Introduction
Coronary atherosclerotic heart disease (CAHD),
coronary heart disease for short, is due to coronary artery
narrowing or obstruction caused by coronary atherosclerotic lesions
and plaque formation in the lumen, that reduce the flow of oxygen
rich blood to the heart, resulting in heart ischemia, anoxia or
necrosis (1). The pathogenesis of
CAHD is excessively complex, but is generally based on lumen
stenosis or occlusion caused by atherosclerosis (AS), thus impeding
normal blood circulation. Recent studies have shown the
inflammatory response plays an important role in the occurrence and
development of AS (2,3). Vascular inflammatory responses can
change the shape of the vascular wall, initiate thrombogenesis and
promote the transformation of AS plaques from a stable to a fragile
state, thus inducing embolism and incurring a variety of
cardiovascular and cerebrovascular diseases (4).
Cytokines are small-inducible proteins synthesized
and secreted by body cells upon stimulation. They are involved in
cell growth and differentiation, regulation of inflammation and
immune response and other processes; there are many kinds of
cytokines including interferon, interleukin, chemokine and tumor
necrosis factor (5). Recent studies
have indicated that chemokine CXC ligand 16 (CXCL16) participates
in the pathologic process of AS through its regulation of the
inflammatory response, lipid metabolism and acceleration of AS
plaque thrombogenesis vulnerability (6–8). Tumor
necrosis factor-α (TNF-α) is secreted by monocyte-macrophages and
can be involved in the immune response and the regulation of
inflammatory responses (9).
CXCL16 and vascular inflammation have been shown to
play important roles in AS lesions (10), so we speculated that its levels in
serum would be closely related to the presence of CAHD. We
determined expression levels of chemokine CXCL16 and TNF-α in
patients with CAHD and analyzed the correlation between those
levels and the severity of disease and clinical markers, so as to
provide new ideas for prognosis and treatment of CAHD.
Patients and methods
Materials
General data
Eighty patients with CAHD diagnosed by the
Department of Cardiology in Jinan Zhangqiu Hospital of Traditional
Chinese Medicine from March 2015 to December 2016 were selected and
set as a CAHD group. This included 52 males and 28 females, with an
average age of 61.73±12.54 years. At the same time, 50 healthy
subjects undergoing physical examinations in the outpatient
department were enrolled and set as the healthy control group, with
31 males and 19 females and an average age of 60.21±10.79 years.
The diagnostic criteria for patients with CAHD met those for the
diagnosis of ischemic heart disease formulated by the World Health
Organization (WHO) in 1979, and were confirmed by computed
tomography (CT) coronary angiography. Patients with a coronary
artery (or any vascular branch) diameter stenosis degree of >50%
were regarded as positive. Patients with diabetes or
hypercholesterolemia were excluded from the CAHD group, while
patients with cardiovascular or endocrine disease were excluded
from the healthy control group. All subjects belonged to the Han
ethnicity and were unrelated individuals and there were no
significant differences in gender ratios or age between the two
groups (p>0.05). The study was approved by the Ethics Committee
of Jinan Zhangqiu Hospital of Traditional Chinese Medicine and
informed consents were signed by the patients and/or guardians.
Main reagents
Assay kits for serum total cholesterol (TC),
triglyceride (TG), ELISA, low-density lipoprotein cholesterol
(LDL-C) and high-density lipoprotein cholesterol (HDL-C) were
purchased from Beijing Solarbio Science and Technology (Beijing,
China). Blood glucose assay kit (Beijing Leadman Biochemical
Technology Co., Ltd., Beijing, China).
Methods
CT coronary angiography and disease
evaluation
Coronary arteriography was performed by the digital
subtraction angiography method. Subjects with vascular diameter
stenosis degrees of >50% of a coronary artery or any of its
branches were classified as CAHD patients. The number of affected
vessels was recorded and the Gensini integral was used to evaluate
the severity of the disease. The CAHD group was divided into plaque
(n=56, 37 males and 19 females, with an average age of 63.45±9.74
years) and no-plaque group (n=24, 15 males and 9 females, with an
average age of 60.33±12.31 years).
Specimen collection
Fasting venous blood samples (3–4 ml) were extracted
from patients with CAHD on the second day of admission and from
healthy individuals on the day of physical examination. The samples
were placed into centrifuge tubes, followed by centrifugation at
2,000 rpm at room temperature for 10 min. Subsequently, the upper
sera were transferred into fresh centrifuge tubes and stored at
−80°C. All the samples were treated together by one-time subsequent
detection after all the collections were completed.
Detection of serum concentrations of
CXCL16 and TNF-α
The serum levels of CXCL16 and TNF-α in each group
were detected by enzyme-linked immunosorbent assay (ELISA),
referring to operation instructions in the serum CXCL16 and serum
TNF-α quantitative assay kits by R&D Systems (Minneapolis, MN,
USA).
Detection of clinical markers
The routine clinical markers, such as blood pressure
and heart rate were detected using standard hospital instruments.
Levels of laboratory biochemical markers, such as seric TC, TG,
LDL-C and HDL-C were determined by following the instructions in
the assay kits as above-mentioned in the CT coronary angiography
and disease evaluation.
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. The t-test was adopted to
analyze the normal distribution of measurement data. Data that
conformed to the normal distribution were represented as mean ±
standard deviation and those that did not conform to the normal
distribution were expressed by median (interquartile range) [M
(Q)]. The independent sample t-test was used to compare the mean of
the two groups and the single factor analysis of variance was
adopted to compare the mean among multiple groups. Spearman's rank
correlation analysis was used to analyze the correlation between
serum levels of CXCL16 and TNF-α and the severity of the disease
and specific clinical signs. P<0.05 was considered to indicate a
statistically significant difference.
Results
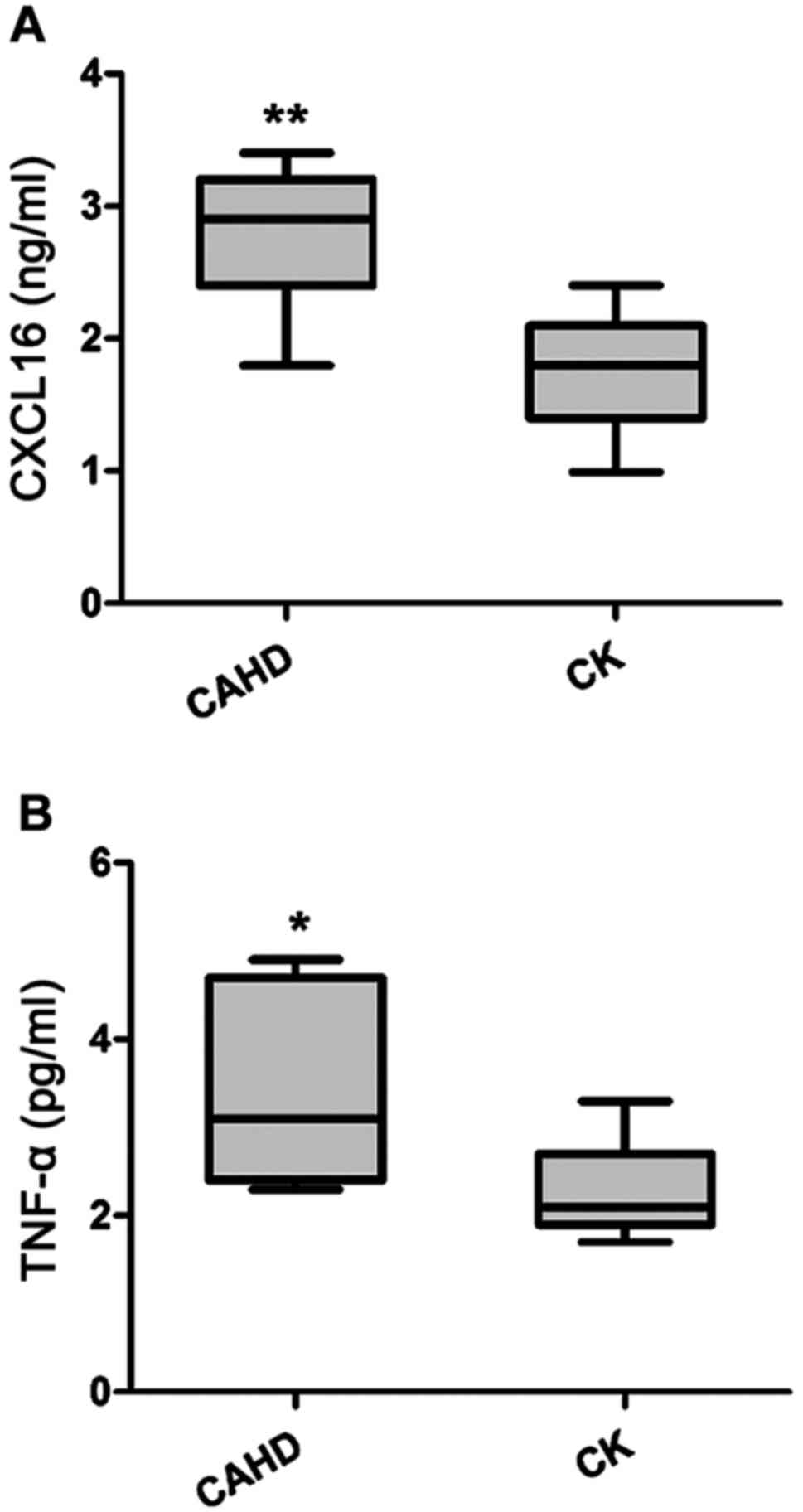
Comparisons of serum levels of CXCL16
and TNF-α between the CAHD and the healthy control groups
The serum levels of CXCL16 and TNF-α were both
significantly higher in the CADH group, when quantified by ELISA
(Fig. 1).
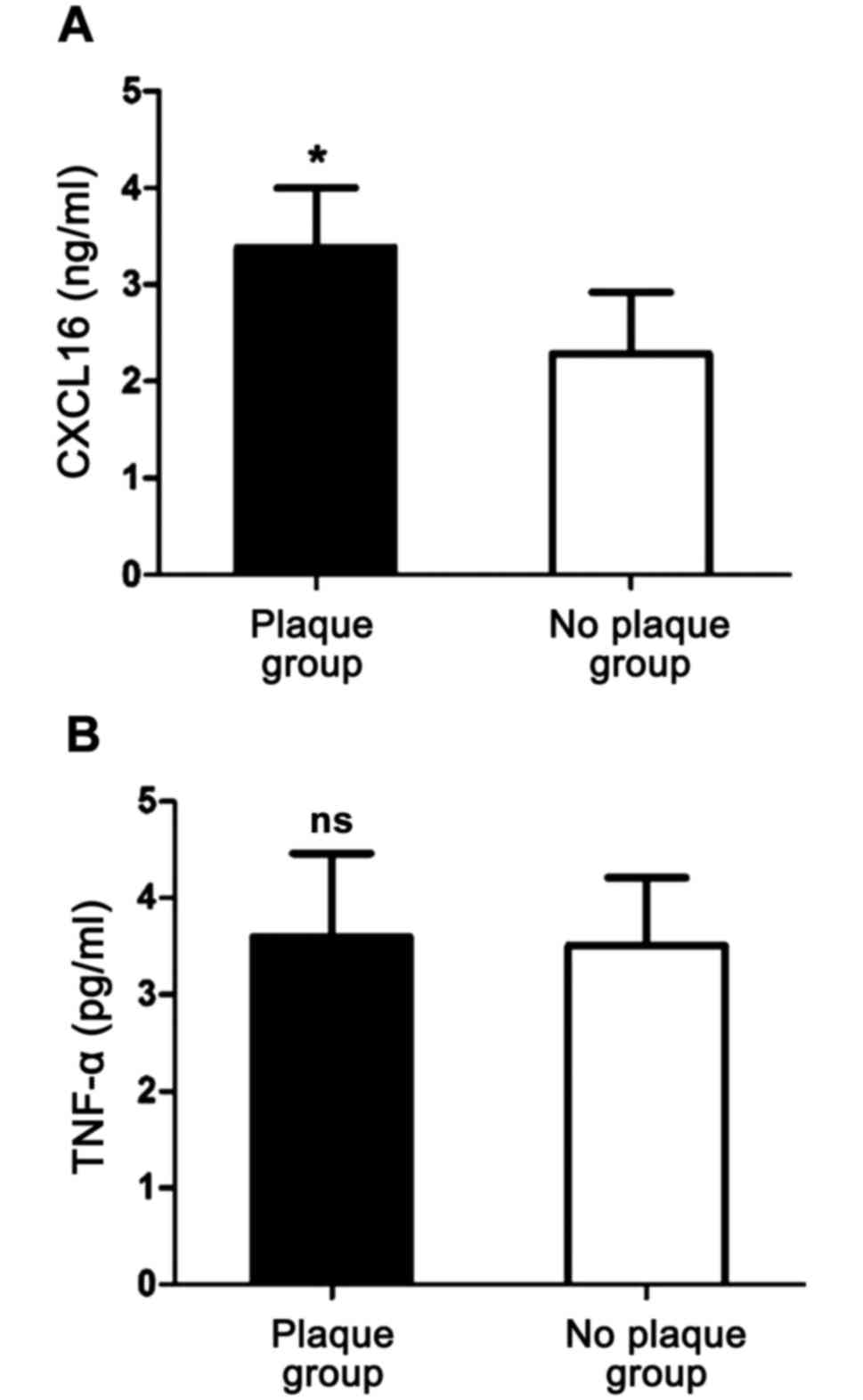
Comparisons of serum levels of CXCL16
and TNF-α between the plaque and no-plaque groups within the CAHD
group
The patients in the CADH group were divided into a
plaque or no-plaque subgroup according to the results of an
ultrasound examination. While the serum CXCL16 levels were
significantly higher in the plaque than that in the no-plaque group
the levels of TNF-α were not significantly different among the
groups (Fig. 2).
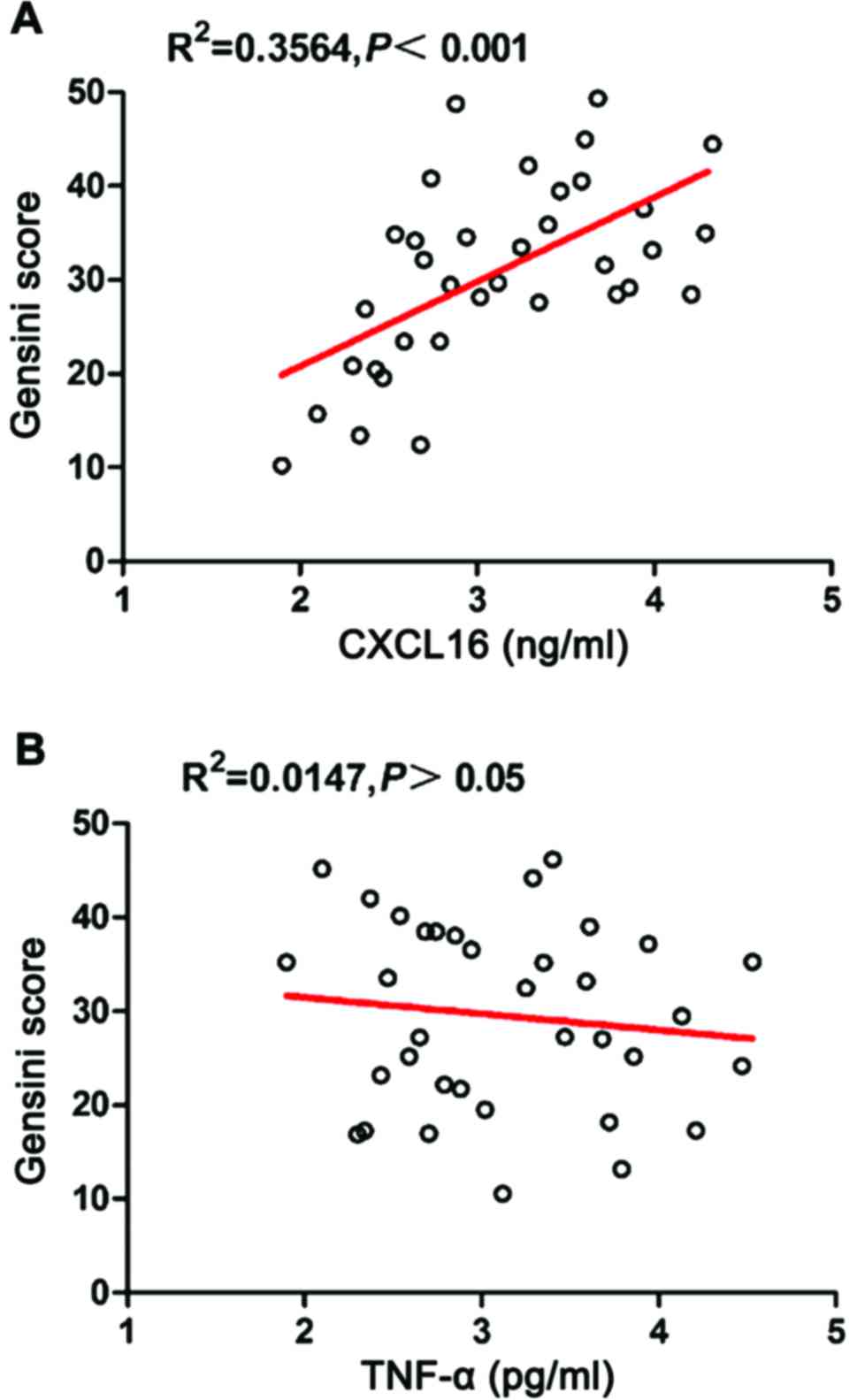
Correlation between serum levels of
CXCL16 and TNF-α and severity of the disease
The Gensini score was used to classify the coronary
lesion in CAHD patients, to assess the severity of CAHD. The
correlation between the Gensini score and the serum levels of
CXCL16 and TNF-α was analyzed, the results are shown in Fig. 3. The serum CXCL16 level has a
significantly positive correlation with the Gensini score
(r=0.5970, p=0.0002); and the serum TNF-α level is not
significantly correlated with Gensini score (r=−0.1214,
p=0.4872).
Correlation between serum levels of
CXCL16 and TNF-α and CAHD clinical variables
The results of correlation analyses between serum
levels of CXCL16 and TNF-α and each clinical variable in patients
with CAHD are shown in Table I. The
serum CXCL16 level is not significantly correlated with systolic
pressure, diastolic pressure, fasting blood glucose, heart rate or
TC, TG and HDL-C levels, but has a significantly positive
correlation with the LDL-C level (r=0.597, p=0.027). There were no
significant correlations between serum TNF-α levels and each
clinical variable.
 | Table I.Correlations between serum levels of
CXCL16 and TNF-α and clinical signs in the CAHD group. |
Table I.
Correlations between serum levels of
CXCL16 and TNF-α and clinical signs in the CAHD group.
|
| CXCL16 | TNF-α |
|---|
|
|
|
|
|---|
| Clinical index | r value | P-value | r value | P-value |
|---|
| Systolic pressure
(mmHg) | 0.198 | 0.124 | −0.208 | 0.412 |
| Diastolic pressure
(mmHg) | −0.168 | 0.156 | 0.166 | 0.241 |
| Fasting blood glucose
(mmol/l) | 0.067 | 0.561 | −0.054 | 0.871 |
| Heart rate
(bp/min) | 0.176 | 0.314 | 0.114 | 0.142 |
| TC (mmol/l) | 0.196 | 0.177 | 0.188 | 0.255 |
| TG (mmol/l) | −0.097 | 0.889 | −0.069 | 0.842 |
| LDL-C (mmol/l) | 0.597 | 0.027a | −0.163 | 0.331 |
| HDL-C (mmol/l) | −0.179 | 0.274 | 0.075 | 0.752 |
Discussion
CAHD patients usually exhibit a variety of heart and
nervous function alterations due to blood flow abnormalities. CADH
greatly endangers the life and health of the middle-aged and
elderly people in any population, which is why it is one of the
most important diseases that need to be better studied. The
pathological mechanism of CAHD is complex, involving oxidative
stress, immune inflammatory reactions, AS plaque formation and
apoptosis. The occurrence of AS and instability of AS plaques are
the basis of CADH pathogenesis. Vascular inflammatory reactions
play an important role in AS plaque formation, which can promote
coronary artery wall lesions thus leading to CAHD.
Chemokines, such as the monocyte chemoattractant
protein-1 and interleukin-8, activate and direct leukocytes to
atherosclerotic lesions. CXCL16 is a recently found CXC family
chemokine. It is expressed in soluble form across the cell
membrane. CXCL16 has been closely related to the pathogenic
formation of AS regulating inflammation and lipid metabolism
(11). Its interaction with CXCR6
receptor on the surface of T lymphocytes directs the migration of
activated T lymphocytes to the AS lesion tissue. T lymphocytes can
promote plaque formation and thromobosis vulnerability by locally
secreting multiple cytokines and matrix metalloproteinases (MMPs)
(12). In addition, CXCL16 combines
with macrophages accelerating the endocytosis of oxidized LDL
(Ox-LDL) to form foam cells (13),
and it acts as an angiogenic factor inducing microvascular
formation in AS plaques (14).
Finally, CXCL16 activates CB8+ T cells, leading to
apoptosis in the surrondings of an AS plaque (15). CXCL16 expression can be upregulated
by IFN-γ in AS plaques, suggesting that it enhances the role of the
inflammatory response in AS lesions by means of a positive feedback
loop mechanism (16). Thus,
inflammation reactions affect the instability of AS plaques and
markers of inflammation can be used to evaluate the risk of
coronary heart disease. The levels of CXCL16 can reflect the
upstream and downstream inflammation pathways, which can be used
for the clinical prognosis and diagnosis of various cardiovascular
and cerebrovascular diseases. Indeed, the soluble CXCL16 has been
utilized as a biological marker of rheumatoid arthritis and
systemic lupus erythematosus and other diseases (17).
TNF-α is a cytokine that can cause tumor cell
necrosis. In recent years, TNF-α has been shown to be of great
importance in the inflammation response (18). It can increase the release of soluble
CXCL16 from endothelial cells and smooth muscle cells (SMCs) and it
is abnormally highly expressed in patients with coronary heart
disease (19). TNF-α is involved in
the pathological process of AS by increasing inflammatory cells in
the injured tissue, assisting vascular smooth muscle remodeling and
exerting a negative inotropic effect on the myocardium, in which
myocardial fiber necrosis and coronary artery ischemia can cause
myocarditis and release of even more inflammatory mediators
(20).
Our study found a significant increase in serum
levels of CXCL16 and TNF-α in CAHD patients, indicating that they
may be involved in the pathological process of CAHD. The levels of
CXCL16 were significantly higher in the plaque group than that in
the no-plaque group and had a significantly positive correlation
with severity of CAHD. But, there were no significant differences
in TNF-α levels between the two CADH groups and TNF-α was not
significantly correlated with the degree of lesion present in CADH
patients. Our findings suggest that CXCL16 can be used as a
clinical diagnostic marker and illness evaluation factor in AS
plaque formation and development of CAHD. Additionally, the TNF-α
level was not significantly correlated with clinical variables
studied, but CXCL16 had a significantly positive correlation with
the plasma LDL-C levels in patients with CAHD, revealing that
CXCL16 can be used as a valuable tool for diagnosis, treatment
assessment and prognosis of CAHD.
In conclusion, in patients with CAHD, CXCL6 acts as
an independent risk factor that can be used as a reliable
biological marker for diagnosis, disease evaluation and clinical
prognosis of CADH.
References
|
1
|
McMillan RL: Coronary atherosclerotic
heart disease. N C Med J. 19:147–149. 1958.PubMed/NCBI
|
|
2
|
Hansson GK: Inflammation, atherosclerosis,
and coronary artery disease. N Engl J Med. 352:1685–1695. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaperonis EA, Liapis CD, Kakisis JD,
Dimitroulis D and Papavassiliou VG: Inflammation and
atherosclerosis. Eur J Vasc Endovasc Surg. 31:386–393. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stoll G and Bendszus M: Inflammation and
atherosclerosis: Novel insights into plaque formation and
destabilization. Stroke. 37:1923–1932. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cannon JG: Inflammatory cytokines in
nonpathological states. News Physiol Sci. 15:298–303.
2000.PubMed/NCBI
|
|
6
|
Barlic J and Murphy PM: Chemokine
regulation of atherosclerosis. J Leukoc Biol. 82:226–236. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abel S, Hundhausen C, Mentlein R, Schulte
A, Berkhout TA, Broadway N, Hartmann D, Sedlacek R, Dietrich S,
Muetze B, et al: The transmembrane CXC-chemokine ligand 16 is
induced by IFN-gamma and TNF-alpha and shed by the activity of the
disintegrin-like metalloproteinase ADAM10. J Immunol.
172:6362–6372. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimaoka T, Kume N, Minami M, Hayashida K,
Kataoka H, Kita T and Yonehara S: Molecular cloning of a novel
scavenger receptor for oxidized low density lipoprotein, SR-PSOX,
on macrophages. J Biol Chem. 275:40663–40666. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schreyer SA, Vick CM and LeBoeuf RC: Loss
of lymphotoxin-alpha but not tumor necrosis factor-alpha reduces
atherosclerosis in mice. J Biol Chem. 277:12364–12368. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nowaczenko M, Sarzyńska-Długosz I and
Członkowska A: Prevalence of carotid arteries atherosclerotic
changes in ischemic stroke patients. Neurol Neurochir Pol.
37:27–36. 2003.(In Polish). PubMed/NCBI
|
|
11
|
Smith C, Halvorsen B, Otterdal K, Waehre
T, Yndestad A, Fevang B, Sandberg WJ, Breland UM, Frøland SS, Oie
E, et al: High levels and inflammatory effects of soluble CXC
ligand 16 (CXCL16) in coronary artery disease:down-regulatory
effects of statins. Cardiovasc Res. 79:195–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Agostini C, Cabrelle A, Calabrese F,
Bortoli M, Scquizzato E, Carraro S, Miorin M, Beghè B, Trentin L,
Zambello R, et al: Role for CXCR6 and its ligand CXCL16 in the
pathogenesis of T-cell alveolitis in sarcoidosis. Am J Respir Crit
Care Med. 172:1290–1298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Minami M, Kume N, Shimaoka T, Kataoka H,
Hayashida K, Yonehara S and Kita T: Expression of scavenger
receptor for phosphatidylserine and oxidized lipoprotein (SR-PSOX)
in human atheroma. Ann N Y Acad Sci. 947:373–376. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhuge X, Murayama T, Arai H, Yamauchi R,
Tanaka M, Shimaoka T, Yonehara S, Kume N, Yokode M and Kita T:
CXCL16 is a novel angiogenic factor for human umbilical vein
endothelial cells. Biochem Biophys Res Commun. 331:1295–1300. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aslanian AM and Charo IF: Targeted
disruption of the scavenger receptor and chemokine CXCL16
accelerates atherosclerosis. Circulation. 114:583–590. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wuttge DM, Zhou X, Sheikine Y, Wågsäter D,
Stemme V, Hedin U, Stemme S, Hansson GK and Sirsjö A:
CXCL16/SR-PSOX is an interferon-gamma-regulated chemokine and
scavenger receptor expressed in atherosclerotic lesions.
Arterioscler Thromb Vasc Biol. 24:750–755. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu T, Xie C, Wang HW, Zhou XJ, Schwartz N,
Calixto S, Mackay M, Aranow C, Putterman C and Mohan C: Elevated
urinary VCAM-1, P-selectin, soluble TNF receptor-1, and CXC
chemokine ligand 16 in multiple murine lupus strains and human
lupus nephritis. J Immunol. 179:7166–7175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carswell EA, Old LJ, Kassel RL, Green S,
Fiore N and Williamson B: An endotoxin-induced serum factor that
causes necrosis of tumors. Proc Natl Acad Sci USA. 72:pp.
3666–3670. 1975; View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang YN, Che SM and Ma AQ: Clinical
significance of serum cytokines IL-1beta, sIL-2R, IL-6, TNF-alpha,
and IFN-γ in acute coronary syndrome. Chin Med Sci J. 19:120–124.
2004.PubMed/NCBI
|
|
20
|
Trepels T, Zeiher AM and Fichtlscherer S:
Acute coronary syndrome and inflammation. Biomarkers for
diagnostics and risk stratification. Herz. 29:769–776. 2004.(In
German).
|