Introduction
Currently there is robust experimental and some
clinical evidence that hypothermia (local or systemic) may target
multiple pathological mechanisms caused by SCI trauma. In
principle, hypothermia reduces metabolic consumption and energy
demands, having beneficial effects on cytoplasmic ATP stores and
the maintenance of normal transmembrane ion and neurotransmitter
gradients (1). Previous
investigations have shown that the degree of ATP preservation
depends on the hypothermic level as well as the severity of the
insult. Erecinska et al (2)
reported that hypothermia lowered the cerebral metabolic rate for
oxygen and glucose two-fold to four-fold per 10°C reduction in
temperature, in addition to reducing cerebral blood flow.
Hypothermia can also provide benefits through its ability to
preserve the blood-spinal cord barrier (BSCB), ameliorate local
edema, suppress axonal swelling and development of gliosis in the
peri-injury zones (3,4); also to reduce oxidative stress,
apoptosis and inflammation (5–8). Spinal
cord injury studies in animals treated with mild systemic
hypothermia for 30 min to 4 h have provided positive results for
locomotor function and neuronal tissue maintenance. The protective
influence of hypothermia has also been reported following its local
application to the CNS. The advantage of local cooling using low
temperature applied directly to the epicenter of injury obviates
many of the risks of systemic hypothermia, such as arterial
hypertension, bradycardia or pneumonia (9,10).
Better outcomes have been achieved when modest cooling was started
as soon as possible after trauma (4,5,8,11,12).
The development of a treatment strategy for acute
spinal cord injury requires reliable and easily reproducible animal
models allowing further characterization and better understanding
of secondary pathophysiological mechanisms which could represent
targets for therapeutic interventions. Preclinical and clinical
trials have demonstrated that no treatment successful in rodents
has been effective in humans so far. With regard to the similarity
of their morphology and anatomical structures with humans, minipigs
have already been selected as a useful SCI animal model for
preclinical therapeutic trials (13,14).
In the current study we used a computer-controlled
chronic compression model in Göttingen-Minesota-Liběchov minipigs
(15) to test local spinal cord
hypothermia treatment with saline or oxygenated culture medium
(4°C) administered to the epicenter of injury beginning 30 min
after traumatic impact. Because previous experimental and human
clinical studies have shown effective transdural diffusion of
epidurally administered analgesics, anesthetics and antibiotics
(16–18), we anticipated that transdural
diffusion of the saline or oxygenated culture medium at the lesion
site without opening the spinal dural sac could lead to a positive
treatment effect by maintaining local cellular homeostasis. Local
hypothermia carried out through a special spinal perfusion chamber,
prepared using 3D scanner technology, provided outright 5 h-long
perfusion of the lesion site (L3) with cold solutions (4°C)
administered by means of a peristaltic pump (flow 2 ml/min), and
allowed stable body temperature to be maintained during the whole
perfusion period. The temperature of solutions in the perfusion
chamber just above the lesion site was maintained at 19°C.
The study provides evidence of considerable loss of
neurofilament immunoreactivity (NF-IR), and reduced tissue
degradation seen at the lesion site and away from the lesion
epicenter (3 cm caudally as well as cranially) nine weeks after SCI
(8N and 15N force), and it supports the beneficial effect of
hypothermia with saline to promote the number of neurofilaments and
gray and white matter preservation, and although without
statistical significance, also functional improvement.
Materials and methods
Animals
Thirty adult female minipigs
(Gottingen-Minnesota-Liběchov crossbred strains; 5–8 months of age,
weighing 25–35 kg) were used in the experiment. The minipigs were
delivered from Liběchov farm 3–4 months after their birth. They
were group housed in connected pens and were maintained in standard
conditions with ad libitum access to food and water. Female
minipigs were used in order to avoid the development of penile
prolapse that can be a serious complication after SCI performed at
lumbar level. No estrus was observed in any experimental animal.
The animals had only a basic feed ration compared with the ration
used by breeders for pubertal gilts to stimulate sexual processes.
This dose restriction therefore did not stimulate early sexual
maturity. This fact, together with the stress (animal transport,
daily manipulation) was probably the reason that no rut was
reported during the period of housing and clinical experiments.
All procedures were carried out in accordance with
the protocols approved by the State Veterinary and Food
Administration in Bratislava (decision no. 1319/13-221) as well as
by the Animal Use Committee at the Institute of Neurobiology,
Slovak Academy of Sciences, and in accordance with the EC Council
Directive (2010/63/EU) regarding the use of animals in research.
All efforts were made to minimize the size of experimental groups
and animal suffering.
Experimental groups
In a pilot study three minipigs were used for
standardization of the compression technique by testing the U
shape/circular aluminum bars, and two other minipigs were assigned
for testing of spinal perfusion chambers. These animals were
excluded from immunohistochemical, histochemical and behavioral
analyses.
Twenty-five animals were randomly divided into six
experimental groups: i) control group without surgical intervention
(n=3); ii and iii) spinal cord compression at the L3 segment
induced by 8N (n=4) or 15N (n=4) force; iv and v) groups that
underwent spinal cord compression (8N force) and local hypothermia
(5 h) with saline (n=4) or oxygenated culture medium (DMEM/F12)
(n=3); vi) group that underwent spinal cord compression (15N force)
and local hypothermia (5 h) with saline (n=7). All experimental
animals survived for 9 weeks.
Pre-surgical procedures
The minipigs were premedicated with an intramuscular
injection of Stresnil (2 mg/kg; Janssen Pharmaceutica, Beerse,
Belgium) and Atropin (0.5 mg/kg; Biotika a.s., Slovenská L'upča,
Slovakia), and then anesthetized with Thiopental (10 mg/kg; Czech
Pharma, Praha, Czech Republic). Prior to surgery, the precise
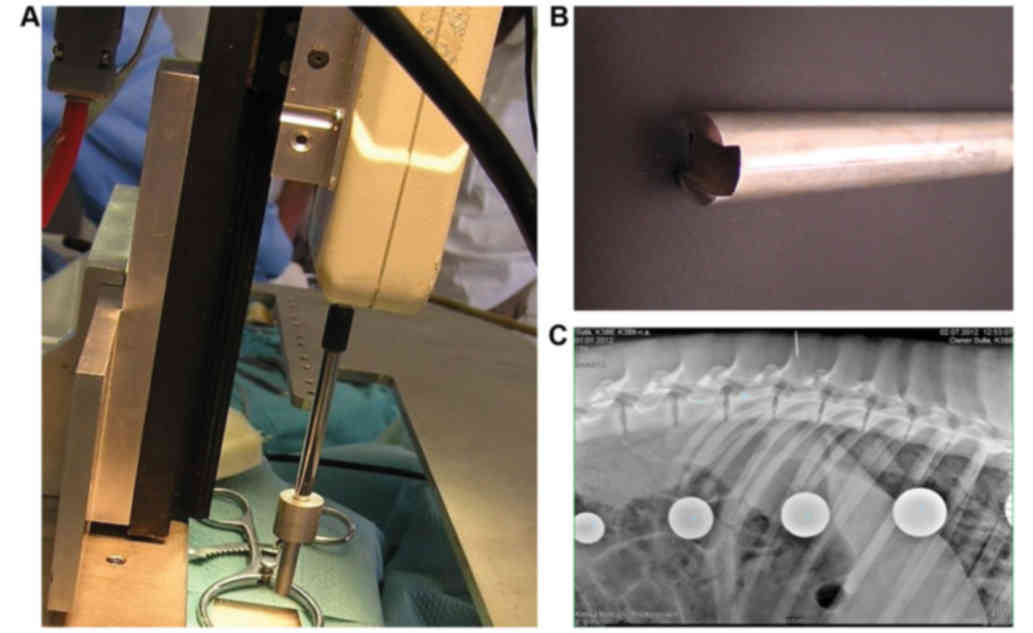
location of the L3 vertebra was identified using plain X-rays in a
lateral projection (Fig. 1)
(19). Afterwards the animals were
intubated with an endotracheal tube (diameter 5.0–6.0 mm) and the
anesthesia was maintained with Sevoflurane (1.5%; Baxter Czech
spol. s r.o., Praha, Czech Republic) mixed with oxygen. For both
the surgical procedure and the duration of hypothermia, the
analgesia was supported by the administration of Butomidor (0.4
mg/kg; i.v.; Richter Pharma AG, Wels, Austria). Catheters for the
administration of infusions and medicaments were inserted
bilaterally into the cephalic and/or auricular veins.
Spinal cord compression
The minipigs were placed in a special immobilization
apparatus consisting of a basal oval stainless steel platform
(50×90 cm) with four horizontal bars (2 cm in diameter) and four
vertical bars (size 3×9 cm and 23 cm length) (Fig. 1A). The apparatus was placed on a
standard operating table. The lumbar portion of the minipigs was
fixed in a prone position with the horizontal bars slid bilaterally
against the lateral portion of their paravertebral muscles. After
disinfection of the lumbar area, a midline skin and subcutaneous
fat incision was made from L2 to L4 vertebrae. Paraspinal muscles
were retracted to access the L2-L4 laminae and a laminectomy was
carried out. The SCI was induced at the L3 segment using a
compression device consisting of a computer-controlled stepping
motor (ViDiTo, Kosice, Slovakia) and a digital force gauge bridged
to a 5-mm diameter circular aluminum bar (Fig. 1B). The computer software (FORCE;
ViDiTo) controlling the compression arrangement permitted
parameters such as compression rate, pressure and force to be
obtained (15). To control the
uniformity of the impact force, the compression curve was recorded
in each animal. After spinal cord compression (8N or 15N force;
velocity: 30 mm/sec.), the paraspinal musculature and skin were
sutured with several absorbable stitches and the animals were
housed in separated cages to recover.
Preparation of perfusion chamber
Two types of chambers were used. In the initial
experiments the closed chamber was fabricated to fit into the
injury site. For that, in two animals the site of laminectomy was
scanned with an eScan 3D laser scanner (3D Digital Corp., Sandy
Hook, CT, USA). Data were then transferred to Rhinoceros 3D
software (MCNeel & Associates, Seattle, WA, USA) where the
scanned data were used to finalize a prearranged model chamber, to
make the bottom surface of the chamber fit perfectly with the bones
over the injury site. The spinal perfusion chamber therefore
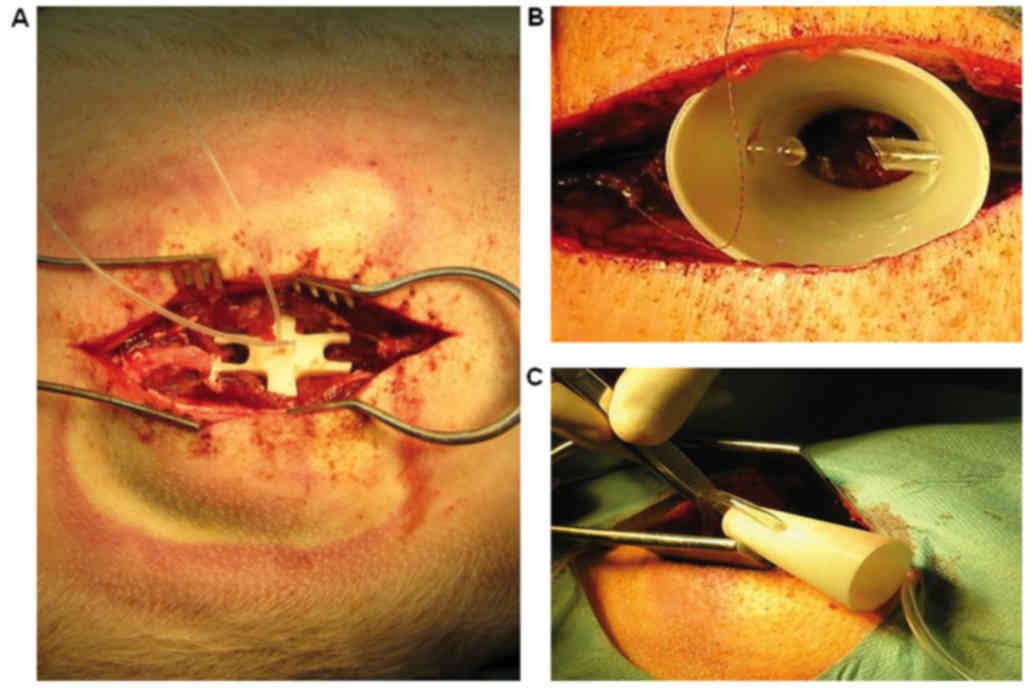
perfectly fitted the injury site (Fig.
2A). The finalized perfusion chamber was then printed in ABS
plastic using a uPrint SE 3D printer (Stratasys, Eden Prairie, MN,
USA). The spinal perfusion chamber was fitted with inflow and
outflow tubing. However, the closed chamber did not allow cleaning
of the spinal spinal dural sac, which was frequently covered with
blood clots, preventing contact between the solution and the
medullary tissue. For this reason these animals were excluded from
the experiment. For all following experiments, an open design of
the chamber was prepared. The chamber in the shape of a cone with
both ends opened (Fig. 2B and C) was
printed from ABS plastic using a uPrint SE 3D printer (Stratasys).
The spinal perfusion chamber had ellipse-shaped openings, the lower
opening measuring 20×14 mm and the upper one 40×30 mm, and the
height of the chamber was 50 mm. The spinal perfusion chamber was
fitted with two tubings (inflow and outflow). An individual chamber
was fabricated for each animal.
Implantation of perfusion chamber
The perfusion chamber was implanted over the exposed
spinal dural sac containing the spinal cord (Fig. 2B and C). The smaller opening was
about the size of the laminectomy, so it could be freely placed
directly on the dura mater with no additional fixation to
surrounding tissue. Only two stitches partially closed the wound,
so the skin edges slightly embraced the chamber. No additional
pressure was generated, since the weight of the chamber was minimal
(4 g), and the paraspinal muscles and subcutaneous fat layer around
conical chamber would lift it, rather than push it down. The
contact of the chamber with dura was checked periodically during
removal of blood clot from the surface of dura mater through
perfusion chamber. No leakage was detected. Since the chamber was
placed freely on the dural surface, without any fixture to the
spine or paravertebral muscles, the edema which developed after SCI
just lifted the chamber, without causing any additional harm.
Hypothermia
Hypothermia with saline or porcine medium (DMEM/F12)
consisting of rh-Transferrin 100 mg/l, rh-Insulin 25 mg/l, Glucose
1.56 g/l, Progesterone 6.3 µg/l, Putrescine 16.1 mg/l, Selenium 5.2
µg/l (Cell Guidance Systems Ltd., Cambridge, UK) was carried out
locally through each spinal perfusion chamber. The inflow tubing of
the perfusion chamber was connected to the tank with sterile ice
cold (4°C) perfusion solution. The cooling of the site of injury
was initiated 30 min after SCI. At the beginning the solution drip
rate was manually adapted to ensure that the temperature of fluid
in the perfusion chamber (just above the SCI) was maintained at
19°C. Given that cooling was applied locally, the temperature of
the solution in the chamber decreased to 19°C very rapidly, within
4–5 min. The temperature of solutions in the perfusion chamber was
measured at 15 min intervals using a needle-tip thermometer (Omega
Bio-Tek, Inc., Norcross, GA, USA) during the whole treatment
procedure (5 h). Continuous inflow of the perfusion solution into
the chamber was regulated by a peristaltic pump (Heidolph, BRD, the
flow 2 ml/min). Outflow tubing was used to drain excess perfusion
solution.
To minimize variability in body temperature during
hypothermic treatment the minipigs were covered with a thermal
blanket. The rectal temperature of each animal was measured at 15
min intervals using a digital predictive thermometer with the use
of lubricant. Each single rectal measurement took 10–20 sec. When
the temperature dropped below 36°C the animals were warmed with a
stream of warm air (a hair dryer blew warm air below the blanket
covering the animal). This heating method was very efficient and
maintained the minipigs at normothermia during the whole procedure.
The rectal temperature varied during hypothermia from 35.4°C±0.4 to
36.6°C±0.4. After finishing the therapy the perfusion chamber was
explanted and the incision was closed with absorbable sutures. The
animals were housed in separated cages to recover.
Post-operative animal care
Post-operative care included bladder expression
twice a day, antibiotic administration (Penstrepten (0.5 ml/30
kg/day; Biotika a.s.) for 10 days, cleaning the hind limbs and
monitoring of skin irritation and development of decubitus ulcers.
Abrasions of the tail and perianogenital region occurred in 18% of
animals (3 out of 4) subjected to 15N SCI and 2 out of 7 treated
with cold saline after 15N SCI. The affected areas (bruises around
the tail) were treated twice a day with 0.1% solution of Rivanol
and with Alamycín spray (Norbrook, Newry, Northern Ireland, UK) for
about one week. The animals survived for an additional 2 months
without further complications. Pressure sores or other skin lesions
were not observed in the 8N SCI group, 8N SCI animals treated with
cold saline or medium, or in the rest of animals after 15N SCI or
15N SCI and treatment.
The 20-point porcine neurological
scale to determine hind-limb motor recovery after spinal cord
compression
Recently, the porcine neurological motor score was
re-graded on a 10-point scale according to Lee et al
(20) or a fourteen-point grading
scale according to Navarro et al (15). Although both protocols are well
validated and easily replicated, the assessment of functional
recovery in this study took into principal consideration the
hind-limb movement for each individual joint, the capability of
each animal to get up by itself, its trunk stability and stepping
coordination. The scoring points range from complete paraplegia
(zero) to normal ambulation (20 points) and represent three
sequential recovery stages which the minipigs attained after SCI
and hypothermic treatment. The 20-point neurological scale for
minipigs is described in detail in Table
I. Porcine neurological motor scores (1–8)
represent slight or extensive movement for each individual joint of
the hind limbs, points (9–11) represent sweeping and the ability to
get up by itself (with or without assistance; these animals were
not able to maintain balance), and points (12–20)
characterize the ability to walk from a few steps without
fore-limb/hind-limb coordination to consistent plantar-hoof
stepping and consistent fore-limb/hind-limb coordination (see
representative pictures in Fig.
3).
 | Table I.20-point neurological scale for
minipigs. |
Table I.
20-point neurological scale for
minipigs.
| Scale | Description |
|---|
| 0 | Complete
paraplegia, no movement in joints of hindlimbs, spontaneous
defecation and urination |
| 1 | Slight movement in
one joint (hip joint) of hindlimbs, not capable of sitting and
plantar placement of hindlimbs during sitting, spontaneous
defecation and urination |
| 2 | Slight movement in
two joints (hip and knee joint) of hindlimbs, not capable of
sitting and plantar placement of during sitting, spontaneous
defecation and urination |
| 3 | Slight movement in
all three joints of hindlimbs, capable of sitting on the side
without plantar placement of hindlimbs during sitting, spontaneous
defecation and urination |
| 4 | Extensive movement
in one joint (hip joint) and slight movement in two joints of
hindlimbs, capable of sitting on the side without plantar placement
of hindlimbs during sitting, spontaneous defecation and
urination |
| 5 | Extensive movement
in twoo joints of hindlimbs and slight movement in third joint of
hindlimbs, capable of straight sitting and plantar placement of
hindlimbs during sitting, spontaneous defecation and urination |
| 6 | Extensive movement
in all three joints of hindlimbs, capable of straight sitting and
plantar placement of hindlimbs during sitting, spontaneous
defecation and urination |
| 7 | Extensive movement
in all three joints of hindlimbs, not capable of standing up,
sweeping with one hindlimb, spontaneous defecation and
urination |
| 8 | Extensive movement
in all three joints of hindlimbs, not capable of standing up,
sweeping with both hindlimbs, spontaneous defecation and
urination |
| 9 | Sweeping with both
hindlimbs, not capable of plantar hoof stepping, attempt to hold
stand with initial help to stand up |
| 10 | Sweeping with both
hindlimbs, capable of weigh support with plantar surface of hoof
with initial help to stand up only, not capable of walking |
| 11 | Capable to stand up
itself, not capable to maintain balance, walking with support only
(falling back on wall), position only on dorsal surface of
hoof |
| 12 | Capable to stand up
itself on hindlimbs and capable standing, able to walk 1–3 steps
with occasional support without forelimb-hindlimb coordination,
occasional plantar-hoof stepping |
| 13 | Capable of standing
up spontaneously and able to take 1–3 steps without support before
losing balance, no forelimb-hindlimb coordination, frequent
plantar-hoof stepping and capable to keep balance between stepping
episodes |
| 14 | Capable of standing
up spontaneously and able to take 3–5 steps, capable to keep
balance between stepping episodes with occasional support only, no
forelimb-hindlimb coorination, occasional to frequent plantar-hoof
stepping |
| 15 | Capable of standing
up spontaneously and able to take 3–5 steps, capable to keep
balance between stepping episodes, frequent plantar-hoof stepping,
no forelimb-hindlimb coorination |
| 16 | Capable of standing
up spontaneously and able to take 5–10 steps, capable to keep
balance, frequent plantar-hoof stepping, no or inconsistent
forelimb-hindlimb coordination |
| 17 | Capable of standing
up spontaneously and able to take 5–10 steps, capable to keep
balance, frequent plantar-hoof stepping, occasional
forelimb-hindlimb coordination |
| 18 | Capable of standing
up spontaneously and able to take 5–10 steps, capable to keep
balance, frequent plantar-hoof stepping, frequent forelimb-hindlimb
coordination |
| 19 | Capable of standing
up spontaneously and able to take more than 10 steps, capable to
keep balance, consistent plantar-hoof stepping, frequent
forelimb-hindlimb coordination |
| 20 | Capable of standing
up and walking spontaneously, consistent plantar-hoof stepping,
consistent forelimb-hindlimb coordination |
Behavioral assessment
Three weeks before the surgery the minipigs
underwent half an hour of training for 5 days/week to walk upon
command up and down a rubber mat (width 0.8 m, length 5.2 m). One
week before SCI the minipigs were videotaped. Post-operative
neurological impairment was tested under blinded outcome assessment
two days after surgical procedures, and continued weekly for nine
weeks of survival. Each minipig was allowed to walk freely on a
rubber mat for 10 min without assistance. A video recording was
made of each evaluation. In order to reduce bias, three independent
observers were involved in the development of the neurological
scale. To blind the outcome assessment, the observers graded the
neurological functions independently without knowledge of the
treatment. To minimize the variability, each camera record was
re-scored by the same three observers. The inter-rater differences
in the scores varied max by 1 point. The discrepancies obtained by
the raters from two independent assessments were discussed and
clarified. Based on direct behavioral observations of neurological
recovery and re-scoring the videos we developed our own porcine
neurological scale to determine hind-limb motor recovery after
spinal cord compression (Table
I).
Spinal cord tissue preparation
At the end of survival, the minipigs were deeply
anesthetized with an intravenous overdose of thiopental and
transcardially perfused with heparinized saline (5l), followed by
4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS, pH
7.4, 5l). The lumbar spinal cord (approximately 10 cm) was
carefully dissected out, cleaned of the dura mater and post-fixed
in the same fixative overnight. Next day, the spinal cord was cut
into one 1 cm block (lesion site) and three 1 cm blocks caudally
(−1, −2, −3) as well as cranially (+1, +2, +3) from the epicenter
of injury. In the next step, the spinal cord pieces were
cryopreserved in a solution of 30% sucrose in PBS at 4°C for 48 h.
Afterwards, each tissue block was cut into transverse serial
sections (30 µm thick) on a cryostat (Leica Microsystems, Wetzlar,
Germany) and stored in PBS. Transverse serial sections were mounted
on slides and were processed in accordance with immunofluorescence
and histochemical procedures.
Immunofluorescence staining
For processing of neurofilament immunoreactivity,
the slices taken from the rostral and caudal blocks were incubated
in blocking solution consisting of 5% normal goat serum in 0.1 M
phosphate-buffered saline (PBS) with 0.3% Triton-X 100 for 1 h at
room temperature. After blocking the slices were incubated
overnight at 4°C with primary monoclonal mouse antibody
anti-neurofilaments (Pan-Axonal Neurofilament Marker SMI312,
1:1,000; cat. no. ab24574; Abcam, Cambridge, UK) diluted in 0.1 M
PBS with 5% normal goat serum and 0.3% Triton-X 100. Next day, the
sections were washed three times in 0.1 M PBS for 10 min and
incubated with secondary anti-mouse antibody (Rhodamine
Red™-X conjugated AffiniPure goat anti-mouse IgG, 1:200;
cat. no. 115-295-003; Jackson ImmunoResearch Laboratories, Inc.,
West Grove, PA, USA) in Tris-buffered saline containing 0.1 M
phosphate buffer (7.4 pH) with 5% normal goat serum and 0.3%
Triton-X 100 for 90 min at room temperature. The sections were
finally rinsed in 0.1 M PBS and cover-slipped with Fluoromount-G
(cat. no. 21644; Serva Electrophoresis. GmbH, Heidelberg, Germany).
Microphotographs were taken using a fluorescence microscope
(Olympus BX51/BX52; Olympus, Hamburg, Germany) with an Olympus DP50
digital camera coupled with a computer equipped with Olympus DP
Image software (version 3.1). Neurofilament immunoreactivity was
evaluated using the Image J graphics software. SMI312 labeled axons
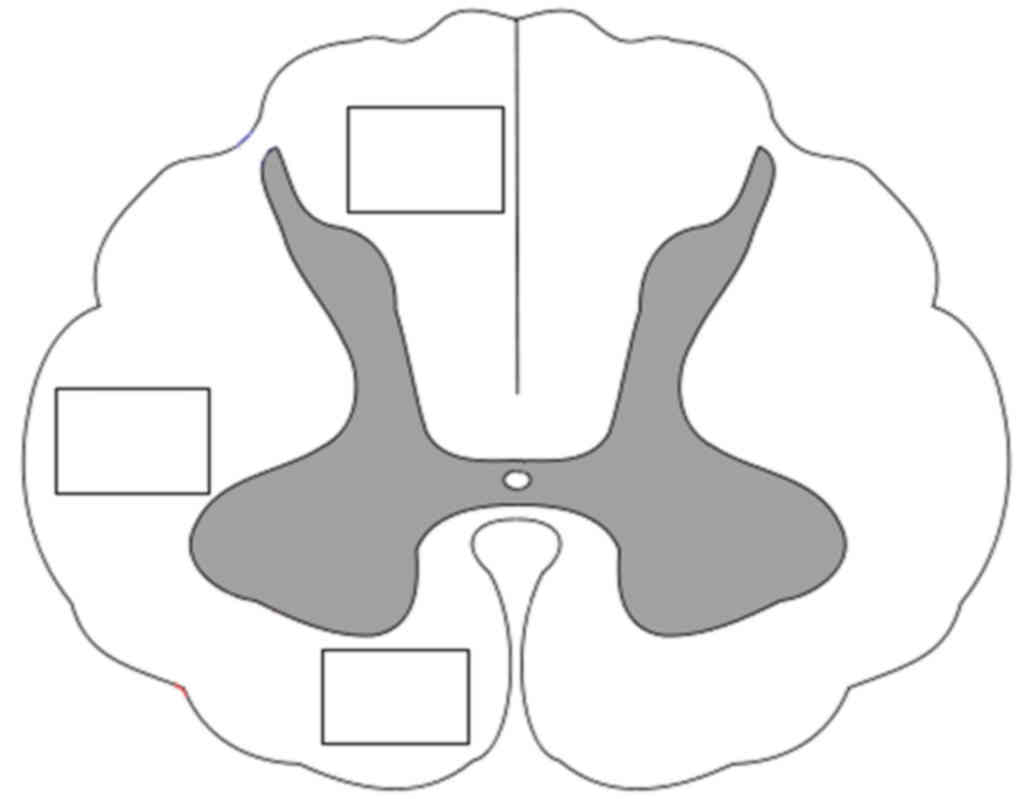
were counted by a blinded observer using a 20× lens in a 800×600 µm
area in three specific regions of the white matter (dorsal, lateral
and ventral columns) (Fig. 4). The
number of neurofilaments (expressed as percentage of
NF/mm2) was evaluated bilaterally from five
randomly-selected transverse slices of each spinal cord block.
Histological analyses
The spinal cord slices (lesion site and 1 cm blocks
in rostral and caudal directions) were stained according to the
protocol for luxol fast blue and cresyl violet staining to
recognize myelinated fibers in white matter and the residual
preserved tissue. The percentage of total volume of preserved
spinal tissue was evaluated using an Olympus (BX51/BX52) light
microscope and ImageJ software.
Data acquisition and statistical
analysis
The results from neurological analyses were assessed
in a blinded manner and statistically evaluated using
Kruskall-Wallis or Mann-Whitney tests. All immunohistochemical and
histological data were analysed in a blinded manner using one-way
ANOVA test. P<0.05 was considered to indicate a statistically
significant difference. All outcomes were expressed as mean values
with standard errors of the mean (SEM). The correlation between
degree of neurological outcome, number of neurofilaments and gray
and white matter preservation was assessed using the Pearson
correlation test. The test was used to evaluate the coefficient of
determination r2.
Results
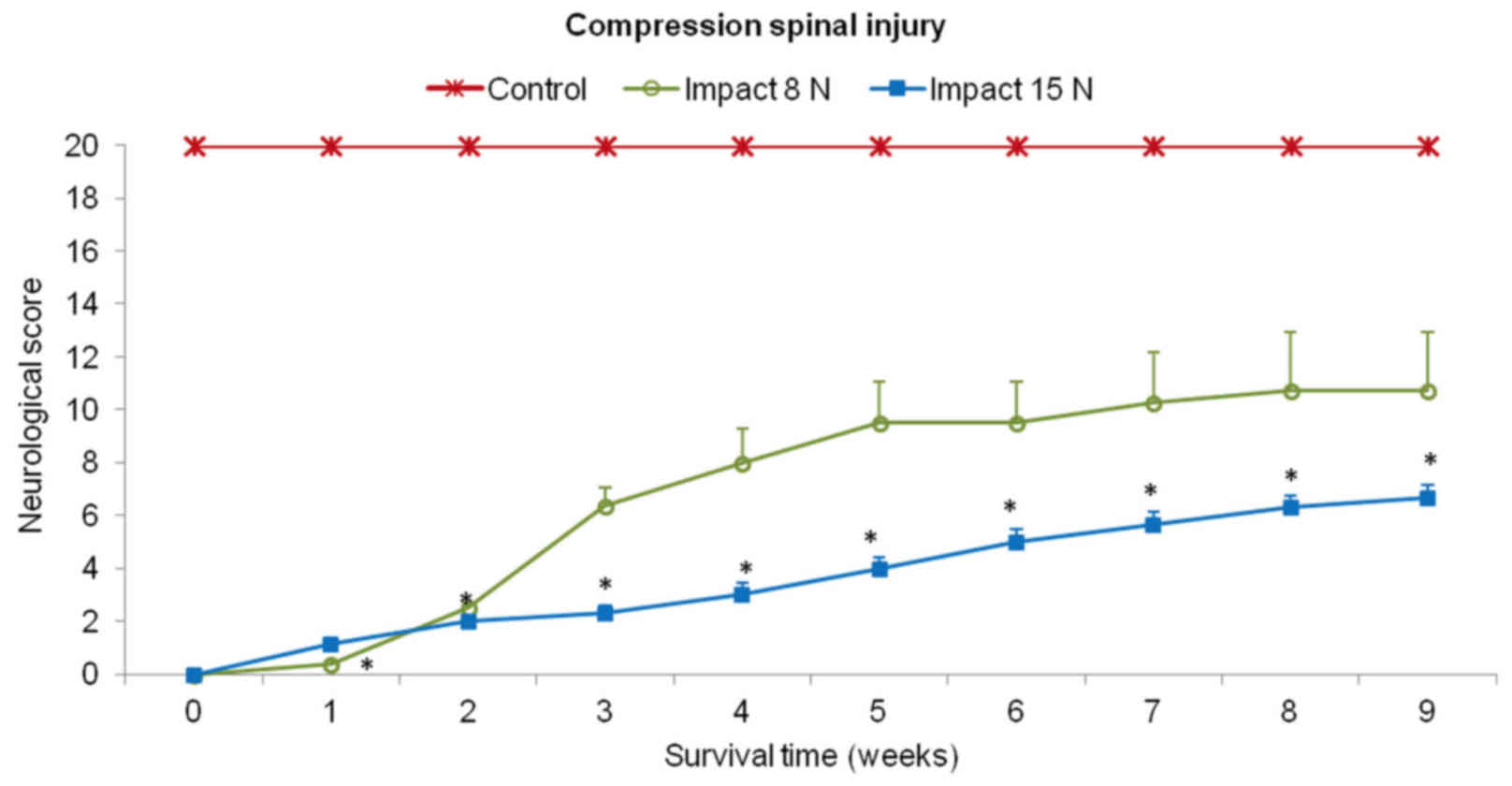
Behavioral outcomes
Recovery of motor function was assessed using a
20-point scoring system representing the recovery stages which the
minipigs attained after SCI and local hypothermia with saline or
porcine medium (DMEM/F12). In the first two weeks after SCI (impact
force 8N and 15N) the behavioral status of each animal was very low
in both experimental groups (Fig.
5). These animals showed total loss of locomotor function in
the hind-limbs or just slight movement in hip and knee joints
(score 0–2). The outcome improved notably from the 2nd to 5th week
in the group of minipigs which underwent impact with 8N force.
Hind-limb movement in this group of animals recovered spontaneously
from the 5th week and later on, during the last two weeks the
outcome was stabilized. In the 9th week the motor score reached
10.8±2.2. In the animals which underwent 15N impact, very slow
spontaneous functional recovery was observed. At the end of the
survival time the mean score for motor outcome in this group was
graded 6.3±0.5.
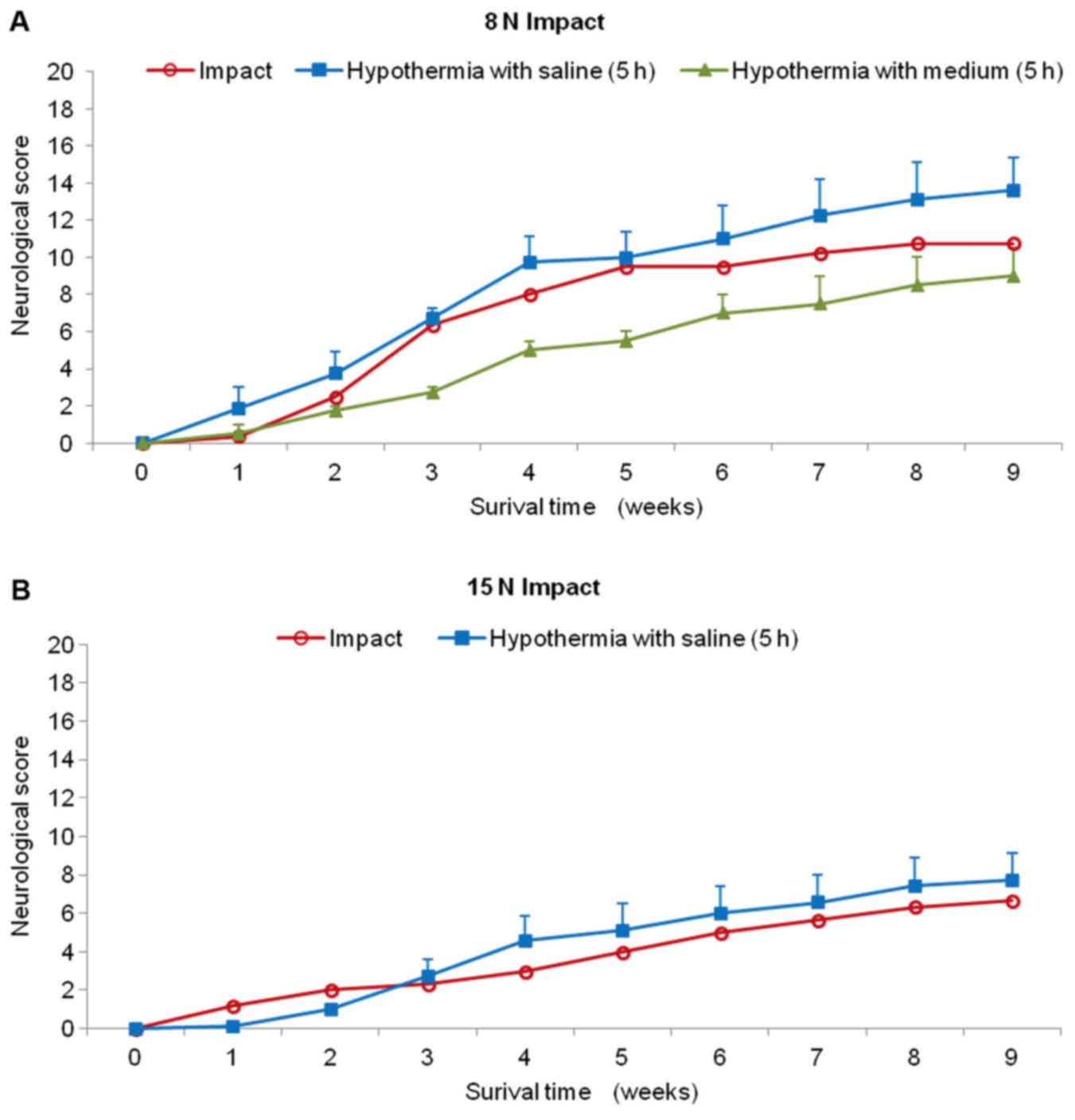
Animals treated with hypothermic saline perfusion
following 8N compression demonstrated continuous slight improvement
during the whole survival period (Fig.
6A). Although the difference in outcome between the two groups
(SCI vs. SCI and hypothermia with saline) was not significant at
the end of the survival period, the improvement after treatment
exceeded compression by 3 scoring points (10.8±2.2 SCI; 13.7±1.8
SCI and hypothermia with saline). The saline-treated animals were
able to take 1–3 steps with initial help to stand up and keep
balance between stepping episodes, but the movement was without
fore-limb/hind-limb coordination. Surprisingly, this group of
animals had substantially better recovery rate than the minipigs
treated with hypothermia using porcine medium (DMEM/F12) containing
a supplement of nutrients. During the first post-injury week, no
animal treated with 4°C medium recovered and complete absence of
movement in the hind-limbs or at most slight movement in only one
joint was observed. Up to the end of the survival period the
medium-treated minipigs showed lower motor function than untreated
animals (8N compression) and at the 9th week the motor score
reached only 9.0±2.3 points. The behavioral study also showed that
recovery in the 15N SCI group treated with cold saline for 5 h was
only slightly better compared with the untreated group (Fig. 6B). From the 4th to 9th week the
minipigs treated with 4°C saline improved their outcome over
compression only by 1 scoring point. This group of animals was not
capable of standing up, but we noted sweeping with one hind
limb.
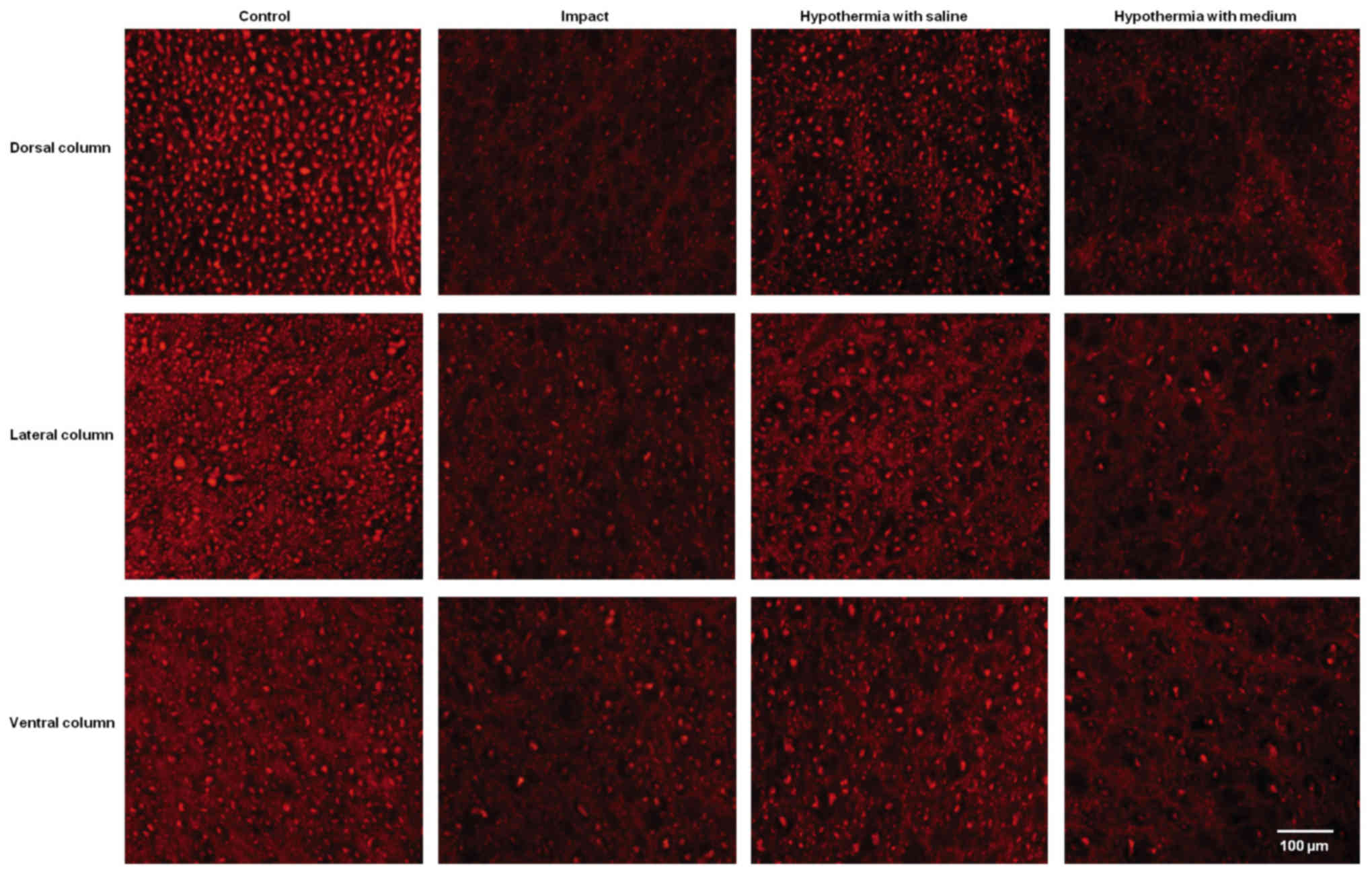
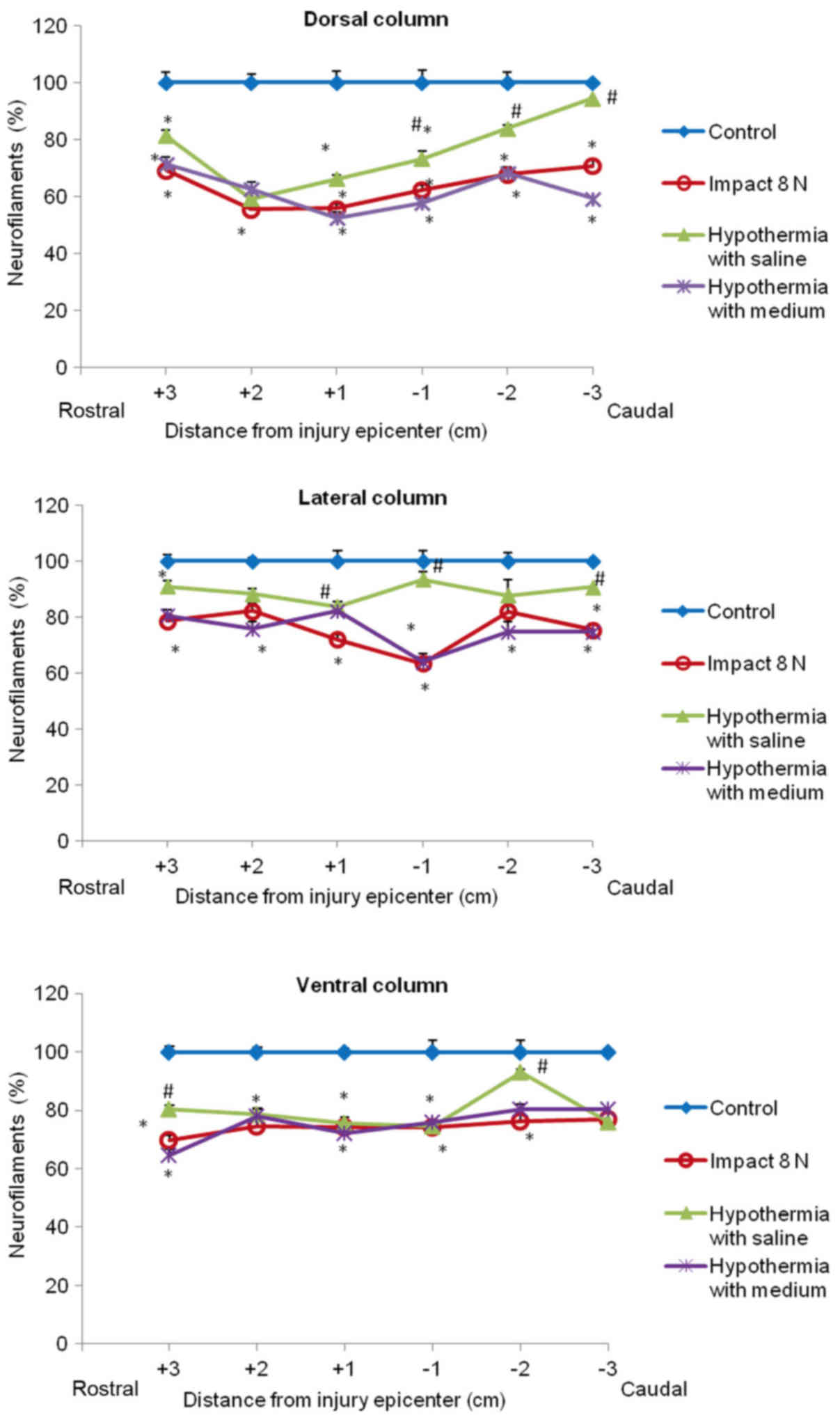
Quantitative analysis of neurofilament
immunoreactivity
The immunoreactivity for neurofilaments was strong
in the dorsal funiculi compared to other white matter columns, and
was equally distributed throughout the lateral and ventral column
axons of control animals (Fig. 7).
Nine weeks after SCI axons in the white matter funiculi were weakly
stained with various degrees of staining. Quantitative analysis
showed significant decrease in NF intensity generally in all
rostral and caudal segments, with the lowest number of NF-IR
profiles near the epicenter of injury (+1 and −1 segments)
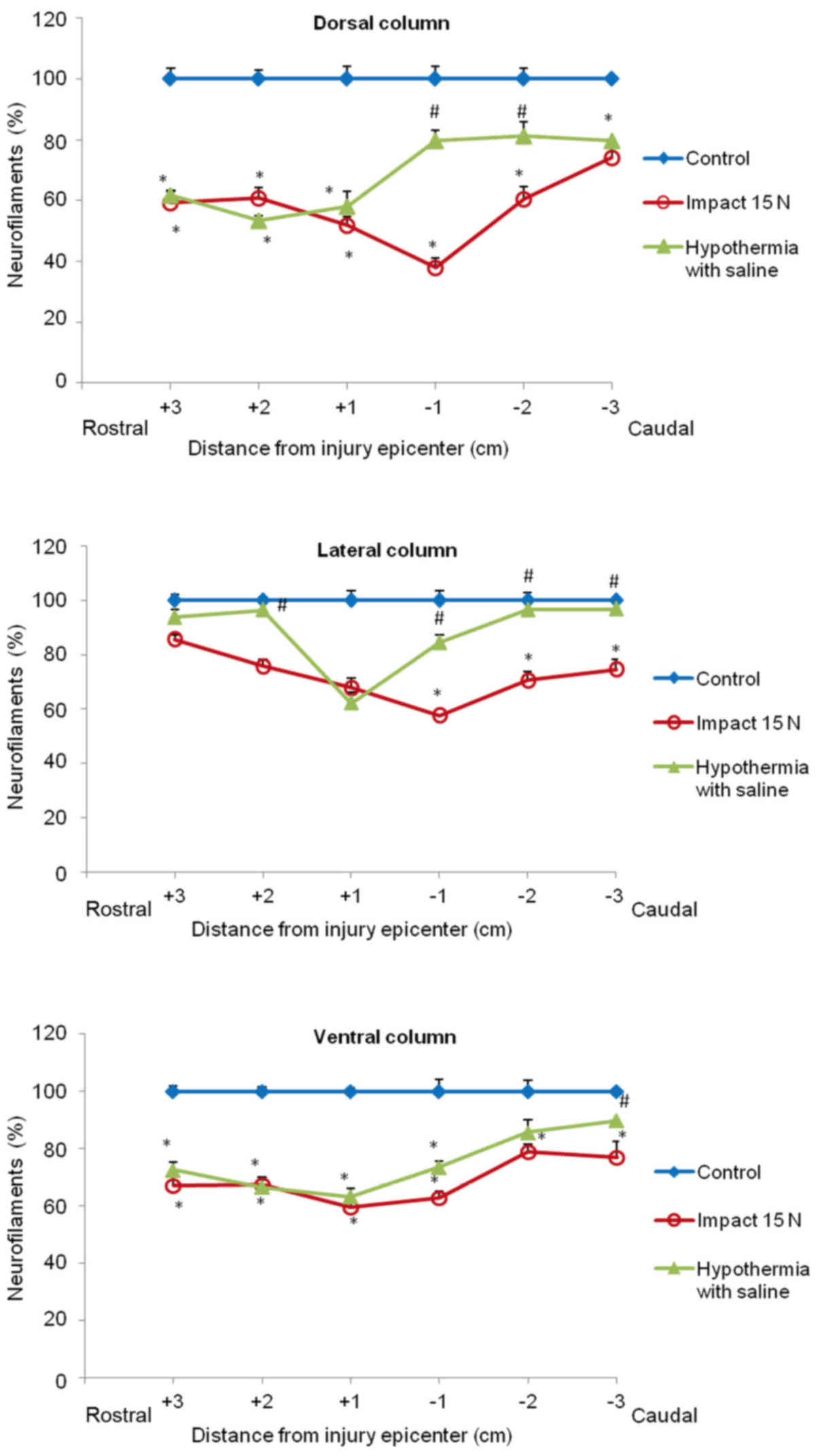
(Figs. 8 and 9). In the group which underwent 15N SCI we
found the most striking alterations at the −1 segment in dorsal and
lateral columns (38, 58%) (Fig. 9).
Hypothermia with saline significantly increased the number of
neurofilaments in each white matter column, with the most extensive
improvement seen in the lateral funiculi (Figs. 8 and 9). The return of NF staining appeared
greater in segments (−1, −2, −3) below the SCI than in the rostral
segments. Extensive improvement was also noticed in the dorsal
column, displaying a similarly high proportion of NF staining in
the caudal segments in saline-treated minipigs. In contrast,
treatment of injured spinal cord with hypothermic porcine medium
(DMEM/F12) solution demonstrated no significant increase in
neurofilament intensity in any of white matter columns in
comparison with the compression group (Fig. 8).
Histological assessment
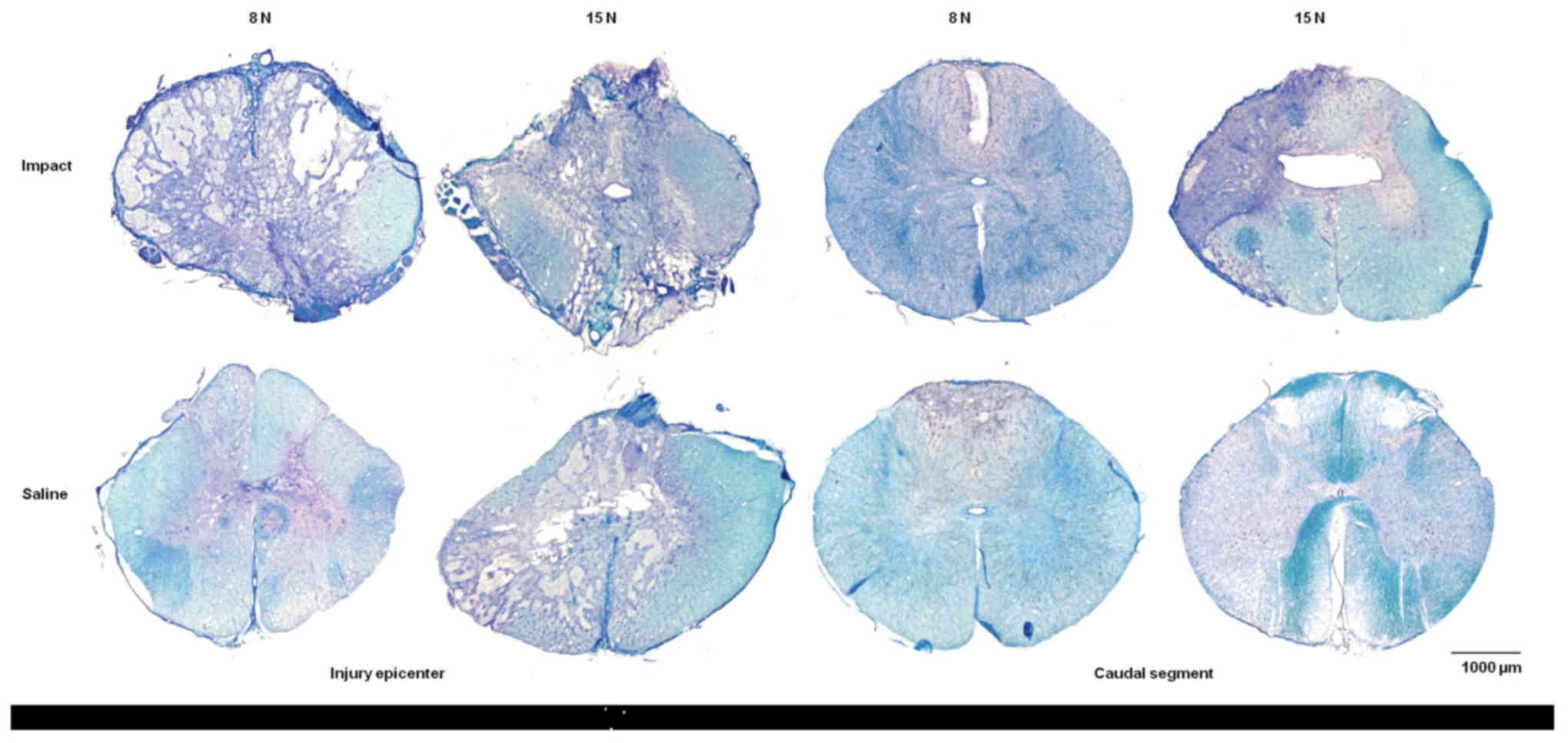
Luxol fast blue combined with cresyl violet staining
of white and gray matter produced a marked contrast between intact
and damaged spinal cord tissue. Histopathological assessment of
transverse serial sections showed congruous loss of myelin sheaths
after trauma inflicted with 8N as well as 15N force and nine weeks
of survival. Tissue degradation was visible in white as well as
gray matter in both compression groups. Major spinal tissue
destruction occurred at the lesion epicenter and in segments near
the site of injury (Fig. 10). The
lesion area extended in both directions cranially and caudally from
the epicenter. With increased compression of the spinal cord the
structural loss of spinal tissue became greater.
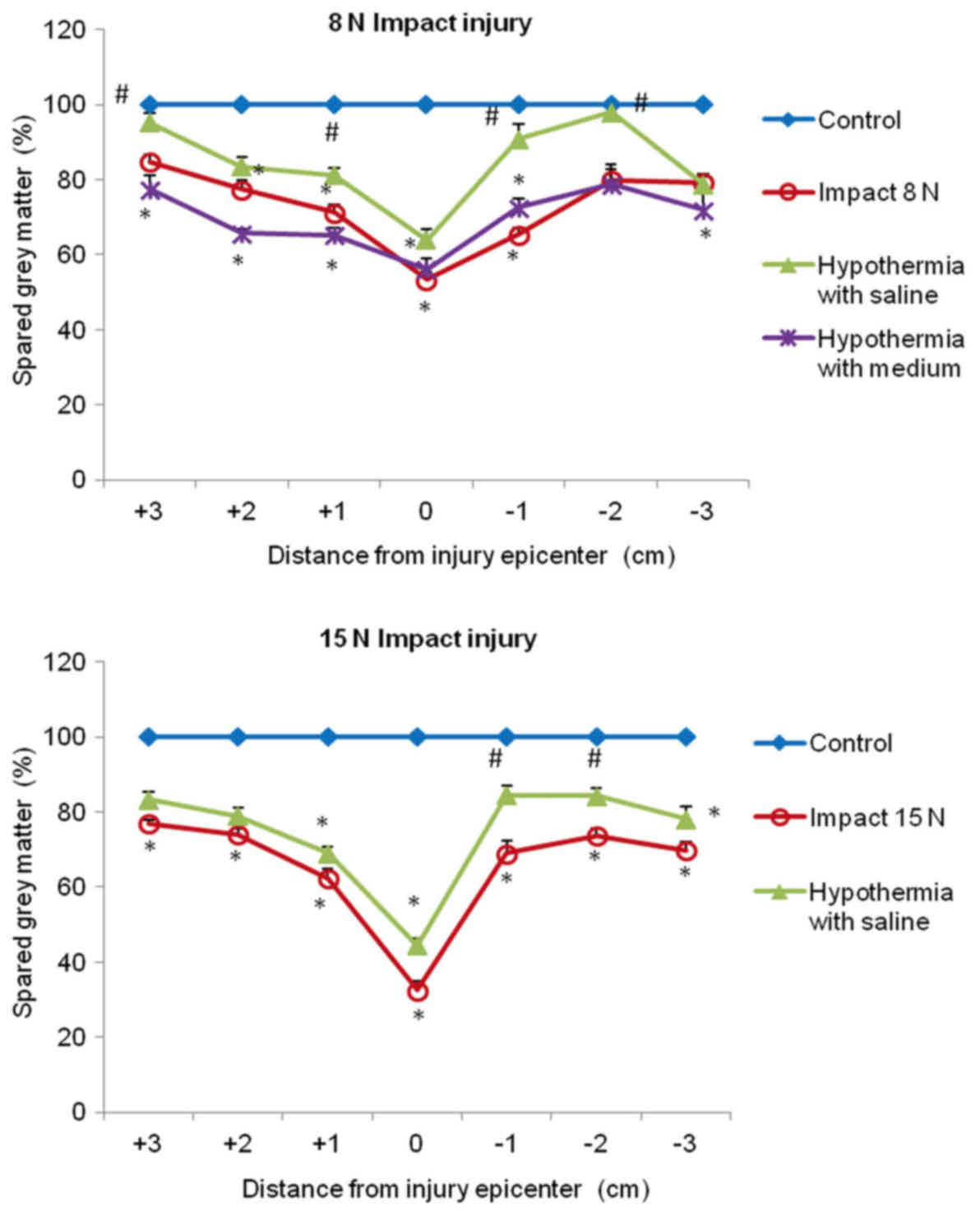
In both compression groups, the gray matter
preservation at the lesion site was quite low (53 and 33%). The
preservation was significantly higher in saline-treated animals in
+3, +1, −1 and −2 segments, compared with untreated 8N SCI
minipigs, and almost no gray matter loss was seen in +3 and −2
segments. In the animals which underwent 15N force impact and were
treated with saline hypothermia, the improvement in gray matter
preservation was significant in the caudal (−1, −2) segments
(Fig. 11).
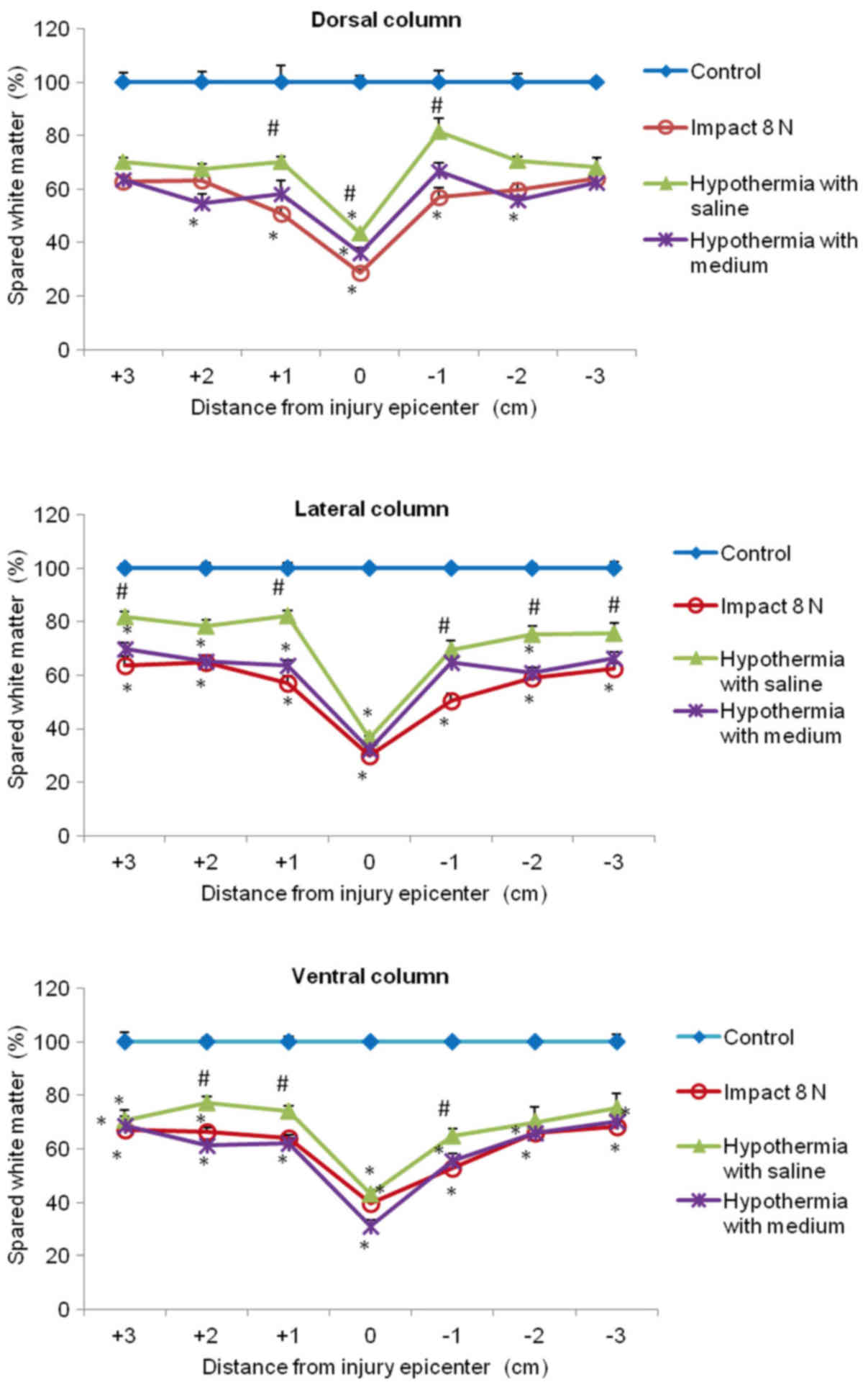
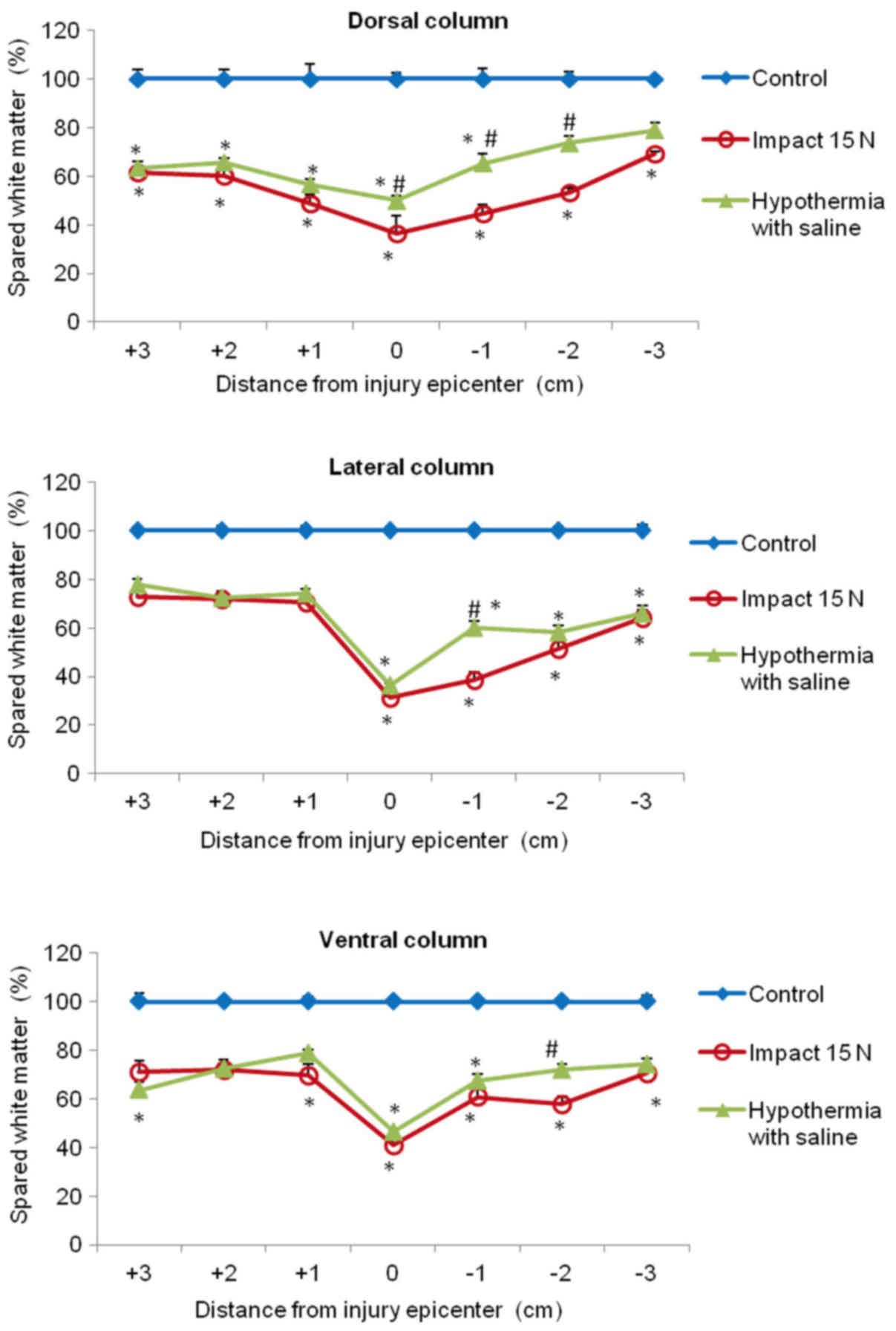
In the white matter columns, the volume of preserved
tissue after SCI (8N and 15N) at the lesion site was less (28–41%)
(Figs. 12 and 13) than in the gray matter. Low
percentages of preserved white matter was also detected in the
section (−1) of the lateral (39%) and dorsal column (45%) after 15N
SCI, while after 8N SCI the preservation in the same segments was
better and in the 9th week of survival it reached 50 and 57%
respectively. Hypothermia with porcine medium solution produced
only minimal difference between untreated and treated animals
(Fig. 12). Quite different results
were noted after perfusion of the injured spinal cord with 4°C
saline. We noted white matter preservation in the rostral and
caudal sections of each white matter column, with substantial
protection seen around the lesion site in the dorsal column. The
most significant attenuation of white matter damage as a whole was
seen after SCI (8N) treatment in the lateral column. In the 15N
compression group the most effective percentage of preserved white
matter was noted in the dorsal column at the site of injury and
caudally (−1, −2) from the lesion site. The differences in
preserved white matter between groups are shown in Figs. 12 and 13.
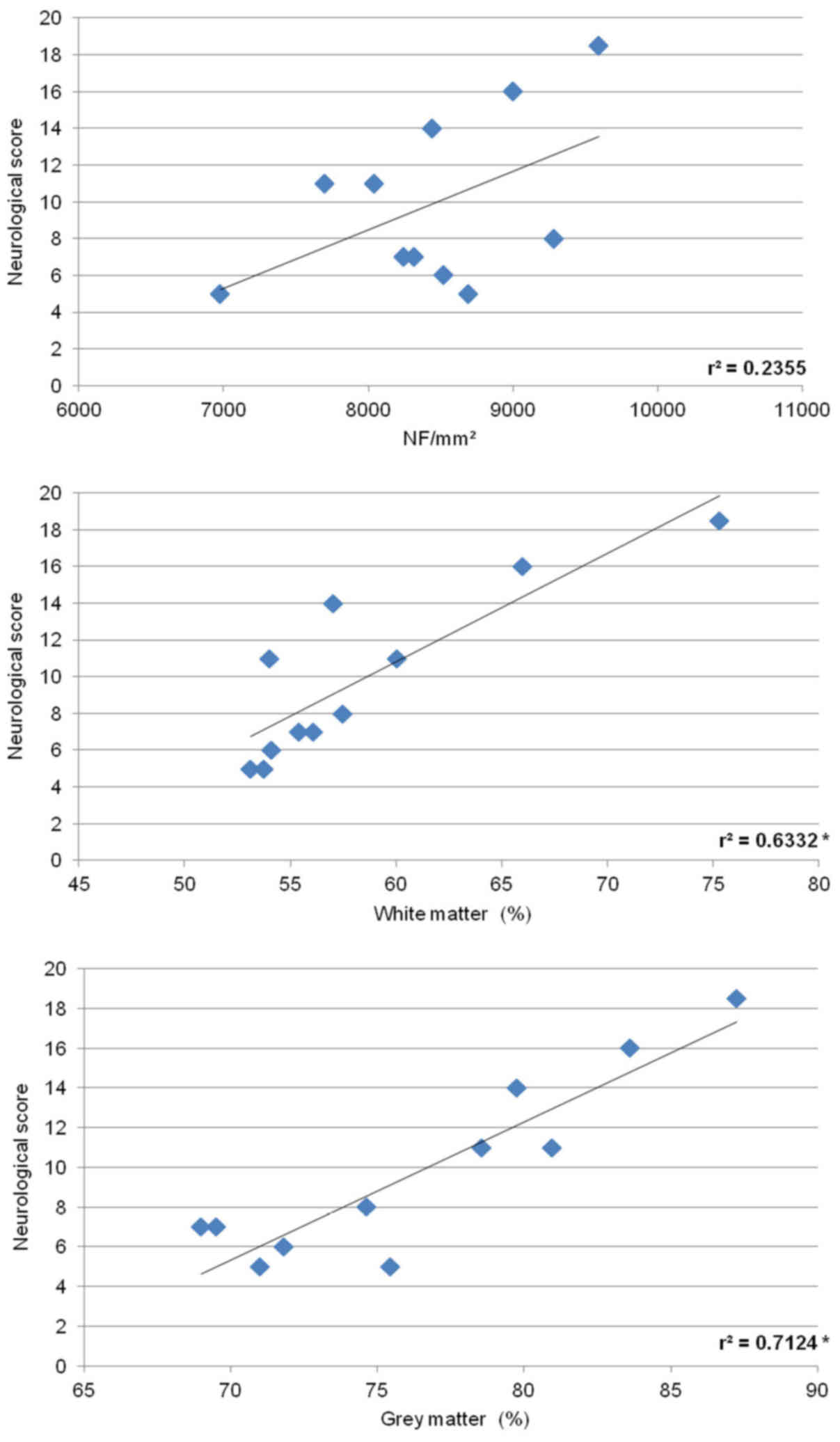
Correlative analyses between
behavioral outcome, neurofilament immunoreactivity and histological
assessment
To determine the relationship between the
histological damage to the spinal cord and behavioral outcome in
animals treated with saline for 5 h, we utilized both SCI groups
(8N, 15N force). As shown in Fig.
14, the number of neurofilaments counted in the white matter
positively correlated with behavioral recovery. Similarly, positive
correlation was observed between behavioral score and cumulative
white and grey matter preservation.
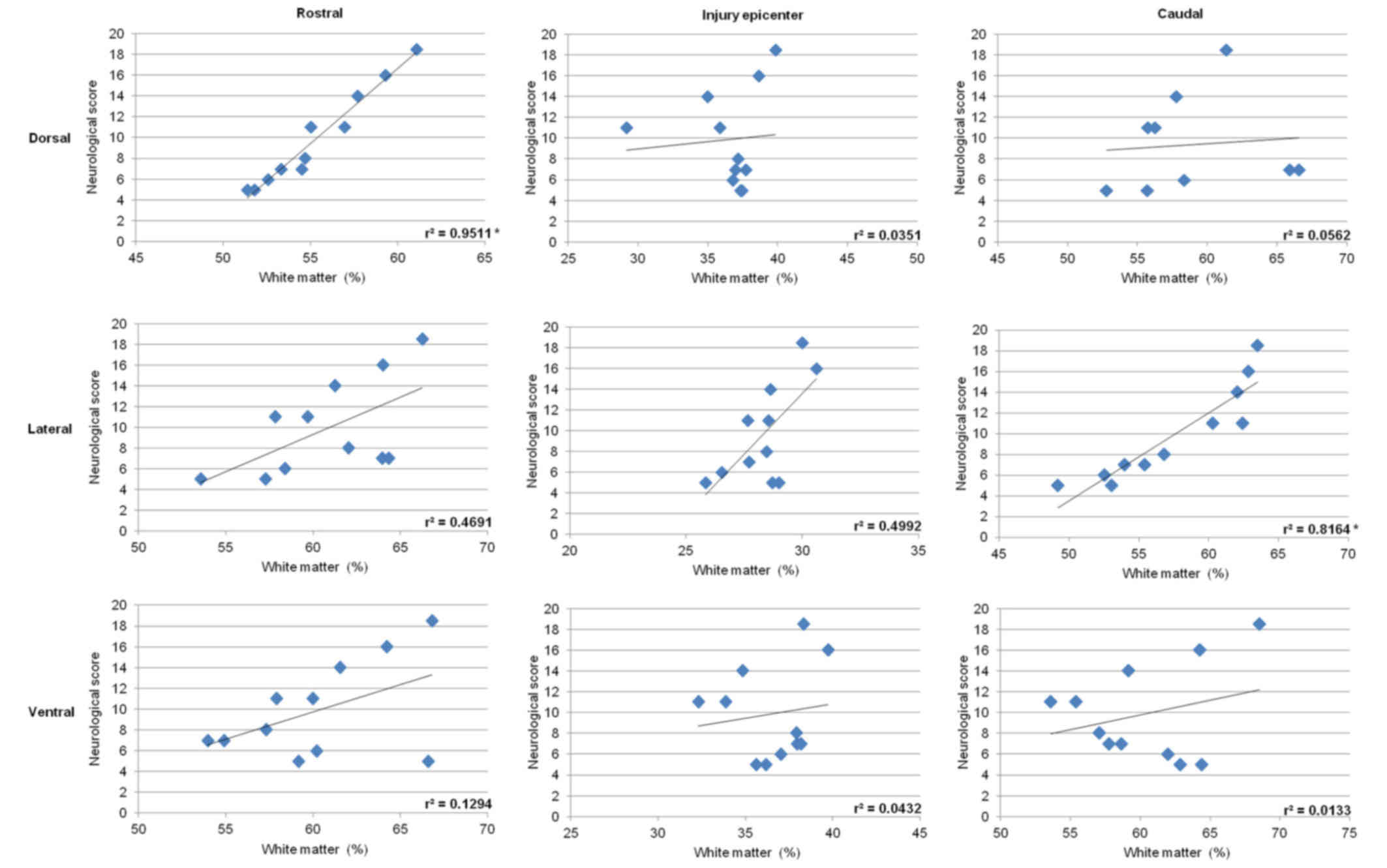
Correlative analysis between the extent of axonal
loss quantified in the dorsal, lateral or ventral funiculi and the
degree of neurological dysfunction demonstrated positive
correlation in the lateral funiculi with values
r2=0.4691 in the rostral section, r2= 0.4992
at the epicenter of injury, and a relatively high degree of
correlation r2= 0.8164 (P<0.05) in the caudal
section. Significant correlation was also found in the dorsal
column, but only in the rostral section (r2=0.9511;
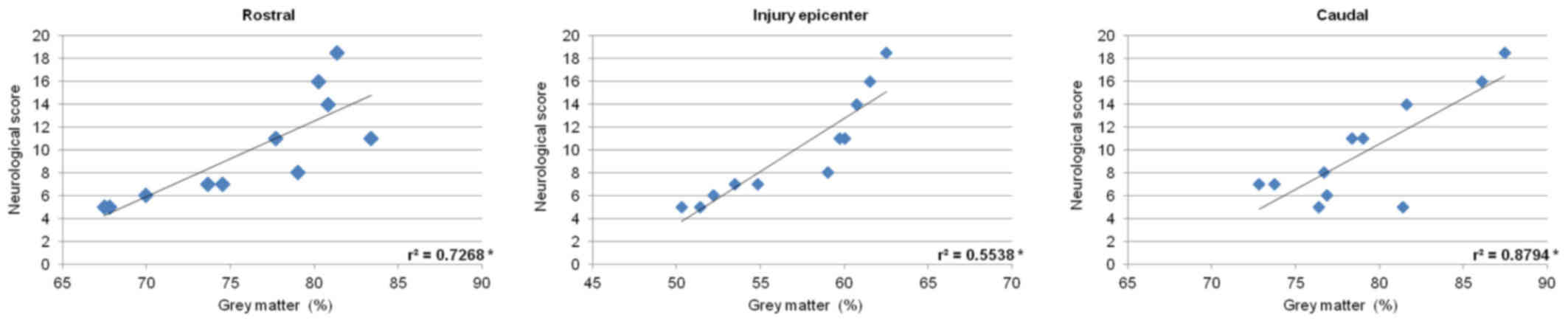
P<0.05) (Fig. 15). Measurements
of gray matter preservation (lesion site, and caudally as well as
cranially from the epicenter of injury) significantly correlated
with the neurological score (Fig.
16).
Discussion
Is the preservation of the lesion site in the
minipig SCI model a predictor of functional outcome?
Hypothermic treatments have been reported to be beneficial for some
recovery of sensory or motor function in a variety of animal SCI
models (21–25). Although animal studies of SCI have
revealed consistent neuroprotective effects of hypothermia applied
either locally or systemically (26), further experimental/clinical studies
are required to determine the optimal cooling parameters
(therapeutic window, optimal temperature and duration) and to test
the effectiveness of hypothermic therapy in individuals afflicted
with this devastating neurological deficit. In the present study,
the computer-controlled compression device produced graded
contusion lesions at L3 level with two different degrees of tissue
preservation and functional recovery, depending upon pre-set impact
parameters. Nine weeks after SCI there was a large contusion lesion
at the epicenter, with 47% reduction of the gray matter after 8N
force impact and 67% reduction in the 15N-force SCI group. Early
and intense local hypothermia applied 30 min after SCI in the
epidural space increased the gray matter preservation at the lesion
site in both SCI groups by ~11%. It seems that neurons whose axons
have been damaged within a few cell diameters of the cell body
upregulate the regeneration-associated genes (27) and may lead to partially enhanced
plasticity and reorganization of preserved neuronal circuits.
Yoshitake et al (28)
demonstrated the neuroprotective effects of local epidural cooling
in pigs which underwent ischemic injury. The hypothermic group
showed better recovery of somatosensory evoked potentials,
behavioral ability and increasing numbers of motor neurons in the
anterior horn.
Earlier studies documented strong correlation
between neurological outcome and the extent of axonal dysfunction
at the lesion epicenter (15,26,29–31).
Dimar et al (32) reported
that local hypothermic treatment at 19°C for 2 h improved
neurological function in the rat spinal cord compression model, and
indicated that directly-applied hypothermia may be beneficial in
preventing injury secondary to ischemic cellular damage. However,
the authors failed to find any improvement in the more severe
contusion model. In this study, local perfusion of the lesion
epicenter with cold saline (19°C just above the lesion) produced
evident protection of white matter integrity in the dorsal columns
(improvement by 13–15%) with minimal 4–7% preservation of tissue in
the lateral and ventral columns. We speculate that, as in other
studies, a small increase in axonal survival at the lesion
epicenter may be one of the parameters which enable improvement in
neurological function. Nout et al (33) reported that the lesion size per se is
not a strict predictor of functional outcome. Our results fully
support this suggestion, showing that early local saline
hypothermia after 8N SCI partially improved tissue integrity at the
epicenter of SCI (by 11% in gray matter and 15, 7 and 4% in the
dorsal, lateral and ventral columns respectively), and that this
improvement was essential for modulation of the key spinal
microcircuits leading to better functional outcome. However, no
functional improvement was seen in the more severe 15N SCI group
treated with saline, although the preservation of both gray and
white matter at the epicenter was very similar in both 8N- and
15N-force experimental groups. This finding raises the question to
what extent the spinal cord must be preserved.
Fawcett (34)
described in his review that in order to produce a two-spinal level
improvement in motor function, corticospinal and other descending
axonal pathways must regenerate over two or more spinal segments,
or for around 2–3 cm. Although extensive spinal relay circuits
exist in rodents, the extent to which this circuitry exists and is
active in large animal models and primates is unknown and is still
controversial. We document that rostro-caudal tissue integrity
causing interconnections throughout the lesion site is needed for
better neurological outcome in the minipig SCI model.
Integrity of the tissue surrounding the
lesion is essential for functional recovery
We attempted to find out to what extent the spinal
cord might be saved/repaired after hypothermia, which could be
beneficial to minipigs with SCI. For a patient with cervical SCI a
return of function over even one segment would improve their
quality of life, while return of function over three or four spinal
segments would transform it (34,35). To
address the potential effect of hypothermic treatment we
systematically analyzed and quantified the histopathological
changes in the spinal sections (rostral +3, +2, +1 and caudal −1,
−2, −3) which are likely to be affected by secondary injury.
Although the promoting of morphological preservation/recovery was
detected up to a 3 cm extent rostro-caudally in both SCI models, we
consider that saline hypothermia (which might be beneficial,
partially returning motor function in minipigs) was strictly
connected with significant tissue preservation, at least within the
immediate rostro-caudal (+1 and −1) segments. Local hypothermia
applied following 8N SCI for 5 h significantly enhanced the gray
and white matter (dorsal, lateral and ventral columns) preservation
near the lesion site (+1 and −1), and improved the preservation of
neurofilaments in the lateral columns of the same segments. This
experimental group achieved faster recovery of hind-limb function
and the ability to walk from one to three steps with consistent
plantar-hoof stepping at nine weeks in comparison with non-treated
animals. It seems that the preservation of axons in the lateral
funiculi in the +1 and −1 segments (improvement by 25 and 19%
respectively) and more rostrally or caudally, might be essential
for favorable neurological outcome.
Axonal injury dispersion in the spinal cord includes
mainly demyelination, loss of neurofilaments and failure of
axolemma permeability. Tomko et al (36) reported that the lesion size after
dorsal column lesion in rats expanded dramatically within the first
two days, when its size reached the maximum (40.2±12.1 mm). Decline
in neurofilament numbers was detected in the dorsal and
ventrolateral white matter funiculi at the lesion epicenter and 5
mm in rostral and caudal directions of injury diffusivity after
spinal compression injury in rats (37). As shown previously, the fundamental
role of neurofilaments is to preserve axonal caliber, so its
breakdown represents deficiency of axonal conduction velocity
(38,39). Schumacher et al (40) reported an association between
neurofilament loss and hind-limb motor function deficiency after
SCI. Recently, Navarro et al (15) detected a loss of NF-positive axons
four and nine months after Th12 contusion in minipigs in areas
heavily infiltrated with IBA-1 positive cells. Nine weeks after SCI
we observed significant reduction in the number of neurofilaments.
Major increase in NF-IR profiles was detected after saline
hypothermia in LC in the +1 and −1 segments, a finding which
strongly correlates with significant white matter preservation. Our
findings confirm the strong correlation between neurological
outcome, the extent of tissue preservation within the immediate
rostro-caudal (+1 and −1) segments, and preservation of
neurofilaments which are almost exclusively found in myelinated
axons. Such improvements were not observed in the saline-treated
animals subjected to more severe (15N) SCI or in the group treated
with DMEM/F12 medium. DMEM/F12 perfusion medium was designed to
support the growth and maintenance of a variety of cells. The lack
of its positive effect may have been due to the use of this medium
at low temperature, which reduces its efficiency.
Saline solution administered over the lesion site in
the 15N SCI group significantly preserved the gray and white matter
and protected neurofilaments in the dorsal and lateral columns,
however the enhancement of tissue integrity was seen only in caudal
segments. Devaux et al (41)
reported that segments caudal to the lesion site hosted a robust
inflammatory process, accompanied at least temporarily by local
synthesis of neuroprotective and regenerative molecules. Most
experimental SCI studies show proliferation and activation of
microglia in the injured spinal cord (42,43).
This proliferation accelerates neuronal damage by releasing toxic
substances, including nitric oxide, super oxide and tumor necrosis
factor-alpha (TNF-α) (44–46). Our previous study revealed strong
increase in NOS activity in the caudal segments immediately
adjacent to the lesion site one day after mid-thoracic spinal cord
constriction in rabbits (47).
Morino et al (48) reported
that peak proliferation of microglia in rats was around 48 h, and
the subsequent production of cytokine and nitric oxide were key
events in accelerating neuronal damage after spinal cord injury.
Mild hypothermia for 48 h inhibited microglia proliferation and
TNF-α production after traumatic SCI and improved hind-limb motor
function (49). In another
computer-controlled minipig SCI model, the presence of high-density
activated microglia and macrophages was identified up to nine
months after SCI (15). This
indicates that if the target of hypothermia treatment is
inflammatory response, the hypothermia treatment in more severe SCI
models should be continued for a longer time after the SCI.
In the present study, we validated the effectiveness
of an implantable spinal perfusion chamber (using 3D scanner
technology) in a computer-controlled spinal cord trauma minipig
model. The perfusion chamber could be successfully used for
application of selective spinal cord hypothermia in large
pre-clinical animal SCI models, aimed at maximum functional benefit
as defined by recovery of motor function. Although the superfusion
of cold saline was restricted to a relatively small spinal cord
region (just above the lesion site), the effectiveness of treatment
spread up to 3 cm both cranially and caudally. The treatment showed
that tissue integrity in both cranial and caudal segments
immediately adjacent to the lesion (+1 and −1) and substantial
preservation of neurofilaments in the lateral funiculi of the same
segments is needed for a better neurological outcome. No
improvement of functional recovery was seen when treatment affected
the tissue integrity only in the caudal segments. These results
suggest that local saline hypothermia (4°C) applied through a
spinal perfusion chamber for 5 h could be a promising therapeutic
method when combined with clinically-proven surgical
(decompression) and medical treatments for SCI. However, further
experimental studies are required to evaluate hypothermia treatment
for its possible utility in human patients with SCI.
Acknowledgements
This study was supported by the project: Formation
and development of a diagnostic procedure in the treatment of
trauma-injured spinal cord (ITMS 26220220127), supported by the
Research and Development Operational Programme funded by the ERDF.
We are grateful to S. Gancarcikova, MVD., PhD. for preparation of
protocols approved by the State Veterinary and Food Administration
and Mrs. A. Kosova for her technical assistance.
References
|
1
|
Arrica M and Bissonnette B: Therapeutic
hypothermia. Semin Cardiothorac Vasc Anesth. 11:6–15. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Erecinska M, Thoresen M and Silver IA:
Effects of hypothermia on energy metabolism in mammalian central
nervous system. J Cereb Blood Flow Metab. 23:513–530. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Westergren H, Farooque M, Olsson Y and
Holtz A: Motor function changes in the rat following severe spinal
cord injury. Dose treatment with moderate systemic hypothermia
improve functional outcome. Acta Neurochir (Wien). 142:567–573.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Westergren H, Farooque M, Olsson Y and
Holtz A: Spinal cord blood flow changes following systemic
hypothermia and spinal cord compression injury: An experimental
study in rat using laser-Doppler flowmetry. Spinal Cord. 39:74–84.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chatzipanteli K, Yanagawa Y, Marcillo AE,
Kraydieh S, Yezierski RP and Dietrich WD: Posstraumatic hypothermia
reduced polymorphonuclear leukocyte accumulation following spinal
cord injury in rats. J Neurotrauma. 17:321–332. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lo TP, Cho KS, Garg MS, Lynch MP, Marcillo
AE, Koivisto DL, Stagg M, Abril RM, Patel S, Dietrich WD and Pearse
DD: Systemic hypothermia improves histological and functional
outcome after cervical spinal cord contusion in rats. J Comp
Neurol. 514:433–448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morino T, Ogata T, Takeba J and Yamamoto
H: Microglia inhibition is a target of mild hypothermic treatment
after the spinal cord injury. Spinal Cord. 46:425–431. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu CG, Jimenez O, Marcillo AE, Weider B,
Bangerter K, Dietrich WD, Castro S and Yezierski RP: Beneficial
effects of modest systemic hypothermia on locomotor function and
histopathological damage following contusion-induced apinal cord
injury in rats. J Neurosurg. 93 1 Suppl:S85–S93. 2000.
|
|
9
|
Kida Y, Takano H, Kitagawa H and Tsuji H:
Effects of systemic or spinal cord cooling on conductive spinal
evoked potentials. Spine (Phila Pa 1976). 19:341–345. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marsala M, Galik J, Ishikawa T and Yaksh
TL: Technique of selective spinal cord cooling in rat: Methodology
and application. J Neurosci Methods. 74:97–106. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Inamasu J and Ichikizaki K: Mild
hypothermia in neurologic emergency: An update. Ann Emerg Med.
40:220–230. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shibuya S, Miyamoto O, Janjua NA, Itano T,
Mori S and Norimatsu H: Post-traumatic moderate systemic
hypothermia reduces TUNEL positive cells following spinal cord
injury in rat. Spinal Cord. 42:29–34. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dath R, Ebinesan AD, Porter KM and Miles
AW: Anatomical measurements of porcine lumbar vertebrae. Clin
Biomech (Bristol, Avon). 22:607–613. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rydevik BL, Pedowitz RA, Hargens AR,
Swenson MR, Myers RR and Garfin SR: Effects of acute, graded
compression on spinal nerve root function and structure: An
experimental study of the pig cauda equina. Spine (Phila Pa 1976).
16:487–493. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Navarro R, Juhas S, Keshavarzi S, Juhasova
J, Motlik J, Johe K, Marsala S, Scadeng M, Lazar P, Tomori Z, et
al: Chronic spinal compression model in minipigs: A systematic
behavioral, quanlitative and quantitative neuropathological study.
J Neurotrauma. 29:499–513. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bernards CM: Understanding the physiology
and pharmacology of epidural and intrathecal opioids. Best Pract
Res Clin Anaesthesiol. 16:489–505. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clément R, Malinovsky JM, Hildgen P, Dollo
G, Estèbe JP, Chevanne F, Le Verge R and LeCorre P: Spinal
disposition and meningeal permeability of local anesthetics. Pharm
Res. 21:706–716. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Durant PA and Yaksh TL: Distribution in
cerebrospinal fluid, blood and lymph of epidurally injected
morphine and inulin in dogs. Anesth Analg. 65:583–592. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sulla I, Boldizar M, Racekova E and Balik
V: Thoracic laminectomy technique in minipigs. Folia Veterinaria.
56:35–39. 2012.
|
|
20
|
Lee JH, Jones CF, Okon EB, Anderson L,
Tigchelaar S, Kooner P, Godbey T, Chua B, Gray G, Hildebrandt R, et
al: A novel porcine model of traumatic thoracic spinal cord injury.
J Neurotrauma. 30:142–159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marsala M, Vanicky I, Galik J, Radonak J,
Kundrat I and Marsala J: Panmyelic epidural cooling protects
against ischemic spinal cord damage. J Surg Res. 55:21–31. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vanicky I, Marsala M, Gálik J and Marsala
J: Epidural perfusion cooling protection against protracted spinal
cord ischemia in rabbits. J Neurosurg. 79:736–741. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grulova I, Slovinska L, Nagyova M, Cizek M
and Cizkova D: The effect of hypothermia on sensory-motor function
and tissue sparing after spinal cord injury. Spine J. 13:1881–1891.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Levi AD, Green BA, Wang MY, Dietrich WD,
Brindle T, Vanni S, Casella G, Elhammady G and Jagid J: Clinical
application of modest hypothermia after spinal cord injury. J
Neurotrauma. 26:407–415. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hansebout RR and Hansebout CR: Local
cooling for traumatic spinal cord injury: outcomes in 20 patients
and review of the literature. J Neurosurg Spine. 20:550–561. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J and Pearse DD: Therapeutic
hypothermia in spinal cord injury: The status of its use and open
questions. Int J Mol Sci. 16:16848–16879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tetzlaff W, Kobayashi NR, Giehl KM, Tsui
BJ, Cassar SL and Bedard AM: Response of rubrospinal and
corticospinal neurons to injury and neurotrophins. Prog Brain Res.
103:271–286. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoshitake A, Mori A, Shimizu H, Ueda T,
Kabei N, Hachiya T, Okano H and Yozu R: Use of an epidural cooling
catheter with a closed countercurrent lumen to protect against
ischemic spinal cord injury in pigs. J Thorac Cardiovasc Surg.
134:1220–1226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eidelberg E, Straehley D, Erspamer R and
Watkins CJ: Relationship between residual hindlimb-assisted
locomotion and surviving axons after incomplete spinal cord
injuries. Exp Neurol. 56:312–322. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fehlings MG and Tator CH: The
relationships among the severity of spinal cord injury, residual
neurological function, axon counts and counts of retrogradely
labeled neurons after experimental spinal cord injury. Exp Neurol.
132:220–228. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Basso DM, Beattie MS and Bresnahan JC:
Graded histological and locomotor outcomes after spinal cord
contusion using the NYU weight-drop device versus transection. Exp
Neurol. 139:244–256. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dimar JR II, Shields CB, Zhang YP, Burke
DA, Raque GH and Glassman SD: The role of directly applied
hypothermia in spinal cord injury. Spine (Phila Pa 1976).
25:2294–2302. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nout YS, Ferguson AR, Strand SC, Moseanko
R, Hawbecker S, Zdunowski S, Nielson JL, Roy RR, Zhong H,
Rosenzweig ES, et al: Methods for functional assessment after C7
spinal cord hemisection in the rhesus monkey. Neurorehabil Neural
Repair. 26:556–569. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fawcett J: Repair of spinal cord injuries:
Where are we, where are we going? Spinal Cord. 40:615–623. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sudo H, Taneichi H and Kaneda K: Secondary
medulla oblongata involvement following middle cervical spinal cord
injury associated with latent traumatic instability in a patient
with ossification of the posterior longitudinal ligament. Spinal
Cord. 44:126–129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tomko P, Farkaš D, Čížková D and Vanický
I: Longitudinal enlargement of the lesion after spinal cord injury
in the rat: A consequence of malignant edema? Spinal Cord Cord
consequence of malignant edema? Spinal Cord. 55:1–263. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ward RE, Huang W, Kostusiak M, Pallier PN,
Michael-Titus AT and Priestley JV: A characterization of white
matter pathology following spinal cord compression injury in the
rat. Neuroscience. 260:227–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sakaguchi T, Okada M, Kitamura T and
Kawasaki K: Reduced diameter and conduction velocity of myelinated
fibers in the sciatic nerve of a neurofilament-deficient mutant
quail. Neurosci Lett. 153:65–68. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu Q, Xie F, Siedlak SL, Nunomura A,
Honda K, Moreira PI, Zhua X, Smith MA and Perry G: Neurofilament
proteins in neurodegenerative diseases. Cell Mol Life Sci.
61:3057–3075. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schumacher PA, Siman RG and Fehlings MG:
Pretreatment with calpain inhibitor CEP-4143 inhibits calpain I
activation and cytoskeletal degradation, improves neurological
function, and enhances axonal survival after traumatic spinal cord
injury. J Neurochem. 74:1646–1655. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Devaux S, Cizkova D, Quanico J, Franck J,
Nataf S, Pays L, Hauberg-Lotte L, Maass P, Kobarg JH, Kobeissy F,
et al: Proteomic analysis of the spatio-temporal based molecular
kinetics of acute spinal cord injury identifies a time- and
segment-specific window for effective tissue repair. Mol Cell
Proteomics. 15:2641–2670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Watanabe T, Yamamoto T, Abe Y, Saito N,
Kumagai T and Kayama H: Differential activation of microglia after
experimental spinal cord injury. J Neurotrauma. 16:255–265. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Saganová K, Burda J, Orendácová J, Cizkova
D and Vanický I: Fluoro-Jade B staining following zymosan
microinjection into the spinal cord white matter. Cell Mol
Neurobiol. 26:1463–1473. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tanaka M, Sotomatsu A, Yoshida T, Hirai S
and Nishida A: Detection of superoxide production by activated
microglia using a sensitive and specific chemiluminescence assay
and microglia-mediated PC12 h cell death. J Neurochem. 63:266–270.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lukácová N, Halát G, Chavko M and Marsala
J: Ischemia-reperfusion injury in the spinal cord of rabbits
strongly enhances lipid peroxidation and modifies phospholipid
profiles. Neurochem Res. 21:869–873. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lukácová N, Kolesárová M, Kuchárová K,
Pavel J, Kolesar D, Radonak J, Marsala M, Chalimoniuk M, Langfort J
and Marsala J: The effect of a spinal cord hemisection on changes
in nitric oxide synthase pools in the site of injury and in regions
located far away from the injured site. Cell Mol Neurobiol.
26:1367–1385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lukácová N, Cízková D, Marsala M, Pavel J,
Jalc P, Sulla I, Kafka J and Marsala J: Effect of midthoracic
spinal cord constriction on catalytic nitric oxide synthase
activity in the white matter columns of rabbit. Neurochem Res.
25:1139–1148. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Morino T, Ogata T, Horiuchi H, Takeba J,
Okumura H, Miyazaki T and Yamamoto H: Delayed neuronal damage
related to microglia proliferation after mild spinal cord
compression injury. Neurosci Res. 46:309–318. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Morizane K, Ogata T, Morino T, Horiuchi H,
Yamaoka G, Hino M and Miura H: A novel thermoelectric cooling
device using Peltier modules for inducing local hypothermia of the
spinal cord: The effect of local electrically controlled cooling
for the treatment of spinal cord injuries in conscious rats.
Neurosci Res. 72:279–282. 2012. View Article : Google Scholar : PubMed/NCBI
|