnopharmacol. 172:44–60. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chinese Pharmacopoeia Commission:
Pharmacopeia of the People's Republic of China. 1. Chemical
Industry Press; Beijing: pp. 50–51. 2010
|
|
28
|
National Institutes for Food and Drug
Control: Zhongguo Minzu Yaozhi. People's Medical Publishing House;
Beijing: pp. 448–449. 1984
|
|
29
|
Shao C, Wang Y and Gao Y: Applications of
urinary proteomics in biomarker discovery. Sci China Life Sci.
54:409–417. 2011. View Article : Google Scholar : PubMed/NCBI
|