Introduction
Thyroid hormones (TH) are critical for brain
development throughout gestation and the first year after birth
(1). However, thyroid dysfunction is
common in preterm infants, and this has been associated with
neurodevelopmental delays and cognitive dysfunction in later life
(2–5). Transient hyperthyrotropinemia (HTT)
with hypothyroxinemia is common in preterm infants. Initial HTT can
lead to transient self-limited hypothyroidism or permanent
hypothyroidism requiring TH replacement therapy. Preterm infants
are at significant risk of congenital hypothyroidism, which is
unpredictable in its evolution to transient or permanent (6). Several physiologic and non-physiologic
factors may contribute to hypothyroidism in premature infants,
including immaturity of the hypothalamic-pituitary-thyroid axis,
limited thyroid capacity to concentrate and synthesize iodine,
increase in TH requirement needs for thermogenesis, iodine
insufficiency, and preterm diseases (7). TH play an important role in myelin
development, in the production and maturation of oligodendrocytes,
and on the expression of genes encoding myelin (8). The period of TH sensitivity with
regards the myelin gene has been reported to extend from about the
end of the first postnatal week in rat brains, which is equivalent
to late gestation in human brains, which is the high-risk period of
periventricular leukomalacia, a major cause of cerebral palsy (CP)
(9). TH deficiency may impair
myelination by alternating the timing of the differentiation of
oligodendrocyte precursor cells and interrupts the proliferation of
precursor cells. The commonest magnetic resonance imaging (MRI)
finding in children with CP is volume reduction of periventricular
white matter (WM), which provided the evidence of myelination
impairment (10).
Brain MRI is thought to be a more sensitive tool to
assess the extent of myelination, as it can detect important
changes in brain structure in patients with hypothyroidism. For
example, neonates with clinically untreated hypothyroidism have
been shown to have abnormal brain metabolite changes on magnetic
resonance spectroscopy (11) and a
reversal of abnormal myelination with thyroxine therapy was
documented using proton magnetic resonance spectroscopy in three
patients with congenital hypothyroidism (12). Diffusion tensor imaging (DTI) makes
it possible to observe WM pathways before myelination is evident on
conventional MRI (13), enabling
measurements of apparent diffusion coefficients (ADC) and
fractional anisotrophy (FA), which are sensitive to the integrity
and organization of the WM microstructure. DTI quantification
requires computation of a tensor, which is a mathematical
description of a three-dimensional ellipsoid depicting the
magnitude and orientation of diffusion in individual voxels. The
tensor is associated with three corresponding orientational vectors
(eigenvectors, λ1, λ2, λ3), describing the diffusion ellipsoid by
its major axes. The eigenvalue average, or trace, reflects the
magnitude of diffusion, referred to as mean diffusivity (MD) or the
ADC. The extent to which one eigenvalue, λ1, dominates the other
two, λ2 and λ3, determines the degree of anisotropy, that is, the
degree of orientational preference within a voxel, typically
measured as FA, ranges between 0 and 1 on a normalized scale
(14). The largest eigenvalue, λ1,
is the axial (or longitudinal) diffusivity and reflects axonal
integrity, whereas λ2 and λ3 quantify radial (or transverse)
diffusivity, λT = (λ2 + λ3)/2, and reflect myelin integrity
(15). Thus, disruption of the WM
microstructure as detected by DTI can reflect compromised myelin,
cytoskeletal structure, and axonal density. Furthermore, MRI with
DTI metrics can provide an opportunity to investigate whether
measures of brain microstructure reflecting the extent of
myelination differ between preterm infants with and without TH
dysfunction. Therefore, we aimed to investigate how alterations in
brain microstructure affect neurodevelopment after 2 years of
follow-up.
Several methods are used determine whether an infant
is developing normally, including neurological examinations, parent
questionnaires, developmental screening techniques such as the
Denver Developmental Screening test II, the CAT/CLAMS, and
neurodevelopmental assessments such as the Bayley Scales of Infant
Development, third edition (BSID-III), the Gesell Developmental
schedules, and the Mullen Scales of Early Learning. Among these
assessment tools, the BSID-III require only 45 min to an hour to
complete, however they require an advanced level of training and
expertise to administer. In clinics that follow the growth and
neurodevelopment of low birth weight infants, the infants may
receive either a neurological examination or a developmental
assessment.
In this study, we aimed to investigate the impacts
of TH in early [gestational age (GA), <30 weeks] and late (GA,
≥30 weeks) preterm infants, and examine correlations among serum TH
level, neurodevelopmental outcomes and MRI with DTI metrics.
Therefore, there were three major aims: i) To assess the
correlation of thyroid stimulating hormone (TSH) levels at
different time points after birth, and neurodevelopmental outcomes
at 2 year follow-up; ii) to further investigate whether thyroid
status was associated with DTI scalars related to brain
microstructure at term equivalence; and iii) to determine whether
DTI metrics are a sensitive marker for adverse neurodevelopmental
outcomes at 2 years of age.
Patients and methods
Patient collection
In this prospective cohort study, we enrolled
preterm infants with a GA of 23–36 weeks who were admitted to our
neonatal intensive care unit (NICU) without perinatal stressful
events or asphyxia based on previous study (16). The exclusion criteria were: i)
Intrapartum distress including placental abruption, thick meconium
amniotic fluid and/or abnormal fetal heart beat (e.g., bradycardia,
<100 beats/min); ii) Apgar score at the 5th min, ≤7; iii)
umbilical cord blood pH, ≤7.15; iv) umbilical artery base excess,
>12; v) those requiring bag and mask ventilation for at least 1
min immediately after birth; vi) multi-organ involvement (brain,
heart, kidney); vii) those requiring resuscitation within 1 h of
birth; viii) abnormal neurological examination results during NICU
stay; ix) abnormal electroencephalography, abnormal head
ultrasound, or abnormal neuroimaging findings during NICU stay; x)
those who presented with any congenital malformation or abnormal
genetic conditions (e.g., trisomy 18, trisomy 21, fragile X
syndrome); and xi) those with inborn errors of metabolism (e.g.,
phenylketonuria, or maple syrup urine disease). The infants of
mothers who had thyroid disease, used thyroxin or anti-thyroid
drugs during pregnancy were also excluded. The Ethics Committee of
Kaohsiung Chang Gung Memorial Hospital (Kaohsiung, Taiwan) approved
the study, and written informed consent was obtained from the
parents or guardians of the newborns (IRB no. 100-2025A3).
The study subjects were divided into two groups
according to GA: The early preterm group (<30 weeks) and late
preterm group (≥30 weeks). Clinical data, including sex, GA, Apgar
score at 1 and 5 min, arterial pH, delivery mode and birth body
weight were collected.
Thyroid function tests in the preterm
infants
A blood sample was collected from each infant in
both groups for gas analysis immediately after admission to the
NICU to determine the level of TSH using a heel stick blood test.
The level of TSH was determined using drops of blood collected on
filter paper on the initial day of admission (range, 1–2 h), and at
18 h (range, 1–2 h) and 24 h of admission (range, 2–4 h) which were
stored at −70°C in sealed bags with desiccant until being
processed. All procedures were performed by the same biochemist who
was blinded to the patients' data. The level of TSH was determined
from the dried blood spots at a university hospital newborn
screening program and expressed in µU/ml. The cut-off value of TSH
used to define hypothyroxinemia in the blood spot tests was 9 µU/ml
based on reference range of Taiwan Newborn Screening Center. The
blood spot analyses were done using Immuchem™ Neonatal TSH-MW ELISA
(bulk kit-20 plates) and RIA kits (500 tubes kit) (MP Biochemicals,
Santa Ana, CA, USA). If the results were abnormal, the level was
rechecked after 2 weeks. The patients with a normal level in the
second exam were defined as having ‘transient’ hypothyroidism, and
those with abnormal results in the second exam were defined as
having ‘persistent’ hypothyroidism requiring thyroxine replacement
therapy.
MRI study protocol
The study group and control group underwent
conventional MRI with DTI at a postmenstrual term-equivalent age.
Parental informed consent was always obtained before the
examination. The data were evaluated retrospectively by an
experienced neuro-radiologist.
Images were acquired using standard scanning
protocols on a 1.5-T GE Echo Speed scanner (GE Medical Systems,
Milwaukee, WI, USA) including T1-W (spin echo, TR/TE 500/11 msec)
and T2-W sequences (spin echo, TR/TE 3,000/120 msec) with a slice
thickness of 4 mm and 0.4-mm gap. For DTI, an echo planar sequence
with diffusion gradients (b=1,000 sec/mm2) applied in 25
non-collinear directions was used with a slice thickness of 3 mm
and no gap. An average of 20 slices was recorded within 4 min using
TR/TE 9,150/98-91 msec. The field of view was 20 cm, the scan
matrix was 128×128, and the reconstruction matrix was 256×256. All
of the infants were scanned using a magnetic resonance-compatible
incubator with a specialized high-sensitivity neonatal head coil
(LMT Medical Systems GmbH, Lübeck, Germany) that allowed for DTI
imaging at high spatial resolution and high signal-to-noise ratio.
The incubator provided controlled temperature and humidity, and the
infants were sedated with chloral hydrate 50 mg/kg 30 min before
the examination and their position was fixed using a vacuum pillow.
This set-up allowed for imaging in this vulnerable patient
population in a stable and safe microenvironment. We used moldable
earplugs and neonatal earmuffs to reduce the noise, and pads around
the infant's head kept movement to a minimum. The DTI scalars we
chose were listed as followings: Radial diffusivity (RD), MD, axial
diffusivity (AD), and FA are DTI scalars which are sensitive
markers for measuring the biological microstructure of WM. AD tends
to be variable in WM pathology and tends to increase with brain
maturation. The tracts selected for quantization in the study
included commissural tracts [corpus callosum (CC): Splenium and
genu], projection tracts including those of the posterior limb of
the internal capsule (PLIC), the anterior limb of the internal
capsule (ALIC), and association tracts [external capsule (EC)].
Regions of interest (ROIs) of the DTI measurements were identified
from multiple ROIs positioned bilaterally within individual WM
tracts. For placement, we used standard-sized, round-shaped
16-pixel ROIs.
Neurodevelopmental evaluation
At 6, 12, 18, and 24 months corrected age, the
BSID-III was administered by experienced testers (17). The BSID-III contains two main scales:
The mental developmental index (MDI) and psychomotor developmental
index (PDI). MDI and PDI scores of 100±15 represent the mean ± 1SD.
BSID-PDI had designed to assess cognition, and the BSID-MDI had
designed to evaluate motor outcome at 2-year follow-up. To assess
whether DTI scores were correlated to motor outcomes, we defined
the infants receiving physical and occupational therapy as the
‘Neuromotor dysfunction’ group, and the other healthy infants as
the ‘without neuromotor dysfunction’ group.
Statistical analysis
Statistical analysis was performed using SPSS
software version 18.0 (SPSS, Inc., Chicago, IL, USA). Differences
in categorical variables between two groups were analyzed using the
independent Student's t-test. TSH levels at different time points
were analyzed using one-way ANOVA with repeat measurements. The
correlations between neurodevelopment, MD and GAs were analyzed by
Pearson's correlation analysis. The correlation among plasma TSH
levels, DTI metrics of ROIs and neurodevelopmental outcomes were
analyzed using bivariate correlation analysis. A P-value <0.05
was considered to indicate statistical significance.
Results
A total of 81 premature infants were enrolled in
this study with a GA ranging from 23–36 weeks. Infants in the late
preterm group were significantly heavier than the early preterm
group (1,287.3±388.1 vs. 1,032.5±32.5 g; P<0.001) and were more
likely to be delivered by cesarean section (CS) (Table I). Caues of CS were summarized in
Table II. We have only 8 patients
with GA <30 weeks receiving CS, and most of the causes were
contributed to maternal health problems. In the contrast, we have
54 patients with GA >30 weeks receiving CS, and the causes were
contributed eventfully to fetal and maternal problems (Table II). There were no significant
differences in sex, Apgar score at 1 and 5 min, and initial
umbilical arterial pH between the two groups.
 | Table I.Clinical information of enrolled
patients (n=81). |
Table I.
Clinical information of enrolled
patients (n=81).
| Variables | ≤30 weeks (n=17) | >30 weeks
(n=64) | P-value |
|---|
| Sex (n) |
| Male | 9 | 30 | 0.786 |
|
Female | 8 | 34 |
|
| Apgare score |
| 1 min
<6 (n) | 6 | 9 |
|
| 5 min
<6 (n) | 0 | 2 |
|
| Delivery mode |
| CS | 8 | 53 | 0.005a |
| NSD | 9 | 11 |
|
| Birth weight (g) | 1,032.5±32.5 | 1,287.3±388.1 | 0.021a |
| Arterial
pH |
| pH
<7.25 | 3 | 13 |
|
 | Table II.Causes of cesarean section in study
subjects. |
Table II.
Causes of cesarean section in study
subjects.
| Causes of cesarean
delivery | GA ≤30 weeks (CS,
n=8) (n, %) | GA >30 weeks (CS,
n=54) (n, %) |
|---|
| Fetal problems
(total) | 3 (37.5) | 26 (48.1) |
| Fetal
distress | 0 (12.5) | 9 (16.7) |
| Premature
delivery | 2 (25) | 9 (16.7) |
| Fetal
malposition | 0 (0) | 3 (5.5) |
| Cord
prolapse | 0 (0) | 1 (1.8) |
| Twin-twin
transfusion syndrome | 1 (12.5) | 0 (0) |
|
Oligohydramnios | 0 (0) | 1 (1.9) |
|
Chorioamnionitis | 0 (0) | 1 (1.9) |
| Fetal
growth arrest | 0 (0) | 2 (3.7) |
| Maternal problems
(total) | 5 (62.5) | 28 (51.9) |
|
Multiple pregnancies | 0 (0) | 10 (18.5) |
|
Placenta problems | 1 (12.5) | 6 (11.1) |
|
Preeclampsia | 4 (50) | 7 (13.0) |
|
Maternal medical crisis | 0 (0) | 5 (9.3) |
There were also no significant differences in mean
TSH values between the groups at 0, 18, or 24 h of admission. The
mean MDI scores were similar between the two groups from 6 months
to 24 months of follow-up, and the mean PDI scores were comparable
between the two groups until 18 months corrected age. The early
preterm group had significantly lower PDI scores than the late
preterm group at 24 months of follow-up (93±31 vs. 107±31). The
rates of HTT were 4.5, 0, and 4.5% at TSH0, TSH18, and TSH 24 in
the early preterm group, and 20.3, 8.5, and 3.4% in the late
preterm group, respectively. All of the infants with abnormal
results had normal results in the second examination, which
indicated all of the preterm with HTT in our study had transient
hypothyroidism (Table III).
Multivariate analysis had been performed to analyze the factors
related to neurodevelopmental outcome. The data demonstrated that
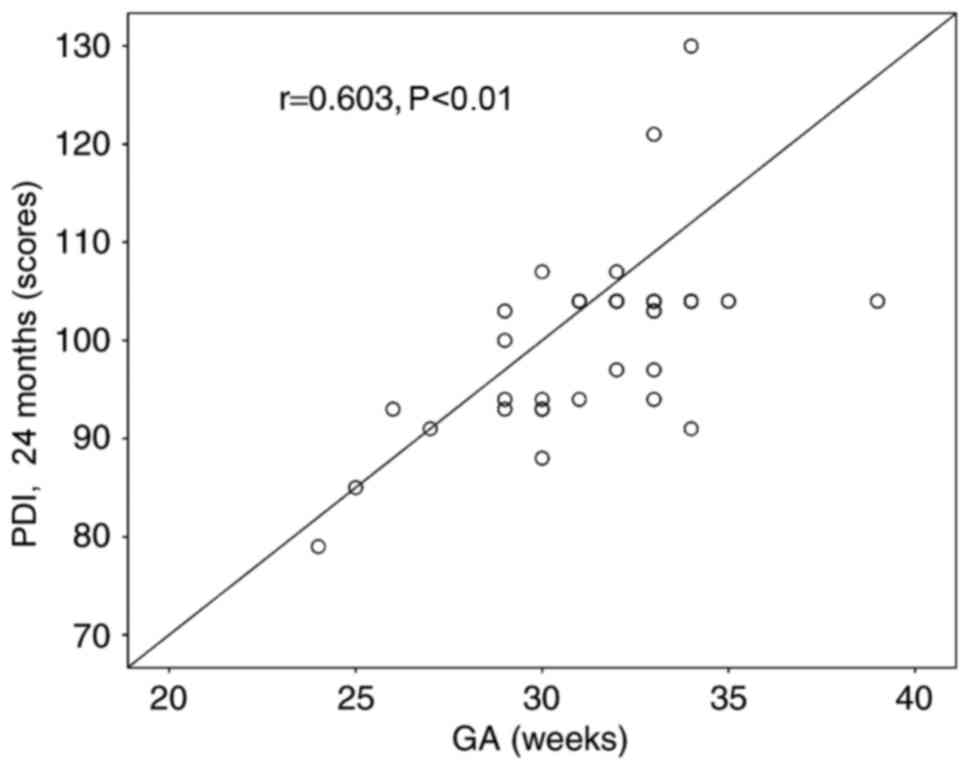
GA is correlated to motor function (PDI) at 2 year follow-up
(Fig. 1). However, neither GA nor
DTI indices is correlated to cognition function (data not
shown).
 | Table III.Illustrations of TSH values, mental
index, and performance index in enrolled patients (n=81). |
Table III.
Illustrations of TSH values, mental
index, and performance index in enrolled patients (n=81).
| Variables | ≤30 weeks
(n=17) | >30 weeks
(n=64) | P-value |
|---|
| TSH |
|
TSH0 | 7.3±0.22 | 9.0±0.1 | 0.283 |
|
TSH18 | 3.6±0.09 | 3.9±0.04 | 0.688 |
|
TSH24 | 3.3±0.14 | 3.5±0.04 | 0.762 |
|
Hyperthyrotropinemia (TSH >9
µU/ml) |
| 0 h [n
(%)] | 1 (4.5) | 12 (20.3) |
|
| 18 h [n
(%)] | 0 (0) | 5 (8.5) |
|
| 24 h [n
(%)] | 1 (4.5) | 2 (3.4) |
|
| MDI (months) |
|
6 | 105±33 | 107±34 | 0.328 |
| 12 | 95±29 | 104±22 | 0.448 |
| 18 | 104±33 | 103±24 | 0.098 |
| 24 | 92±31 | 101±30 | 0.071 |
| PDI (months) |
|
6 | 85±27 | 97±31 | 0.496 |
| 12 | 91±27 | 97±21 | 0.851 |
| 18 | 97±31 | 102±24 | 0.105 |
| 24 | 93±31 | 107±31 | 0.049a |
To investigate the association between TH function
and neurodevelopmental outcomes in later life, we analyzed
correlations between TSH levels at 0, 18, and 24 h of admission
(TSH0, TSH18, TSH24) and MDI/PDI scores using bivariate correlation
analysis. There were no significant correlations between TSH levels
and developmental indices. Some r-values for TSH time point are
positive and some are negative in Table
IV. This was possibly due to the randomness of result but not
biological relationship since the r-value was small and the
distribution of positive or negative data was not related to
chronologic age. BSID-III MDI scoreswere positively correlated with
GA at 2 years of follow-up. The late preterm group had higher MDI
scores compared to the early preterm group. Furthermore, BSID-III
PDI scoreswere positively correlated with GA throughout the 2 years
of follow-up. The late preterm group had higher PDI scores compared
to the early preterm group, suggesting that GA but not thyroid
function determined the long-term neurological outcomes in the
premature infants (Table IV).
 | Table IV.Correlation between TSH levels, GA
and developmental index by bivariate correlation analysis. |
Table IV.
Correlation between TSH levels, GA
and developmental index by bivariate correlation analysis.
|
| Bivariate
correlation analysis r |
|---|
|
|
|
|---|
| Developmental index
(postnatal age) |
TSH0 |
TSH18 |
TSH24 | GA |
|---|
| MDI (months) |
|
6 | −0.138 | −0.071 | −0.042 | 0.000 |
| 12 | 0.035 | 0.066 | −0.029 | 0.340a |
| 18 | 0.008 | −0.009 | 0.080 | 0.150 |
| 24 | 0.114 | 0.158 | 0.132 | 0.626b |
| PDI (months) |
|
6 | 0.156 | −0.164 | −0.007 | 0.238c |
| 12 | 0.086 | 0.012 | 0.028 | 0.414b |
| 18 | −0.013 | −0.054 | −0.083 | 0.431b |
| 24 | −0.025 | 0.099 | 0.022 | 0.608b |
Nineteen cases withdrew from MRI study because of
difficulty in sedating with oral chloral hydrate. Ten poor-quality
records were discarded because of motion artifact. Twenty four
cases terminated participation for fear of side effect of chloral
hydrate. Another twenty cases lost of follow-up from prematurity
follow-up program. Only 8 reliable DTI indices data could be
available. Our data showed that AD (λ1) was significantly decreased
in the splenium of the CC in the early preterm group, and that FA
was also significantly decreased in the early preterm group in the
anterior and posterior limbs of the internal capsule and the EC. In
our observation, FA and AD decrease in these ROIs did not correlate
to adverse neuromotor in our study (Table V). As for the correlation between TSH
and DTI scalars, our data demonstrated no significant correlation
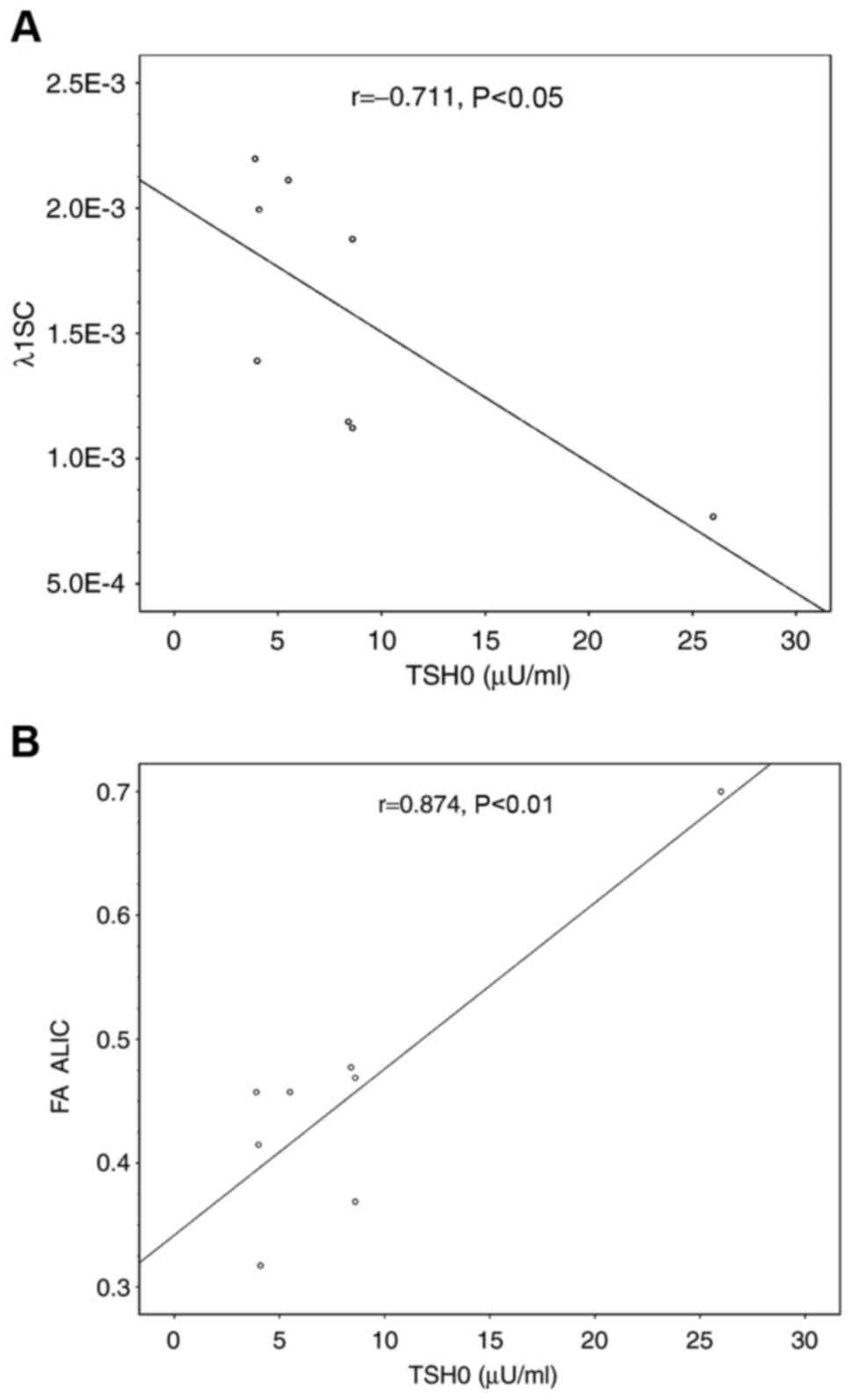
between TSH and DTI scalars except the AD (λ1) value over the
splenium of the CC, which showed negatively correlated with TSH0
(Fig. 2A), while the FA value over
the anterior limb of the internal capsule was positively correlated
with TSH0 (Fig. 2B). However, these
changes in DTI scalars in ROIs did not show clinical significance
in predicting the long-term neuromotor outcomes. MD is an inverse
measure of the membrane density, and is sensitive to cytoedema and
cellular necrosis. We found that MD in the ROIs of the genus and
splenium of the CC were significantly higher in the infants with
motor dysfunction group. In addition, MD in the ROIs of the right
side ALIC was relatively higher in the motor dysfunction group,
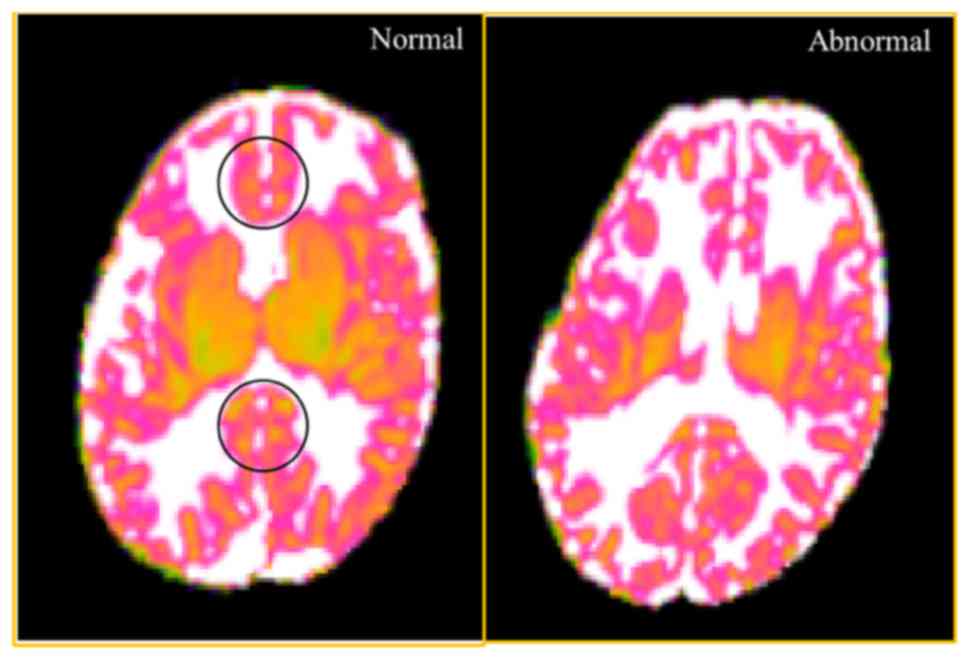
although without statistical significance (Table VI). The illustrative fig. was
demonstrated in Fig. 3.
 | Table V.Comparison of diffusion scalars
between premature babies (n=8) with gestational age <30 weeks
and ≤30 weeks. |
Table V.
Comparison of diffusion scalars
between premature babies (n=8) with gestational age <30 weeks
and ≤30 weeks.
| Variables | ≤30 weeks
(n=3) | >30 weeks
(n=5) | P-value |
|---|
| Radial
diffusivity |
| Genum
of CC |
0.001158±0.000669 |
0.001313±0.000667 | 0.279 |
|
Splenium of CC |
0.001173±0.000677 |
0.001439±0.00072 | 0.212 |
|
ALIC |
0.002044±0.00118 |
0.002234±0.00117 | 0.619 |
|
PLIC |
0.001713±0.000989 |
0.001729±0.00865 | 0.929 |
| EC |
0.001944±0.001123 |
0.002451±0.001225 | 0.255 |
| Mean
diffusivity |
| Genum
of CC |
0.001538±0.000769 |
0.001233±0.000712 | 0.106 |
|
Splenium of CC |
0.001634±0.000817 |
0.001433±0.000827 | 0.297 |
|
ALIC |
0.002701±0.00135 |
0.002389±0.001379 | 0.394 |
|
PLIC |
0.002476±0.001238 |
0.001685±0.000973 | 0.369 |
| EC |
0.002787±0.001393 |
0.002387±0.001378 | 0.297 |
| Axial diffusivity
(λ1) |
| Genum
of CC |
0.001594±0.00092 |
0.001871±0.000936 | 0.076 |
|
Splenium of CC |
0.001638±0.000946 |
0.002163±0.001082 | 0.027a |
|
ALIC |
0.002694±0.001555 |
0.00311±0.001562 | 0.160 |
|
PLIC |
0.002828±0.001633 |
0.003124±0.001562 | 0.160 |
| EC |
0.002591±0.001496 |
0.003274±0.001637 | 0.087 |
| Fractional
anisotrophy |
| Genum
of CC |
0.151736±0.087605 |
0.227086±0.113543 | 0.078 |
|
Splenium of CC |
0.177009±0.102196 |
0.2450878±0.1225439 | 0.105 |
|
ALIC |
0.264688±0.152818 |
0.3678843±0.1839421 | 0.045a |
|
PLIC |
0.339957±0.196274 |
0.551738±0.275869 | 0.043a |
| EC |
0.288964±0.166833 |
0.3782525±0.1891263 | 0.041a |
 | Table VI.Correlation between DTI indices and
neuromotor function. |
Table VI.
Correlation between DTI indices and
neuromotor function.
|
| Without neuromotor
dysfunction (n=5) | With neuromotor
dysfunction (n=3) | P-value |
|---|
| MD GC | 0.0014
(0.0012–0.0015) | 0.0016
(0.00156–0.00162) | 0.024a |
| MD SC | 0.0016
(0.0013–0.0016) | 0.0017
(0.0017–0.00171 | 0.024a |
| MD ALICR | 0.0011
(0.00100–0.00135) | 0.00138
(0.00133–0.00139) | 0.051 |
| AD(λ1) SC | 0.00148
(0.0010–0.0015) | 0.0011
(0.0007–0.0015) | 0.456 |
| FA ALIC | 0.4573
(0.3688–0.4591) | 0.4573
(0.3660–0.4589) | 0.546 |
| FA GC | 0.2656
(0.1919–0.3544) | 0.1825
(0.1825–0.1936) | 0.294 |
| FA SC | 0.2878
(0.2076–0.3797) | 0.1975
(0.1975–0.2037) | 0.177 |
Discussion
In this study, we recorded TSH levels of infants in
early and late preterm groups and analyzed the association between
TSH levels and neurodevelopmental outcomes according to the
BSID-III. All of our patients with HTT had transient changes not
requiring thyroxine therapy, and none of the infants had
detrimental neurodevelopmental outcomes at 2 years of age, and GA
but not TSH level determined neuromotor outcomes in the infants at
2 years of age. We also analyzed the association between regional
WM microstructural development and TSH level, and found that FA
increased with TSH0 levels over the ALIC, while AD (λ1) decreased
with TSH0 levels over the SC. We further found that FA values
increased at the genus and splenium of the CC, and that MD value
tended to decrease with increasing age, which is consistent with a
previous report (18). We also found
lower MD scores in the patients with motor dysfunction.
TH are known to play a critical role in the growth,
development, and maturation of the nervous system. Animal
experiments have suggested that TH are also necessary for
myelination, a process essential for motor function. Congenital
hypothyroidism in newborns has been reported to have an adverse
impact on intelligent quotient even after thyroxine replacement
therapy. Thus, even transiently low levels of thyroxine can be a
risk factor for adverse neurodevelopmental outcomes in preterm
infants (19,20). Three screening strategies are used
for congenital hypothyroidism: A primary TSH method, a primary
thyroxine method, and a combined primary TSH and thyroxine method.
The newborn screening test in Taiwan uses a primary TSH method
instead of free thyroxine level. The cut-off value of TSH is
defined as 9 µU/ml, which has been reported to be sensitive for
detecting newborns with congenital hypothyroidism (21). Transient HTT with an elevated TSH
level is common in preterm infants, and can lead to transient or
permanent hypothyroidism. Although our study provided only TSH
value without free thyroxin level for sake of facility limitation,
all of our cases encountered transient HTT were assumed to have
only transient hypothyroidism since none of our enrolled cases had
permanent hypothyroidism requiring thyroxine supplements. The
fertility rate in Taiwan is 0.9 which is one of the lowest
globally, and that is why only 81 cases were enrolled in this
study.
Dammann and Leviton proposed that both, a
two-component model, play an important role in WM damage in preterm
infants (22). The insult model
suggests that hypoxic-ischemic and/or infection causes elevated
inflammatory responses, which consequently results in WM injury.
The developmentally-regulated endogenous protection theory includes
three components of vulnerability associated with immaturity:
vascular/ependymal factors, oligodendroglial development factors,
and the lack of certain developmentally-regulated endogenous
protectors such as neurotrophins, oligotrophins (such as nerve
growth factor, neurotrophin-3, and ciliary-neurotrophic factor),
hormones (of which TH are best known), and cytokines (interleukin-6
family) in preterm infants (22). TH
promotes the maturation of oligodendrocytes past a period of
vulnerability, and may protect against WM injury due to
hypoxic-ischemic insults in developing brains (23). HTT is only a surrogate for thyroid
deficiency, however, it has been reported to be linked to
inflammation and thyroid deficiency in neonates (24). It is possible that some of our
patients still had HTT at 24 h postnatal age, indicating that they
may have had inflammation severe enough to cause brain damage.
Another possibility is that HTT is an expression of
immaturity/vulnerability. TSH levels tend to be higher in newborns
with a lower GA which reverts to normality at re-examination after
2 weeks (25,26). Although one multicenter study
reported tranisient HTT is a risk factor of developing persistent
HTT in children with neurodevelopment delays (27), our results revealed that HTT in our
study reverts to normality without neurodevelopmental consequences.
It was GA, but not thyroid function, determined the long-term
neurological outcomes in our premature infants.
MRI can detect the extent of myelination in brain
structures in hypothyroidism, and DTI metrics make it possible to
observe WM pathways before myelination is evident on conventional
MRI (11) Ng et al reported
no differences in brain MRI at term equivalence in DTI metrics
either in FA or MD in preterm infants who received LT4 supplements
compared with those who had not. However, low ADC values were
associated with better WM organization among infants with higher
plasma levels of thyroxine (28). In
contrast to previous studies, we found that AD (λ1) values over the
splenium of the CC were negatively correlated to TSH0, while FA
values over the anterior limb of the internal capsule were
positively correlated to TSH0, although neither reached
significance. In addition, our data should be interpreted with
caution because there was an outlier case with extremely high TSH
level in the analysis. If this case was excluded from the analysis,
there was no association between TSH level and DTI metrics.
Rogers et al demonstrated a pattern of
changes in diffusion measurements during WM development, with
increases in FA and decreases in MD with increasing postmenstrual
age (18). In this study, the FA
slope in the PLIC was steeper than in other WM regions, which
likely reflects early myelination. In addition, ALIC, CC and optic
radiations have been demonstrated to have steeper FA slopes with
increasing GA (18). Consistent with
the study by Roger et al, we also found that FA increased
with age in a regionally-specific manner over regions of the ALIC,
PLIC and EC. The data indicated the late preterm group had more
intact WM integrity over the anterior and posterior limbs of the
internal capsule and EC in FA than the early preterm group. To the
best of our knowledge, this is the first study to report increases
in FA with age in the EC. We also showed that MD tends to decrease
with increasing GA, although there was no statistical significance.
AD measures water diffusion along the axis of the axon and
increases during axonal organization as the axons become more
coherently arranged along the main axis. It is largely stable
during true myelination as the hydrophobic myelin sheathing
restricts diffusion in the perpendicular direction. We found
significantly increased AD values with increasing GA in the
splenium of the CC.
We next investigated whether the changes in DTI
metrics in this study were clinically relevant to the
neurodevelopmental outcomes. Lower FA and higher MD values in the
genu of the CC have been reported to be correlated with slower gait
velocity, and lesion studies have indicated that the genus WM
mediates motor coordination (29,30). Our
findings are partially consistent with the previous results. The
patients with motor dysfunction who needed rehabilitation in our
study had significantly higher MD values at the ROIs of the genu
and splenium of the CC than those who did not need rehabilitation.
Of note, relatively higher MD values were obtained at the right
side of the ALIC in the patients with motor dysfunction, although
the results did not reach statistical significance. In addition,
Rose et al reported that near-term ALIC MD values were
correlated with BSID-III fine motor scores (29). We did not observe increases in FA in
the genu and splenium of the CC in the patients with motor
dysfunction, which may be due to the limited number of patients.
There are four major limitations to this study. First, we lacked
information about thyroxine levels at the time TSH levels were
determined, and thus we could not differentiate HTT due to
thyroxine deficiency or host medical conditions. Second, the
children had to survive until 2 years of age and return for a
developmental assessment to be included in this study, and the
patients who died with preterm comorbidities were not included. As
these children differed from the survivors, the requirements for
survival may have introduced bias. Third, only eight patients
participated in the MRI study at a postmenstrual age of 40 weeks
due to concerns of guardians with regards the risk of sedation for
the MRI studies. In addition, a Dixon's Q test was run and
identified the outlier data in Fig.
2 (P=0.002). We still kept the outlier data in Fig. 2 since babies possessing TSH with an
outlier value at initial of admission needed to be double checked
at term-equivalent age. This is current newborn screening policy in
Taiwan. We need more study subjects to reach a significant
statistical power. Fourth, we should be cautious to interpret the
association among TSH0, AD and FA since removing the outlier from
the analyses leads to non-significant findings. In conclusion, GA
determined the neurodevelopmental outcomes of the premature infants
in this study. Transient hypothyroidism is a risk factor for
neurodevelopmental outcome, however, it did not play a critical
role in neuromotor dysfunction in preterm babies. Our novel
findings demonstrated that the infants with neuromotor dysfunction
had significantly higher MD values at the ROIs in the genu and
splenium of the CC. A further investigation with a larger sample
size is necessary to validate our results.
Acknowledgements
This study was supported by the Research Support
Scheme of Chang Gung Memorial Hospital (grant no. CMRPG8B0721). We
thank the Biostatistics Center of Kaohsiung Chang Gung Memorial
Hospital for assistance with the statistics. The funding source
played no role in the study design, in the collection, analysis and
interpretation of data; in the writing of the manuscript; and in
the decision to submit the manuscript for publication.
References
|
1
|
Patel J, Landers K, Li H, Mortimer RH and
Richard K: Thyroid hormones and fetal neurological development. J
Endocrinol. 209:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meijer WJ, Verloove-Vanhorick SP, Brand R
and van den Brande JL: Transient hypothyroxinaemia associated with
developmental delay in very preterm infants. Arch Dis Child.
67:944–947. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahmed OM, El-Gareib AW, El-Bakry AM, Abd
El-Tawab SM and Ahmed RG: Thyroid hormones states and brain
development interactions. Int J Dev Neurosci. 26:147–209. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahmed RG: Hypothyroidism and brain
developmental players. Thyroid Res. 8:22015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Den Ouden AL, Kok JH, Verkerk PH, Brand R
and Verloove-Vanhorick SP: The relation between neonatal thyroxine
levels and neurodevelopmental outcome at age 5 and 9 years in a
national cohort of very preterm and/or very low birth weight
infants. Pediatr Res. 39:142–145. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vigone MC, Caiulo S, Di Frenna M,
Ghirardello S, Corbetta C, Mosca F and Weber G: Evolution of
thyroid function in preterm infants detected by screening for
congenital hypothyroidism. J Pediatr. 164:1296–1302. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Wassenaer AG and Kok JH:
Hypothyroxinaemia and thyroid function after preterm birth. Semin
Neonatol. 9:3–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schoonover CM, Seibel MM, Jolson DM, Stack
MJ, Rahman RJ, Jones SA, Mariash CN and Anderson GW: Thyroid
hormone regulates oligodendrocyte accumulation in developing rat
brain white matter tracts. Endocrinology. 145:5013–5020. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sugisaki T, Noguchi T and Tsukada Y:
Cerebral myelinogenesis in the Snell dwarf mouse: Stimulatory
effects of GH and T4 restricted to the first 20 days of postnatal
life. Neurochem Res. 10:767–778. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Korzeniewski SJ, Birbeck G, DeLano MC,
Potchen MJ and Paneth N: A systematic review of neuroimaging for
cerebral palsy. J Child Neurol. 23:216–227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gupta RK, Bhatia V, Poptani H and Gujral
RB: Brain metabolite changes on in vivo proton magnetic resonance
spectroscopy in children with congenital hypothyroidism. J Pediatr.
126:389–392. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jagannathan NR, Tandon N, Raghunathan P
and Kochupillai N: Reversal of abnormalities of myelination by
thyroxine therapy in congenital hypothyroidism: Localized in vivo
proton magnetic resonance spectroscopy (MRS) study. Brain Res Dev
Brain Res. 109:179–186. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berman JI, Mukherjee P, Partridge SC,
Miller SP, Ferriero DM, Barkovich AJ, Vigneron DB and Henry RG:
Quantitative diffusion tensor MRI fiber tractography of
sensorimotor white matter development in premature infants.
Neuroimage. 27:862–871. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Basser PJ and Pierpaoli C: Microstructural
and physiological features of tissues elucidated by
quantitative-diffusion-tensor MRI. J Magn Reson B. 111:209–219.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song SK, Sun SW, Ramsbottom MJ, Chang C,
Russell J and Cross AH: Dysmyelination revealed through MRI as
increased radial (but unchanged axial) diffusion of water.
Neuroimage. 17:1429–1436. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pereira DN and Procianoy RS: Effect of
perinatal asphyxia on thyroid-stimulating hormone and thyroid
hormone levels. Acta Paediatr. 92:339–345. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
D'Agostino JA: An evidentiary review
regarding the use of chronological and adjusted age in the
assessment of preterm infants. J Spec Pediatr Nurs. 15:26–32. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rogers CE, Smyser T, Smyser CD, Shimony J,
Inder TE and Neil JJ: Regional white matter development in very
preterm infants: Perinatal predictors and early developmental
outcomes. Pediatr Res. 79:87–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Simic N, Asztalos EV and Rovet J: Impact
of neonatal thyroid hormone insufficiency and medical morbidity on
infant neurodevelopment and attention following preterm birth.
Thyroid. 19:395–401. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reuss ML, Paneth N, Pinto-Martin JA,
Lorenz JM and Susser M: The relation of transient hypothyroxinemia
in preterm infants to neurologic development at two years of age. N
Engl J Med. 334:821–827. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Olivieri A, Fazzini C and Medda E; Italian
Study Group for Congenital Hypothyroidism, : Multiple factors
influencing the incidence of congenital hypothyroidism detected by
neonatal screening. Horm Res Paediatr. 83:86–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dammann O and Leviton A: Brain damage in
preterm newborns: Might enhancement of developmentally regulated
endogenous protection open a door for prevention? Pediatrics.
104:541–550. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hung PL, Huang CC, Huang HM, Tu DG and
Chang YC: Thyroxin treatment protects against white matter injury
in the immature brain via brain-derived neurotrophic factor.
Stroke. 44:2275–2283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soto-Rivera CL, Fichorova RN, Allred EN,
Van Marter LJ, Shah B, Martin CR, Agus MS and Leviton A: The
relationship between TSH and systemic inflammation in extremely
preterm newborns. Endocrine. 48:595–602. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaye C; Committee on Genetics, ; Accurso
F, La Franchi S, Lane PA, Northrup H, Pang S and Schaefer GB:
Introduction to the newborn screening fact sheets. Pediatrics.
118:1304–1312. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gaudino R, Garel C, Czernichow P and Léger
J: Proportion of various types of thyroid disorders among newborns
with congenital hypothyroidism and normally located gland: A
regional cohort study. Clin Endocrinol (Oxf). 62:444–448. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cuestas E, Gaido MI and Capra RH:
Transient neonatal hyperthyrotropinemia is a risk factor for
developing persistent hyperthyrotropinemia in childhood with
repercussion on developmental status. Eur J Endocrinol.
172:483–490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ng SM, Turner MA, Gamble C, Didi M, Victor
S, Atkinson J, Sluming V, Parkes LM, Tietze A, Abernethy LJ and
Weindling AM: Effect of thyroxine on brain microstructure in
extremely premature babies: Magnetic resonance imaging findings in
the TIPIT study. Pediatr Radiol. 44:987–996. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rose J, Cahill-Rowley K, Vassar R, Yeom
KW5, Stecher X, Stevenson DK, Hintz SR and Barnea-Goraly N:
Neonatal brain microstructure correlates of neurodevelopment and
gait in preterm children 18–22 mo of age: An MRI and DTI study.
Pediatr Res. 78:700–708. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Caillé S, Sauerwein HC, Schiavetto A,
Villemure JG and Lassonde M: Sensory and motor interhemispheric
integration after section of different portions of the anterior
corpus callosum in nonepileptic patients. Neurosurgery. 57:50–59.
2005. View Article : Google Scholar : PubMed/NCBI
|