Introduction
Idiopathic chylopericardium (CP) is a rare clinical
entity that was first reported by Hasebroek in 1888 (1). The term primary CP was initially used
in a study by Groves and Effler in 1954 (2), which described a case of isolated
accumulation of chyle in the pericardium without any evident cause.
Secondary CP typically refers to complications due to
cardiothoracic surgery, trauma or a thrombus in the jugular vein,
infection, radiotherapy, mediastinal tumors, hygromas, bone
lymphangiomas, lymphoma, acute necrotic pancreatitis and other
malignancies (3–5). Dib et al (6) analyzed the CP cases reported in the
literature between 1996 and 2006, and observed that the idiopathic
cause of CP accounted for 56% of cases. The possible mechanisms of
idiopathic CP include the following: i) Impaired lymphatic valves
on branches that are connected to the thoracic duct and pericardium
lymphatic vessel; ii) elevated pressure in the thoracic duct that
may occur in lymphangiectasia; iii) abnormal communication of
lymphatic vessels to the pericardial lymphatics resulting in
chylous reflux; and iv) congenital malformation.
Generalized lymphatic anomaly (GLA) is a rare
congenital disease resulting in malformation of lymphatic vessels.
GLA is known by multiple terms, including lymphangiomatosis,
generalized lymphangioma, systemic cystic angiomatosis, multiple
lymphangiectasia and generalized lymphatic malformation (7,8).
Lymphatic anomalies are considered to arise from disordered
lymphatic development resulting in the proliferation of lymphatic
channels (8). Lymphatic anomalies
can also cause chylopericardium (CP), which is very rare. In the
present case, lymphatic abnormality was confirmed by
lymphangiography and surgery.
The present study reports a case of CP in an Asian
male patient, who presented with no specific symptoms and with
occasional detection of pericardial effusion by ultrasound.
Furthermore, a literature review was performed to improve the
understanding on the diagnosis and treatment of primary CP.
Case report
In June 2008, a 57-year-old man was admitted to the
Cardiology Care Unit of Beijing Hospital (Beijing, China) with
symptoms of dyspnea and chronic cough that persisted for 10 months.
The patient did not present fever or chills at home. Workup
examination included transthoracic echocardiography, which was
performed 2 months prior to this admission, which demonstrated a
large cardiac effusion. Upon physical examination on admission, the
blood pressure was 135/80 mmHg and the pulse was at 95 bpm. The
patient was alert and oriented, and a neck exam did not detect
jugular venous distention (JVD). The lungs were clear upon
bilateral auscultation, without wheezing or crackles.
Cardiovascular examination demonstrated a regular heart rhythm
without murmurs, and the heart sound was distant. His abdomen was
soft and non-tender on palpation, and the patient did not present
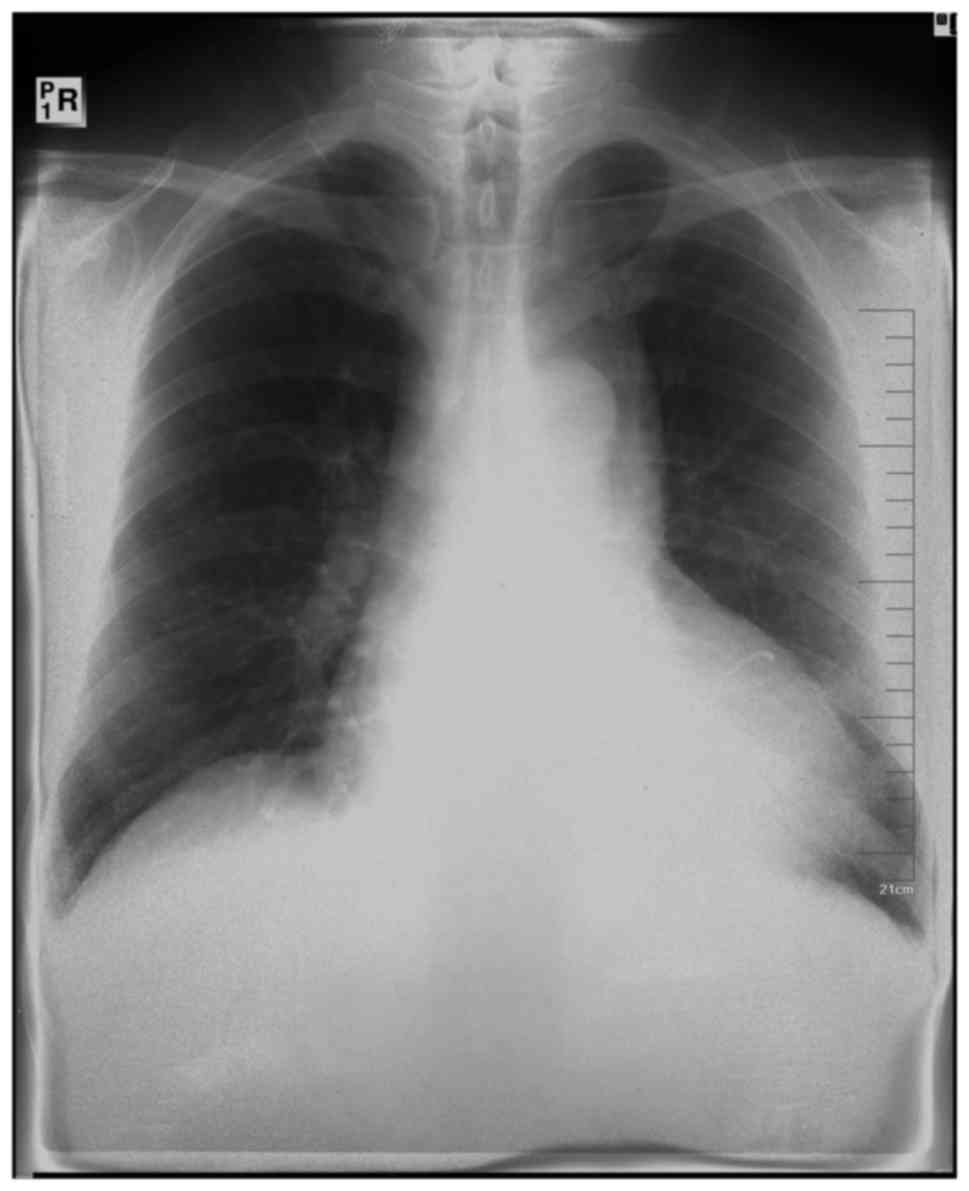
hepatosplenomegaly. A chest X-ray scan demonstrated an enlarged
cardiac silhouette, as shown in Fig.
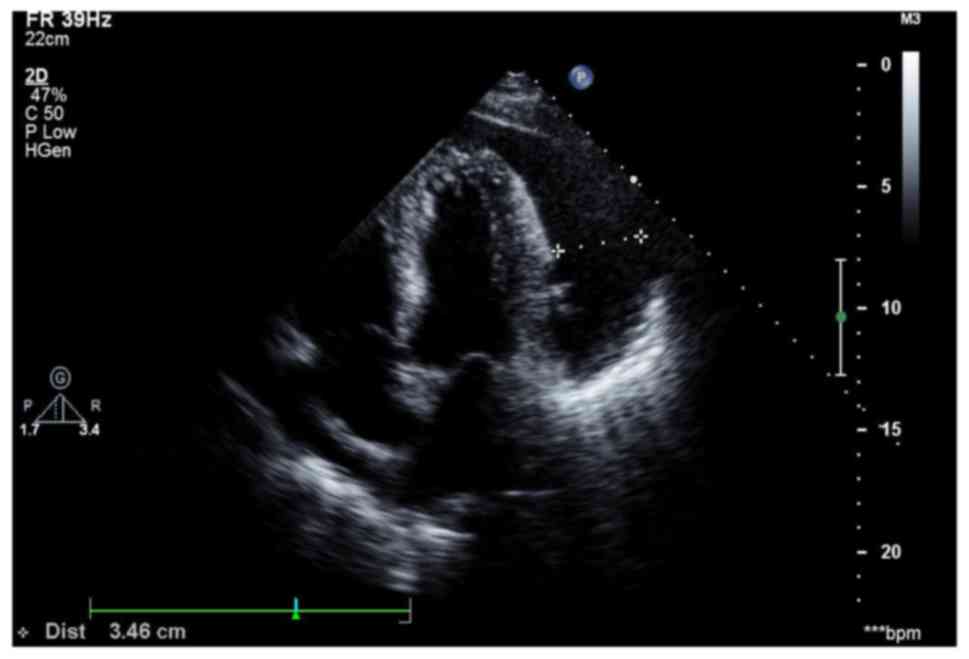
1. Transthoracic echocardiography was repeated and demonstrated
a large cardiac effusion with 6–34 mm of fluid in the apex and
basal area (Fig. 2). Laboratory
testing revealed an erythrocyte sedimentation rate of 12 mm/h
(normal range, <15 mm/h). A purified protein derivative skin
test was negative.
To alleviate the symptom of dyspnea and clarify the
reason for it, the patient underwent diagnostic and therapeutic
pericardiocentesis with the removal of 3,080 ml milky fluid with
symptomatic relief (Fig. 3). The
pericardial effusion was confirmed to be chylous with the following
contents: Specific gravity ≥1.030; red blood cell count,
2,880/mm3; white blood cell count, 350/mm3;
monocytes, 90%; neutrophils, 10%; glucose, 7 mmol/l; total protein,
82 g/l; albumin, 27 g/l; lactate dehydrogenase, 150 U/l;
triglycerides (TG), 13.4 mmol/l; total cholesterol (TC), 3.4
mmol/l. The pericardial effusion was negative for tuberculosis via
polymerase chain reaction analysis, while bacterial culture was
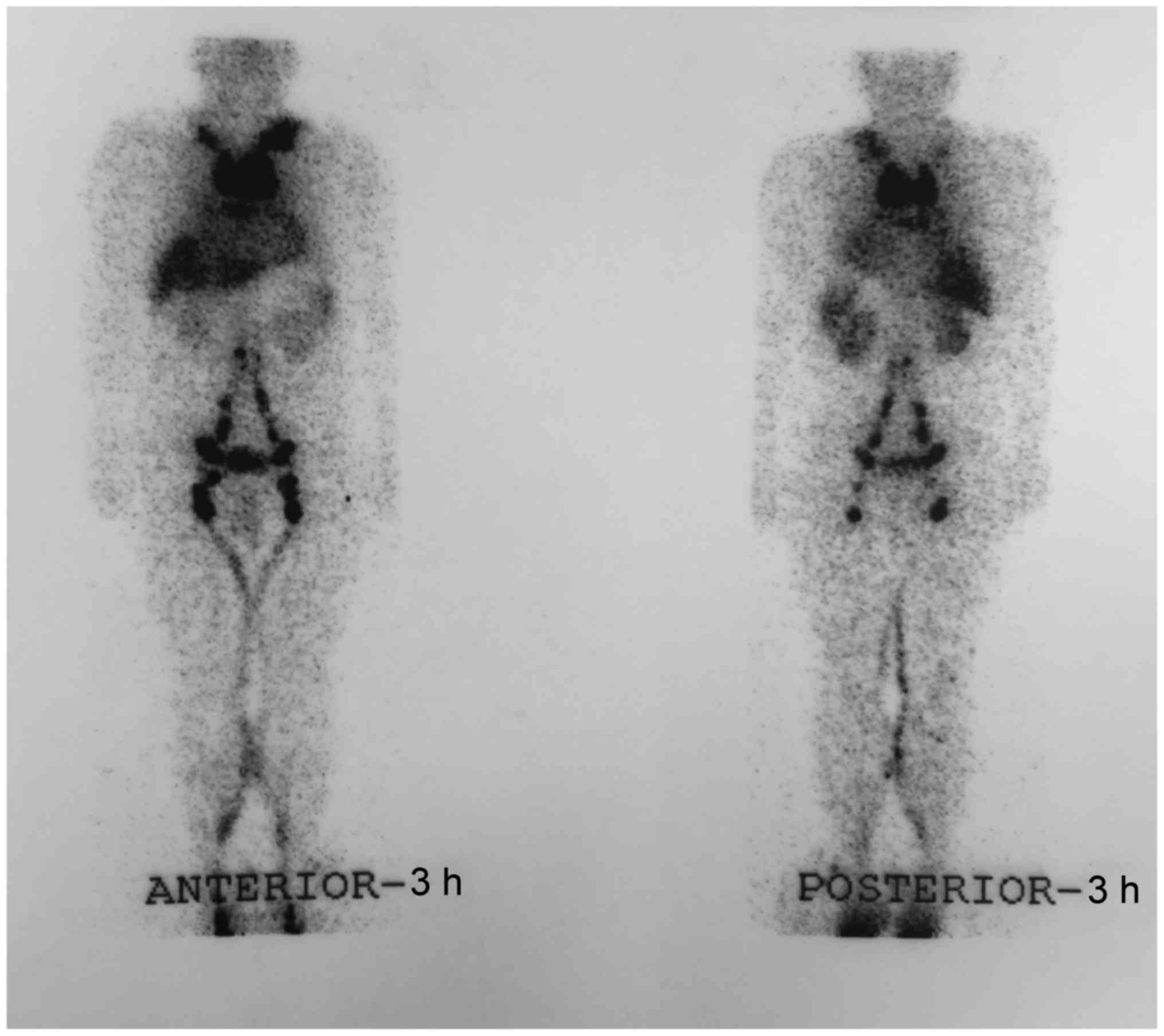
also negative. Lymphangioscintigraphy with 99mTc-DX demonstrated
obstruction of the upper part of the thoracic duct and abnormal
accumulation of radiotracer was observed in the pericardium after 3
h of injection (Fig. 4).
The patient was subsequently transferred to the
Beijing Shijitan Hospital (Beijing, China) for surgery.
Lymphangiography was conducted prior to surgery, which revealed
four lymphatic collaterals below the left sternoclavicular joint
divided from the thoracic duct. These collaterals communicated with
the left supraclavicular vein, cervical lymph nodes and right
supraclavicular vessel. Certain other communicating braches from
the thoracic duct were connected with the left bronchomediastinal
trunks abutting the pericardial sac. Chylous regurgitation was
observed from the thoracic duct toward the left bronchomediastinal
trunks. The patient was identified to have congenital anomalies of
the thoracic duct. The end of thoracic duct was not converged to
the left subclavian vein as usual, but branched with multiple small
vessels and connected with other lymphatic vessels. Thus, the
patient underwent thoracic duct-left internal jugular vein
anastomosis and ligation of communicating branches between the
thoracic duct and left bronchomediastinal trucks.
At 1 month after surgery, follow-up echocardiography
revealed recurrence of a pericardial effusion. The patient
underwent further pericardiocentesis with removal of 200 ml chylous
fluid, and conservative treatment including low-fat diet and
medium-chain triglyceride (MCT) diet was continued from last
admission. The patient responded well to treatment and presented a
good quality of life without dyspnea on exertion upon follow-up at
6 months and 7 years.
Discussion
The pericardial space usually contains 25–35 ml of
fluid, which is identical to lymph and is produced by the
epicardium (9). The pericardium
itself does not produce a large amount of fluid. Lymphatic vessels
of the heart drain the pericardial fluid into the left subclavian
vein via the mediastinal lymph vessels, lymph nodes and the
thoracic duct (10). The main
lymphatic flow from the pleura, the lower portion of the left lung,
and the entire right lung and pericardium meet at the
bronchomediastinal lymphatics (11,12).
Therefore, lymphatic flow regurgitation can occur simultaneously in
multiple sites in the thoracic cavity, such as the pleura, lungs
and pericardium. Obstruction of the upper area of the thoracic duct
may then cause CP, pulmonary lymphedema and chylothorax.
Furthermore, the intestinal lymph will be affected by the
obstruction and result in chylous ascites.
Primary CP is a rare clinical entity in which
chylous fluid containing high concentrations of TG accumulate in
the pericardial cavity. Causes of CP include congenital mediastinal
lymphangiectasia, iatrogenic development following cardiac surgery,
malignant tumors, blunt or penetrating trauma, infection and
congenital lymphatic anomalies (6,11,13–15).
It has been reported that ~56% of cases are idiopathic (6), and only ~100 CP cases have been
reported worldwide (16). In the
present study, a systematic review of the literature was performed
to analyze the etiology, symptoms, physical examination features,
laboratory findings, imaging, type of surgery and follow-up data of
primary CP cases. A relatively large number of cases reported
within the past 60 years were included in this systematic review.
The current study aimed to provide further evidence for the
recognition of primary CP.
A systematic PubMed (www.ncbi.nlm.nih.gov/pubmed) and Wanfang (www.wanfangdata.com.cn) database search was conducted
in order to identify English and Chinese studies describing primary
or idiopathic CP reported between January 1, 1950 and December 31,
2015. The search terms included the following: ‘primary
chylopericardium’ or ‘idiopathic chylopericardium’. The
bibliographies of all articles were subsequently independently
reviewed by two of the authors. A total of 104 cases were
identified from 92 studies (2–5,7,11–87).
Patient demographics, clinical presentation, diagnosis and
treatment were analyzed. Patients lacking laboratory data (such as
the TG concentration), imaging or surgical findings that supported
their primary or idiopathic CP definitive diagnosis were excluded
from the present study. The mean ± standard deviation was used to
express count variables, which were statistically analyzed by SPSS
version 20 (IBM Corp., Armonk, NY, USA).
According to the literature review conducted in the
present study, 53 male (50.96%) and 51 female (49.03%) CP patients
were identified. The age at diagnosis ranged between 6 weeks and 79
years, with a mean 27.95±16.50 years, while 5 cases were <3
years old. The time from onset of symptoms to the diagnosis
demonstrated a wide variation, ranging between a few hours and
several years.
The clinical manifestations, physical examination
features, electrocardiogram and X-ray finding are listed in
Table I. Dyspnea was the most common
initial presenting symptom, followed by coughing. Other symptoms
included palpitation, chest pain, gastrointestinal (GI) symptoms,
syncope, fatigue and edema. In addition, cardiac tamponade occurred
in certain patients as a result of decreased cardiac output due to
their chronic pericardial effusion. In the present review,
approximately 39.42% of patients had no symptoms, although X-ray
plain film demonstrated an enlarged cardiac silhouette. Signs of
pericardial effusion were also identified during physical
examination, including enlarged heart border, distant heart sounds,
JVD and edema. JVD and a distant heart sound were only identified
in <50% of the included cases. Furthermore, 16 cases presented
with low voltage in the limb lead upon electrocardiographic
examination. Cardiomegaly was easily identified by X-ray screening
in 93.27% of cases.
 | Table I.Clinical data of 104 chylopericardium
cases identified in the literature. |
Table I.
Clinical data of 104 chylopericardium
cases identified in the literature.
|
Characteristics | Number of cases
(n) | Percentage (%) |
|---|
| Symptom |
|
|
|
Asymptomatic | 41 | 39.42 |
|
Dyspnea | 48 | 53.90 |
|
Coughing | 11 | 10.58 |
|
Palpitation | 10 | 9.62 |
| Cardiac
tamponade | 7 | 6.73 |
| Chest
pain | 4 | 3.85 |
| GI
symptoms | 4 | 3.85 |
|
Syncope | 3 | 3.40 |
|
Fatigue | 3 | 2.88 |
|
Edema | 2 | 1.92 |
| Physical
examination |
|
|
|
JVD | 23 | 22.12 |
| Distant
heart sound | 34 | 32.69 |
|
Electrocardiogram |
|
|
| Low
voltage in limb lead | 16 | 15.38 |
| X-ray scanning |
|
|
|
Cardiomegaly | 97 | 93.27 |
The diagnosis of chylous pericardium relied upon the
pericardial fluid analysis. Regarding the appearance of the fluid,
a milky white pericardial effusion (78 cases, 72.89%) was typically
observed, followed by pink or bloody fluid (4 cases, 3.85%). The
level of TG was tested in 63 cases and was in the range of 181–4299
mg/dl, with a median of 1305.43±865.34 mg/dl, while the TC level
was tested in 42 cases and was within the range of 42–1021 mg/dl,
with a mean value of 132.42±148.22 mg/dl. The TC/TG ratio in all CP
patients included in the current study was <1.
Imaging served an important role in the exploration
of the leak site of CP, as shown in Table II. In total, 42 (40.38%) cases
underwent lymphangioscintigraphy and 24 of these cases presented
abnormal accumulation within the pericardial sac. In addition, 24
cases (23.07%) underwent lymphangiography and 18 of them had
abnormal finding; 35 (33.65%) cases underwent chest
contrast-enhanced computed tomography (CT) scanning without any
secondary disease finding.
 | Table II.Imaging data. |
Table II.
Imaging data.
| Type of
imaging | Number of cases
(n) | Percentage (%) | Abnormal finding
(n) |
|---|
| Chest X-ray | 97 | 93.27 | 97 |
|
Echocardiography | 79 | 75.96 | 79 |
|
Lymphangioscintigraphy | 42 | 40.38 | 24 |
|
Lymphangiography | 24 | 23.07 | 18 |
| Computed
tomography | 35 | 33.65 | 0 |
Of the 104 identified cases, treatment information
was provided for 102 patients. A total of 62 cases were subjected
to initial conservative management, including dietary control
involving total parenteral nutrition (TPN), low-fat diet and MCT
diet. Among these patients, 36 cases did not improve and underwent
subsequent surgery, while 26 cases were successfully treated
following conservative management. In total, 71 cases (71.2%) were
treated by surgery. Formation of a pericardial window was used in
50 cases (48.08%) and ligation of the thoracic duct was performed
in 46 cases (44.23%). There were also 4 cases (3.85%) that received
embolization. Embolization of the thoracic duct was an uncommon
type of surgery for CP, which was performed in weak patients or
subsequent to failure of ligation. Furthermore,
pericardioperitoneal shunt was conducted in 3 cases (3.88%). Only 1
case underwent reconstruction of the thoracic duct, which is rarely
performed.
Follow-up data were recorded in 64 cases, with a
median follow-up time of 12 months (1 month to 9 years) and all
patients had a positive outcome. Only 1 case was complicated by
constrictive pericarditis. No patients succumbed to the condition
during the follow-up period.
A scoring system has been suggested by Dib et
al (6) to assist in establishing
the diagnosis of CP. The score is calculated according to the
following features: i) Milky yellowish appearance of fluid; ii) TG
level of >500 mg/dl; iii) TC/TG ratio of <1; and iv) negative
fluid bacterial culture and lymphocytic predominance on fluid cell
count. Each feature is given 1 point, and a score of 2 (specificity
and sensitivity of 100%) is required for the diagnosis of CP
(6). The patient in the current
study had a score of 4, which could be confirmed as CP.
Other conditions that may mimic a chylous
pericardium include cholesterol pericarditis (88,89) and
purulent pericarditis (90,91), which can be complicated by a
pericardial effusion with a positive chylous test. The pericardial
effusion in cholesterol pericarditis typically appears orange and
full of cholesterol crystals. Clinically, patients with purulent
pericarditis typically have more severe presentation compared with
patients with CP. Diagnosis of pericardial effusion secondary to
cholesterol and purulent pericarditis depends on the cell count,
biochemistry, cytology and fluid culture findings.
Echocardiography is helpful in diagnosing
pericardial effusion and helps guide pericardiocentesis. Enlarged
cardiac silhouette is often observed on chest X-ray scans in
patients with CP (14). The main
diagnostic methods for CP include lymphoscintigraphy and
lymphangiography. However, diagnosis is highly variable depending
on the device and technique. Lymphangiography is performed to
assess the thoracic duct anatomy by administering a contrast agent
directly into catheterized lymph vessels (20). When successful, this test is able to
demonstrate the connection between the pericardial capacity and
lymphatic system. However, lymphangiography is invasive and
requires insertion of a catheter into a lymphatic vessel and use of
a contrast agent. Combining lymphangiography and CT has also been
reported to be useful for detecting lymphatic vessels and abnormal
communications within the pericardial space (92). Lymphoscintigraphy is a noninvasive
alternative to lymphangiography, which utilizes radionuclides as
imaging agents and can be performed using oral or subcutaneous
administration of radiotracers (93). Chest CT scanning is also helpful to
detect the association between the thoracic duct and surrounding
organs (94).
Regardless of the etiology of CP, conservative
therapy in the absence of hemodynamic compromise is the first line
treatment. MCT diet or TPN can also be attempted; however, the
majority of patients will require pericardiocentesis for symptom
alleviation (95).
Pericardiocentesis followed by drainage and MCT diet should be
considered as a primary management strategy since the mortality
rate of CP is not high (6).
Definitive treatment with surgery is recommended in cases where the
daily pericardial drainage is at >1,500 ml/day, drainage does
not decrease after 5 days (>500 ml/day) or malnutrition occurs
(96). Management with
pericardiocentesis alone rarely prevents recurrence.
Pericardiocentesis followed by pericardiostomy s another reasonable
approach, particularly when the patient is reluctant to undergo
surgery or has a concomitant life-limiting disease. Conservative
treatment failed in 57.1% of the cases as reported previously
(1), and in 58.06% of cases as
reported in the present study. However, surgical treatment was
curative in all cases. For patients presenting a recurrent chylous
pericardial effusion within 3-month, surgical treatment is
recommended.
There are different approaches for the surgical
treatment of CP (6). Pericardiectomy
is performed to ensure complete drainage and to prevent secondary
constrictive pericarditis. Creation of a pericardial window alone
is insufficient since it has a high recurrence rate, as it does not
close the communication between the thoracic duct and the
pericardial sac (11). Ligation of
the thoracic duct above the level of the diaphragm is the best
choice and is recommended by the majority of surgeons (81). Video-assisted thoracoscopic surgery
is also becoming more favorable by cardiothoracic surgeons since it
is less invasive and affects the pulmonary function to a lesser
extent. Furthermore, embolism of the thoracic duct is used in
certain situation, and is an alternative type of surgery,
particularly for patients who refuse to be subjected or
re-subjected to more invasive surgery, or those that cannot
tolerate anesthetization (10).
Another novel surgical therapy is the reconstruction of the
occluded thoracic duct (97). This
approach is most consistent with the restoration of nearly normal
physiology. Although thoracic duct reconstruction results in
substantial symptomatic improvement and volume reduction, CP may
recur. Recurrences should be managed conservatively since full
restoration of the normal lymphatic flow may require a certain
time.
The patient in the present case study underwent
thoracic duct reconstruction surgery and required several months
for complete restoration. However, the short term and long term
prognosis of this patient was good. Based on the literature review
conducted, the current case is the first reported case of primary
CP that underwent lymphatic reconstructive surgery and had a 7-year
follow up. This case aims to provide further insight on this
condition to assist in the treatment of primary CP.
In conclusion, the present study reported the case
of a 57-year-old Chinese male who presented with nonspecific
symptoms of primary CP, which is a rare condition with only ~100
cases reported worldwide to date. The current patient underwent a
rare type of surgery of the thoracic duct, namely left internal
jugular vein anastomosis, which was only reported by Melduni et
al from the Mayo clinic in 2008 (97). In addition, long-term follow-up
findings subsequent to thoracic duct anastomosis surgery were
reported for the present patient. A systematic literature review
was also performed, analyzing the etiology, symptoms, physical
examination findings, laboratory results, imaging, type of surgery
and follow-up data of primary CP. A relative large number of cases
reported during the past 60 years were included in this systematic
review. Correct diagnosis can be achieved with analysis of the
pericardial effusion, while lymphoscintigraphy and lymphangiography
are useful for locating the chyle leak site. Surgical management is
the most successful treatment and is associated with a favorable
prognosis. The current study provided an overall perspective on the
etiology, symptoms, physical examination and laboratory findings,
imaging, type of surgery and follow-up data of primary CP, and this
evidence will assist in improving the understanding and recognition
of this rare disease.
References
|
1
|
Hasebroek K: Analyse einer chylösen
pericardialen Flüssigkeit (Chylopericardium). Biol Chem.
12:289–294. 1888.(In German).
|
|
2
|
Groves LK and Effler DB: Primary
chylopericardium. N Engl J Med. 250:520–523. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raza M and Matar K: Idiopathic chylothorax
and chylopericardium resistant to conservative and surgical
management. Heart Lung Circ. 18:229–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Courtney M and Ayyagari RR: Idiopathic
chylopericardium treated by percutaneous thoracic duct embolization
after failed surgical thoracic duct ligation. Pediatr Radiol.
45:927–930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ossiani MH, McCauley RG and Patel HT:
Primary idiopathic chylopericardium. Pediatr Radiol. 33:357–359.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dib C, Tajik AJ, Park S, Kheir ME,
Khandieria B and Mookadam F: Chylopericardium in adults: A
literature review over the past decade (1996–2006). J Thorac
Cardiovasc Surg. 136:650–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Faul JL, Berry GJ, Colby TV, Ruoss SJ,
Walter MB, Rosen GD and Raffin TA: Thoracic lymphangiomas,
lymphangiectasis, lymphangiomatosis, and lymphatic dysplasia
syndrome. Am J Respir Crit Care Med. 161:1037–1046. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rostom AY: Treatment of thoracic
lymphangiomatosis. Arch Dis Child. 83:138–139. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luisada AA: Development and structure of
the cardiovascular system. Am Heart J. 64:4351962. View Article : Google Scholar
|
|
10
|
Chen E and Itkin M: Thoracic duct
embolization for chylous leaks. Semin Intervent Radiol. 28:63–74.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dunn RP: Primary chylopericardium: A
review of the literature and an illustrated case. Am Heart J.
89:369–377. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Naef AP: Primary chylopericardium and its
surgical treatment. Dis Chest. 30:160–167. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Svedjeholm R, Jansson K and Olin C:
Primary idiopathic chylopericardium-a case report and review of the
literature. Eur J Cardiothorac Surg. 11:387–390. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harada K, Takigawa I, Toyoda H, Okada T
and Usami H: Primary chylopericardium recovered without surgical
treatment. Report of a case and review of the literature. Jpn Circ
J. 46:162–171. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akamatsu H, Amano J, Sakamoto T and Suzuki
A: Primary chylopericardium. Ann Thorac Surg. 58:262–266. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han Z, Li S, Jing H and Liu H: Primary
idiopathic chylopericardium: A retrospective case series. BMC Surg.
15:612015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yankopoulos NA, Akbarian M, Starkey GW and
Abelmann WH: Isolated chylopericardium. Diagnosis, hemodynamic
studies and surgical treatment. Am J Cardiol. 19:440–446. 1967.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fawal IA, Kirkland L, Dykes R and Foster
GL: Chronic primary chylopericardium. Report of a case and review
of the literature. Circulation. 35:777–782. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Puig-Massana M, Murtra M and Calbet JM:
Idiopathic massive chylopericardium. Br Heart J. 34:431–433. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chavez CM, Rodriquez GR and Conn JH:
Isolated chylopericardium. Lymphographic findings and surgical
treatment. Am J Cardiol. 32:352–355. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gallant TE, Hunziker RJ and Gibson TC:
Primary chylopericardium: The role of lymphangiography. AJR Am J
Roentgenol. 129:1043–1045. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Charnilas Y, Levo Y, Weiss A, Glaser J and
Levy MJ: Isolated idiopathic chylopericardium. J Thorac Cardiovasc
Surg. 73:719–721. 1977.PubMed/NCBI
|
|
23
|
Toltzis RJ, Rosenthal A, Fellows K,
Castaneda AR and Nadas AS: Chylous reflux syndrome involving the
pericardium and lung. Chest. 74:457–458. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Haan HP, Kolff J and Buis B: Isolated
chylopericardium due to lymphangiomatous dysplasia of the thymus.
Eur Heart J. 5:846–849. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mask WK, Penido JR and Printup C: Primary
idiopathic chylopericardium. J Thorac Cardiovasc Surg. 99:569–571.
1990.PubMed/NCBI
|
|
26
|
Itoh T, Tanaka S, Nakanishi M, Nishiyama
K, Kitajima N, Kinoshita Y, Inatome T, Inoh T and Fukuzaki H:
Primary chylopericardium with pulmonary shadow and large granular
lymphocytosis: A case report. Lymphology. 24:168–173.
1991.PubMed/NCBI
|
|
27
|
de Winter RJ, Bresser P, Romer JW,
Kromhout JG and Reekers J: Idiopathic chylopericardium with
bilateral pulmonary reflux of chyle. Am Heart J. 127:936–939. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scholten C, Staudacher M, Girsch W, Wolf
G, Grimm M, Binder T, Lang I and Stefenelli T: A novel therapeutic
strategy for the management of idiopathic chylopericardium and
chylothorax. Surgery. 123:369–370. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mewis C, Kühlkamp V, Sokiranski R and
Karsch KR: Primary chylopericardium due to partial aplasia of the
thoracic duct. Eur Heart J. 18:880–881. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baratella MC, Montesello M and Marinato P:
Images in cardiology. Idiopathic chylopericardium. Heart.
80:3761998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang CH, Yen TC, Ng KK, Lee CM, Hung MJ
and Cherng WJ: Pedal (99m)Tc-sulfur colloid lymphoscintigraphy in
primary isolated chylopericardium. Chest. 117:598–601. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sakata S, Yoshida I, Otani Y, Ishikawa S
and Morishita Y: Thoracoscopic treatment of primary
chylopericardium. Ann Thorac Surg. 69:1581–1582. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rizzello V, Colizzi C and Falappa P:
Primary chylopericardium due to lymphangiectasias: The crucial role
of lymphangiography. Eur Heart J. 29:19742008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cho BC, Kang SM, Lee SC, Moon JG, Lee DH
and Lim SH: Primary idiopathic chylopericardium associated with
cervicomediastinal cystic hygroma. Yonsei Med J. 46:439–444. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mitsui K, Namiki K, Matsumoto H, Konno F,
Yoshida R and Miura S: Thoracoscopic treatment for primary
chylopericardium: Report of a case. Surg Today. 35:76–79. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miyoshi K, Nakagawa T, Kokado Y, Matsuoka
T, Kameyama K and Okumura N: Primary chylopericardium with
pulmonary lymphedema. Thorac Cardiovasc Surg. 56:306–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schild HH, Simon B, Kuhl CK, Nelles M,
Koerfer R, Skowasch D, Kuntz-Hehner S and Haude M: Percutaneous
treatment of idiopathic chylopericardium. J Vasc Interv Radiol.
20:842–846. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Itkin M, Swe NM, Shapiro SE and Shrager
JB: Spontaneous chylopericardium: Delineation of the underlying
anatomic pathology by CT lymphangiography. Ann Thorac Surg.
87:1595–1597. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Madison WM Jr and Logue B: Isolated
(primary) chylopericardium due to anomalous communications with the
thoracic duct, of unknown causation. Am J Med. 22:825–830. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Glasser SP, Cheitlin MD, Serfas LS and
Sbar SS: Isolated massive chylopericardium. Am Heart J. 75:663–672.
1968. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee CY, Di Loreto PC and Kim S: Isolated
primary chylopericardium in pregnancy. Obstet Gynecol. 43:586–591.
1974.PubMed/NCBI
|
|
42
|
Jenner R and Oo H: Isolated
chylopericardium due to mediastinal lymphangiomatous hamartoma.
Thorax. 30:113–117. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Savran SV, Ratshin RA, Shirley JH, Naguwa
SM and Goodman L: Idiopathic chylopericardium: 131-I-triolein scan
for noninvasive diagnosis. Ann Intern Med. 82:663–665. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ross P, Joseph S and Walker D: A case of
isolated primary chylopericardium. Br Heart J. 41:508–511. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rankin RN, Raval B and Finley R: Primary
chylopericardium: Combined lymphangiographic and CT diagnosis. J
Comput Assist Tomogr. 4:869–870. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Torrington KG and Youkey JR: Simultaneous
idiopathic chylopericardium and chylothorax. South Med J.
74:357–359. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bewick DJ, Johnstone DE and Landrigan PL:
Primary chylopericardium associated with allergic alveolitis. Can
Med Assoc J. 130:1577–1579. 1984.PubMed/NCBI
|
|
48
|
Morishita Y, Taira A, Furoi A, Arima S and
Tanaka H: Constrictive pericarditis secondary to primary
chylopericardium. Am Heart J. 109:373–375. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Musemeche CA, Riveron FA, Backer CL, Zales
VR and Idriss FS: Massive primary chylopericardium: A case report.
J Pediatr Surg. 25:840–842. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yoon YS, Shim WH, Chung TS and Lee YS:
Primary idiopathic chylopericardium: Report of a case and review of
the literature. Yonsei Med J. 34:98–108. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chiu CH, Su WJ, Chang JP and Chang CH:
Primary chylopericardium: Report of a case. J Formos Med Assoc.
92:468–471. 1993.PubMed/NCBI
|
|
52
|
Taggart SC, Roberts TE and Marshall DA:
Chylopericardium complicating pericardiocentesis for acute
idiopathic pericardial effusion. J Thorac Cardiovasc Surg.
108:388–389. 1994.PubMed/NCBI
|
|
53
|
Yang MF, Long MQ, Tian J and Li ZJ: A case
of chylopericardium diagnosed by lymphoscintigraphy. Zhonghua
Heyixue Zazhi. 14:157–158. 1994.(In Chinese).
|
|
54
|
Furrer M, Hopf M and Ris HB: Isolated
primary chylopericardium: Treatment by thoracoscopic thoracic duct
ligation and pericardial fenestration. J Thorac Cardiovasc Surg.
112:1120–1121. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chinushi M, Watanabe Y, Aizawa Y, Hanawa
H, Yamazoe M, Osman Y, Shibata A and Shinonaga M: Suppression of
fluid accumulation following pericardial inflammation in a patient
with primary chylopericardium. Jpn Heart J. 37:271–274. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dogan R, Demircin M, Sarigül A, Celiker A,
Güngen Y and Paşaoglu I: Isolated chylopericardium secondary to
intrathymic lymphangiomatous malformation. Pediatr Cardiol.
17:413–415. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yüksel M, Yildizeli B, Zonüzi F and
Batirel HF: Isolated primary chylopericardium. Eur J Cardiothorac
Surg. 12:319–321. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Akashi H, Tayama K, Ishihara K, Tanaka A,
Fujino T, Okazaki T and Aoyagi S: Isolated primary
chylopericardium. Jpn Circ J. 63:59–60. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
López-Castilla JD, Soult JA, Falcón JM,
Muñoz M, Santos J, Gavilan JL and Rodríguez A: Primary idiopathic
chylopericardium in a 2 month old successfully treated without
surgery. J Pediatr Surg. 35:646–648. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bhat P, Ananthakrishna R, Panneerselvam A,
Yalagudri SD, Rao PS and Nanjappa MC: Recurrent chylopericardium.
BMJ Case Rep. 2011:pii: bcr07201145202011. View Article : Google Scholar
|
|
61
|
Sleilaty G, Rassi I, Alawi A and Jebara
VA: Primary isolated chronic chylopericardium. Interact Cardiovasc
Thorac Surg. 1:86–87. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Anil SR, Manoj P, Hejmadi A and Kumar RK:
Massive primary chylopericardium in an infant. Indian Heart J.
54:295–296. 2002.PubMed/NCBI
|
|
63
|
Watanabe S, Kariatsumari K, Sakasegawa K,
Imagama I, Yotsumoto G and Sakata R: Primary chylopericardium
treated with video-assisted thoracoscopic surgery. Thorac
Cardiovasc Surg. 50:360–361. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mahon NG, Nölke L, McCann H, Sugrue D and
Hurley J: Isolated chylopericardium. Surgeon. 1:236–238. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Fan SH, Ge MJ, Xiang XY and Wang B:
Primary chylopericardium: A case report. Zhonguo Xiongxinxueguan
Waike Linchuang Zachi. 17:3522010.(In Chinese).
|
|
66
|
Nanjo S, Yamazaki J, Tsubuku M, Ohyama T,
Ohtsuka T and Nakano H: Primary idiopathic chylopericardium: Report
of two cases. Ann Nucl Med. 18:537–539. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yokusoglu M, Savasoz BS, Baysan O, Erinc
K, Gunay C and Isik E: Primary chylopericardium. Thorac Cardiovasc
Surg. 53:386–388. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zisis C, Rontogianni D, Charalambous E and
Bellenis I: Lymphangiomatous hamartoma: Cause or bystander of the
isolated chylopericardium? J Thorac Cardiovasc Surg. 130:1201–1202.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mehrotra S, Peeran NA and Bandyopadhyay A:
Idiopathic chylopericardium: An unusual cause of cardiac tamponade.
Tex Heart Inst J. 33:249–252. 2006.PubMed/NCBI
|
|
70
|
Cui FH, Xie MY and Huang JP: A primary
chylopericardium misdiagnosed as tuberculous pericarditis. Zhongguo
Wuzhenxue Zazhi. 9:1825–1826. 2006.(In Chinese).
|
|
71
|
Cervantes-Salazar JL, Calderón-Colmenero
JE and Ramírez-Marroquín S: Idiopathic chylopericardium. A case in
point. Rev Esp Cardiol. 60:884–885. 2007.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Silva MA, Martins AS, Campos NL, Andrade
RR, Tohi LM and Hueb JC: Primary idiopathic chylopericardium-case
report. Arq Bras Cardiol. 92:e40–e43. e67–e70. 2009.(In English,
Multiple languages). PubMed/NCBI
|
|
73
|
Timóteo AT, Albino JP, Branco LM, Banazol
N, Colarinha P, Jalles NT and Ferreira R: Primary idiopathic
chylopericardium. Rev Port Cardiol. 28:325–332. 2009.(In English,
Portuguese). PubMed/NCBI
|
|
74
|
Barbetakis N, Asteriou C, Konstantinou D,
Giannoglou D, Tsilikas C and Giannoglou G: Spontaneous chylous
cardiac tamponade: A case report. J Cardiothorac Surg. 5:112010.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Luaces M, Perales I, Núñez-Gil IJ and
Cabezudo J: Non-invasive diagnosis of chylopericardium by cardiac
magnetic resonance imaging. Eur Heart J. 31:8232010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Novitzky D and Guglin M: Chylopericardium
presenting with tamponade and cardiogenic shock. J Card Surg.
25:522–524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tan JX, Fu Y and Chen J: Primary
idiopathic chylopericardium: A case report. Cardiol Young.
23:773–775. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Mandarry MT, Ru XH, Wei ZQ and Ge MJ:
Primary idiopathic chylopericardium: A rare case with a synopsis of
the literature. Singapore Med J. 53:e156–e158. 2012.PubMed/NCBI
|
|
79
|
Gilmore D and Colson YL: Recurrent chylous
pericardial effusion and left neck mass. Thorac Cardiovasc Surg. 60
Suppl 2:e25–e27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kwon JB, Choi SY, Kim CK and Park CB:
Primary idiopathic silent chylopericardium. J Cardiothorac Surg.
8:282013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Rivera-Beltrán S, Ortíz VN, Díaz R and
Hernández JA: Transabdominal ligation of the thoracic duct with
pericardial-peritoneal shunting in a case of primary idiopathic
chylous pericardial effusion. J Pediatr Surg. 48:1434–1437. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Rosa GM and Campisi C, Bioccardo F,
Dorighi U, Parodi A, Molinari L, Spinaci S, Dessalvi S, Brunelli C
and Campisi C: Chylopericardium: A case report demonstrating
utility of lymphography combined with 3D computed tomography for
corrective surgical treatment using VATS. Lymphology. 47:40–43.
2014.PubMed/NCBI
|
|
83
|
Vinayakumar D, Arunkumar G, Sajeev CG,
Rajesh G, Muneer K, Haridasan V, Babu K and Krishnan MN: Cystic
lymphangioma of pericardium presenting as isolated
chylopericardium-a case report. Indian Heart J. 66:119–121. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Karakurt C, Celik SF, Celik RM, Elkiran O,
Ulutaş H and Kuzucu A: Primary idiopathic chylopericardium
presenting with cardiac tamponade. Herz. 39:644–646. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Chew H, Shetty P, Elahi M and Akhunji Z: A
case of spontaneous chylous pericardial effusion in Poland
syndrome. Gen Thorac Cardiovasc Surg. 62:386–389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Huang JW, Zhao D, Han F, Qi BL, Zhang SQ,
Liu GT and Lin Y: A primary idiopathic chylopericardium. Zhongguo
Yaowu Jingjixue. 10:255–256. 2014.(In Chinese).
|
|
87
|
Atkinson C and Banks K: Imaging idiopathic
chylopericardium with 99mTc-SC lymphoscintigraphy and SPECT/CT.
Clin Nucl Med. 40:e508–e510. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Fernandes F, Vieira GS, Arteaga E, Ianni
BM, Pêgo- Fernandes P and Mady C: Cholesterol pericarditis. A
specific but rare cause of pericardial disease. Arq Bras Cardiol.
76:391–394. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Alsemgeest F, Spiegelenberg SR and Kamp O:
Cholesterol pericarditis with massive pericardial cholesterol cyst.
Eur Heart J. 33:15542012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Cilloniz C, Rangel E, Barlascini C,
Piroddi IM, Torres A and Nicolini A: Streptococcus
pneumoniae-associated pneumonia complicated by purulent
pericarditis: Case series. J Bras Pneumol. 41:389–394. 2015.(In
English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Sagristà-Sauleda J, Barrabés JA,
Permanyer-Miralda G and Soler-Soler J: Purulent pericarditis:
Review of a 20-year experience in a general hospital. J Am Coll
Cardiol. 22:1661–1665. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Liu DY, Shao Y and Shi JX: Unilateral
pedal lymphangiography with non-contrast computerized tomography is
valuable in the location and treatment decision of idiopathic
chylothorax. J Cardiothorac Surg. 9:82014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chen FC, Huang JL, Lin WY and Ting CT:
Pedal Tc-99m phytate lymphoscintigraphy in primary
chylopericardium. Int J Cardiol. 90:341–343. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Sachs PB, Zelch MG, Rice TW, Geisinger MA,
Risius B and Lammert GK: Diagnosis and localization of laceration
of the thoracic duct: Usefulness of lymphangiography and CT. AJR Am
J Roentgenol. 157:703–705. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Pitol R, Pederiva JR, Pasin F and Vitola
D: Isolated chylopericardium after cardiac surgery. Arq Bras
Cardiol. 82:384–389. 2004.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Denfield SW, Rodriguez A, Miller-Hance WC,
Stein F, Ott DA, Jefferson LS and Bricker JT: Management of
postoperative chylopericardium in childhood. Am J Cardiol.
63:1416–1418. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Selle JG, Snyder WH III and Schreiber JT:
Chylothorax: Indications for surgery. Ann Surg. 177:245–249. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Melduni RM, Oh JK, Bunch TJ, Sinak LJ and
Gloviczki P: Reconstruction of occluded thoracic duct for treatment
of chylopericardium: A novel surgical therapy. J Vasc Surg.
48:1600–1602. 2008. View Article : Google Scholar : PubMed/NCBI
|