Introduction
Cystic diseases of kidney (CDK) refers to a variety
of complex diseases with the same morphological features of renal
cysts, including simple renal cysts, multiple/multilocular renal
cysts, parapelvic cyst, medullary sponge kidney, calyceal
diverticulum and other non-hereditary diseases, and autosomal
dominant hereditary polycystic kidney disease, multiple
malformations with renal cyst and other genetic diseases (1). Clinical treatment includes puncture
drainage and sclerotherapy injection, laparoscopic surgery,
percutaneous nephrolithotomy, ureteroscopy and open surgery
(1,2).
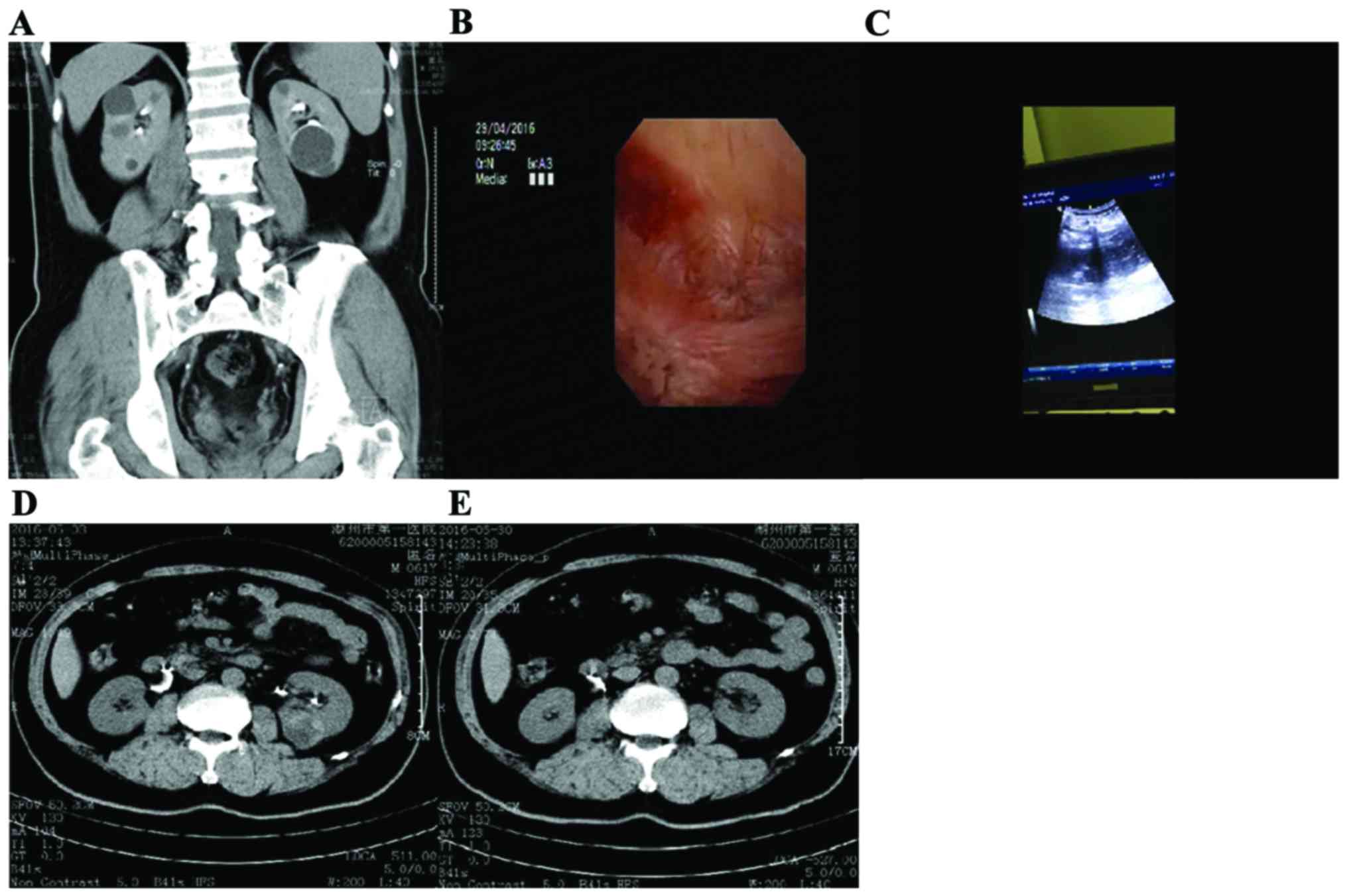
Renal cyst incision and drainage under ureteroscope
is developed on the basis of the wide use of ureteroscope in
examination and lithotripsy, with the advantage of passing the
natural cavity path and is minimally invasive (1). Intraoperative ultrasound can provide
precise real-time positioning for cysts, especially thick-walled
cysts, guiding the incision direction of ureteroscope (2). The renal cyst adjacent to the calyx on
the transverse position of two-dimensional CT cannot be fully
positioned, which reduces the success rate of fenestration
drainage, increases the risk of peripheral organ damage and other
complications (3). Computed
tomography (CT) urography (CTU) multiphase enhanced scan can obtain
the three-dimensional image of the entire urinary system including
the calyces, renal pelvis, ureter and bladder (4). By combining with multi-planar
reconstruction (MPR) post-processing technology, the location of
the cyst and its relationship with the collection system can be
comprehensively assessed (5). The
aim of this study was to evaluate the safety and efficacy of
MPR-CTU combined with intraoperative ultrasonography to guide
ureteroscopy in the treatment of renal cystic disease.
Materials and methods
Object data
A total of 68 patients diagnosed with CDK charged
into the First Affiliated Hospital of Huzhou Teacher's College from
June 2014 to September 2016 was continuously selected, inclusion
criteria: i) Recurrent or persistent lumbar pain, the conservative
treatment was invalid; ii) repeated urinary tract infection or
merged with calculus, poor effect after conservative treatment;
iii) imaging examination suggested cysts endogenous growth, kidney
collection system compression, maximum diameter >4 cm, normal
renal function or mild to moderate damage iv) blood pressure and
blood sugar can be controlled in the normal range; and v) complete
clinical data, informed consent obtained. Exclusion criteria: i)
Past kidney surgery history, combined with renal abscess and kidney
tumors; ii) severe heart, liver, lung, brain and other organ
dysfunction, intolerance to the risk of surgery and anesthesia; and
iii) deformities in urinary system development, ureteroscope cannot
pass.
The patients were divided into control and
observation group by random number method (n=34). The baseline data
of the two groups were comparable (Table
I).
 | Table I.Baseline data of the two groups. |
Table I.
Baseline data of the two groups.
| Groups | Control, (n=34) | Observation,
(n=34) | t/χ2 | P-value |
|---|
| Male/Female | 20/14 | 18/16 | 0.239 | 0.625 |
| Age (years) | 53.2±8.9 | 54.6±11.2 | 0.196 | 0.867 |
| Unilateral/bilateral
cyst | 22/12 | 20/14 | 0.249 | 0.618 |
| Simple
cysts/polycystic kidney | 25/9 | 23/11 | 0.283 | 0.595 |
| Cyst position, n
(%) |
|
| 0.340 | 0.987 |
| Renal pelvis | 16 (47.1) | 18 (52.9) |
|
|
| Superior pole | 5 (14.7) | 5 (14.7) |
|
|
| Medium pole | 5 (14.7) | 4 (11.8) |
|
|
| Inferior pole | 3 (8.8) | 3 (8.8) |
|
|
| Multiple calices | 5 (14.7) | 4 (11.8) |
|
|
| Cyst diameter
(cm) | 5.5±1.4 | 5.6±1.5 | 0.086 | 0.924 |
| Combined disease, n
(%) |
|
|
|
|
| Kidney stones | 8 (23.5) | 9 (26.5) | 0.078 | 0.779 |
| Localized calyceal
water | 6 (17.6) | 8 (23.5) | 0.360 | 0.549 |
| Hypertension | 10 (29.4) | 6 (17.6) | 1.308 | 0.253 |
| Diabetes | 5 (14.7) | 4 (11.8) | 0.000 | 1.000 |
| Coronary heart
disease | 3 (8.8) | 3 (8.8) | 0.000 | 1.000 |
The study was approved by the Ethics Committee of
the First Affiliated Hospital of Huzhou Teacher's College and
informed consents were signed by the patients and/or guardians.
Research methods
Two groups of patients both underwent ureteroscopic
renal cysts incision and drainage, which was performed by the same
surgical and nursing team according to the standard surgical
procedure. The control group was under ultrasound guidance, the
observation group also combined with MPR-CTU technology, the
procedures were as follows: The location of collection system and
cyst was observed under ureteroscope, cyst wall was blue and
transparent and processed to the collection system, cyst wall was
generally foggy after incision, the wall folds were
diverticulum-like; the wall of hydronephrosis was smooth, the
mucosa was pink, the capillaries were obvious; the wall was cut
about 2.5–3.5 cm with 200 µm holmium laser fiber, so that the cysts
and the collection system were connected. Low energy (0.8 J) and
low frequency (10–15 Hz) was used for thin wall cyst, low energy
(0.8 J) and high frequency (20–30 Hz) was used for thick wall cyst,
high energy (1.0–1.5 J) and low frequency (10–15 Hz) was used for
hemostasis, holmium laser irradiation distance should not be too
close or too far (2 mm).
The cut wall was burnt and coagulated, in order to
avoid the hyperplasia and closure of the wall; holmium laser
lithotripsy was used for combined ipsilateral kidney stones, double
J tube was indwelled after operation, the proximal end was
indwelled in the cysts for drainage, 1 month after the operation,
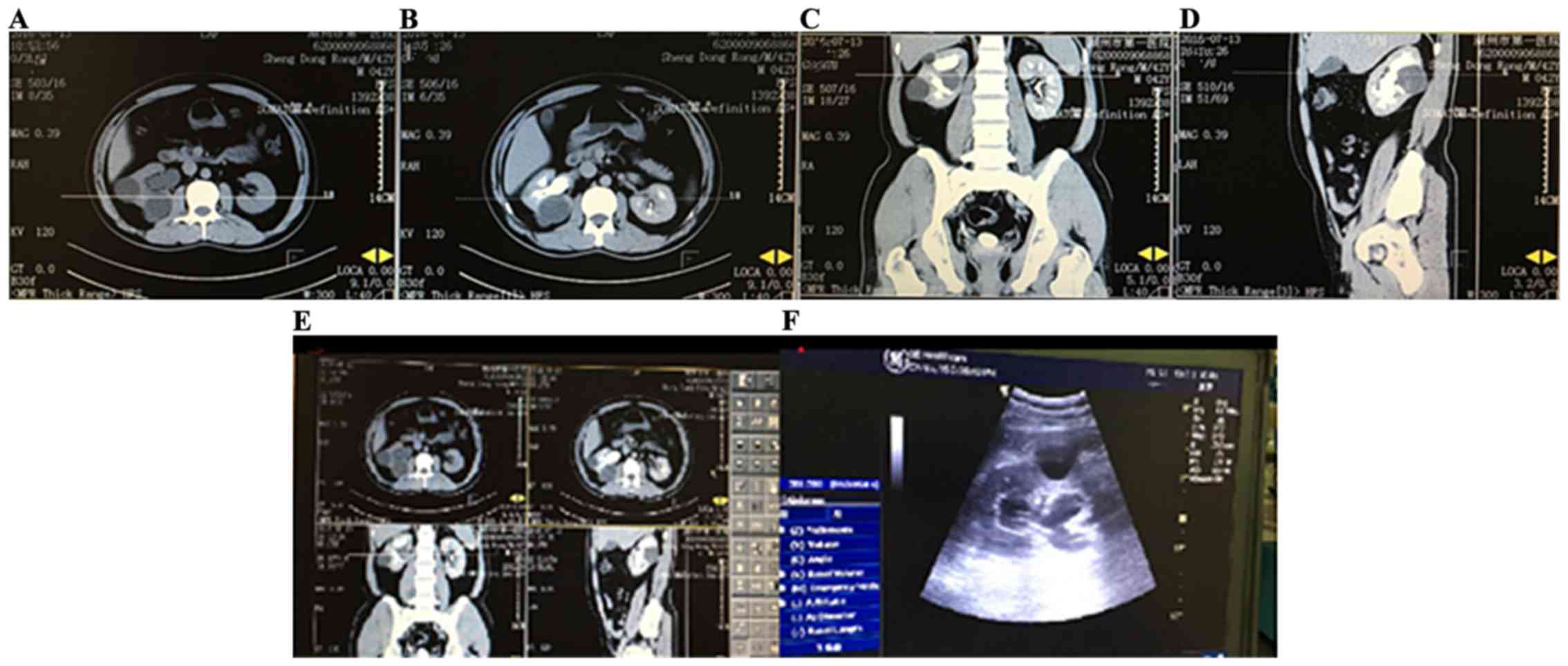
reexamined by CT and double J tube was removed. CTU were scanned in
the renal arterial phase, renal parenchyma and excretion phase. The
original images were processed by computer and then
three-dimensionally reconstructed. The stereo images with high
special resolution were obtained. The position relationship between
the endogenous renal cysts and the front and the rear of renal
pelvis, the adjacency relation with the collection system was
accurately identified (6). MPR was
adjusted by transverse axial, coronal, sagittal position and
multi-directionally: The marginal zone nearest to the calyx and the
cyst was taken, the fenestration drainage site was decided when the
calyx and the cyst was located the closest to the three axial
planes by locating simultaneously in the transverse axis, coronal,
sagittal position (7). The cursor
line perpendicular to each other in the coronal, sagittal and axial
images can be adjusted to show real-time position and plane images,
providing multi-directional and multi-angle observation, a series
of axial image was reorganized along the line, the reconstruction
of the cyst on the coronal plane, sagittal plane or any slope can
be done, providing the target calyx incision position safely and
accurately (Figs. 1 and 2).
The observed indicators
The operative success rate and mean cystic treatment
time, intraoperative and postoperative complications, the total
effective rate after 1 month follow-up were compared between the
two groups. Treatment effect was divided into cure, improved and
ineffective, in which cure was complete disappearance of clinical
symptoms and signs, no complications, no recurrence after 6 months
of follow-up; improved was clinical symptoms and signs
significantly alleviated, no obvious complications, no recurrence
after 6 months of follow-up; invalid was surgical failure or no
significant relief or deterioration for clinical symptoms and
signs, or associated with serious complications. Total efficiency =
(cure + improved)/total number of cases × 100%.
Statistical analysis
Statistical analysis was performed using SPSS20.0
software (SPSS Inc., Chicago, IL, USA). The measurement data were
expressed as mean ± standard deviation, and the independent samples
t-test was used for comparison between groups. The enumeration data
were expressed as the number of cases or (%), comparison between
groups using χ2 test; A P<0.05 was considered to
indicate a statistically significant difference.
Results
The success rate of surgery and the
average cyst treatment time
There was no significant difference in the operative
success rate and cyst treatment time between the two groups
(P>0.05) (Table II).
 | Table II.The success rate of surgery and the
average cyst treatment time. |
Table II.
The success rate of surgery and the
average cyst treatment time.
| Groups | Success, n (%) | Cyst not found, n
(%) | Cyst excision
difficult, n (%) | Average cyst
treatment time, (min) |
|---|
| Control (n=34) | 28 (82.4) | 3 (8.8) | 3 (8.8) | 36.7±12.5 |
| Observation
(n=34) | 30 (88.2) | 1 (2.9) | 3 (8.8) | 32.2±10.3 |
| t/χ2 | 0.469 |
|
| 1.625 |
| P-value | 0.493 |
|
| 0.239 |
Intraoperative and postoperative
complications
The incidence of intraoperative and postoperative
complications was significantly lower in the observation group than
in the control group, the difference was statistically significant
(P<0.05). (Table III).
 | Table III.Intraoperative and postoperative
complications, n (%). |
Table III.
Intraoperative and postoperative
complications, n (%).
| Groups | Intracapsular
hematoma | Perforation | Urinary fistula | Infection | Renal failure | Total
complications |
|---|
| Control (n=34) | 3 (8.8) | 3 (8.8) | 2 (5.9) | 1 (2.9) | 1 (2.9) | 10 (29.4) |
| Observation
(n=34) | 1 (2.9) | 1 (2.9) | 1 (2.9) | 0 | 0 | 3 (8.8) |
| χ2 |
|
|
|
|
| 4.660 |
| P-value |
|
|
|
|
| 0.031 |
Follow-up effect
The total effective rate of the observation group
was significantly higher than the control group, the difference was
statistically significant (P<0.05) (Table IV).
 | Table IV.Follow-up treatment effect, n (%). |
Table IV.
Follow-up treatment effect, n (%).
| Groups | Cure | Improved | Invalid | Total efficiency |
|---|
| Control (n=34) | 10 (29.4) | 10 (9.4) | 14 (41.2) | 20 (58.8) |
| Observation
(n=34) | 19 (55.9) | 9 (26.5) | 6 (17.6) | 28 (82.4) |
| χ2 |
|
|
| 4.533 |
| P-value |
|
|
| 0.033 |
Discussion
The theory basis of renal cystic disease are: i)
Polycystic kidney disease is occult, with long duration, a large
number of cysts increase chronically and progressively causing
renal parenchyma squeezed and degenerated, gradually replaced by
fibrous tissue, renal function is gradually diminished, ultimately
progress to chronic renal failure (8); ii) due to decreased renal filtration
rate, water and sodium retention, renal cortex ischemia, renin –
aldosterone system is activated, leading to secondary hypertension,
further exacerbate renal function deterioration (9); iii) with the cysts increasing, the
obstruction factors inside and outside the cavity cause the
oppression of functional nephrons and blood vessels, resulting in
renal ischemic damage and functional renal unit reduction; iv)
polycystic kidney capsule has epidermal growth factor, cytokines
and other factors, which can promote renal epithelial cell
proliferation, change the renal interstitial, playing an important
role in the formation and development of cysts (10).
Ultrasound guided percutaneous drainage is the most
common treatment of renal cystic disease, with minimum surgical
trauma; but the cyst disappearance rate is <50%, the recurrence
rate is as high as 17 to 44% (11);
especially not ideal for cyst with diameter >10 cm; it is
restricted by the location of the cyst, with difficulties of
puncture on the cyst located at the renal ventral side and the
superior pole (12); for the cyst
close to the collection system, sclerosing agent may penetrate into
the collection system, causing serious complications (13); obvious pain may occur after the
injection of cysts; renal-derived cyst, localized hydronephrosis
are contraindications; it cannot handle kidney stones, the
malignant transformation is difficult to be identified (14). Laparoscopic renal cyst decompression
has significantly reduced recurrence rate, the surgical effect is
better than ultrasound-guided puncture, and is similar to open
surgery (15); drawbacks are
traumatic bleeding and other complications; difficult to operate on
parapelvic cyst, endogenous cyst (16); has high incidence of postoperative
urinary fistula after localized hydronephrosis, poor repeatability
and often needs re-operation (17).
Internal drainage combined with decompression can make the larger
cysts combined and connected to the collection system, and perform
decompression to deeper cysts or connect them to larger cysts, then
communicate with the connection system, therefore most of the cyst
fluid is excreted from the urinary tract, the drainage is more
reasonable and smooth, which is confirmed by a number of studies
(18,19) has better clinical safety and
effectiveness. It is also concluded from this study that there is
no significant difference between the two groups in terms of the
incidence of surgery and operation time on cysts, the incidence of
operative and postoperative complications in the observation group
was significantly lower than that in the control group. After
1-month follow-up, the total effective rate of the observation
group was significantly higher than that of the control group. The
three-dimensional image of the entire urinary system, including the
calyx, renal pelvis, ureter and bladder can be obtained through CT
urography (CTU) multi-phase dynamic enhanced scan. By combining
with MPR image post-processing technique, the location of cysts and
their relationship with the collection system can be evaluated
comprehensively. Therefore, we believe that MPR-CTU combined with
renal cyst incision and drainage under intraoperative ultrasound
guided ureteroscope has a higher safety and effectiveness in the
treatment of renal cystic disease, it is worthy of clinical
application.
Studies found that (20,21) when
treating endogenous cyst with flexible ureteroscope, the best
incision position cannot be precisely determined; it was difficult
to find the best laser rupture position for the cyst in the calyx
and pelvis by ureteroscope; the incision cannot be found because of
the thick cyst wall. Therefore, effective assessment of whether the
flexible ureteroscope can handle specific endogenous cysts before
surgery and how to effectively locate the cyst is a key factor for
the success of surgery. The main innovative points of this study
are: flexible ureteroscope technology is currently the most
minimally invasive technique, treating the cyst via the urinary
approach is most in line with the natural cavity path, with
characteristics of repeatable operating, suitable for recurrence
patients after open or laparoscopic surgery. There is no clinical
report of MPR-CTU assisted ultrasonography in the treatment of
renal cystic disease with ureteroscope localization within renal
calyx. The deficiency of this research is the small sample size,
short follow-up time, it still needs further verification.
Acknowledgements
The present study was supported by the Public
Welfare Marjor Project of the Bureau of Science & Technology
(no. 2014GZB03).
References
|
1
|
Mao X, Xu G, Wu H and Xiao J:
Ureteroscopic management of asymptomatic and symptomatic simple
parapelvic renal cysts. BMC Urol. 15:482015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu L, Rong Y, Wang W, Lian H, Gan W, Yan
X, Li X and Guo H: Percutaneous radiofrequency ablation with
contrast-enhanced ultrasonography for solitary and sporadic renal
cell carcinoma in patients with autosomal dominant polycystic
kidney disease. World J Surg Oncol. 14:1932016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rahbari-Oskoui F, Mittal A, Mittal P and
Chapman A: Renal relevant radiology: Radiologic imaging in
autosomal dominant polycystic kidney disease. Clin J Am Soc
Nephrol. 9:406–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramanathan S, Kumar D, Khanna M, Al
Heidous M, Sheikh A, Virmani V and Palaniappan Y: Multi-modality
imaging review of congenital abnormalities of kidney and upper
urinary tract. World J Radiol. 8:132–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chapman AB and Wei W: Imaging approaches
to patients with polycystic kidney disease. Semin Nephrol.
31:237–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pallwein-Prettner L, Flöry D, Rotter CR,
Pogner K, Syré G, Fellner C, Frauscher F, Aigner F, Krause FS and
Fellner F: Assessment and characterisation of common renal masses
with CT and MRI. Insights Imaging. 2:543–556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rule AD, Sasiwimonphan K, Lieske JC,
Keddis MT, Torres VE and Vrtiska TJ: Characteristics of renal
cystic and solid lesions based on contrast-enhanced computed
tomography of potential kidney donors. Am J Kidney Dis. 59:611–618.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ta MH, Rao P, Korgaonkar M, Foster SF,
Peduto A, Harris DC and Rangan GK: Pyrrolidine dithiocarbamate
reduces the progression of total kidney volume and cyst enlargement
in experimental polycystic kidney disease. Physiol Rep.
2:e121962014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dell KM: The spectrum of polycystic kidney
disease in children. Adv Chronic Kidney Dis. 18:339–347. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saigusa T and Bell PD: Molecular pathways
and therapies in autosomal-dominant polycystic kidney disease.
Physiology (Bethesda). 30:195–207. 2015.PubMed/NCBI
|
|
11
|
Ali TA, Abdelaal MA, Enite A and Badran
YA: Ultrasound-guided percutaneous sclerotherapy of simple renal
cysts with n-butyl cyanoacrylate and iodized oil mixture as an
outpatient procedure. Urol Ann. 8:51–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akoh JA: Current management of autosomal
dominant polycystic kidney disease. World J Nephrol. 4:468–479.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu JH, Du Y, Li Y, Yang HF, Xu XX and
Zheng HJ: CT-guided sclerotherapy for simple renal cysts: Value of
ethanol concentration monitoring. Korean J Radiol. 15:80–86. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ars E, Bernis C, Fraga G, Martínez V,
Martins J, Ortiz A, Rodríguez-Pérez JC, Sans L and Torra R: Spanish
working group on inherited kidney disease: Spanish guidelines for
the management of autosomal dominant polycystic kidney disease.
Nephrol Dial Transplant. 29:95–105. 2014. View Article : Google Scholar
|
|
15
|
Agarwal M, Agrawal MS, Mittal R and Sachan
V: A randomized study of aspiration and sclerotherapy versus
laparoscopic deroofing in management of symptomatic simple renal
cysts. J Endourol. 26:561–565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Riella C, Czarnecki PG and Steinman TI:
Therapeutic advances in the treatment of polycystic kidney disease.
Nephron Clin Pract. 128:297–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Millar M, Tanagho YS, Haseebuddin M,
Clayman RV, Bhayani SB and Figenshau RS: Surgical cyst
decortication in autosomal dominant polycystic kidney disease. J
Endourol. 27:528–534. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Papatsoris AG, Kachrilas S, Howairis ME,
Masood J and Buchholz N: Novel technologies in flexible
ureterorenoscopy. Arab J Urol. 9:41–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo Q, Zhang X, Chen H, Liu Z, Chen X, Dai
Y and Zhao Z: Treatment of renal parapelvic cysts with a flexible
ureteroscope. Int Urol Nephrol. 46:1903–1908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao Q, Huang S, Li Q, Xu L, Wei X, Huang
S, Li S and Liu Z: Treatment of parapelvic cyst by internal
drainage technology using ureteroscope and Holmium laser. West
Indian Med J. 64:230–235. 2015.
|
|
21
|
Basiri A, Hosseini SR, Tousi VN and
Sichani MM: Ureteroscopic management of symptomatic, simple
parapelvic renal cyst. J Endourol. 24:537–540. 2010. View Article : Google Scholar : PubMed/NCBI
|