Introduction
Brain injury in preterm infants is related to
inflammatory responses and viral infections (1). Herpes simplex virus (HSV) infection is
one of the common types of intrauterine viral infection, with ~2.5%
incidence in pregnant women and 0.04% incidence in neonates
(2). Many studies have shown that
the cytokine-induced inflammatory response is the main mechanism of
perinatal infection-induced brain injury. Pathogens are able to
enter the fetus via maternal systems, thus increasing the risk of
fetal inflammation syndrome, and inflammatory cytokines can enter
the fetal brain via the circulation, contributing to adverse
neurodevelopmental outcomes. Accordingly, effective and
prophylactic intervention in pregnant women with perinatal
intrauterine viral infections is critical for protecting the
cerebral neurofunction of preterm neonates (3). In the past, recombinant human
erythropoietin (rhEPO) was generally used for treating anemia,
including gestational anemia, with a high degree of safety
(4). However, the non-hematopoietic
functions of rhEPO have recently attracted clinical attention,
focusing especially on its neuroprotective capabilities. Indeed,
the ability of rhEPO to protect against brain injuries has been
investigated in neonatal rats (5).
At this stage though, the majority of studies have focused on
neonates and animal models, with no clinical investigation of
prophylactic rhEPO intervention. The present study, having
understood the reported safety of rhEPO intervention in pregnant
women, investigated the neuroprotective capabilities of rhEPO
intervention as an adjuvant therapy in pregnant women infected with
intrauterine herpes virus.
Patients and methods
Patient information
One hundred and twenty women infected with perinatal
intrauterine herpes virus treated at the Second People's Hospital
of Liaocheng between July, 2012 and August, 2014 were selected. The
inclusion criteria were defined in accordance with ‘Chinese
Obstetrics and Gynecology’ (6).
Selected patients presented fever (>37.5°C), heart rate >100
bpm, fetal heart rate >160 bpm, abnormal amniotic fluid smell,
uterine tenderness, CRP >8 mg/l, WBC >15×109/l.
All selected patients were required to provide voluntary informed
written consent prior to enrollment in the study. Participant
exclusion criteria were as follows: i) allergy to the selected
medicine; ii) having pregnancy-induced hypertension, diabetes, or
preeclampsia, other pregnancy-related complications, or serious
internal diseases; iii) refusal to provide consent.
Fetus inclusion criteria were as follows: i)
gestational age <37 weeks, in line with diagnostic criteria for
preterm children; ii) presence of maternal pathogens in fetal
pharyngeal secretions, urine, or blood. Fetus exclusion criteria
were as follows: i) allergy to the selected medicine; ii) presence
of any other congenital diseases. Participants were randomly
assigned into four groups of 30 each: A, B, C and D. Maternal and
fetal baseline data for all four groups are shown in Tables I and II. No significant differences in any
parameter were found between any of the groups (P>0.05). The
study was approved by the Ethics Committee of the Second People's
Hospital of Liaocheng.
 | Table I.Maternal participant baseline
data. |
Table I.
Maternal participant baseline
data.
| Variable | Group A | Group B | Group C | Group D | P-value |
|---|
| Age (years) |
26.3±4.2 |
26.7±3.9 |
27.1±4.8 |
26.9±5.2 | >0.05 |
| Gestational age
(weeks) |
32.4±2.2 |
31.5±3.5 |
31.8±1.8 |
32.6±2.8 | >0.05 |
| Body weight (kg) |
72.6±10.5 |
75.6±11.4 |
73.2±12.6 |
73.7±13.0 | >0.05 |
 | Table II.Fetal baseline data. |
Table II.
Fetal baseline data.
| Variable | Group A | Group B | Group C | Group D | P-value |
|---|
| Gender
(male/female) | 16/14 | 18/12 | 15/15 | 14/16 | >0.05 |
| Gestational age
(weeks) | 31.2±1.8 | 31.4±2.2 | 30.1±2.1 | 31.7±1.9 | >0.05 |
| Weight (g) | 1725.2±159.4 | 1746.5±161.7 | 1722.5±154.9 | 1736.5±171.5 | >0.05 |
| 1-min Apgar
score | 9.5±1.4 | 9.7±1.6 | 9.7±1.8 | 9.4±1.6 | >0.05 |
Intervention methodology
Participants in all four groups were given the same
baseline intrauterine infection treatment, with rhEPO
administration only after their infections were under control.
Participants in group A were treated with 1,500 IU rhEPO doses
(S20030068; Shanghai HKB Co., Ltd., Shanghai, China) via slow
intravenous injection every 3 days until delivery. Participants in
group B underwent the same procedure except the dosage was 3,000 IU
rhEPO. In group C, neonates were intravenously administered 250
IU/kg rhEPO 3 times/week from 2–3 days post-natal until 1 month.
Participants in group D were not given rhEPO at any time. Hb levels
were closely monitored throughout the study period, with Hb levels
>220 g/l resulting in termination of rhEPO administration to
prevent hyper-erythropoiesis.
Measured parameters
The following parameters were measured immediately
after delivery (T0) and 1 (T1), 2
(T2), and 4 weeks after delivery (T3):
hemoglobin (Hb), reticulocyte (Ret), hematocrit (Hct),
neuronspecific enolase (NSE), myelin basic protein (MBP), and S100
calcium-binding protein B (S100B). The linear relationships and the
correlation coefficient r between Hb, Ret and Hct with NSE,
MBP and S100B were analyzed. Receiver operating characteristic
(ROC) curve models were established to determine the evaluation
values of Hb, Ret and Hct for brain injury in preterm infants and
their optimal cut-off values.
Statistical analysis
SPSS 19.0 (IBM, Armonk, NY, USA) was used in data
analysis. Measurement data were recorded as (mean ± SD), and the
comparison of multiple time-points between the four groups was
calculated via repeated measures analysis of variance (rANOVA).
Multivariate analysis of variance (MANOVA) was used for single
time-point comparisons while least significant difference (LSD) was
used for pair-comparisons. Odds ratio (%) was calculated and
Mann-Whitney U test was used to compare pair-data. Chi-square test
was applied in mortality and bacterial eradication calculations,
data were analyzed via linear correlation and P<0.05 indicated
statistical significance.
Results
Incidence of brain injury
The incidence of brain injury in groups A, B, C and
D were 10% (3/30), 6.7% (2/30), 26.7% (8/30), and 33.3% (10/30),
respectively. The difference between group A and groups B and C
were not statistically significant (χ2=0.218, P=0.640;
χ2=2.783, P=0.095, respectively). However, group A was
significantly different than group D (χ2=4.812,
P=0.028). group B was also significantly different from groups C
and D (χ2=4.320, P=0.038; χ2=6.667, P=0.010,
respectively), while groups C and D were not statistically
significant (χ2=0.318, P=0.573).
Multiple time-point Hb, Ret and Hct
level comparisons between groups
Hb levels in all four groups showed a decreasing
trend over time (Table III). At
T0, Hb levels in groups A and B were significantly
higher than in groups C and D (P<0.05) while no differences were
observed between groups C and D (P>0.05). At T1,
T2 and T3, Hb levels were not significantly
different between the four groups (P>0.05), although groups A, B
and C all showed higher levels than group D. Ret levels were not
different between groups A and B at any time-point (P>0.05), but
Ret levels in groups A and B were higher than those in groups C and
D at T0 (P<0.05). In addition, Ret levels in groups
A, B and C were higher than in group D at T1-T3
(P<0.05). Finally, Hct levels were higher in group B than group
A at T0, but the difference was not statistically
significant (P>0.05). However, Hct levels in groups A and B were
significantly higher than in groups C and D (P<0.05). At
T1, T2 and T3, Hct levels in
groups A, B and C were significantly higher than in group D
(P<0.05).
 | Table III.Multiple time-point Hb, Ret and Hct
level comparisons. |
Table III.
Multiple time-point Hb, Ret and Hct
level comparisons.
| Variable | Time-point | Group A (n=30) | Group B (n=30) | Group C (n=30) | Group D (n=30) |
|---|
| Hb (g/l) | T0 | 171.6±14.8 | 173.8±15.3 |
159.9±17.2a,b |
165.2±16.8a,b |
|
| T1 | 156.3±20.4 | 158.5±19.5 | 161.8±13.4 |
142.6±20.2a–c |
|
| T2 | 135.5±18.7 | 137.3±20.5 | 141.3±13.3 |
112.5±21.6a–c |
|
| T3 | 109.9±16.5 | 112.5±15.4 | 115.4±12.9 |
90.4±14.3a–c |
| F-value |
| 6.369 | 5.493 | 5.894 | 7.610 |
| P-value |
| 0.024 | 0.049 | 0.039 | 0.014 |
| Ret
(×109) | T0 | 22.4±9.7 | 24.1±12.9 |
18.4±10.2a,b | 18.1±9.5a,b |
|
| T1 | 24.5±10.3 | 24.6±13.4 | 27.5±14.2 |
17.3±10.2a–c |
|
| T2 | 22.7±11.5 | 21.2±10.3 | 26.2±13.5 |
17.7±11.2a–c |
|
| T3 | 24.5±12.4 | 20.4±11.7 | 24.8±11.3 |
18.6±12.7a–c |
| F-value |
| 3.169 | 2.693 | 4.775 | 3.031 |
| P-value |
| 0.136 | 0.271 | 0.069 | 0.142 |
| Hct (%) | T0 | 31.4±6.2 | 32.5±5.8 |
27.1±4.9a,b |
26.6±5.3a,b |
|
| T1 | 28.6±5.4 | 31.7±5.6 | 32.5±5.5 |
25.4±4.3a–c |
|
| T2 | 33.6±5.8 | 34.1±6.5 | 31.4±6.3 |
24.1±5.9a–c |
|
| T3 | 34.1±5.6 | 37.5±7.5 | 38.4±7.4 |
23.4±6.3a–c |
| F-value |
| 7.893 | 9.963 | 5.336 | 4.116 |
| P-value |
| 0.001 | <0.01 | 0.056 | 0.089 |
Differences in specific brain injury
indicators in preterm infants
As shown in Table
IV, NSE, MBP and S100B levels were significantly lower in
groups A and B vs. groups C and D at T0 (P<0.05),
while at T1, T2 and T3, there was
no significant difference among groups A, B and C (P>0.05), but
all three showed significantly lower NSE, MBP and S100B levels than
group D (P<0.05).
 | Table IV.Differences in specific brain injury
indicators in preterm infants. |
Table IV.
Differences in specific brain injury
indicators in preterm infants.
| Indicator | Time-point | Group A | Group B | Group C | Group D |
|---|
| NSE (pg/ml) | T0 | 1.9±0.5 | 1.5±0.2 |
2.5±0.5a,b |
2.6±0.6a,b |
|
| T1 | 1.9±0.3 | 1.8±0.5 | 2.0±0.4 |
2.5±0.4a–c |
|
| T2 | 1.8±0.2 | 1.9±0.2 | 1.8±0.3 |
2.3±0.4a–c |
|
| T3 | 1.3±0.1 | 1.5±0.1 | 1.4±0.2 |
2.2±0.4a–c |
| F-value |
| 13.007 | 15.960 | 27.582 | 8.369 |
| P-value |
| <0.01 | <0.01 | <0.01 | 0.001 |
| MBP (pg/ml) | T0 | 0.7±0.2 | 0.6±0.1 |
0.8±0.2a,b |
0.9±0.2a,b |
|
| T1 | 0.5±0.3 | 0.5±0.3 | 0.5±0.2 |
0.9±0.2a–c |
|
| T2 | 0.3±0.4 | 0.4±0.2 | 0.4±0.1 |
0.8±0.1a–c |
|
| T3 | 0.2±0.09 | 0.3±0.1 | 0.3±0.1 |
0.8±0.2a–c |
| F-value |
| 28.693 | 17.525 | 8.582 | 4.696 |
| P-value |
| <0.01 | <0.01 | 0.041 | 0.121 |
| S100B | T0 | 4.2±1.0 | 4.1±0.9 |
5.4±1.4a,b |
5.6±2.3a,b |
|
| T1 | 3.9±1.6 | 4.0±1.5 | 5.1±1.3 |
5.5±2.4a–c |
|
| T2 | 3.8±1.9 | 4.0±1.8 | 4.2±1.2 |
4.9±1.9a–c |
|
| T3 | 4.1±1.2 | 3.9±1.3 | 3.7±1.1 |
4.8±2.0a–c |
| F-value |
| 10.960 | 5.916 | 29.398 | 5.226 |
| P-value |
| <0.01 | 0.067 | <0.01 | 0.089 |
Correlation between blood indicators
and specific brain injury indicators
As shown in Table V,
there was a negative correlation between Hb and NSE, MBP and S100B
(P<0.05), while Ret was not significantly correlated with NSE,
MBP or S100B (P>0.05). Hct was negatively correlated with NSE,
MBP and S100B (P<0.05).
 | Table V.Correlation between blood indicators
and specific brain injury indicators. |
Table V.
Correlation between blood indicators
and specific brain injury indicators.
| Independent
variables | NSE (r-,
P-value) | MBP (r-,
P-value) | S100B (r-,
P-value) |
|---|
| Hb | −0.341, 0.009 | −0.625,
<0.01 | −0.442,
<0.01 |
| Ret | −0.125, 0.576 | −0.046, 0.889 | −0.133, 0.421 |
| Hct | −0.844,
<0.01 | −0.136, 0.042 | −0.643,
<0.01 |
ROC brain injury prediction model
using umbilical vein blood immediately after delivery
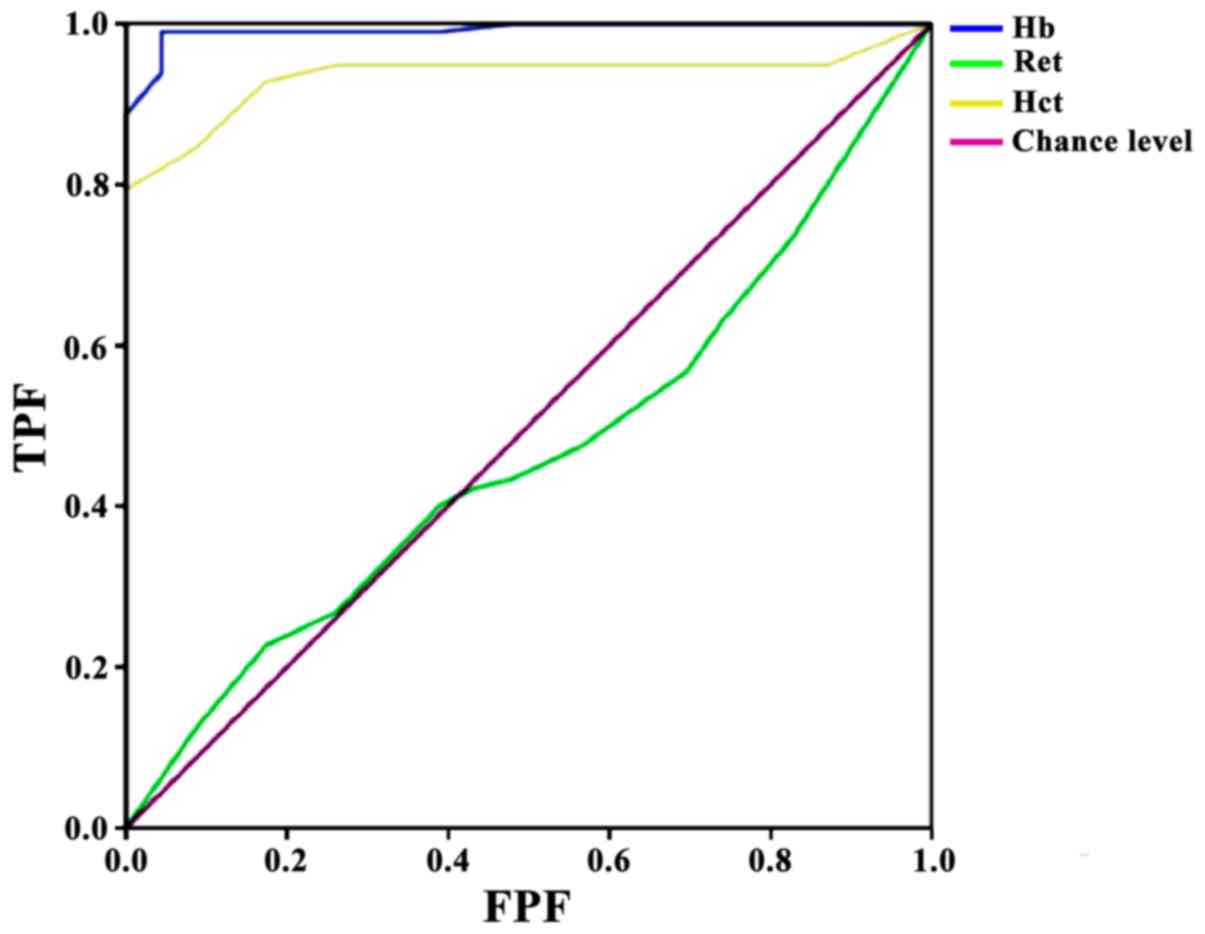
ROC model results presented an area under the curve
(AUC) of 0.992 for Hb, with standard error 0.006, asymptotic
significance P<0.01, 95% CI, 0.980–1.000, optimal cut-off value
170, and sensitivity 99%/specificity 95.7%. The Ret ROC model
presented an AUC of 0.465, with standard error 0.062, asymptotic
significance P=0.608, and 95% CI, 0.344–0.587; this indicated that
Ret had no predictive value for brain injury. Finally, Hct
presented an AUC of 0.934, with standard deviation 0.023,
asymptotic significance P<0.01, 95% CI, 0.889–0.980, optimal
cut-off value 28.5, and sensitivity 79.4%/specificity 100%
(Fig. 1).
Discussion
It is clinically recognized today that preterm
infant brain injury is mainly related to hypoxia/ischemia and
intrauterine infections, with the most common type of brain injury
being white matter lesions (7). HSV
is one of the common types of intrauterine infection, and studies
have confirmed that infants can be infected with HSV at birth,
manifesting as lesions of the central nervous system and skin
(8). Different viral infections have
affinity for different organs, thus leading to significant
individual differences in lesion profile. Recent research indicates
that HSV-infected infants present microcephaly, heart abnormalities
and neuropsychiatric disorders (9).
Indeed, research has indicated that ~30% of preterm deliveries are
intrauterine infection-related (10). The main mechanism of intrauterine
infection-induced brain injury is fetal blood-brain barrier
permeability induced by circulating microorganism-stimulated
inflammatory factors. This allows microorganisms to enter the fetal
brain, causing injury and fever (11,12).
Some researchers believe that fever at delivery could aggravate
hypoxia/ischemia in fetal brain, and cerebral palsy incidence has
been found to increase 9-fold (13).
In addition, studies have demonstrated that 24–32 weeks of
gestational age was the most vulnerable period for fetal white
matter. This neurodevelopment stage is roughly equivalent to 2–5
day old neonatal rats, which have been found to be susceptible to
infection and hypoxia-induced inflammation (14,15).
Therefore, it is entirely possible that the intrauterine infected
fetus may have already developed brain injury before delivery.
Apart from its hematopoietic function, EPO also has
a neuro-protective role. Preliminary findings have indicated that
it may be involved in brain cytokine-manipulated biological
regulation, although the mechanism is unclear (16,17).
rhEPO administration in an intrauterine fetal hypoxia/ischemia rat
model was detected in the pups, indicating that rhEPO breached the
blood-brain barrier and protected the fetal brain in the third
trimester. However, this particular study investigated uterine
hypoxia/ischemia (18) and
contradicted earlier studies suggesting that rhEPO could not breach
the blood-brain barrier (19).
Studies have indicated that brain hypoxia/ischemia can be induced
by anemia (20), and thus, we
investigated blood condition indicators such as Hb, Ret and Hct.
Indeed, we found that maternal rhEPO administration resulted in
elevated Hb, Ret and Hct levels in infants immediately after
delivery. Infants given post-natal rhEPO showed slow decline in Hb,
Ret and Hct levels over time. ROC modeling demonstrated that the Hb
and Hct levels measured immediately after delivery could predict
brain injury incidence that could not be corrected with
post-delivery administration, indicating hysteresis and that
infants may have already developed irreversible brain injury by
post-delivery administration. In addition, we found that the
aforementioned fetus blood indicators were correlated with the
specific brain injury indicators NSE, MBP and S100B, suggesting a
probable relationship between preterm infant brain injury and
anemia (21). Although animal
experiments have indicated that low Hb levels can increase stroke
risk (22), no studies regarding the
relationship between Hb and brain injury have been reported until
this study. The ROC model established in this study found that the
optimal cut-off value for Hb was 170 and for Hct was 28.5,
suggesting an increasing rate of brain injury when Hb and Hct
levels were lower than their cutoff values in preterm infants after
delivery. In addition, early administration of rhEPO before
delivery may correct low immediately post-delivery Hb and Hct
levels, thereby reducing brain injury risk.
In conclusion, maternal rhEPO intervention in
intrauterine HSV-infected women during pregnancy has predictive
value on preterm infant brain injury, with a dose-effect
relationship. However, to further prove this predictive
relationship, animal experiments and studies with a large quantity
of sample are still required.
References
|
1
|
Barton SK, Tolcos M, Miller SL, Roehr CC,
Schmölzer GM, Davis PG, Moss TJ, LaRosa DA, Hooper SB and Polglase
GR: Unraveling the links between the initiation of ventilation and
brain injury in preterm infants. Front Pediatr. 3:972015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pichler M, Staffler A, Bonometti N,
Messner H, Deluca J, Thuile T, Kluge R, Schmuth M and Eisendle K:
Premature newborns with fatal intrauterine herpes simplex virus-1
infection: First report of twins and review of the literature. J
Eur Acad Dermatol Venereol. 29:1216–1220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi J and Mu D: Intrauterine infection and
neonate brain injury. J Clin Pediatr. 33:767–770. 2015.
|
|
4
|
Kashiwagi M, Breymann C, Huch R and Huch
A: Hypertension in a pregnancy with renal anemia after recombinant
human erythropoietin (rhEPO) therapy. Arch Gynecol Obstet.
267:54–56. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan XS, Bian XX, Wei WF, Tang Y and Bao
Q: Effect of recombinant human erythropoietin expressions of
apoptosis genes in rats following traumatic brain injury. Trop J
Pharm Res. 15:695–699. 2016. View Article : Google Scholar
|
|
6
|
Cao Z: Chinese Obstetrics and Gynecology.
2nd. People's Medical Publishing House Co., Ltd.; Beijing: pp.
3792004
|
|
7
|
Kidokoro H, Anderson PJ, Doyle LW,
Woodward LJ, Neil JJ and Inder TE: Brain injury and altered brain
growth in preterm infants: Predictors and prognosis. Pediatrics.
134:e444–e453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Al-Fadhli M and Saraya M: Herpes Zoster
infection in an infant. J Kuwait Med Assoc. 46:256–257. 2014.
|
|
9
|
Fleiss B, Guillot PV, Titomanlio L, Baud
O, Hagberg H and Gressens P: Stem cell therapy for neonatal brain
injury. Clin Perinatol. 41:133–148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang L and Li W: Intrauterine infection
and preterm infant disease. J Appl Clin Pediatr. 28:1041–1043.
2013.
|
|
11
|
Zhang Z, Li A and Xiao X: Risk factors for
intrauterine infection with hepatitis B virus. Int J Gynaecol
Obstet. 125:158–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kemp MW: Preterm birth, intrauterine
infection, and fetal inflammation. Front Immunol. 5:574. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bennet L, Booth L and Gunn AJ: Potential
biomarkers for hypoxic-ischemic encephalopathy. Semin Fetal
Neonatal Med. 15:253–260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Northam GB, Liégeois F, Chong WK, Wyatt JS
and Baldeweg T: Total brain white matter is a major determinant of
IQ in adolescents born preterm. Ann Neurol. 69:702–711. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thiebaugeorges O, Fresson J, Audibert F,
Guihard-Costa AM, Frydman R and Droulle P: Diagnosis of
small-for-gestational-age fetuses between 24 and 32 weeks, based on
standard sonographic measurements. Ultrasound Obstet Gynecol.
16:49–55. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uchikura Y, Matsubara K, Matsubara Y, Mori
M, Nabeta M, Hashimoto H, Fujioka T, Hamada K and Nawa A: Nucleated
red blood cells are involved in endothelial progenitor cell
proliferation in umbilical venous blood of preeclamptic patients.
Hypertens Res Pregnancy. 1:46–51. 2013. View Article : Google Scholar
|
|
17
|
Lund A, Lundby C and Olsen NV: High-dose
erythropoietin for tissue protection. Eur J Clin Invest.
44:1230–1238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang W, Cheng Z and Dai H: Calcium
concentration response to uterine ischemia: A comparison of uterine
fibroid cells and adjacent normal myometrial cells. Eur J Obstet
Gynecol Reprod Biol. 174:123–127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhiyuan Q, Qingyong L, Shengming H and Hui
M: Protective effect of rhEPO on tight junctions of cerebral
microvascular endothelial cells early following traumatic brain
injury in rats. Brain Inj. 30:462–467. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Osredkar D, Thoresen M, Maes E, Flatebø T,
Elstad M and Sabir H: Hypothermia is not neuroprotective after
infection-sensitized neonatal hypoxic-ischemic brain injury.
Resuscitation. 85:567–572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blackburn S: Brain injury in preterm
infants: Pathogenesis and nursing implications. Newborn Infant Nurs
Rev. 16:8–12. 2016. View Article : Google Scholar
|
|
22
|
Lieber BA, Taylor B, Appelboom G, Prasad
K, Bruce S, Yang A, Bruce E, Christophe B and Connolly ES Jr:
Meta-analysis of telemonitoring to improve HbA1c levels: Promise
for stroke survivors. J Clin Neurosci. 22:807–811. 2015. View Article : Google Scholar : PubMed/NCBI
|