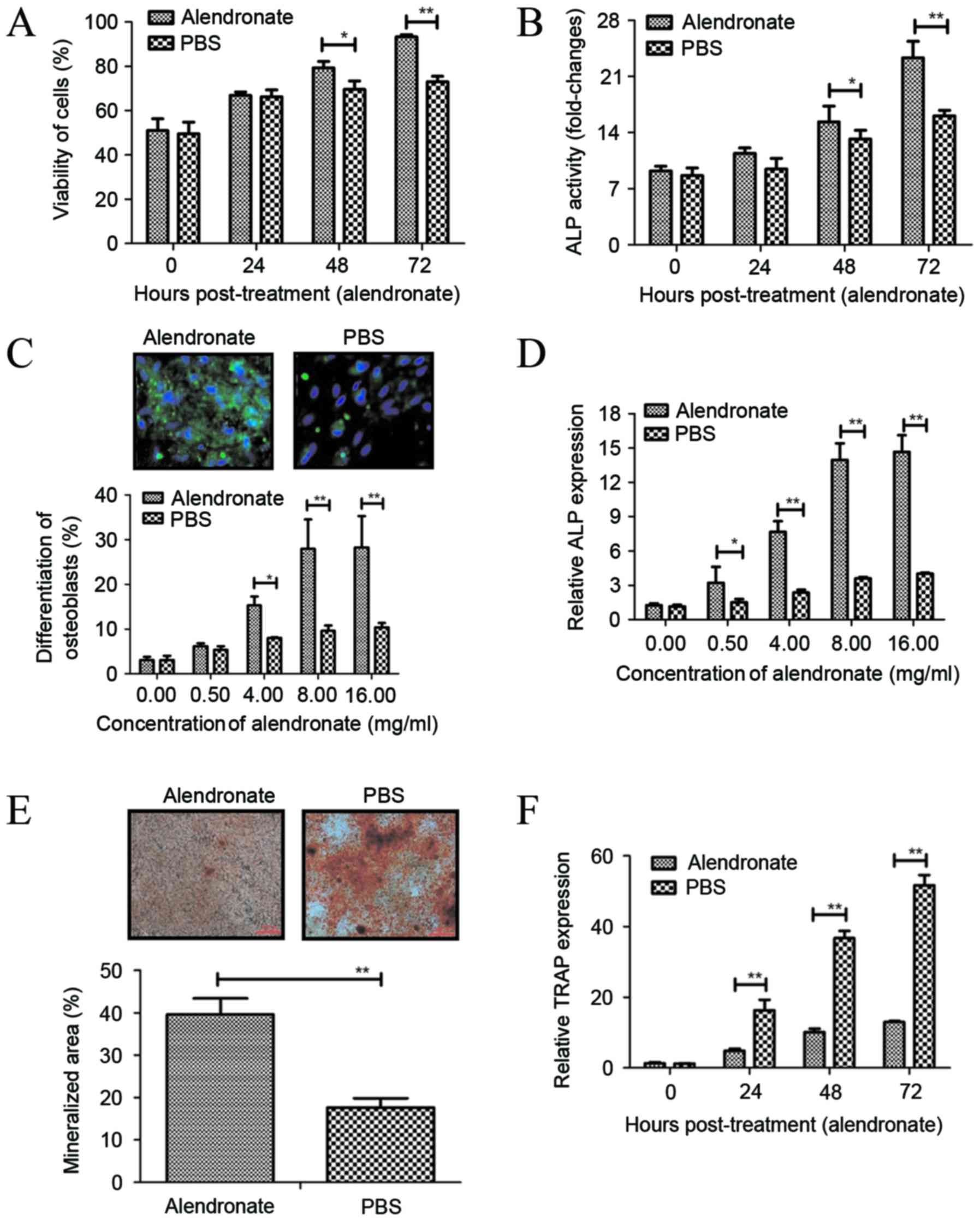
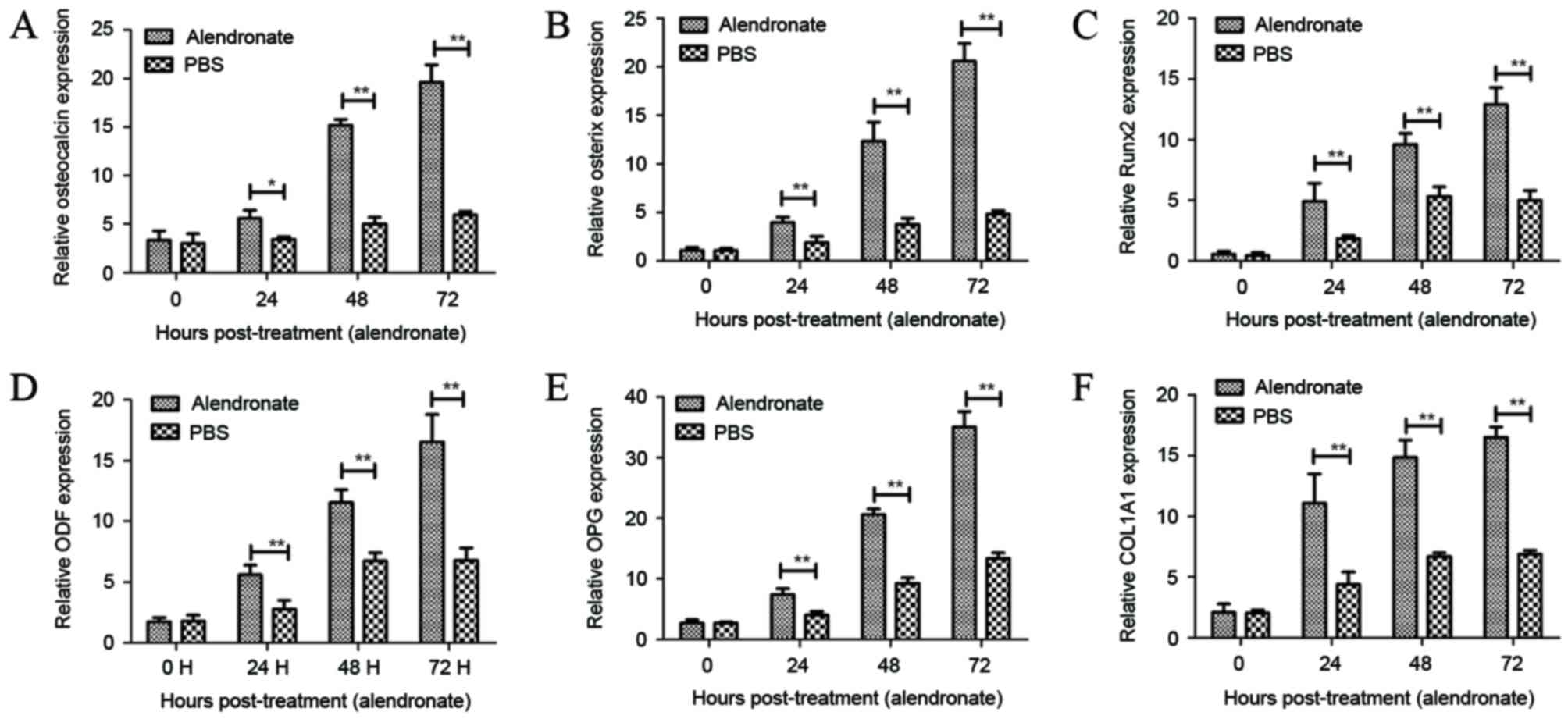
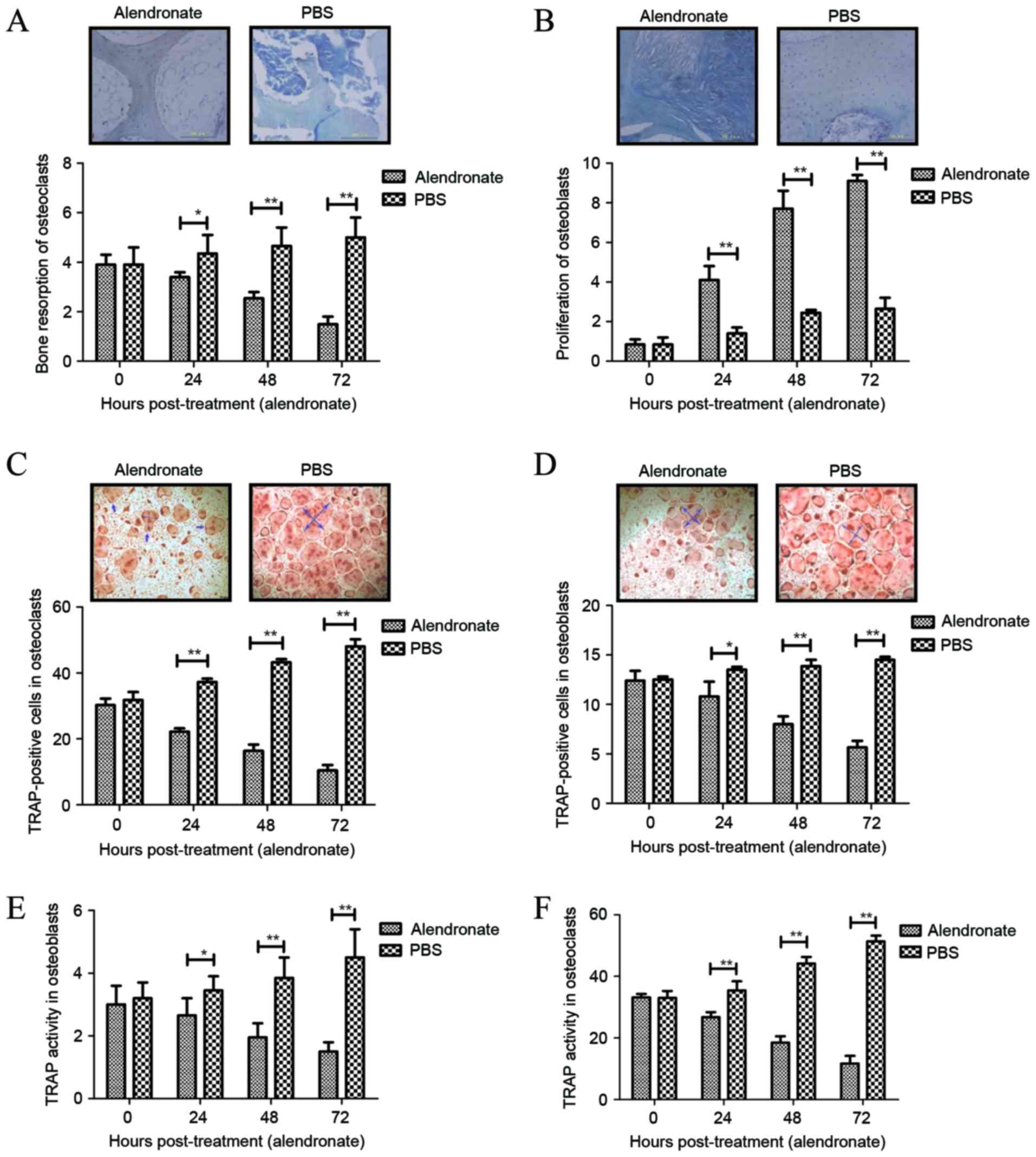
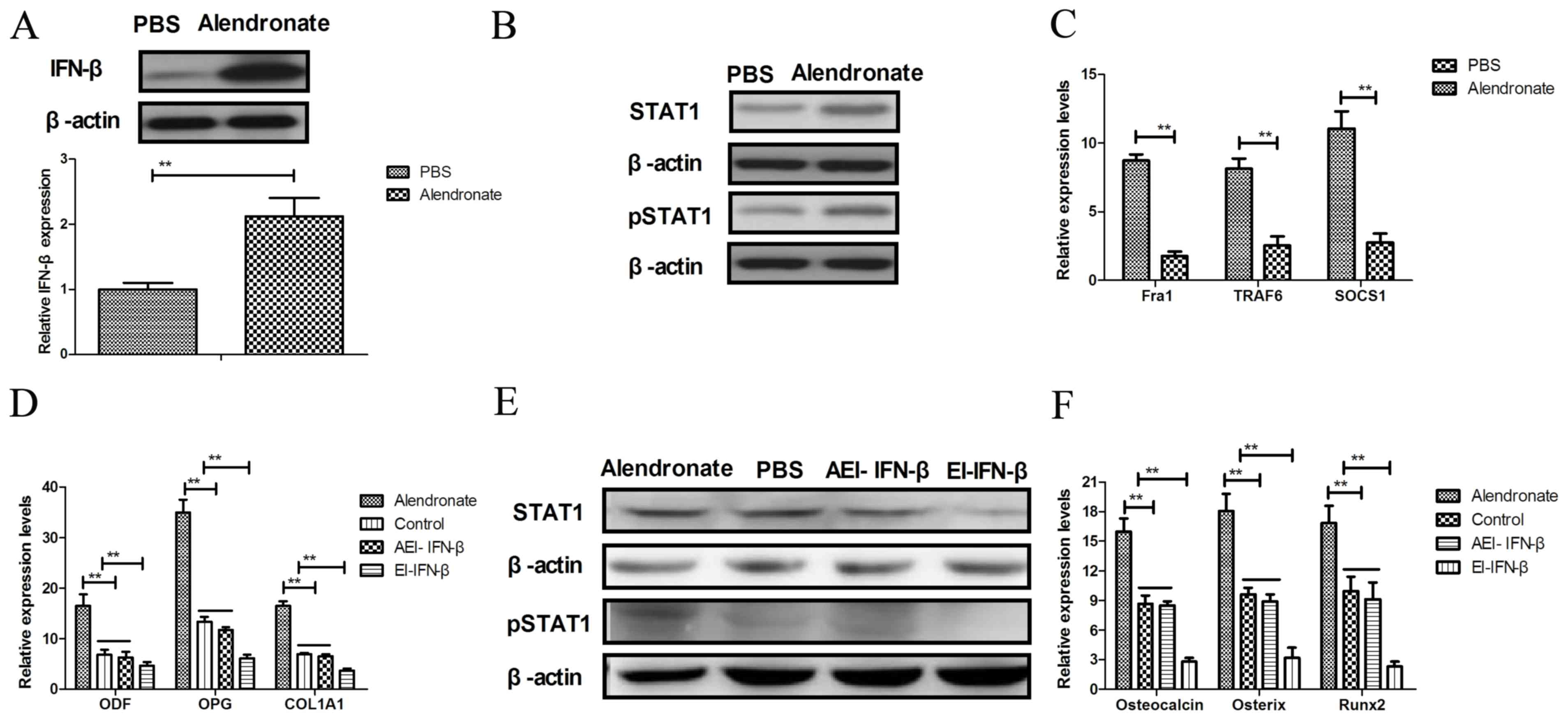
|
1
|
Wong SK, Chin KY, Suhaimi FH, Ahmad F and
Ima-Nirwana S: The Relationship between metabolic syndrome and
osteoporosis: A Review. Nutrients. 8:pii: E3472016. View Article : Google Scholar
|
|
2
|
Petty SJ, Wilding H and Wark JD:
Osteoporosis associated with epilepsy and the use of
anti-epileptics-a review. Curr osteoporos Rep. 14:54–65. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Newman M, Lowe C Minns and Barker K:
Spinal orthoses for vertebral osteoporosis and osteoporotic
vertebral fracture: A systematic review. Arch Phys Med Rehabil.
97:1013–1025. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gennari L, Merlotti D, De Paola V, Calabrò
A, Becherini L, Martini G and Nuti R: Estrogen receptor gene
polymorphisms and the genetics of osteoporosis: A HuGE review. Am J
Epidemiol. 161:307–320. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bushardt RL, Turner JL, Ragucci KR and
Askins DG Jr: Non-estrogen treatments for osteoporosis: An
evidence-based review. JAAPA. 19:25–30. 2006.PubMed/NCBI
|
|
6
|
Ziablitsev DS and Larin OS: Influence of
single nucleotide polymorphisms of vitamin D receptor-gene on the
level of osteoassociated hormones linkage with postmenopausal
osteoporosis. Fiziol Zh. 61:21–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dempster DW, Zhou H, Recker RR, Brown JP,
Bolognese MA, Recknor CP, Kendler DL, Lewiecki EM, Hanley DA, Rao
SD, et al: A longitudinal study of skeletal histomorphometry at 6
and 24 months across four bone envelopes in postmenopausal women
with osteoporosis receiving teriparatide or zoledronic acid in the
SHOTZ Trial. J Bone Miner Res. 31:1429–1439. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iba K, Sonoda T, Takada J, Dohke T and
Yamashita T: Further significant effects of eldecalcitol on bone
resorption markers and bone mineral density in postmenopausal
osteoporosis patients having undergone long-term bisphosphonate
treatment. J Bone Miner Metab. 35:171–176. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Darba J, Kaskens L, Vilela F Sorio and
Lothgren M: Cost-utility of denosumab for the treatment of
postmenopausal osteoporosis in Spain. Clinicoecon Outcomes Res.
7:105–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Inderjeeth CA, Glendenning P, Ratnagobal
S, Inderjeeth DC and Ondhia C: Long-term efficacy, safety, and
patient acceptability of ibandronate in the treatment of
postmenopausal osteoporosis. Int J Womens Health. 7:7–17. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang ZB, Wan SL, Lu YJ, Ning L, Liu C and
Fan SW: Does vitamin K2 play a role in the prevention and treatment
of osteoporosis for postmenopausal women: A meta-analysis of
randomized controlled trials. Osteoporos Int. 26:1175–1186. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ouyang L, Zhang Q, Ruan X, Feng Y and Wang
X: Treatment effect of Bushen Huayu extract on postmenopausal
osteoporosis in vivo. Exp Ther Med. 7:1687–1690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhdan VM, Kitura O, Kitura IeM, Babanina M
and Tkachenko MV: Treatment of postmenopausal osteoporosis in the
general medical practice (clinical case). Lik Sprava. 1–89.
2013.
|
|
14
|
Massafra U, Integlia D, Broccoli S and
Migliore A: Mixed treatment comparison to rank antiresorptive
agents in preventing new non vertebral fractures in postmenopausal
osteoporosis. Value Health. 18:A6362015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hassler N, Gamsjaeger S, Hofstetter B,
Brozek W, Klaushofer K and Paschalis EP: Effects of long-term
alendronate treatment on postmenopausal osteoporosis bone material
properties. Osteoporos Int. 26:339–352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Igase M, Kohara K, Tabara Y, Ohara M,
Takita R, Ochi M, Okada Y and Miki T: Change in arterial stiffness
associated with monthly bisphosphonate treatment in women with
postmenopausal osteoporosis. Menopause. 21:962–966. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Celer O, Akalin A and Oztunali C: Effect
of teriparatide treatment on endothelial function, glucose
metabolism and inflammation markers in patients with postmenopausal
osteoporosis. Clin Endocrinol (Oxf). 85:556–560. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rugpolmuang L and Waikakul S: Effect of a
short-term treatment with once-a-week medication of alendronate 70
Mg on bone turnover markers in postmenopausal women with
osteoporosis. J Med Assoc Thai. 98 Suppl 8:S70–S75. 2015.PubMed/NCBI
|
|
19
|
Schultz TC, Valenzano JP, Verzella JL and
Umland EM: Odanacatib: An emerging novel treatment alternative for
postmenopausal osteoporosis. Womens Health (Lond). 11:805–814.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seeliger C, Schyschka L, Kronbach Z,
Wottge A, van Griensven M, Wildemann B and Vester H: Signaling
pathway STAT1 is strongly activated by IFN-β in the pathogenesis of
osteoporosis. Eur J Med Res. 20:12015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen XD, Xiao P, Lei SF, Liu YZ, Guo YF,
Deng FY, Tan LJ, Zhu XZ, Chen FR, Recker RR and Deng HW: Gene
expression profiling in monocytes and SNP association suggest the
importance of the STAT1 gene for osteoporosis in both Chinese and
Caucasians. J Bone Miner Res. 25:339–355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kushwaha P, Khedgikar V, Ahmad N, Karvande
A, Gautam J, Kumar P, Maurya R and Trivedi R: A neoflavonoid
dalsissooal isolated from heartwood of Dalbergia sissoo Roxb. Has
bone forming effects in mice model for osteoporosis. Eur J
Pharmacol. 788:65–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Papamanthos M, Varitimidis S, Dailiana ZH,
Kogia E and Malizos K: Computer-assisted evaluation of Mandibular
Cortical Width (MCW) index as an indicator of osteoporosis.
Hippokratia. 18:251–257. 2014.PubMed/NCBI
|
|
24
|
Shum LC, White NS, Nadtochiy SM, Bentley
KL, Brookes PS, Jonason JH and Eliseev RA: Cyclophilin D Knock-out
mice show enhanced resistance to osteoporosis and to metabolic
changes observed in aging bone. PLoS One. 11:e01557092016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao H, Nazarian A, Ackerman JL, Snyder BD,
Rosenberg AE, Nazarian RM, Hrovat MI, Dai G, Mintzopoulos D and Wu
Y: Quantitative (31)P NMR spectroscopy and (1)H MRI measurements of
bone mineral and matrix density differentiate metabolic bone
diseases in rat models. Bone. 46:1582–1590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McCann RM, Colleary G, Geddis C, Clarke
SA, Jordan GR, Dickson GR and Marsh D: Effect of osteoporosis on
bone mineral density and fracture repair in a rat femoral fracture
model. J Orthop Res. 26:384–393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leamy LJ, Kelly SA, Hua K, Farber CR and
Pomp D: Quantitative trait loci for bone mineral density and
femoral morphology in an advanced intercross population of mice.
Bone. 55:222–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jonason JH and O'Keefe RJ: Isolation and
culture of neonatal mouse calvarial osteoblasts. Methods Mol Biol.
1130:295–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saiyed ZM, Sharma S, Godawat R, Telang SD
and Ramchand CN: Activity and stability of alkaline phosphatase
(ALP) immobilized onto magnetic nanoparticles (Fe3O4). J
Biotechnol. 131:240–244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Subramanian K, Geraerts M, Pauwelyn KA,
Park Y, Owens DJ, Muijtjens M, Ulloa-Montoya F, Jiang Y, Verfaillie
CM and Hu WS: Isolation procedure and characterization of
multipotent adult progenitor cells from rat bone marrow. Methods
Mol Biol. 636:55–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Idris AI, Landao-Bassonga E and Ralston
SH: The TRPV1 ion channel antagonist capsazepine inhibits
osteoclast and osteoblast differentiation in vitro and ovariectomy
induced bone loss in vivo. Bone. 46:1089–1099. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hiemer B, Ziebart J, Jonitz-Heincke A,
Grunert PC, Su Y, Hansmann D and Bader R: Magnetically induced
electrostimulation of human osteoblasts results in enhanced cell
viability and osteogenic differentiation. Int J Mol Med. 38:57–64.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiao S, Wang J and Xiao N: MicroRNAs as
noninvasive biomarkers in bladder cancer detection: A diagnostic
meta-analysis based on qRT-PCR data. Int J Biol Markers.
31:e276–e285. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wai-Hoe L, Wing-Seng L, Ismail Z and
Lay-Harn G: SDS-PAGE-based quantitative assay for screening of
kidney stone disease. Biol Proced Online. 11:145–160. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Johnson RW, White JD, Walker EC, Martin TJ
and Sims NA: Myokines (muscle-derived cytokines and chemokines)
including ciliary neurotrophic factor (CNTF) inhibit osteoblast
differentiation. Bone. 64:47–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takahashi M, Kushida K, Kawana K, Hoshino
H and Inoue T: Discrimination ability of pyridinoline crosslinks
related markers for bone resorption in postmenopause and
osteoporosis. Endocr Res. 23:105–117. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma J, Ma Y, Liu X, Chen S, Liu C, Qin A
and Fan S: Gambogic acid inhibits osteoclast formation and
ovariectomy-induced osteoporosis by suppressing the JNK, p38 and
Akt signalling pathways. Biochem J. 469:399–408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Song SH, Wang D, Mo YY, Ding C and Shang
P: Antiosteoporotic effects of naringenin on ovariectomy-induced
osteoporosis in rat. Yao Xue Xue Bao. 50:154–161. 2015.(In
Chinese). PubMed/NCBI
|
|
39
|
Lee SN, Cho JY, Eun YM, Song SW and Moon
KW: Associations between osteoporosis and coronary artery disease
in postmenopausal women. Climacteric. 19:458–462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alami S, Hervouet L, Poiraudeau S, Briot K
and Roux C: Barriers to effective postmenopausal osteoporosis
treatment: A qualitative study of patients' and practitioners'
views. PLoS One. 11:e01583652016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Karlsson L, Lundkvist J, Psachoulia E,
Intorcia M and Ström O: Persistence with denosumab and persistence
with oral bisphosphonates for the treatment of postmenopausal
osteoporosis: A retrospective, observational study, and a
meta-analysis. Osteoporos Int. 26:2401–2411. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim MJ, Kim SN, Lee IS, Chung S, Lee J,
Yang Y, Lee I and Koh SE: Effects of bisphosphonates to treat
osteoporosis in children with cerebral palsy: A meta-analysis. J
Pediatr Endocrinol Metab. 28:1343–1350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Diringer MN, Dhar R, Scalfani M, Zazulia
AR, Chicoine M, Powers WJ and Derdeyn CP: Effect of high-dose
simvastatin on cerebral blood flow and static autoregulation in
subarachnoid hemorrhage. Neurocrit Care. 25:56–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dauletbaev N, Cammisano M, Herscovitch K
and Lands LC: Stimulation of the RIG-I/MAVS Pathway by
polyinosinic: Polycytidylic acid upregulates IFN-β in airway
epithelial cells with minimal costimulation of IL-8. J Immunol.
195:2829–2841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Grumbach IM, Fish EN, Uddin S, Majchrzak
B, Colamonici OR, Figulla HR, Heim A and Platanias LC: Activation
of the Jak-Stat pathway in cells that exhibit selective sensitivity
to the antiviral effects of IFN-beta compared with IFN-alpha. J
Interferon Cytokine Res. 19:797–801. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Imaizumi T, Yoshida H, Hayakari R, Xing F,
Wang L, Matsumiya T, Tanji K, Kawaguchi S, Murakami M and Tanaka H:
Interferon-stimulated gene (ISG) 60, as well as ISG56 and ISG54,
positively regulates TLR3/IFN-β/STAT1 axis in U373MG human
astrocytoma cells. Neurosci Res. 105:35–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ma JS, Kim WJ, Kim JJ, Kim TJ, Ye SK, Song
MD, Kang H, Kim DW, Moon WK and Lee KH: Gold nanoparticles
attenuate LPS-induced NO production through the inhibition of
NF-kappaB and IFN-beta/STAT1 pathways in RAW264.7 cells. Nitric
Oxide. 23:214–219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yun CH, Yang JS, Kang SS, Yang Y, Cho JH,
Son CG and Han SH: NF-kappaB signaling pathway, not IFN-beta/STAT1,
is responsible for the selenium suppression of LPS-induced nitric
oxide production. Int immunopharmacol. 7:1192–1198. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bustamante MF, Morcillo-Suárez C, Malhotra
S, Rio J, Leyva L, Fernández O, Zettl UK, Killestein J, Brassat D,
García-Merino JA, et al: Pharmacogenomic study in patients with
multiple sclerosis: Responders and nonresponders to IFN-β. Neurol
Neuroimmunol Neuroinflamm. 2:e1542015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Damiano S, Sasso A, De Felice B,
Terrazzano G, Bresciamorra V, Carotenuto A, Orefice NS, Orefice G,
Vacca G, Belfiore A, et al: The IFN-β 1b effect on Cu Zn superoxide
dismutase (SOD1) in peripheral mononuclear blood cells of
relapsing-remitting multiple sclerosis patients and in
neuroblastoma SK-N-BE cells. Brain Res Bull. 118:1–6. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Takahashi T, Watanabe T, Nakada H,
Tanimoto Y, Kimoto S, Mijares DQ, Zhang Y and Kawai Y: Effect of a
dietary supplement on peri-implant bone strength in a rat model of
osteoporosis. J Prosthodont Res. 60:131–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li X, Niu QT, Warmington KS, Asuncion FJ,
Dwyer D, Grisanti M, Han CY, Stolina M, Eschenberg MJ, Kostenuik
PJ, et al: Progressive increases in bone mass and bone strength in
an ovariectomized rat model of osteoporosis after 26 weeks of
treatment with a sclerostin antibody. Endocrinology. 155:4785–4797.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Brennan O, Kuliwaba JS, Lee TC, Parkinson
IH, Fazzalari NL, McNamara LM and O'Brien FJ: Temporal changes in
bone composition, architecture, and strength following estrogen
deficiency in osteoporosis. Calcif Tissue Int. 91:440–449. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ying SH, Kao HC, Chang MC, Yu WK, Wang ST
and Liu CL: Fixation strength of PMMA-augmented pedicle screws
after depth adjustment in a synthetic bone model of osteoporosis.
Orthopedics. 35:e1511–e1516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Simon JA, Recknor C, Moffett AH Jr, Adachi
JD, Franek E, Lewiecki EM, McClung MR, Mautalen CA, Ragi-Eis S,
Nicholson GC, et al: Impact of denosumab on the peripheral skeleton
of postmenopausal women with osteoporosis: Bone density, mass, and
strength of the radius, and wrist fracture. Menopause. 20:130–137.
2013.PubMed/NCBI
|
|
56
|
Mashiba T: Morphological analysis of bone
dynamics and metabolic bone disease. Histological findings in
animal fracture model-effects of osteoporosis treatment drugs on
fracture healing process. Clin Calcium. 21:551–558. 2011.(In
Japanese).
|
|
57
|
Delling G, Ritzel H and Werner M:
Histological characteristics and prevalence of secondary
osteoporosis in systemic mastocytosis. A retrospective analysis of
158 cases. Pathologe. 22:132–140. 2001.(In German).
|
|
58
|
Saag KG, Agnusdei D, Hans D, Kohlmeier LA,
Krohn KD, Leib ES, MacLaughlin EJ, Alam J, Simonelli C, Taylor KA
and Marcus R: Trabecular bone score in patients with chronic
glucocorticoid-induced osteoporosis treated with alendronate or
teriparatide. Arthritis Rheumatol. 68:2122–2128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ryu TK, Kang RH, Jeong KY, Jun DR, Koh JM,
Kim D, Bae SK and Choi SW: Bone-targeted delivery of
nanodiamond-based drug carriers conjugated with alendronate for
potential osteoporosis treatment. J Control Release. 232:152–160.
2016. View Article : Google Scholar : PubMed/NCBI
|