Introduction
Lower back pain (LBP) is one of the most common
orthopedic problems, and is associated with high cost for medical
health and social care (1,2). Intervertebral disc degeneration (IDD)
has long been considered to have an important role in the
pathogenesis of LBP (3), although
other causes have also been suggested (4,5). Several
factors are thought to cause IDD, including genetic factors
(6), abnormal mechanical loading
(7) and poor disc cell nutrition
(8). Intervertebral discs (IVDs)
have three major components, namely nucleus pulposus, annulus
fibrosus and two thin hyaline cartilage endplates (CEPs) situated
between the disc and the adjacent vertebral bodies. IVD scontain
the largest amount of avascular tissue in the body and nutrient
supply to mature disc cells is almost entirely reliant on the
diffusion through CEPs (9).
Therefore, the morphological integrity and physiological function
of the CEPs are essential for the maintenance of the IVDs, and
adequate transport is critical for their health. It is increasingly
recognized that functional disorders of CEPs have an important role
in the process of IDD (10).
However, the role of CEP degeneration in this process and the
underlying mechanisms have remained elusive.
Apoptosis is a type of programmed cell death, which
has an important role in homeostasis, normal development and
elimination of potentially pathological cells from the organism
(11). Inappropriate regulation of
apoptosis may lead to various pathological conditions, including
cancer, stroke, ischemia and neurodegenerative diseases (12). Gruber and Hanley (13) assessed apoptosis in human
intervertebral disc annulus fibrosus by a terminal deoxynucleotidyl
transferase deoxyuridine triphosphate nick end labeling assay and
identified that apoptosis was associated with patient age. Ariga
et al (14) confirmed that
apoptosis also occurred in cartilage endplates and that mechanical
stress was an important factor affecting disc cell apoptosis and
disc degeneration. However, the mechanisms of stress-induced
apoptosis in intervertebral discs have remained to be fully
elucidated.
A previous study by our group identified stem cells
in human degenerated CEPs, which were named as CEP stem cells
(CESCs) (15). The present study
investigated the effects of cyclic tensile stretch on the apoptosis
of CESCs and investigated the underlying molecular mechanisms.
Materials and methods
Ethics statement
Institutional Review Board approval and informed
consent for sample collection were obtained prior to the collection
of all samples. All of the procedures specified below were approved
by the Ethical Committee of Xinqiao Hospital (the Third Military
Medical University, Chongqing, China) and were in accordance with
the Helsinki Declaration.
Cell culture and investigation of
surface markers
Human intervertebral disc cartilage endplate cells
and CESCs were obtained from 6 patients (3 men, 3 women; 39–50
years old), following the protocol of a previous study (15). Cells were derived from surgically
resected intervertebral disc cartilage endplate. CESCs at passage 3
were used in the present study. The expression profile of cell
surface antigens was determined by flow cytometry. In brief, the
cells were seeded in a 6-well culture plate and grown until they
reached 90% confluence. After washing with PBS, the cells were
stained with the following fluorescein isothiocyanate (FITC),
phycoerythrin (PE) or peridinin chlorophyll protein
(PerCP)-conjugated antibodies: CD73-FITC (cat. no. 11-0739-41),
CD14-FITC (cat. no. 11-0149-41), CD19-FITC (cat. no. 11-0199-41),
CD90-FITC (cat. no. 11-0909-41), CD34-FITC (cat. no. 11-0349-41),
CD45-FITC (cat. no. 11-9459-41), CD105-PE (cat. no. 12-1057-41) or
human leukocyte antigen – antigen D related (HLA-DR)-PerCP(cat. no.
9043-4724-025) (1:500 dilution, all from eBioscience; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Immunoglobulin G (eBioscience;
Thermo Fisher Scientific, Inc.) was used as an isotype control.
After incubation for 30 min at 37°C, cells were washed 3 times with
PBS and resuspended in 200 µl PBS. Finally, labeled cells were
subjected to single-channel flow cytometric analysis (BD
Biosciences, Franklin Lakes, NJ, USA). The percentage of stained
cells was calculated relative to the isotype control.
Application of cyclic tension to
cultured cells
For cyclic tension loading, CESCs were placed on an
elastic silicone membrane coated with collagen I at a density of
2×105 cells per well. After reaching 80–90% confluence,
cells were serum-starved in Dulbecco's modified Eagle's medium
(DMEM)/F12 (Gibco; Thermo Fisher Scientific, Inc.) containing 1%
fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.)
for 24 h for synchronization, followed by stretching using a
Flexercell™ Tension Plus system (FX-4000T; Flexcell
International Corp., Burlington, NC, USA) at 37°C in a 5%
CO2 incubator in DMEM/F12 with 10% FBS. According to the
experimental protocol, a 20% stretch elongation at a frequency of 1
Hz was applied for 12, 24, 36 or 48 h.
Cell viability detection and caspase-3
repression assay
After stretching, cells were seeded into 96-well
plates at a density of 5×103 cells per well. After 24 h,
the cell viability was assessed using a Cell Counting Kit-8 (CCK-8)
according to the manufacturer's instructions. In brief, 10 µl CCK-8
solution was added to each well and the plate was then incubated at
37°C for 4 h. The optical density was measured at a wavelength of
450 nm with a microplate reader. In another experiment prior to
stretching, caspase-3 inhibitor Z-VAD-FMK (20 µM; Beyotime
Institute of Biotechnology, Haimen, China) was added to the cell
culture medium and the plate was incubated at 37°C for 20 min.
Detection of apoptotic rate by flow
cytometry
The apoptotic rate was measured with an Annexin
V-FITC apoptosis detection kit I (BD Pharmingen, Franklin Lakes,
NJ, USA) according to the manufacturer's instructions. In brief,
cells were washed twice with cold PBS and then resuspended in 300
µl binding buffer at a concentration of 1×106 cells per
ml. Annexin V-FITC solution (5 µl) and propidium iodide (PI; 5 µl
of a 1 µg/ml solution) were then added to these cells, followed by
incubation for 30 min at 37°C. The apoptotic incidence was detected
by flow cytometry within 1 h.
Caspase-3 activity detection
assay
Caspase-3 activity was detected with a caspase assay
kit (Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. In brief, 2×106 cells were
lysed with 100 µl lysis buffer on ice for 15 min and the cell
lysates were then centrifuged at 16,000 × g for 10 min at 4°C.
Reaction buffer (80 µl) and acetyl-DEVD-p-nitroanilin (pNA; 10 µl)
was mixed with 10 µl lysate and the mixture was then incubated for
2 h at 37°C. The optical density of free pNA was measured at an
absorption wavelength of 405 nm using a microplate
spectrophotometer (Dynex Technologies, Chantilly, VA, USA).
Caspase-3 activity was expressed as the relative absorbance ratio
of samples vs. control group.
Western blot analysis
Cells were lysed using lysis buffer containing 1 mM
phenylmethylsulfonylfluoride (Beyotime Institute of Biotechnology),
Protein concentration was determined with the BCA Protein Assay kit
(Beyotime Institute of Biotechnology). Equal amounts of protein (30
µg) were separated by 12% SDS-PAGE and transferred onto
polyvinylidene diflouride membranes (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Subsequently, the membrane was blocked in 5%
non-fat milk and 0.1% Tween-20 containing Tris-buffered saline
(TBST) at room temperature for 1 h and then incubated with the
respective primary antibodies overnight at 4°C. After washing with
TBST, the membrane was then incubated with appropriate horseradish
peroxidase-conjugated secondary antibodies at room temperature for
2 h. Immunoblotting was detected by enhanced chemiluminescence
(BeyoECLPlus, Beyotime, Beijing, China) and images of blots were
captured on a ImageQuant LAS4010 (GE Healthcare, Little Chalfont,
UK). Western blot bands were quantified using ImageJ software
(ImageJ 1.41; National Institutes of Health, Bethesda, NJ, USA).
The primary antibodies used for western blot analysis were as
follows: B-cell lymphoma 2 (Bcl-2)/adenovirus E1B 19 kDa
interacting protein 3 (BNIP3) (cat. no. ab10433; mouse monoclonal),
Bcl-2 (cat. no. ab59348; rabbit monoclonal), Bcl-2 homologous
antagonist killer (Bak; cat. no. ab69404; rabbit monoclonal),
Bcl-2-associated X protein (Bax; cat. no. ab53154; rabbit
monoclonal) and Bcl extra large (Bcl-xl; cat. no. ab32370; rabbit
monoclonal) (all used at 1:500 dilution; Abcam, Cambridge, MA, USA)
and GAPDH (cat. no. TA-08, 1:2,000 dilution; Beijing Zhongshan
Goldenbridge Biotechnology Co., Ltd., Beijing, China). The
secondary antibodies used in the present study were as follows:
Horse radish peroxidase (HRP)-labeled goat anti-mouse (cat. no.
A0216) and anti-rabbit (cat. no. A0208) Immunoglobulin G (IgG)
(H+L) (all 1:1,000 dilution; Beyotime Institute of
Biotechnology).
Statistical analysis
Data were analyzed using SPSS 13.0 statistical
software (SPSS, Inc., Chicago, IL, USA). Values are expressed as
the mean ± standard deviation. Differences between groups were
assessed using the one-way analysis of variance and the Least
Significant Difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
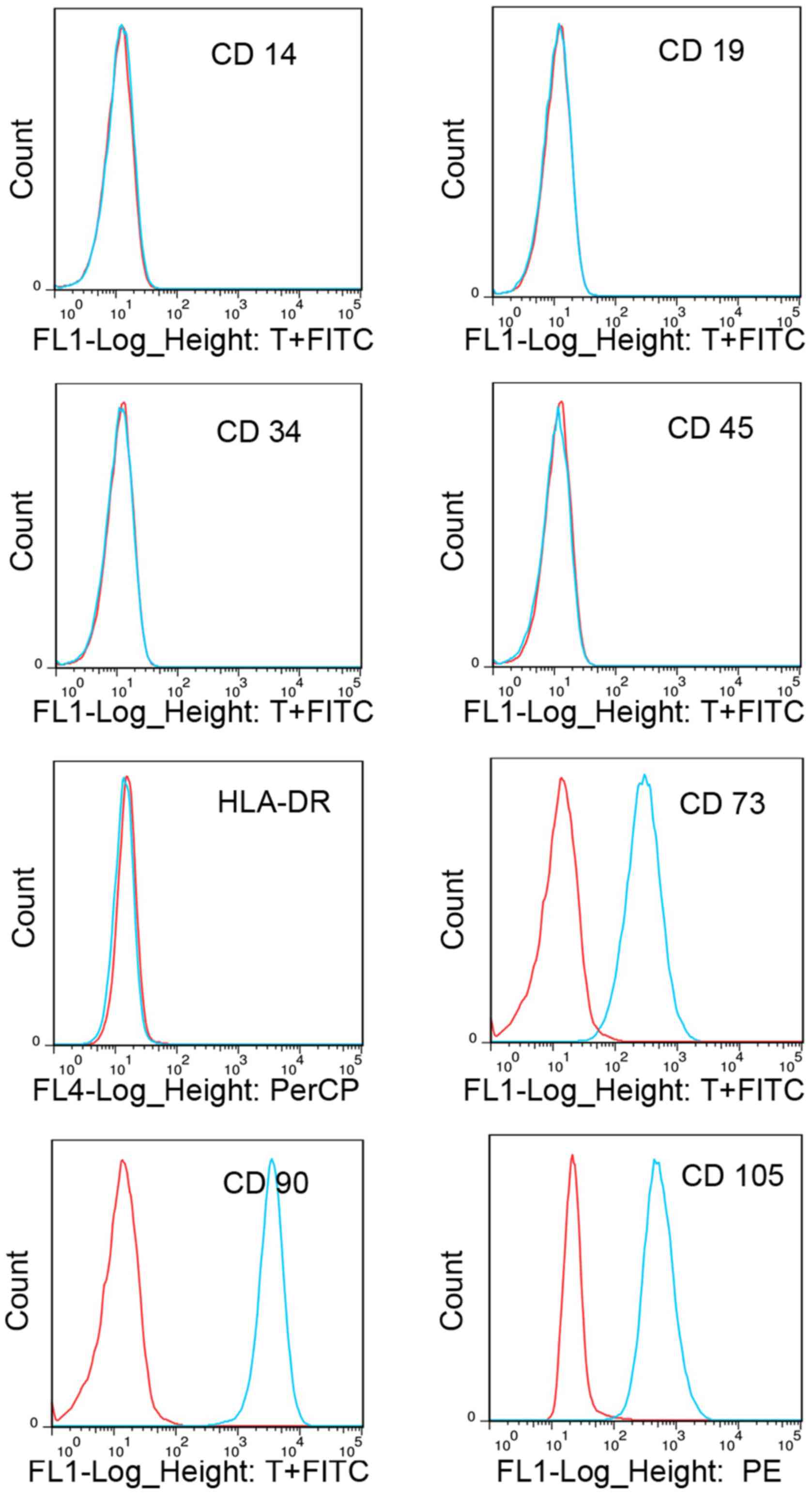
Surface antigen profile of CESCs
The surface antigens of CESCs were investigated in
passage 3 cells. Flow cytometric analysis revealed that the CESCs
were negative for CD14, CD19, CD34, CD45 and HLA-DR, but positive
for CD73, CD90 and CD105 (Fig. 1),
which was similar to the result of a previous study by our group
(15). Considering that the
pluripotency of CESCs has already been demonstrated in a previous
study (15), these results indicated
that the cells used in the present study possessed the properties
of stem cells.
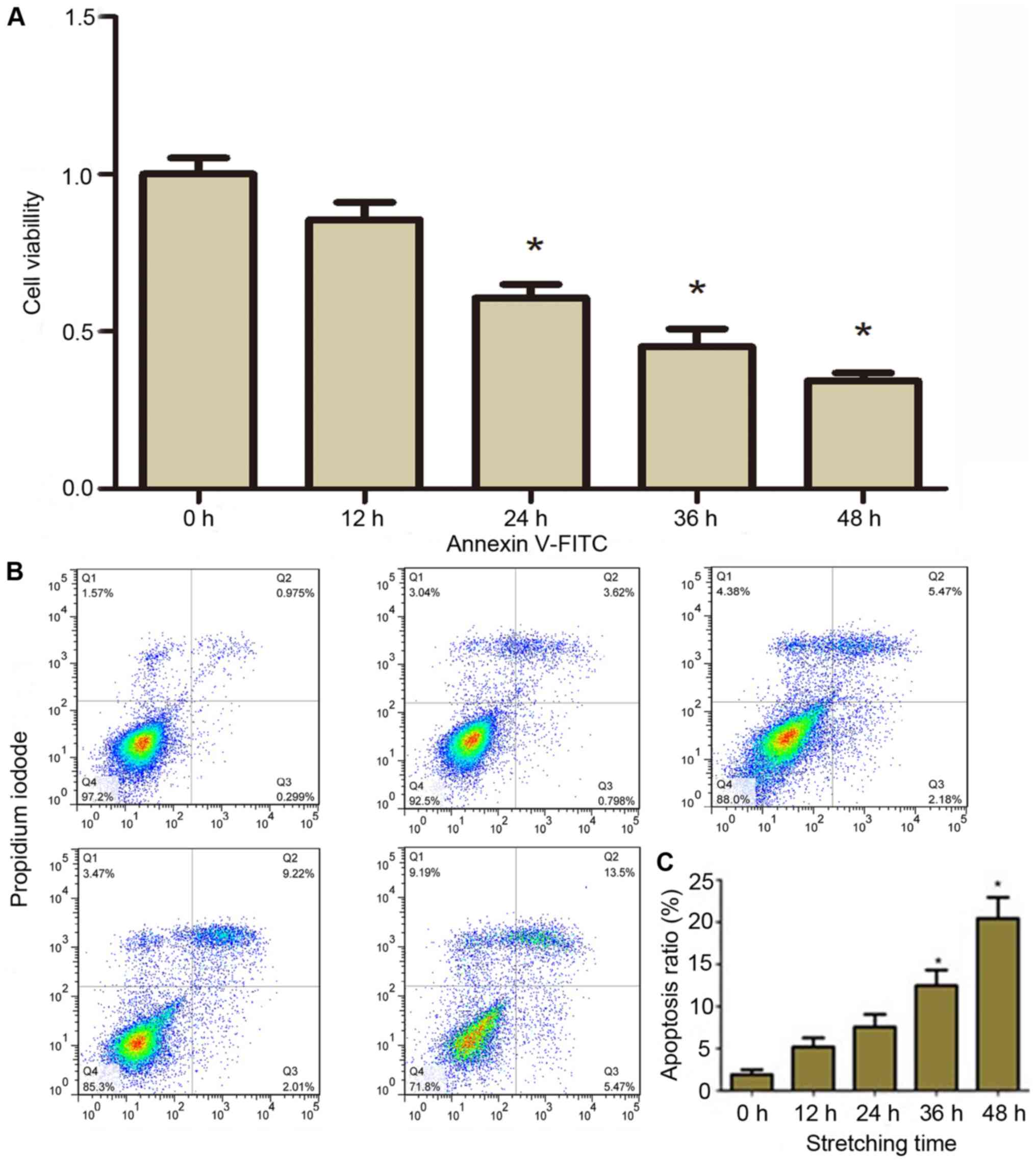
Effects of stretch on the viability
and apoptosis of CESCs
The effects of a tensile stretch on the viability of
CESCs were assessed. After 20% stretch elongation at a frequency of
1 Hz for 12, 24, 36, or 48 h, the viability of CESCs was assessed
using a CCK-8. The results demonstrated that compared with static
control cells (0 h), the viability of CESCs decreased with the
increase of stretching time (P<0.05; Fig. 2A).
Furthermore, it was assessed whether the decrease in
viability of CESCs was due to apoptosis. The apoptotic rate was
detected by flow cytometry with Annexin V and PI double labeling.
PI labels all dead cells, including necrotic cells and those at the
final stages of apoptosis, whereas cells in early apoptosis were
only stained with Annexin V. Representative graphs obtained by flow
cytometric analysis of cells after Annexin V-FITC and PI double
staining are presented in Fig. 2B.
The apoptotic incidence in the static control group was 1.27%. The
results demonstrated that the apoptotic incidence was significantly
increased in CESCs subjected to cyclic stretch compared to that in
the control group in a time-dependent manner (Fig. 2B). Hence, the apoptotic incidence in
the CESCs was increased to 5.12, 7.06, 12.06 and 20.00% after
cyclic stretch for 12, 24, 36 and 48 h, respectively (Fig. 2C). These results demonstrated that a
cyclic tensile stretch decreased the viability and increased the
apoptotic rate of CESCs.
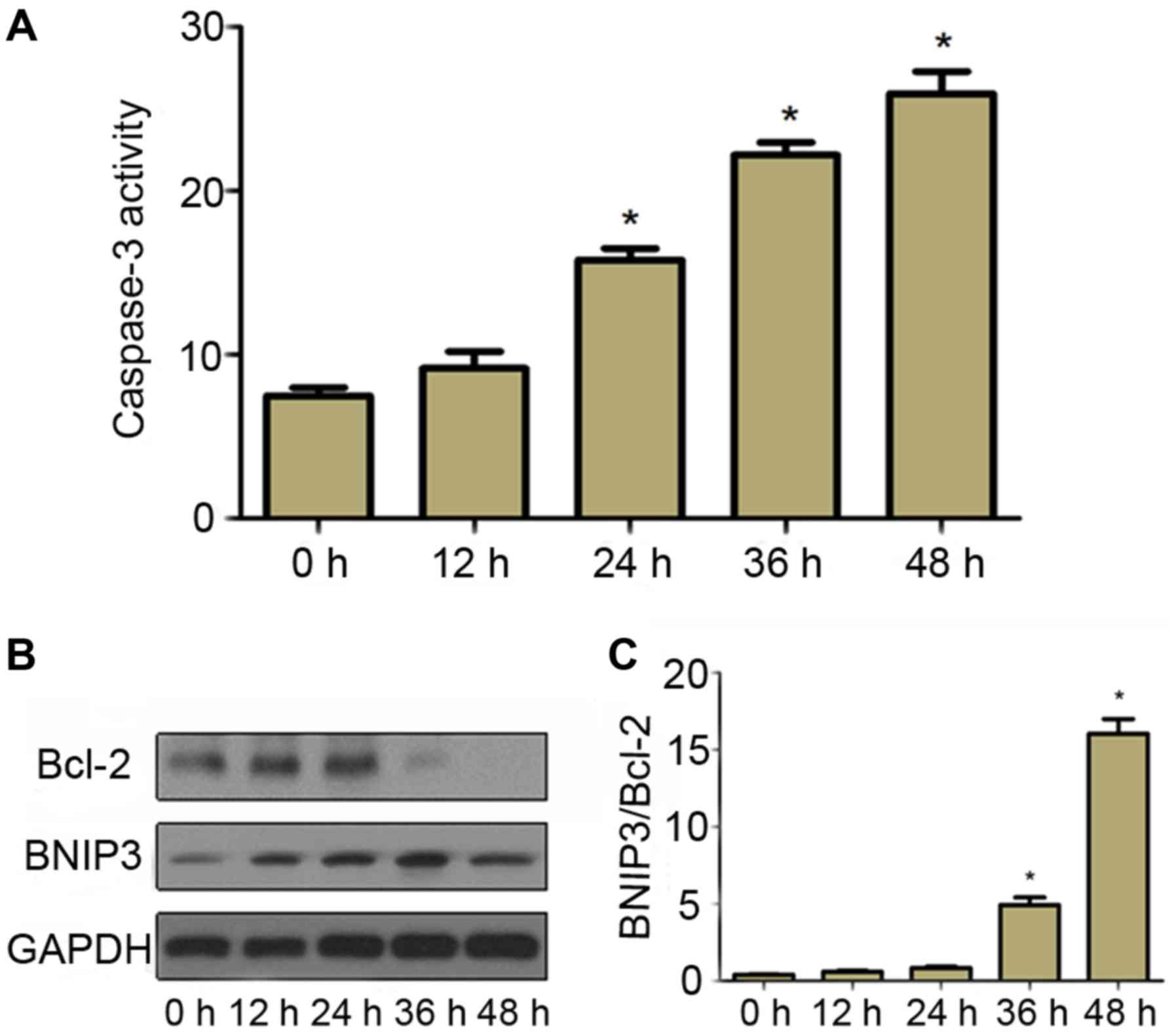
Effect of stress on the activity of
caspase-3
Since cyclic stretch increased cell apoptosis in a
time-dependent manner, the apoptotic mechanism in CESCs was then
assessed. In order to understand whether apoptosis induced by
cyclic stretch was caspase-dependent, the activity of caspase-3 was
measured. The results indicated that the activity of caspase-3 in
cells subjected to cyclic stretch increased in a time-dependent
manner compared with that in static control cells (Fig. 3A), which was consistent with the
apoptosis incidence (Fig. 3B and C).
This finding suggested that cyclic stretch activated caspase-3 in
CESCs, which finally induced cell apoptosis.
Effect of stress on the expression of
BNIP3 and Bcl-2 in CESCs
To further investigate the molecular mechanism of
cyclic stretch-induced apoptosis, the expression of BNIP3 and Bcl-2
in CESCs was assessed. As presented in Fig. 3B, compared with the static control
cells, the expression of pro-apoptotic protein BNIP3 increased in
cells subjected to 20% stretch elongation at a frequency of 1 Hz
for 12, 24, 36 and 48 h. Furthermore, the expression of
anti-apoptotic protein Bcl-2 began to decrease after cells had been
subjected to a 20% stretch elongation for 36 h (Fig. 3C). These results suggested that
upregulation of BNIP3 and downregulation of Bcl-2 probably
contributed to apoptosis induced by cyclic stretch in CESCs.
Caspase-3 inhibitor prevents
stretch-induced apoptosis
To identify the role of apoptosis in cells exposed
to cyclic stretch, caspase-3 was repressed to evaluate cell
viability and apoptosis. As presented in in Fig. 4A and B, pro-apoptotic protein Bak and
Bax were significantly increased after cells were subjected to a
20% stretch for 36 h, while Bcl-xl was decreased, which indicated
marked apoptosis in the cells. When the caspase-3 inhibitor was
added to the cell culture medium, the caspase-3 activity in the
stretched cells restored to near basal levels (Fig. 4C), and the cell viability in the
stretched cells (Fig. 4D) was also
restored comparing with stretched cells without caspase-3
inhibitor. Cell apoptosis (Fig. 4E and
F) was also significantly reduced compared with those in
stretched cells without caspase-3 inhibitor treatment.
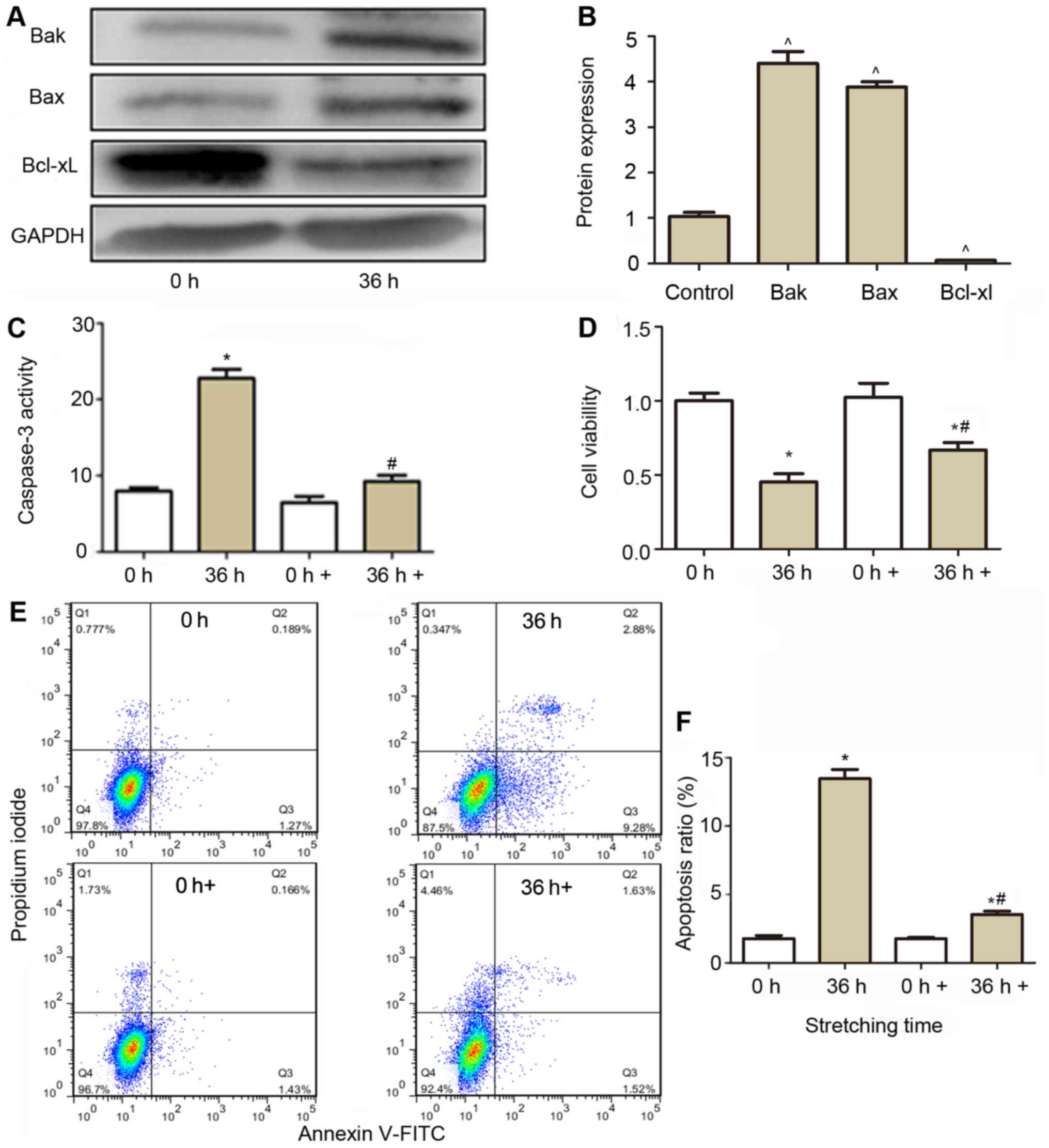 | Figure 4.Effect of caspase-3 inhibitor on the
activity of caspase-3, cell viability and apoptosis of CESCs
subjected to a 20% stretch at a frequency of 1 Hz for 36 h. (A)
Western blot analysis was performed to analyze the expression of
Bak, Bax and Bcl-xl. GAPDH was used as a loading control. (B)
Quantification of the expression of Bak, Bax and Bcl-xl from (A).
(C) Effect of caspase-3 inhibition (0 and 36 h+) on the caspase-3
activity in normal (white column) and stretched cells (brown
column). (D) The cell viability was assessed after caspase-3 was
inhibited (0 and 36 h+), using a Cell Counting Kit-8 assay in
normal (white column) and stretched cells (brown column). (E)
Apoptosis was detected using aby flow cytometric Annexin V/PI
assay. Representative flow cytometry dot plots of CESCs after
double staining with Annexin V-FITC and PI are displayed. (F)
Apoptotic indices of CESCs from E. Static CESCs (0 h) were used as
a control. +, group incubated with caspase inhibitor Z-VAD-FMK.
^P<0.05 vs. control group; *P<0.05 vs. 0 h group;
#P<0.05 vs. 36 h group. CESCs, cartilage
endplate-derived stem cells; Bcl-2, B-cell lymphoma 2; FITC,
fluorescein isothiocyanate; PI, propidium iodide; Bak, Bcl-2
antagonist killer protein; Bax, Bcl-2-associated X protein; Bcl-xl,
Bclextra large protein. |
Discussion
Intervertebral discs have an important buffering
function in the human body, and are also the basic structure
facilitating spinal movement. Homeostasis in the synthesis and
degradation of extracellular matrix is necessary for the normal
physiological structure and function of intervertebral discs. When
this balance is broken, intervertebral disc degeneration occurs
(16). The cartilage endplate is a
thin and transparent type of cartilage, which maintains the
integrity and physiological function of the intervertebral disc. A
previous study indicated that with increasing age of individuals,
the morphology of the cartilage endplate changes, which is closely
associated with pathological changes in the nucleus pulposus and
annulus fibrosus (17). Sowa and
Agarwal (18) reported that a
moderate cyclic stretch loading decreased the catabolism level and
protected intervertebral discs, while an excessive stretch induced
cell apoptosis and intervertebral disc degeneration. However, the
mechanisms of stretch-induced cell apoptosis in intervertebral
discs have remained elusive.
BNIP3 is a member of Bcl-2 protein family and has a
single Bcl-2 homology 3 (BH3) domain and is a pro-apoptotic protein
in the mitochondrial apoptotic pathway. BNIP3 expression is low in
various cell types, where it is primarily localized to the
cytoplasm. However, under hypoxia, BNIP3 expression increases,
followed by translocation from the cytoplasm to the mitochondria
through inserting its C-terminus in the outer mitochondrial
membrane (19). After membrane
insertion, BNIP3 mediates mitochondrial dysfunction to induces cell
apoptosis (20). While BNIP3 is
overexpressed in hypoxia, knockdown of BNIP3 expression was
reported to reduce hypoxic death of glioblastoma, which are
malignant brain tumor cells (21).
Another study reported that BNIP3 knockout cells are protected from
mitochondrial damage and cell death induced by mutant huntingtin, a
key protein associated with Huntington's disease (HD). Deletion of
the C-terminal transmembrane domain-encoding region from the BNIP3
gene suppressed mitochondrial depolarization and fragmentation in a
cell culture model of HD (22).
Bcl-2 family members may interact with the C-terminal domain of
BNIP3 to form heterodimers, which was observed to inhibit the
translocation of BNIP3 and induce apoptosis (23). BNIP3 inhibits the anti-apoptotic
effects of Bcl-2 by interacting with it through its BH3 domain
(20). Caspases are a family of
cysteinyl aspartate-specific proteases that are highly
evolutionarily conserved in multicellular organisms and function as
central regulators of apoptosis. As a member of this family,
caspase-3 has been identified as a key mediator of apoptosis in
most cells (11). The extrinsic and
intrinsic apoptotic pathways converge at caspase-3, which
orchestrates the dismantling of diverse cell structures through
cleavage of specific substrates.
The present study confirmed that BNIP3 was expressed
at a low level in static CESCs, while a cyclic stretch upregulated
BNIP3 expression and downregulated Bcl-2 expression in a
time-dependent manner. CCK-8 and flow cytometric assays
demonstrated that a cyclic tensile stretch decreased the cell
viability and increased apoptosis of CESCs, which was consistent
with the results of previous studies (24). Furthermore, the present study
demonstrated that a 20% cyclic stretch activated the expression of
caspase-3 in CESCs, which probably contributed to the increase in
apoptosis. Caspase-3 inhibitor abrogated the stretch-induced
decrease in cell viability and increase in apoptosis at 36 h, when
cell apoptosis was at a high level. However, no unstretched control
group at time-points 12–48 h that was kept in the stretching device
was included, and therefore, the contribution of the effect of
being kept in the stretching device to cell apoptosis and decrease
in viability could not be assessed. This should be assessed in a
future study.
Taken together, the present study suggested that
BNIP3 contributed to the regulation of cyclic stretch-induced
apoptosis of CESCs in an in vitro model. These results
implied that blocking of BNIP3 may improve the tolerance of CESCs
to abnormal stretching and delay the process of intervertebral disc
degeneration as a potential preventive or treatment strategy.
Acknowledgements
This study was funded by the National Natural
Science Fund (grant no. 81272028).
References
|
1
|
Haldeman S, Kopansky-Giles D, Hurwitz EL,
Hoy D, Erwin W Mark, Dagenais S, Kawchuk G, Stromqvist B and Walsh
N: Advancements in the management of spine disorders. Best Pract
Res Clin Rheumatol. 26:263–280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wieser S, Horisberger B, Schmidhauser S,
Eisenring C, Brügger U, Ruckstuhl A, Dietrich J, Mannion AF,
Elfering A, Tamcan O and Müller U: Cost of low back pain in
Switzerland in 2005. Eur J Health Econ. 12:455–467. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Freemont AJ: The cellular pathobiology of
the degenerate intervertebral disc and discogenic back pain.
Rheumatology (Oxford). 48:5–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schwarzer AC, Aprill CN, Derby R, Fortin
J, Kine G and Bogduk N: The relative contributions of the disc and
zygapophyseal joint in chronic low back pain. Spine (Phila Pa
1976). 19:801–806. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schwarzer AC, Aprill CN and Bogduk N: The
sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976).
20:31–37. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Battié MC, Videman T, Levalahti E, Gill K
and Kaprio J: Genetic and environmental effects on disc
degeneration by phenotype and spinal level: A multivariate twin
study. Spine (Phila Pa 1976). 33:2801–2808. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stokes IA and Iatridis JC: Mechanical
conditions that accelerate intervertebral disc degeneration:
Overload versus immobilization. Spine (Phila Pa 1976).
29:2724–2732. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boos N, Weissbach S, Rohrbach H, Weiler C,
Spratt KF and Nerlich AG: Classification of age-related changes in
lumbar intervertebral discs: 2002 Volvo Award in basic science.
Spine (Phila Pa 1976). 27:2631–2644. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Urban JP, Smith S and Fairbank JC:
Nutrition of the intervertebral disc. Spine (Phila Pa 1976).
29:2700–2709. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ariga K, Miyamoto S, Nakase T, Okuda S,
Meng W, Yonenobu K and Yoshikawa H: The relationship between
apoptosis of endplate chondrocytes and aging and degeneration of
the intervertebral disc. Spine (Phila Pa 1976). 26:2414–2420. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fiers W, Beyaert R, Declercq W and
Vandenabeele P: More than one way to die: Apoptosis, necrosis and
reactive oxygen damage. Oncogene. 18:7719–7730. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carson DA and Ribeiro JM: Apoptosis and
disease. Lancet. 341:1251–1254. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gruber HE and Hanley EN Jr: Analysis of
aging and degeneration of the human intervertebral disc. Comparison
of surgical specimens with normal controls. Spine (Phila Pa 1976).
23:751–757. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ariga K, Yonenobu K, Nakase T, Hosono N,
Okuda S, Meng W, Tamura Y and Yoshikawa H: Mechanical
stress-induced apoptosis of endplate chondrocytes in organ-cultured
mouse intervertebral discs: An ex vivo study. Spine (Phila Pa
1976). 28:1528–1533. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang B, Liu LT, Li CQ, Zhuang Y, Luo G,
Hu SY and Zhou Y: Study to determine the presence of progenitor
cells in the degenerated human cartilage endplates. Eur Spine J.
21:613–622. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cappello R, Bird JL, Pfeiffer D, Bayliss
MT and Dudhia J: Notochordal cell produce and assemble
extracellular matrix in a distinct manner, which may be responsible
for the maintenance of healthy nucleus pulposus. Spine (Phila Pa
1976). 31:873–883. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sowa G and Agarwal S: Cyclic tensile
stress exerts a protective effect on intervertebral disc cells. Am
J Phys Med Rehabil. 87:537–544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee H and Paik SG: Regulation of BNIP3 in
normal and cancer cells. Mol Cells. 21:1–6. 2006.PubMed/NCBI
|
|
20
|
Sassone F, Margulets V, Maraschi A,
Rodighiero S, Passafaro M, Silani V, Ciammola A, Kirshenbaum LA and
Sassone J: Bcl-2/adenovirus E1B 19-kDa interacting protein (BNip3)
has a key role in the mitochondrial dysfunction induced by mutant
huntingtin. Hum Mol Genet. 24:6530–6539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Y, Henson ES, Xiao W, Shome E, Azad
MB, Burton TR, Queau M, Sathya A, Eisenstat DD and Gibson SB: Bcl-2
family member Mcl-1 expression is reduced under hypoxia by the E3
ligase FBW7 contributing to BNIP3 induced cell death in glioma
cells. Cancer Biol Ther. 17:604–613. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Landes T, Emorine LJ, Courilleau D, Rojo
M, Belenguer P and Arnauné-Pelloquin L: The BH3-only Bnip3 binds to
the dynamin Opa1 to promote mitochondrial fragmentation and
apoptosis by distinct mechanisms. EMBO Rep. 11:459–465. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ray R, Chen G, Velde C Vande, Cizeau J,
Park JH, Reed JC, Gietz RD and Greenberg AH: BNIP3 heterodimerizes
with Bcl-2/Bcl-X(L) and induces cell death independent of a Bcl-2
homology 3 (BH3) domain at both mitochondrial and nonmitochondrial
sites. J Biol Chem. 275:1439–1448. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu C, Hao Y, Wei B, Ma J, Li J, Huang Q
and Zhang F: Apoptotic gene expression by human periodontal
ligament cells following cyclic stretch. J Periodontal Res.
46:742–748. 2011. View Article : Google Scholar : PubMed/NCBI
|