Introduction
Esophageal cancer is one of the eight most common
human tumors worldwide, which commonly occurs in the esophageal
mucosal epithelium or gland, and its mortality rate ranked sixth
worldwide (1,2). Approximately 482,300 patients were
newly diagnosed and 406,800 patients succumbed to esophageal cancer
worldwide in 2008 (3). This type of
cancer has a high incidence in developing countries, mainly in East
Asia, East Africa and South Africa. There are two main histological
types of esophageal cancer, including the esophageal adenocarcinoma
and the esophageal squamous cell carcinoma (ESCC), which accounts
for >90% of cases in China (4).
Esophageal cancer is one of the malignant diseases that severely
endanger the life and health of individuals in China. According to
the investigation of the cancer incidence in China, esophageal
cancer is the fifth most common type of cancer and the fourth
highest cause of cancer-associated mortality (5).
In recent years, although advances have been
achieved in surgery, chemotherapy and other treatment methods of
esophageal cancer, the worldwide overall survival rate is <10%
and the 5-year survival rate is only 14% due to the high recurrence
rate of esophageal cancer and poor prognosis (6,7).
Therefore, at present, the research on the pathogenesis and
treatment of esophageal squamous cell carcinoma is receiving
increasing attention.
Yes-associated protein (YAP), a 65 kD molecule, is
the nuclear effector of the Hippo signaling pathway which is a
newly discovered cell signal transduction pathway that serves
essential roles in regulating the volume and size of organs
(8,9). YAP participates in tumor progression
via regulating cell growth, proliferation and apoptosis, and is
regarded as an oncogene (10).
Previous studies have demonstrated that YAP is overexpressed in a
variety of tumor tissues, including breast, liver, prostate, colon
and lung cancer (11–15). It has also been indicated that YAP
overexpression is frequently observed in ESCC and it may serve as
an oncogene in ESCC (16).
The exact role of YAP in ESCC remains largely
unclear. Therefore, the present study aimed to investigate the
effects of YAP inhibition on ESCC by small interfering RNA (siRNA)
transfection in ECA-109 cells, and to explore the underlying
molecular mechanism.
Materials and methods
Cell culture
Human ECA-109 cells were obtained from the American
Type Culture Collection (cat. no. EY-X0635; Manassas, VA, USA). The
ECA-109 cells were grown in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin
solution. Cells were incubated in a humidified atmosphere with 5%
CO2 at 37°C.
Cell transfection
At 24 h prior to transfection, ECA-109 cells
(5×104 cells per well) were seeded into a 6-well plate
and cultured at 37°C in 5% CO2 humidified air. When the
cell confluence reached 60–70%, YAP-siRNA or control-siRNA (Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) was transfected into the
cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
cells were initially transfected for 12 h, followed by further
incubation for 24 h at 37°C in 5% CO2. Subsequently, the
culture medium was refreshed. Cells transfected with YAP-siRNA were
considered as the siRNA group (siRNA), cells transfected with
control-siRNA were considered to be the negative control group
(NC), and cells without any treatment were considered to be the
blank control group (BL).
MTT assay
Cells were seeded in a 96-well culture plate (~200
µl at 1×104 cells/ml), and an MTT assay was performed at
24 h after transfection to determine the cell proliferation ability
of cells. Briefly, MTT solution (20 mg/ml; Amresco LLC, Solon, OH,
USA) was added into the culture medium in each well and incubated
for 4 h at 37°C. Next, the optical density (OD) at 570 nm was
detected by a microplate reader.
Apoptosis analysis assay
At 24 h after transfection, cells were washed three
times with cold phosphate-buffered saline (PBS) solution, fixed
with 70% ethanol at room temperature for 15 min and rinsed again
with PBS. Subsequently, the cells were labeled with Annexin V-FITC
and propidium iodide (PI) (cat. no. 6592; Cell Signaling
Technology, Danvers, MA, USA) following the manufacturer's
protocol, and incubated at room temperature for 30 min. Flow
cytometry (FCM; BD Biosciences, Franklin Lakes, NJ, USA) was then
applied to analyze the cell apoptosis. Experiments were performed
in triplicate.
Cell cycle assay
At 24 h after transfection, the transfected ECA-109
cells were collected using 0.25% trypsin, washed three times with
PBS solution and then fixed with 70% ethanol at 4°C overnight.
Following centrifugation (1,000 × g, 4°C for, 5 min) and washing,
the cells were dyed with RNAse A and PI and incubated at 4°C for 30
min. The cell cycle distribution was then analyzed using a flow
cytometer (FACSort; FACSCanto II; BD Biosciences, Franklin Lakes,
NJ, USA).
Western blot analysis
The protein expression levels of YAP, B-cell
lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax), p53, caspase
3, extracellular signal-regulated kinase (Erk) and phosphorylated
Erk (p-Erk) were determined by western blot analysis. Briefly, the
total cell protein was collected from the transfected cells using a
lysis buffer (Cell Signaling Technology). Next, a BCA assay (Thermo
Fisher Scientific, Inc.) was applied to detect the protein
concentrations of the cell lysates, and bovine albumin was used as
standard. Protein samples were then separated by 10% SDS-PAGE and
then blotted onto polyvinylidene difluoride membranes, followed by
blocking with 5% fat-free powdered milk for 2 h at room
temperature. Subsequently, the membranes were incubated overnight
at 4°C with the primary antibodies against YAP (cat. no. 14074),
Bcl-2 (cat. no. 4223), Bax (cat. no. 5023), p53 (cat. no. 2527),
caspase 3 (cat. no. 9665), Erk (cat. no. 4695) and p-Erk (cat. no.
8510; all 1:1,000; all from Cell Signaling Technology). β-actin
(cat. no. 4970; 1:5,000; Cell Signaling Technology) was used as an
internal control. Following washing three times with Tris-buffered
saline/Tween-20, the samples were then incubated with horseradish
peroxidase-conjugated secondary antibodies for 2 h at room
temperature. Finally, the membranes were developed with an enhanced
chemiluminescence detection kit (cat. no. 6883; Cell Signaling
Technology) according to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the transfected cells
using TRIzol regent (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's protocol. The RNA concentration was
quantified by using a Nanodrop spectrophotometer (Thermo Fisher
Scientific, Inc.) at 260 nm. cDNAs were generated using the
PrimeScript reverse transcription reagent kit (Takara Biotechnology
Co., Ltd., Dalian, China) according to the manufacturer's
instructions. cDNAs were subsequently analyzed using qPCR with a
TaqMan Universal PCR Master Mix kit and the ABI PRISM 7900 HT
sequence-detection system (Thermo Fisher Scientific, Inc.). The
amplification conditions were as follows: 95°C for 10 min, followed
by 40 cycles of 95°C for 10 sec and 60°C for 60 sec. The primer
sequences used in qPCR were as follows: YAP forward,
5′-ACGTTCATCTGGGACAGCAT-3′ and reverse, 5′-GTTGGGAGATGGCAAAGACA-3′;
GAPDH forward, 5′-GGCATTGCTCTCAATGACAA-3′ and reverse,
5′-TGTGAGGGAGATGCTCAGTG-3′. GAPDH was used as an internal control.
The relative gene expressions were quantified using the
2−ΔΔCq method (17).
Statistical analysis
Experiments were performed for at least three times.
SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, USA) was
performed for all statistical analyses. The results are expressed
as the mean ± standard deviation, and were compared by Student's
t-test or one-way analysis of variance followed by Tukey's test.
Statistically significant differences were indicated by
P<0.05.
Results
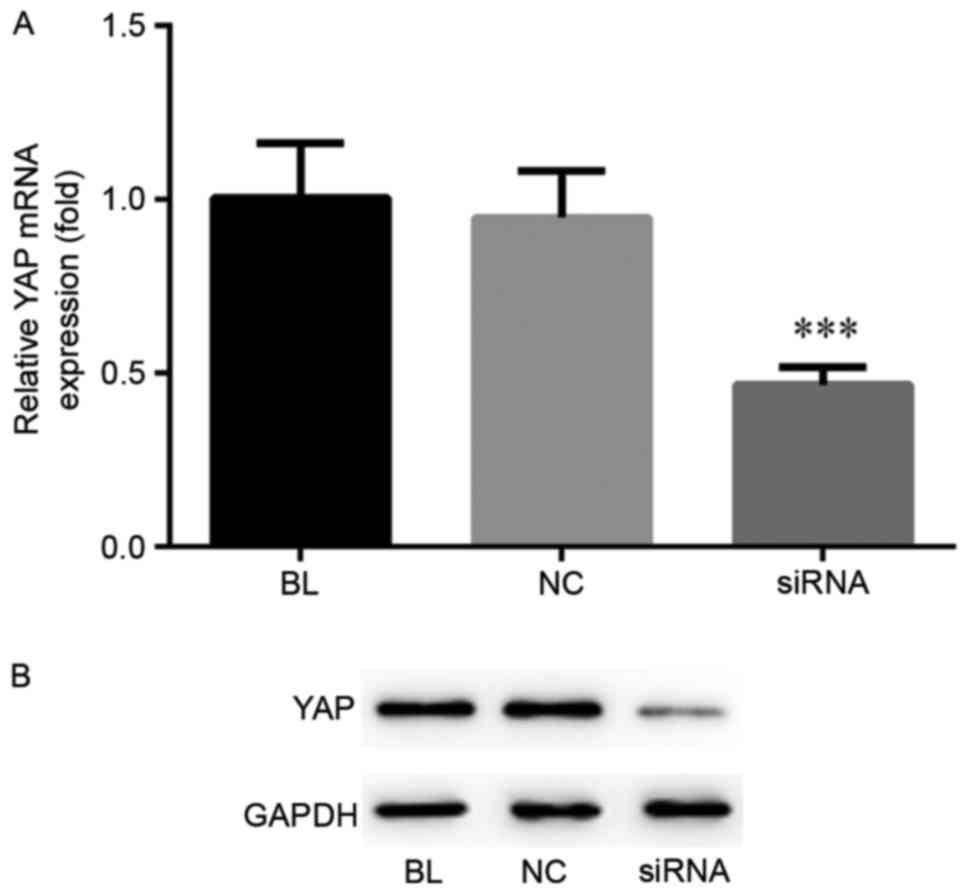
YAP expression is inhibited by YAP
siRNA transfection in ECA-109 cells
In order to investigate the exact role of YAP in
ESCC cells, a stable YAP low-expression ESCC cell line was
established using YAP siRNA transfection. RT-qPCR and western blot
analysis were performed to detect the mRNA and protein YAP
expression levels, respectively (Fig.
1). The results indicated that the mRNA and protein levels of
YAP were significantly inhibited following YAP siRNA transfection
in Eca-109 cells.
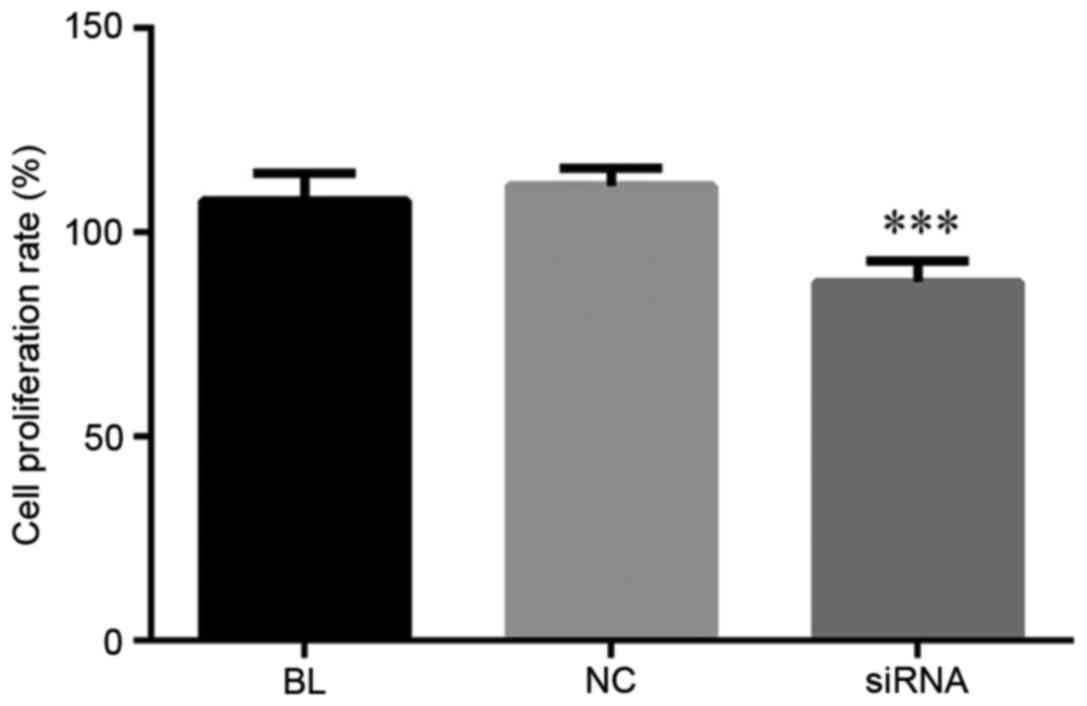
YAP inhibition suppresses cell
proliferation in ECA-109 cells
At 24 h after transfection, the cell proliferation
ability of Eca-109 cells was determined by MTT assay. As shown in
Fig. 2, compared with the controls,
the Eca-109 cell proliferation ability was notably decreased in
cells transfected with YAP siRNA. These data indicated that YAP
inhibition was able to suppress the proliferation in Eca-109
cells.
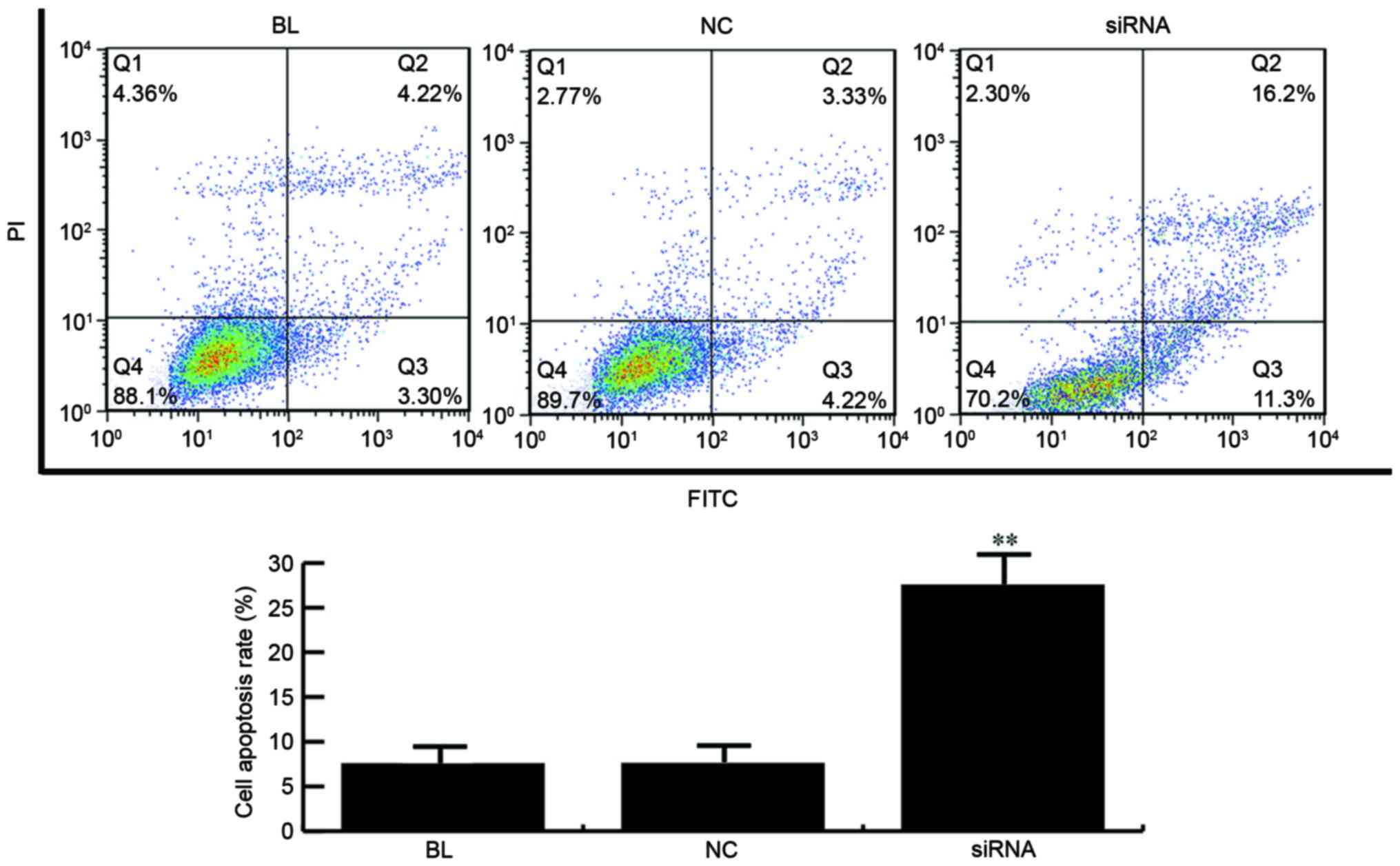
YAP inhibition induces cell apoptosis
in ECA-109 cells
The effects of YAP on cell apoptosis were detected
using FCM. YAP siRNA or control siRNA was transfected into Eca-109
cells, and the cell apoptosis was detected at 24 h after
transfection. As observed in Fig. 3,
the apoptosis rate of Eca-109 cells was significantly increased in
the YAP siRNA-transfected group. Thus, these results indicated that
YAP inhibition induced cell apoptosis in Eca-109 cells.
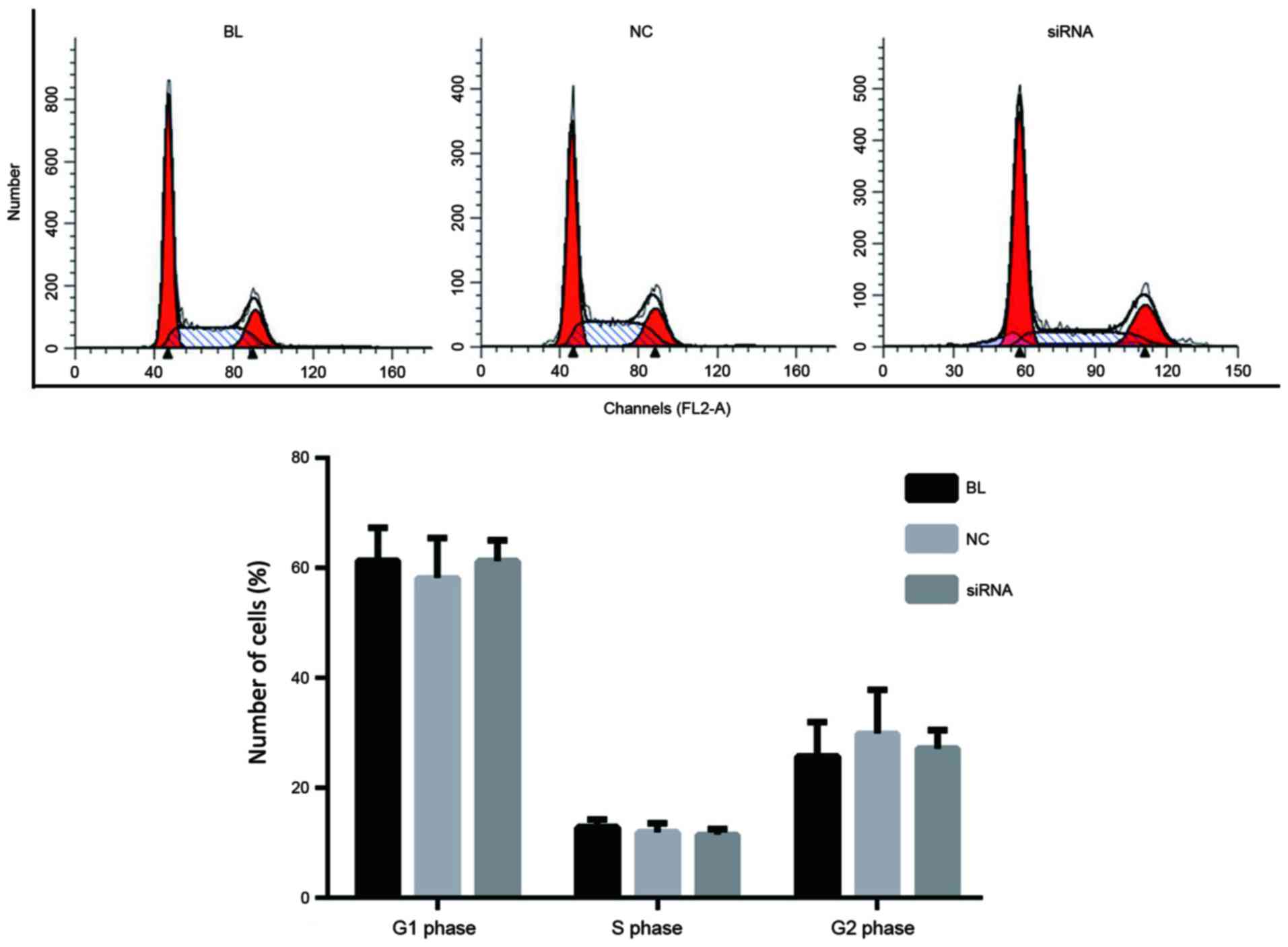
YAP inhibition exerts no effects on
the cell cycle distribution in ECA-109 cells
At 24 h after transfection, the effects of YAP
inhibition on the cell cycle were determined. As shown in Fig. 4, no significant difference was
detected between the controls and the YAP siRNA group with regard
to the percentage of cells at each cell cycle phase.
Effects of YAP inhibition on the
expression of proteins associated with cell proliferation and
apoptosis
In order to investigate the underlying molecular
mechanisms of YAP-induced regulation of cell proliferation and
apoptosis, western blot analysis was conducted to detect the
expression levels of proteins associated with cell proliferation
and apoptosis. The results suggested that, compared with the
controls, the expression levels of Bcl-2 and p-Erk were evidently
decreased in the YAP siRNA-transfected group, while the levels of
Bax, caspase 3 and p53 were notably increased (Fig. 5).
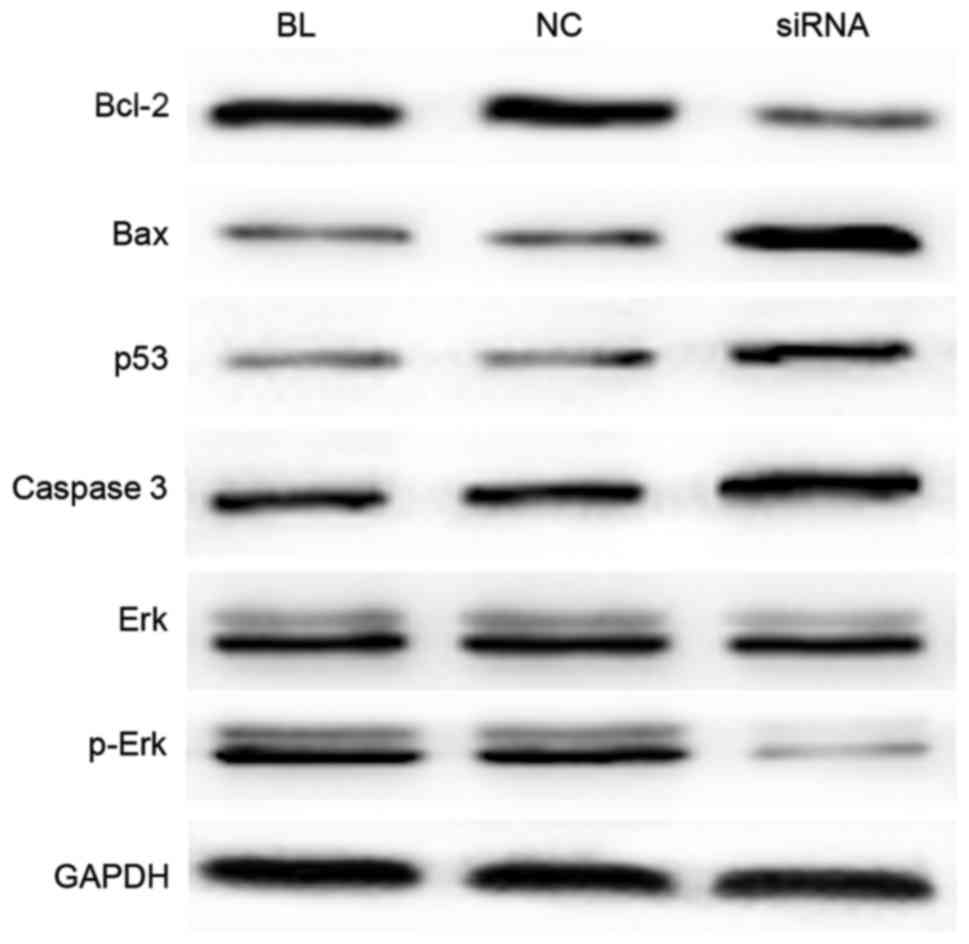 | Figure 5.YAP inhibition alters the expression
of proteins associated with cell proliferation and apoptosis. At 24
h after transfection, western blot analysis was applied to detect
the expression levels of the Bcl-2, Bax, Erk, p-Erk, caspase 3 and
p53 proteins. YAP, Yes-associated protein; Bcl-2, B-cell lymphoma
2; Bax, Bcl-2-associated X protein; Erk, extracellular
signal-regulated kinase; p-, phosphorylated. |
Discussion
The Hippo signaling pathway regulates the organ size
in various species, and dysregulation of this pathway may induce
the development of tumors (18).
Since the disruption of any factor in this pathway can lead to
tumorigenesis, YAP is recognized as an oncogene and the major
downstream effector of the Hippo signaling pathway. Upregulation of
the YAP gene and its nuclear localization have been observed in a
variety of cancer types, including hepatocellular carcinoma (HCC),
as well as lung, ovarian, prostate and colon cancer (19–22). It
has been observed that YAP is an independent prognostic marker for
the overall survival and disease-free survival of HCC patients
(21). Furthermore, a previous study
demonstrated that the overexpression and nuclear localization of
YAP in ESCC tissues was associated with a poor prognosis (16).
RNA interference, a process of post-transcriptional
regulation of gene expression, has been widely used in experiment
studies (23,24). In the present study, this process was
used to investigate the effects of YAP inhibition on ESCC cells
using YAP siRNA transfection. Subsequent to transfection with YAP
siRNA, the mRNA and protein expression levels of YAP in Eca-109
cells were significantly decreased. MTT assay was also applied to
determine the effect of YAP inhibition on Eca-109 cell
proliferation, and the results suggested that YAP inhibition
prevented the proliferation ability of the Eca-109 cells. The
Eca-109 cell apoptosis and cell cycle distribution were also
detected using FCM, which revealed that YAP inhibition by YAP siRNA
induced Eca-109 cell apoptosis; however, no significant alterations
were identified in the cell cycle distribution in Eca-109 cells in
different groups.
To further examine the mechanisms underlying the
effects of YAP inhibition on ESCC cells, the levels of proteins
associated with cell proliferation and apoptosis were analyzed. The
Bcl-2 family, which is a dominant regulator of programmed cell
death in mammalian cells (25),
consists of the apoptosis regulator Bcl-2 and its homologues. These
proteins govern the mitochondrial outer membrane permeabilization
and can be pro-apoptotic (such as Bax, Bcl-2-associated death
promoter, Bcl-2 antagonist/killer and Bcl-2 related ovarian killer)
or anti-apoptotic (including Bcl-2, Bcl-extra large and Bcl-2-like
protein 2). Caspase 3 is the main terminal shear enzyme in the
process of cell apoptosis and its activation is prevented by Bcl-2.
In the present study, it was observed that the protein expression
level of Bcl-2 was significantly decreased in Eca-109 cells
transfected with YAP siRNA, while the levels of Bax and caspase 3
were increased. Erk1/2, a member of the mitogen-activated protein
kinase family, serves critical roles in regulating cell
differentiation, proliferation, migration and angiogenesis through
the phosphorylation of phosphatases, cytoskeletal protein and
transcriptional factors (26). In
addition, p53 serves a crucial role in preventing cancer formation
(27). The present study confirmed
that YAP inhibition notably enhanced p53 expression, while the
level of p-Erk1/2 was markedly decreased. These results indicate
that YAP inhibition affect ESCC cell proliferation and apoptosis at
least partly via regulating the expression of Bcl-2, Bax, p-Erk,
caspase 3 and p53.
In conclusion, the results presented in the current
study demonstrate that YAP gene inhibition suppressed proliferation
and induced apoptosis in ECA-109 cells. Furthermore, YAP gene
silencing decreased Bcl-2 and p-Erk expression, while the
expression of Bax, caspase 3 and p53 was increased. These results
indicated that the YAP gene may serve as a novel target in ESCC
treatment in the future.
References
|
1
|
Kranzfelder M, Büchler P and Friess H:
Surgery within multimodal therapy concepts for esophageal squamous
cell carcinoma (ESCC): The MRI approach and review of the
literature. Adv Med Sci. 54:158–169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen ZL, Zhao XH, Wang JW, Li BZ, Wang Z,
Sun J, Tan FW, Ding DP, Xu XH, Zhou F, et al: microRNA-92a promotes
lymph node metastasis of human esophageal squamous cell carcinoma
via E-cadherin. J Biol Chem. 286:10725–10734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma S, Bao JYJ, Kwan PS, Chan YP, Tong CM,
Fu L, Zhang N, Tong AHY, Qin YR, Tsao SW, et al: Identification of
PTK6, via RNA sequencing analysis, as a suppressor of esophageal
squamous cell carcinoma. Gastroenterology. 143:675–686.e12. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen W, He Y, Zheng R, Zhang S, Zeng H,
Zou X and He J: Esophageal cancer incidence and mortality in China,
2009. J Thorac Dis. 5:19–26. 2013.PubMed/NCBI
|
|
6
|
Lagergren J and Lagergren P: Oesophageal
cancer. BMJ. 341:c62802010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sant M, Allemani C, Santaquilani M, Knijn
A, Marchesi F and Capocaccia R; EUROCARE Working Group, :
EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999.
Results and commentary. Eur J Cancer. 45:931–991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu AM, Xu Z and Luk JM: An update on
targeting Hippo-YAP signaling in liver cancer. Expert Opin Ther
Targets. 16:243–247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hong L, Cai Y, Jiang M, Zhou D and Chen L:
The Hippo signaling pathway in liver regeneration and
tumorigenesis. Acta Biochim Biophys Sin (Shanghai). 47:46–52. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Overholtzer M, Zhang J, Smolen GA, Muir B,
Li W, Sgroi DC, Deng CX, Brugge JS and Haber DA: Transforming
properties of YAP, a candidate oncogene on the chromosome 11q22
amplicon. Proc Natl Acad Sci USA. 103:pp. 12405–12410. 2006;
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Su L and Ou Q: Yes-associated
protein promotes tumor development in luminal epithelial derived
breast cancer. Eur J Cancer. 48:1227–1234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ge L, Smail M, Meng W, Shyr Y, Ye F, Fa
KH, Li X, Zhou HM and Bhowmick NA: Yes-associated protein
expression in head and neck squamous cell carcinoma nodal
metastasis. PLoS One. 6:e275292011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui ZL, Han FF, Peng XH, Chen X, Luan CY,
Han RC, Xu WG and Guo XJ: Yes-associated protein 1 promotes
adenocarcinoma growth and metastasis through activation of the
receptor tyrosine kinase Axl. Int J Immunopathol Pharmacol.
25:989–1001. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Avruch J, Zhou D and Bardeesy N: YAP
oncogene overexpression supercharges colon cancer proliferation.
Cell Cycle. 11:1090–1096. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Ma L, Weng W, Qiao Y, Zhang Y, He
J, Wang H, Xiao W, Li L, Chu Q, et al: Mutual interaction between
YAP and CREB promotes tumorigenesis in liver cancer. Hepatology.
58:1011–1020. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muramatsu T, Imoto I, Matsui T, Kozaki K,
Haruki S, Sudol M, Shimada Y, Tsuda H, Kawano T and Inazawa J: YAP
is a candidate oncogene for esophageal squamous cell carcinoma.
Carcinogenesis. 32:389–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu AM, Wong KF, Jiang X, Qiao Y and Luk
JM: Regulators of mammalian Hippo pathway in cancer. Biochim
Biophys Acta. 1826:357–364. 2012.PubMed/NCBI
|
|
19
|
Zender L, Spector MS, Xue W, Flemming P,
Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et
al: Identification and validation of oncogenes in liver cancer
using an integrative oncogenomic approach. Cell. 125:1253–1267.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Steinhardt AA, Gayyed MF, Klein AP, Dong
J, Maitra A, Pan D, Montgomery EA and Anders RA: Expression of
Yes-associated protein in common solid tumors. Hum Pathol.
39:1582–1589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT,
Zender L, Lowe SW, Poon RT and Luk JM: Yes-associated protein is an
independent prognostic marker in hepatocellular carcinoma. Cancer.
115:4576–4585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Dong Q, Zhang Q, Li Z, Wang E and
Qiu X: Overexpression of yes-associated protein contributes to
progression and poor prognosis of non-small-cell lung cancer.
Cancer Sci. 101:1279–1285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dubreuil G, Magliano M, Dubrana MP, Lozano
J, Lecomte P, Favery B, Abad P and Rosso MN: Tobacco rattle virus
mediates gene silencing in a plant parasitic root-knot nematode. J
Exp Bot. 60:4041–4050. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li JC, Yang XR, Sun HX, Xu Y, Zhou J, Qiu
SJ, Ke AW, Cui YH, Wang ZJ, Wang WM, et al: Up-regulation of
Krüppel-like factor 8 promotes tumor invasion and indicates poor
prognosis for hepatocellular carcinoma. Gastroenterology.
139:2146–2157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsujimoto Y and Shimizu S: Bcl-2 family:
Life-or-death switch. FEBS Lett. 466:6–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Surget S, Khoury MP and Bourdon JC:
Uncovering the role of p53 splice variants in human malignancy: A
clinical perspective. Onco Targets Ther. 7:57–68. 2013.PubMed/NCBI
|