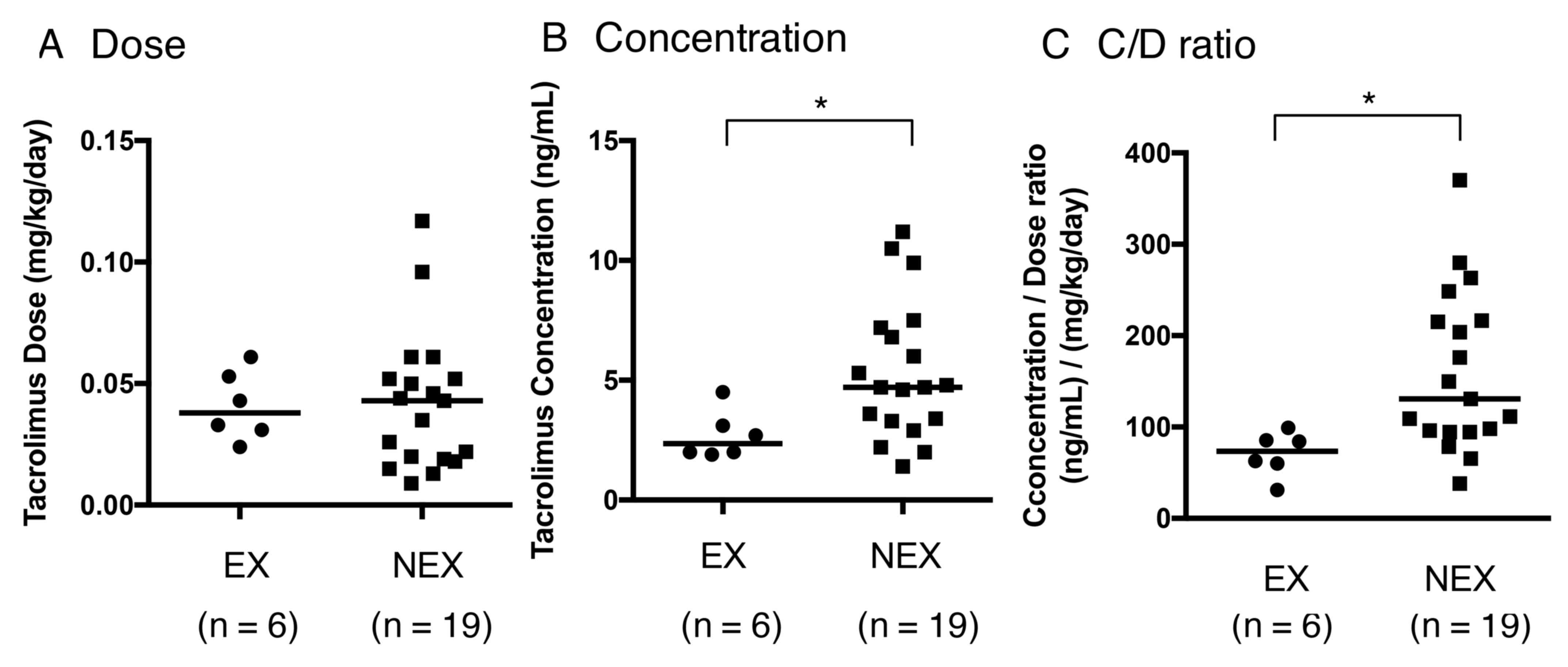
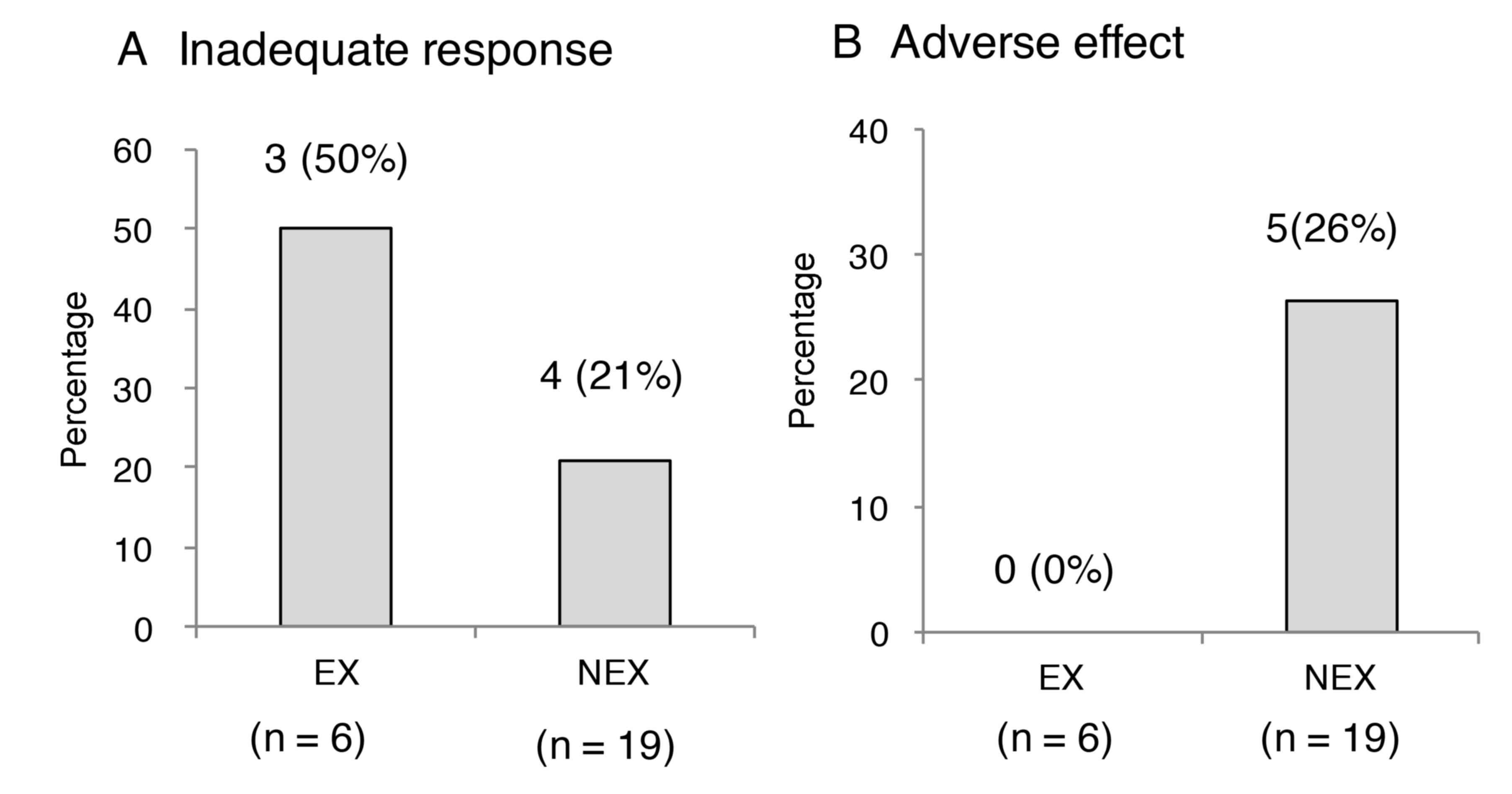
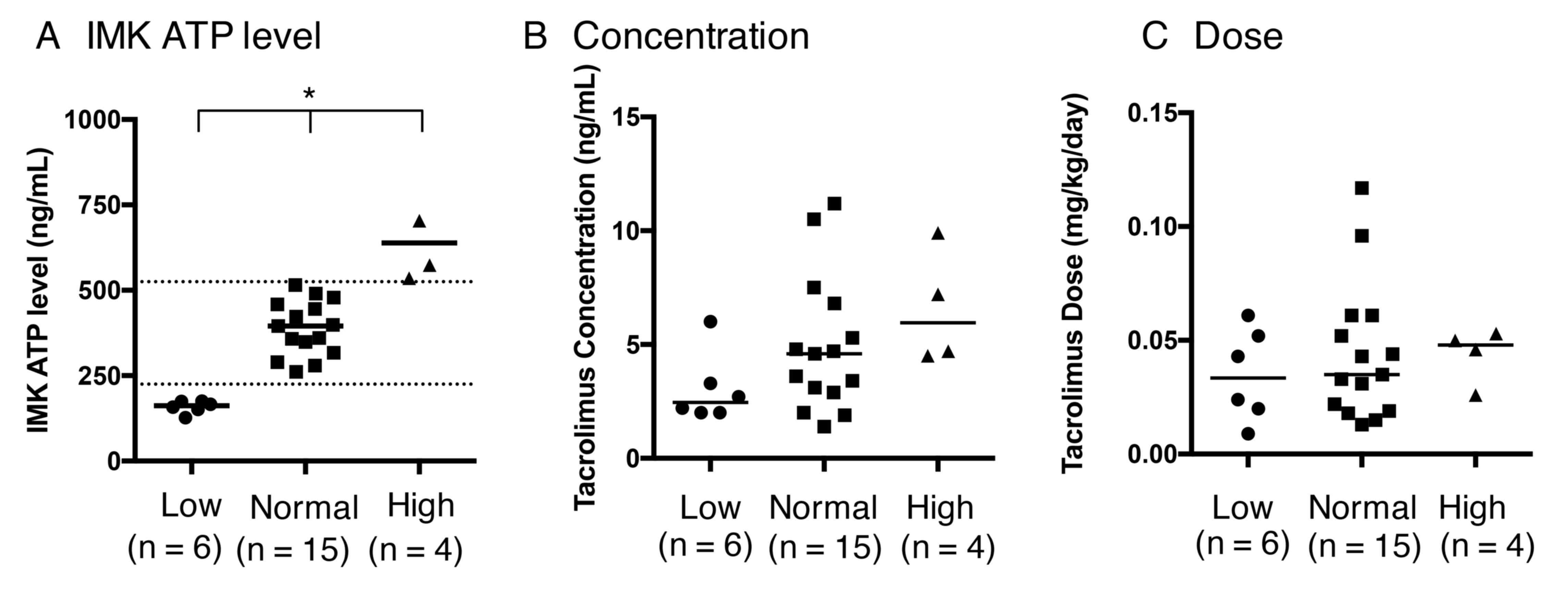
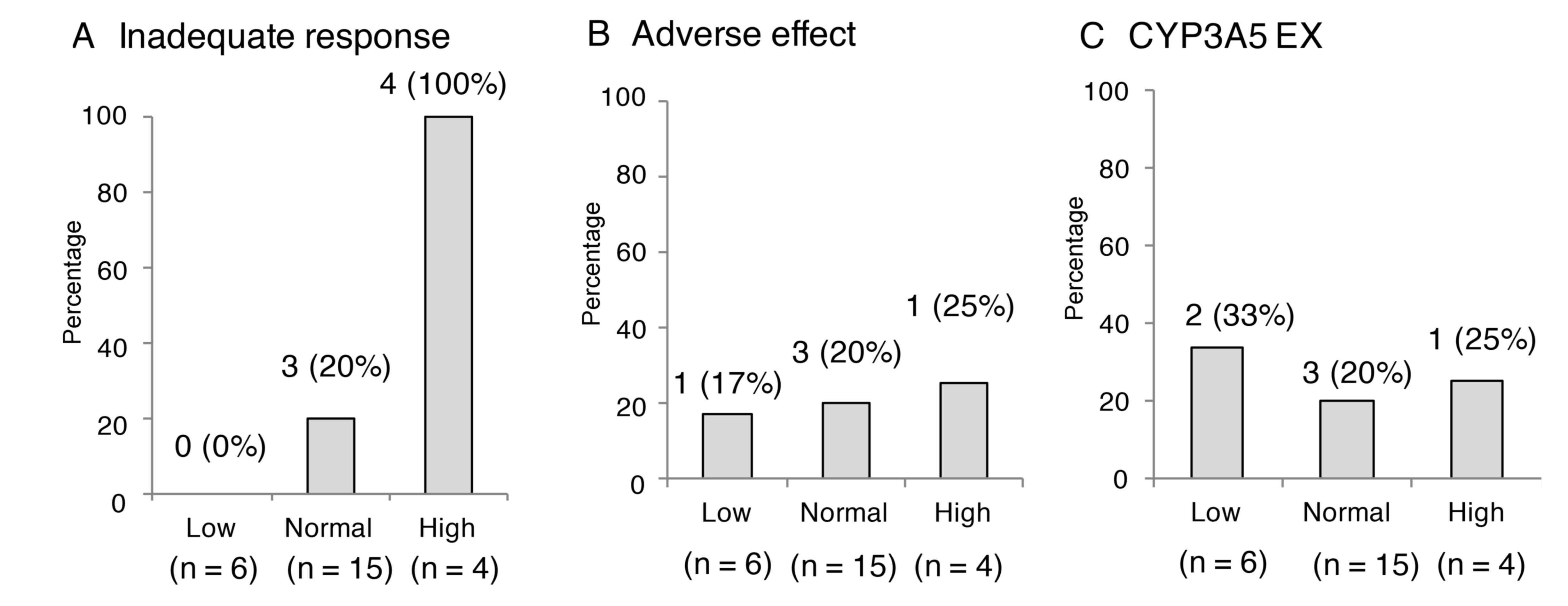
|
1
|
Kowalski R, Post D, Schneider MC, Britz J,
Thomas J, Deierhoi M, Lobashevsky A, Redfield R, Schweitzer E,
Heredia A, et al: Immune cell function testing: An adjunct to
therapeutic drug monitoring in transplant patient management. Clin
Transplant. 17:77–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mizuno S, Hamada T, Nakatani K, Kishiwada
M, Usui M, Sakurai H, Tabata M, Sakamoto Y, Nishioka J, Muraki Y,
et al: Monitoring peripheral blood CD4+ adenosine triphosphate
activity after living donor liver transplantation: Impact of
combination assays of immune function and CYP3A5 genotype. J
Hepatobiliary Pancreat Sci. 18:226–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kowalski RJ, Post DR, Mannon RB, Sebastian
A, Wright HI, Sigle G, Burdick J, Elmagd KA, Zeevi A, Lopez-Cepero
M, et al: Assessing relative risks of infection and rejection: A
meta-analysis using an immune function assay. Transplantation.
82:663–668. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Israeli M, Yussim A, Mor E, Sredni B and
Klein T: Preceeding the rejection: In search for a comprehensive
post-transplant immune monitoring platform. Transpl Immunol.
18:7–12. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muraki Y, Usui M, Isaji S, Mizuno S,
Nakatani K, Yamada T, Iwamoto T, Uemoto S, Nobori T and Okuda M:
Impact of CYP3A5 genotype of recipients as well as donors on the
tacrolimus pharmacokinetics and infectious complications after
living-donor liver transplantation for Japanese adult recipients.
Ann Transplant. 16:55–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawakami K, Inoue T, Murano M, Narabayashi
K, Nouda S, Ishida K, Abe Y, Nogami K, Hida N, Yamagami H, et al:
Effects of oral tacrolimus as a rapid induction therapy in
ulcerative colitis. World J Gastroenterol. 21:1880–1886. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagaishi A, Yukitake M and Kuroda Y:
Long-term treatment of steroid-dependent myasthenia gravis patients
with low-dose tacrolimus. Intern Med. 47:731–736. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
The US Food and Drug Administration, .
Class II Special Controls Guidance Document: cyclosporine and
tacrolimus assays; guidance for industry and FDA. 2002, http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm092778.htm
|
|
9
|
Nobuoka Y, Mizuno S, Nishikawa K, Nakatani
K, Muraki Y, Yamada T, Okuda M, Nobori T, Sugimura Y and Isaji S:
Immune response following liver transplantation compared to kidney
transplantation: Usefulness of monitoring peripheral blood CD4+
adenosine triphosphate activity and cytochrome P450 3A5 genotype
assay. Clin Dev Immunol. 2013:9360632013. View Article : Google Scholar
|
|
10
|
Tanaka H, Tsuruga K, Aizawa-Yashiro T,
Watanabe S and Imaizumi T: Treatment of young patients with lupus
nephritis using calcineurin inhibitors. World J Nephrol. 1:177–183.
2012. View Article : Google Scholar
|
|
11
|
Asamiya Y, Uchida K, Otsubo S, Takei T and
Nitta K: Clinical assessment of tacrolimus therapy in lupus
nephritis: One-year follow-up study in a single center. Nephron
Clin Pract. 113:c330–c336. 2009. View Article : Google Scholar
|
|
12
|
Fukuen S, Fukuda T, Maune H, Ikenaga Y,
Yamamoto I, Inaba T and Azuma J: Novel detection assay by PCR-RFLP
and frequency of the CYP3A5 SNPs, CYP3A5*3 and *6, in a Japanese
population. Pharmacogenetics. 12:331–334. 2002. View Article : Google Scholar
|
|
13
|
Denton MD, Magee CC and Sayegh MH:
Immunosuppressive strategies in transplantation. Lancet.
353:1083–1091. 1999. View Article : Google Scholar
|
|
14
|
Kuehl P, Zhang J, Lin Y, Lamba J, Assem M,
Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, et al:
Sequence diversity in CYP3A promoters and characterization of the
genetic basis of polymorphic CYP3A5 expression. Nat Genet.
27:383–391. 2001. View
Article : Google Scholar
|
|
15
|
Uesugi M, Masuda S, Katsura T, Oike F,
Takada Y and Inui K: Effect of intestinal CYP3A5 on postoperative
tacrolimus trough levels in living-donor liver transplant
recipients. Pharmacogenet Genomics. 16:119–127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mizuno S, Muraki Y, Nakatani K, Tanemura
A, Kuriyama N, Ohsawa I, Azumi Y, Kishiwada M, Usui M, Sakurai H,
et al: Immunological aspects in late phase of living donor liver
transplant patients: Usefulness of monitoring peripheral blood CD4+
adenosine triphosphate activity. Clin Dev Immunol. 2013:9821632013.
View Article : Google Scholar : PubMed/NCBI
|