Introduction
Bone formation in the developing embryo is governed
by intramembranous and endochondral ossification initiating from
mesenchymal condensations, in which mesenchymal progenitor cells
differentiate (1). During
endochondral ossification, mesenchymal progenitors differentiate
into chondrocytes to form the cartilage template of the bone, which
is required for bone elongation. Chondrocyte hypertrophy is
required for endochondral bone formation; elongated chondrocytes
exit the cell cycle to become prehypertrophic chondrocytes prior to
terminally differentiating to form hypertrophic chondrocytes
(2). These chondrocytes subsequently
secrete factors required for osteoblast differentiation and
maturation (3).
It has previously been reported that the Indian
hedgehog (Ihh) signaling pathway is a key regulator of skeletal
development and homeostasis (4) and
that Ihh signaling must be enhanced to increase bone formation
(5). In a previous study,
overexpression of Ihh ameliorated short stature homeobox 2 (shox2)
overexpression-associated reduction of extracellular matrix
components; however, it did not inhibit the increase in matrix
metalloproteinase (MMP)9 and MMP13, or apoptosis in the
temporomandibular joint, which suggests that overexpression of Ihh
only partially inhibits shox2 overexpression-associated congenital
dysplasia of the temporomandibular joint (6). Dysregulation of Ihh signaling results
in multiple bone diseases, including progressive osseous
heteroplasia (7). Treatment with an
Ihh signaling inhibitor has been reported to reduce the occurrence
of chondroma-like lesions, including enchondromas and
osteochondromas, adjacent to disordered growth plates in fibroblast
growth factor receptor 3-deficient mice (8). Ihh deletion also induces symphalangism,
characterized by initial cartilaginous fusion preventing epiphyseal
growth plate formation, resulting in abnormal directionality of
chondrocyte differentiation in mutant mice (9). Ihh signaling serves an important role
in the mineralization process of fibrocartilaginous entheses
(10). Inhibiting Hh signaling
reduces mineralized fibrocartilage, leading to less collagen
embedded within mineralized tissue (11). Understanding the signaling mechanisms
and functions of Ihh signaling in bone development may provide
important insights into bone disease prevention and
therapeutics.
In the present study, Ihh was knocked down in
chondrocytes using short hairpin (sh)RNA to investigate the
function of Ihh signaling in chondrocyte proliferation and
differentiation. The present study also aimed to explore the
potential mechanism by which Ihh induces chondrocyte apoptosis and
cell cycle arrest.
Materials and methods
Cell line and cell transfection
Mouse chondrocyte cells (CP-M087) were purchased
from Procell Life Science & Technology Co., Ltd. (Wuhan,
China). Cells were grown routinely in Dulbecco's modified Eagle's
medium/F-12 medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) and cultured for 24 h before
refreshing the medium in a 37°C humidified atmosphere containing 5%
CO2. Knockdown of Ihh in chondrocyte cells was achieved by
transfection with 5 µl (109 U) lentivirus containing Ihh
shRNA (Ihh-shRNA; Shanghai GenePharma Co., Ltd., Shanghai, China)
for 48 h using Lipofectamine 3000 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Cells transfected with
empty lentivirus were used as a negative control (NC), and
untreated cells were used as blank control. Cells (5×105
cells/ml) were seeded in 6-well clusters (1 ml) or 96-well plates
(0.2 ml) and transfected for 48 h. Transfected cells were used in
further assays or RNA/protein extraction.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. RevertAid First Strand cDNA Synthesis
Kit (Thermo Fisher Scientific, Inc.) was used to reverse transcribe
the mRNA to cDNA according to the manufacturer's protocol. Briefly,
0.5 ng of the template RNA, 1 µl oligo(dT)18 primer and 12 µl RNase
free water were mixed, and incubated at 65°C for 5 min, then cooled
down on ice. The mixture was added to Reaction Buffer (4 µl),
RiboLock RNase inhibitor (1 µl), 10 mM dNTP mix (2 µl) and
RevertAid Reverse Transcriptase (1 µl), and incubated at 42°C for 1
h. Finally, the mixture was heated to 70°C for 5 min to obtain the
cDNA. Ihh, parathyroid hormone-related protein (PTHrP),
transforming growth factor (TGF)-β, osteocalcin (OCN), runt-related
transcription factor 2 (Runx2), osteoprotegerin (OPG), mothers
against decapentaplegic homolog 2 (Smad2), Smad3, collagen type X
α1 chain (COL10A) and receptor activator of nuclear factor-κB
ligand (RANKL) mRNA expression was measured using SYBR Fast qPCR
Mix (cat. no. RR430A; Takara Biotechnology Co., Ltd., Dalian,
China) under the following conditions: 95°C for 30 sec, followed by
40 cycles of 95°C for 5 sec and 60°C for 10 sec, and then for
melting curves (one cycle of 95°C for 5 sec and 60°C for 60 sec,
followed by cooling to 50°C in 30 sec) β-actin was used as an
endogenous control. Primers sequences used for qPCR were as
follows: Ihh, forward 5′-CTCAGCCTGCTCTCACTACG-3′ and reverse
5′-AAGCACATCCAACCCACCTC-3′; PTHrP, forward
5′-CGAGGTTCAAAGGTTTGCCTC-3′ and reverse 5′-GGCCAGAGAAGCCTGTTACC-3′;
TGF-β, forward 5′-AGGGCTACCATGCCAACTTC-3′ and reverse
5′-TGACACAGAGATCCGCAGTC-3′; OCN, forward 5′-TCCTTTGGGGTTTGGCCTAC-3′
and reverse 5′-CTTGGACACAAAGGCTGCAC-3′; RUNX2, forward
5′-CGCCTCACAAACAACCACAG-3′ and reverse 5′-TCACTGTGCTGAAGAGGCTG-3′;
OPG, forward 5′-CTGGAACCCCAGAGCGAAAT-3′ and reverse
5′-GCGTTTACTTTGGTGCCAGG-3′; Smad2, forward
5′-GTTCCTTTCCTCCTCCGCTC-3′ and reverse 5′-AGTCTCTTCACAACTGGCGG-3′;
Smad3, forward 5′-TTCACTGGTGCTGGGGTTAG-3′ and reverse
5′-GGTAGGGATTCACGCAGACC-3′; COL10A, forward
5′-CTCCCAGCACGCAGAATC-3′ and reverse: 5′-TTCCCTACAGCTGATGGTCC-3′;
RANKL, forward 5′-GGAGTTGGCCGCAGACAAGA-3′ and reverse
5′-TGATGTGCTGTGATCCAACGA-3′; and β-actin, forward
5′-GCAGGAGTATGACGAGTCCG-3′ and reverse
5′-AACAACGCATCTCATATTTGGAA-3′. Data were processed using the
2−ΔΔCq method (12).
Cell Counting Kit-8 (CCK-8) cell
proliferation assay
Cell proliferation rates were measured using CCK-8
(Beyotime Institute of Biotechnology, Haimen, China). A total of
0.5×104 cells were seeded in 96-well plates for 24 h,
transfected with the indicated lentivirus, and further incubated
for 24, 48 and 72 h at 37°C, respectively. A total of 10 µl CCK-8
reagents were added to each well at 1 h prior to the endpoint of
incubation (23, 47 and 71 h). The optical density of each well was
subsequently determined using a microplate reader at a wavelength
of 490 nm.
Flow cytometric analysis of apoptosis
with Annexin-V/propidium iodide (PI) double staining
An Annexin V Apoptosis Detection kit (Thermo Fisher
Scientific, Inc.) was used to assess apoptosis. Following
incubation for 24, 48 or 72 h at 37°C, the cells were trypsinized
for 2 min at 37°C, collected by centrifugation (960 × g) for 5 min
at room temperature and resuspended in 2 ml medium. Approximately
2×105 cells were harvested, washed twice with cold PBS
and resuspended in 500 µl binding buffer provided in the kit. A
total of 10 µl Annexin V-fluorescein isothiocyanate and 10 µl PI
was added to the solution and mixed well. Following incubation for
15 min at 37°C, cells were analyzed using a flow cytometer (BD
Biosciences, San Jose, CA, USA).
Cell cycle analysis assay
Cell cycle analysis was determined by flow cytometry
using a Cell Cycle and Apoptosis Analysis kit (cat no. C1052,
Beyotime Institute of Biotechnology) and analyzed the data using
Flowjo (7.6 version; Flowjo LLC, Ashland, OR, USA). Briefly,
~1×104 cells were seeded in each well of a 6-well plate.
Following 48 h transfection with Ihh-shRNA or empty lentivirus,
cells were harvested and fixed in 70% ice-cold ethanol for 24 h,
followed by staining with PI for 10 min at room temperature. The
different phases of the cell cycle were analyzed using a
FACSCalibur flow cytometer (BD Biosciences).
Alkaline phosphatase (ALP) assay
Alkaline Phosphatase Assay Kit (cat no. P0321,
Beyotime Institute of Biotechnology) was used to detect ALP
activity according to the manufacturer's protocol. Black cobaltous
sulfide staining indicated a positive signal. Cells were
counterstained with nuclear fast red for 5 min at room temperature
to label the nucleus and cytoplasm of cells. Images were captured
using an inverted microscope (magnification, ×100; Nikon Eclipse TC
100; Nikon Corporation, Tokyo, Japan).
Determination of mineralization using
von Kossa staining
Von Kossa staining (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was used to determine the extent of minerals
deposited on chondrocyte cells. Silver nitrate solution was added
to the cells and the plate was exposed to ultraviolet light for 30
min. Cells were subsequently washed with PBS and the reaction was
stopped with the addition of 500 µl of 5% sodium thiosulfate
(Sigma-Aldrich; Merck KGaA). The positive mineral depositions were
stained in black. Cells were counterstained with nuclear fast red
for 5 min at room temperature to label the nucleus and cytoplasm.
Images were captured using an inverted microscope (magnification,
×100; Nikon Eclipse TC 100; Nikon Corporation).
Western blot analysis
Immunoblotting was performed to detect the
expression of Ihh, PTHrP, TGF-β, OCN, Runx2, OPG, Smad2, Smad3,
COL10A and RANKL in chondrocyte cells. Cultured or transfected
cells were lysed in radioimmunoprecipitation assay buffer (Wuhan
Boster Biological Technology, Ltd., Wuhan, China) with 1%
phenylmethylsulfonyl fluoride. The concentration of protein was
determined using the BCA Protein Assay kit (cat no. P0011; Beyotime
Institute of Biotechnology, Haimen, China) according to the
manufacturer's protocol. A total of 60 µg/lane of proteins were
separated by 12% SDS-PAGE and transferred onto polyvinyl difluoride
membranes. The membranes were blocked in non-fat milk for 1 h at
37°C. Membranes were incubated at 4°C overnight with the following
primary antibodies: Anti-Ihh, (cat. no. SAB1403965; dilution,
1:1,000); anti-PTHrP (cat. no. SAB5300029; dilution, 1:1,000);
anti-TGF-β (cat. no. SAB4502958; dilution, 1:2,000); anti-OCN (cat.
no. SAB1306277; dilution, 1:2,000; all from Sigma Aldrich; Merck
KGaA); anti-RUNX2 (cat. no. 12556; dilution, 1:1,000); anti-OPG
(cat. no. 4816; dilution, 1:1,000); anti-Smad2 (cat. no. 5339;
dilution, 1:1,000), anti-Smad3 (cat. no. 9523; dilution, 1:1,000;
all from Cell Signaling Technology, Inc., Danvers, MA, USA);
anti-COL10A (cat. no. ab58632; dilution, 1:1,000); anti-RANKL (cat.
no. ab9957; dilution, 1:1,000) and anti-GAPDH (cat. no. ab9485;
dilution, 1:3,000; all from Abcam, Cambridge, UK). The blots were
subsequently incubated with goat anti-rabbit IgG (cat. no. ab7090)
and goat anti-mouse (cat. no. ab97040; both 1:5,000; Abcam)
horseradish peroxidase-conjugated secondary antibodies for 1 h at
37°C. Signals were visualized using enhanced chemiluminescence
substrates (EMD Millipore, Billerica, MA, USA). The densitometry of
bands was analyzed using Quantity One software (version 4.6.9;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). GAPDH was used as
an endogenous protein for normalization.
Statistical analysis
All data are expressed as the mean ± standard
deviation of three independent experiments. Statistical analyses
were performed using SPSS v. 17.0 (SPSS, Inc., Chicago, IL, USA).
Differences between groups were analyzed using Student's t-test or
one-way analysis of variance with Tukey's post hoc test dependent
on the conditions. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of Ihh on chondrocyte cell
proliferation, apoptosis and cell cycle
To investigate the biological role of Ihh in
chondrocyte cell growth, chondrocytes transfected with Ihh shRNA
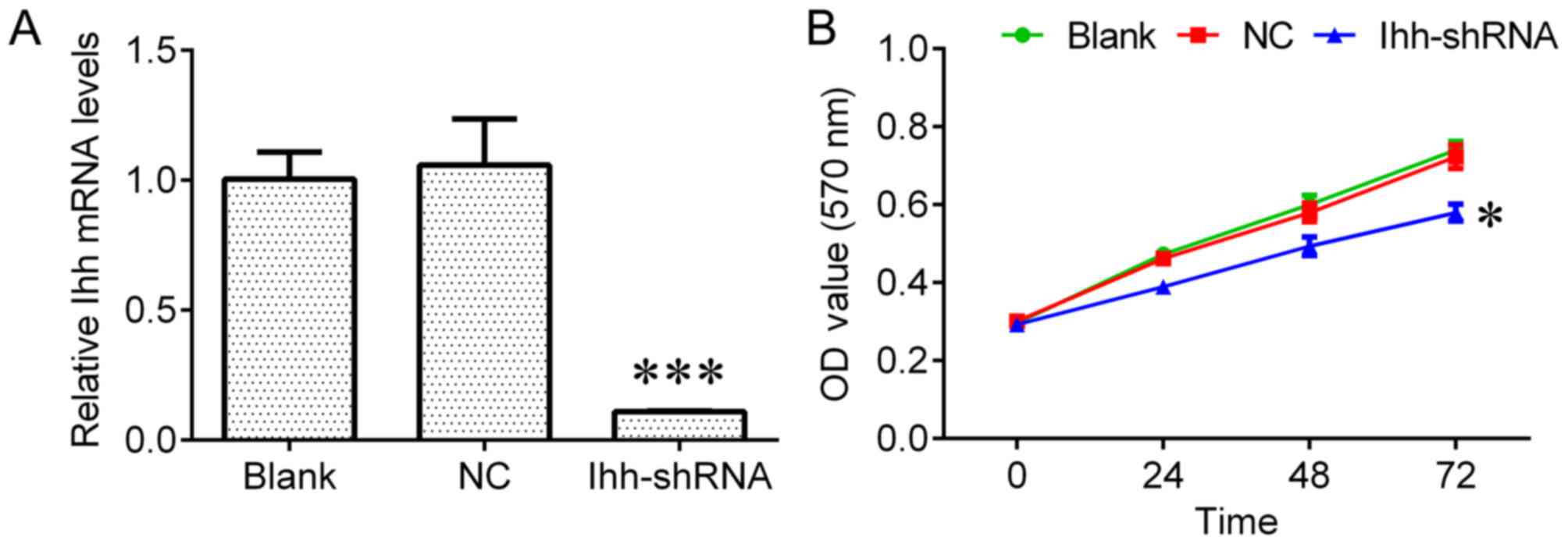
were used in the present study. The results of RT-qPCR revealed
that Ihh expression was significantly reduced in transfected cells
compared with NC cells (P<0.001; Fig.
1A). Ihh downregulation significantly inhibited cell growth
(P<0.05; Fig. 1B) and
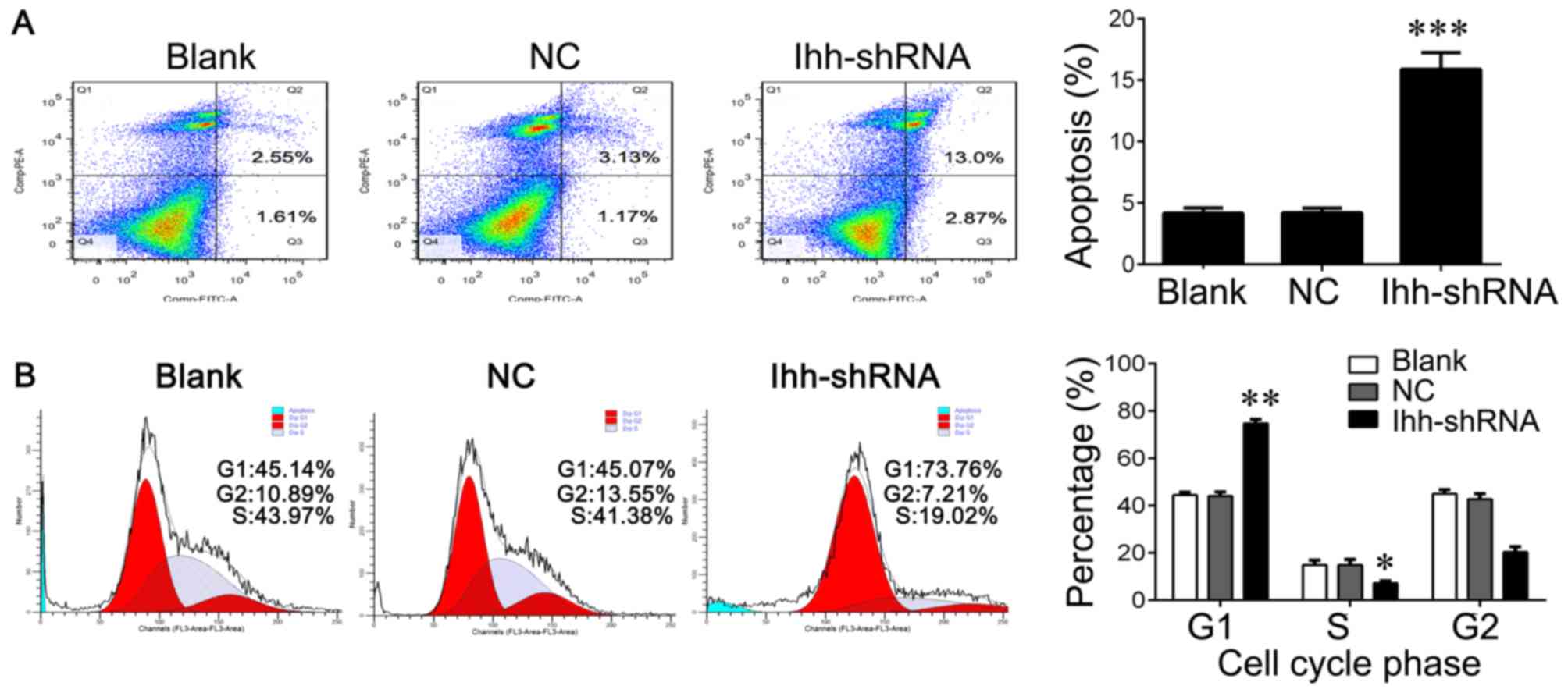
significantly increased the apoptosis rate compared with the NC
group (P<0.001; Fig. 2A). In
addition, Ihh knockdown significantly reduced the percentage of
cells in S phase (P<0.05) and increased the percentage of cells
in G1 phase compared with the NC group (P<0.01), indicating cell
cycle arrest at G1 to S phase (Fig.
2B).
Effects of Ihh on chondrocyte cell
differentiation
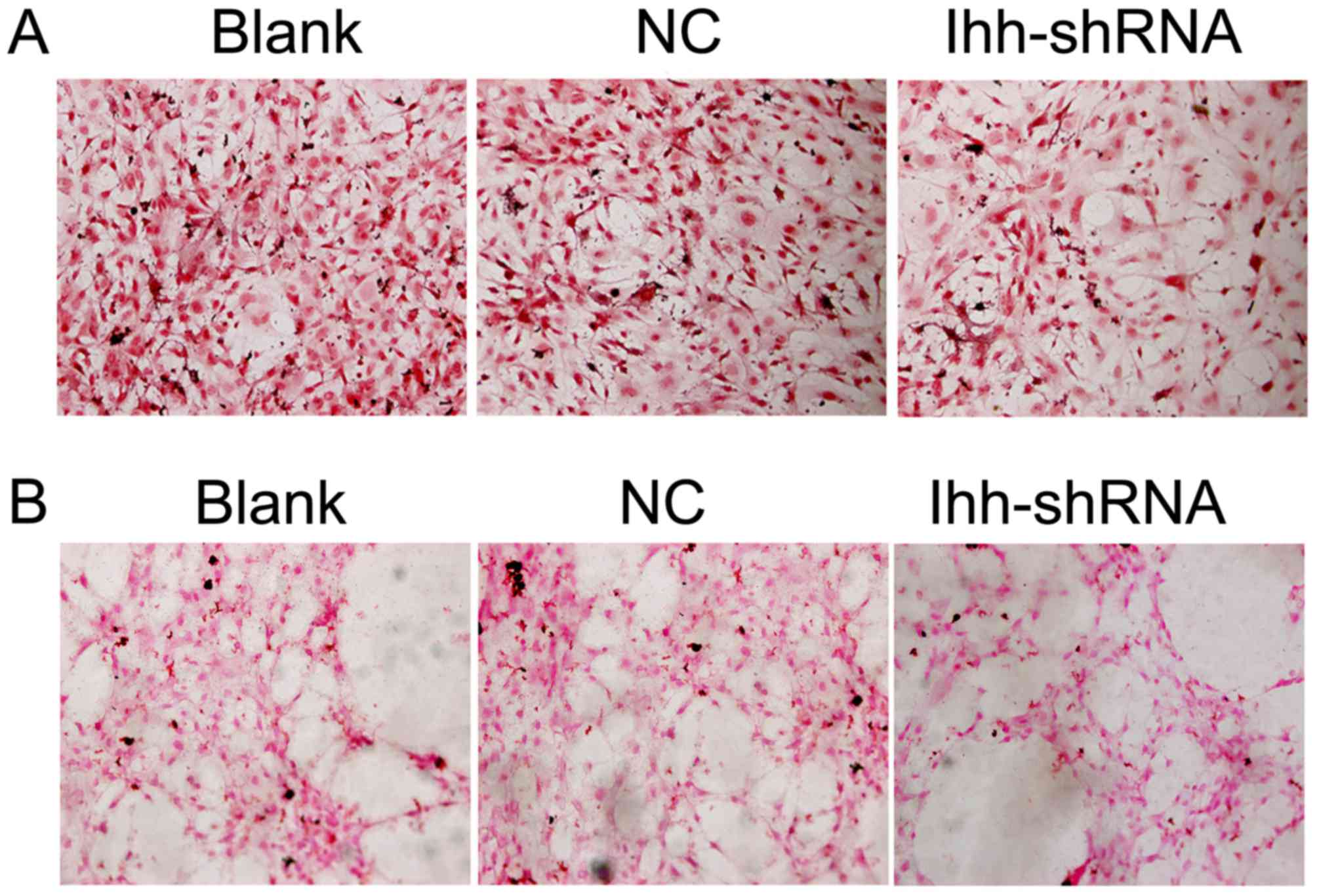
ALP is an enzyme that is required for bone
mineralization (13). ALP activity
was assessed in the present study and was markedly decreased in Ihh
knockdown cells compared with the NC group (Fig. 3A). The calcification and
mineralization of bone matrix are essential for the strength and
rigidity of the spinal skeletal system (14). To estimate mineralization and
calcification, von Kossa staining was performed following Ihh
knockdown. Representative images of von Kossa staining were
obtained by bright field microscopy (Fig. 3B). Compared with the NC cells,
mineral deposition was markedly decreased in the Ihh-shRNA
group.
Effects of Ihh on TGF-β/Smads and
OPG/RANKL signaling
The effects of Ihh on TGF-β/Smads and OPG/RANKL
signaling were also investigated. The mRNA expression of COL10A,
RANKL, Samd2 and Smad3 was significantly upregulated in the
Ihh-shRNA group compared with the NC cells (P<0.001; Fig. 4), whereas the levels of OCN, OPG,
PTHrP, Runx2 and TGF-β were significantly downregulated in the
Ihh-shRNA compared with the NC group (P<0.001; Fig. 4). Protein expression of these genes
was further assessed by western blot analysis (Fig. 5). The protein expression of COL10A,
RANKL, Samd2 and Smad3 were upregulated, whereas levels of OCN,
OPG, PTHrP, Runx2 and TGF-β were significantly downregulated in Ihh
knockdown cells compared with the NC group (P<0.05; Fig. 5). These findings suggest that the
inhibitory effects of Ihh knockdown on bone differentiation and
generation are achieved via the TGF-β/Smads and OPG/RANKL signaling
pathway.
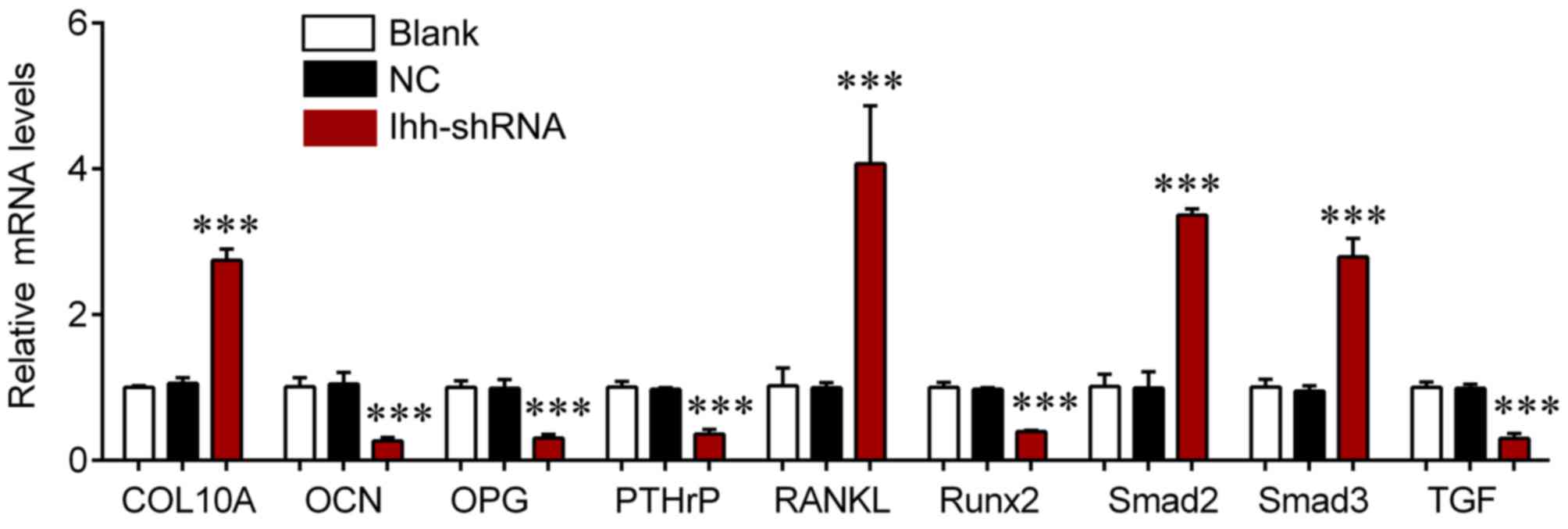 | Figure 4.Reverse transcription-quantitative
polymerase chain reaction was used to detect the mRNA expression of
COL10A, RANKL, Smad2, Smad3, OCN, OPG, PTHrP, Runx2 and TGF-β in
chondrocytes following transfection with Ihh shRNA or empty
lentivirus. ***P<0.001 vs. NC. COL10A, collagen type X α1 chain;
RANKL, receptor activator of nuclear factor-κB ligand; Smad,
mothers against decapentaplegic; OCN, osteocalcin; OPG,
osteoprotegerin; PTHrP, parathyroid hormone-related protein; Runx2,
runt-related transcription factor 2; TGF, transforming growth
factor; Ihh, Indian hedgehog; shRNA, short hairpin RNA; NC,
negative control. |
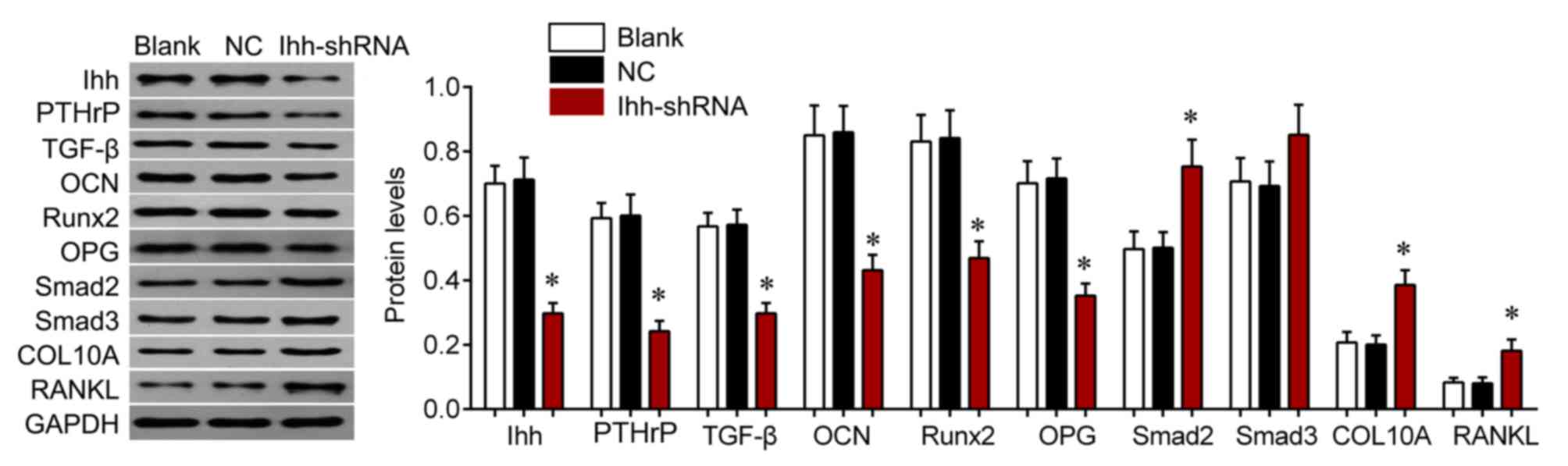 | Figure 5.Western blot analysis was used to
detect the protein expression of COL10A, RANKL, Smad2, Smad3, OCN,
OPG, PTHrP, Runx2 and TGF-β in chondrocytes following transfection
with Ihh shRNA or empty lentivirus. *P<0.05 vs. NC. COL10A,
collagen type X α1 chain; RANKL, receptor activator of nuclear
factor-κB ligand; Smad, mothers against decapentaplegic homolog;
OCN, osteocalcin; OPG, osteoprotegerin; PTHrP, parathyroid
hormone-related protein; Runx2, runt-related transcription factor
2; TGF, transforming growth factor; Ihh, Indian hedgehog; shRNA,
short hairpin RNA; NC, negative control. |
Discussion
In the present study, Ihh knockdown was demonstrated
to significantly inhibit cell growth and increase the apoptosis
rate compared with NC cells. Downregulation of Ihh resulted in cell
cycle arrest at G1 to S phase in chondrocytes. In addition, Ihh
knockdown decreased the ALP activity and mineral deposition of
chondrocytes. These results suggest that the inhibitory roles of
Ihh downregulation on chondrocyte growth and differentiation may be
associated with the TGF-β/Smads and OPG/RANKL signaling
pathways.
Ihh signaling is essential for chondrocyte
differentiation and endochondral ossification (15). Deletion of Ihh in postnatal
chondrocytes in a temporal/spatial-specific manner results in the
loss of ectopic hypertrophic chondrocyte formation in the growth
plate (16). In a previous study,
mutant mice with Ihh deletion exhibited a continuous loss of
trabecular bone over time, which indicates that postnatal
chondrocyte-derived Ihh is essential for skeletal growth,
maintaining the growth plate and sustaining trabecular bone growth
(16). A previous report
demonstrated that treating chondrogenic cells with an Ihh inhibitor
inhibited chondrocyte differentiation and secondary ossification
center formation (17). Furthermore,
Gualeni et al (18)
previously demonstrated that diastrophic dysplasia mouse
proteoglycan undersulfation resulted in reduced chondrocyte
proliferation in the proliferative zone via the Ihh pathway,
contributing to reduced long bone growth. Ihh expression has been
reported to be significantly increased in humans with the modic
degeneration I and II groups and is positively correlated with the
severity of degeneration (19).
Overexpression of Ihh signaling promotes abnormal chondrocyte
differentiation in endochondral ossification and enhances bone
formation in posterior longitudinal ligaments (20). Ihh is synthesized by chondrocytes and
is required for the synthesis of PTHrP (21). Ihh acts with PTHrP in a negative
feedback loop to regulate early chondrocyte differentiation and
hypertrophic differentiation (22).
Blocking Ihh signaling with cyclopamine has been reported to delay
chondrocyte hypertrophy in PTHrP knockout embryos, whereas
upregulating Ihh signaling in the postnatal cartilage led to
accelerated chondrocyte hypertrophy during secondary ossification,
indicating that Ihh signaling promotes chondrocyte hypertrophy
independently of PTHrP, which may be mediated by Wnt/β-catenin
signaling (23,24). As chondrocytes go through a program
of proliferation and subsequent differentiation into hypertrophic
chondrocytes, PTHrP maintains chondrocyte proliferation and delays
their further differentiation (25).
The differentiation-delaying action of PTHrP is mediated by
suppressing the synthesis of Runx2, which is a transcription factor
integral to osteoblast differentiation (26). In the absence of Ihh, the osteoblast
fails to activate the expression of Runx2 (27). However, forced expression of Runx2 in
the skeletogenic cells restores bone formation in the Runx2-null
embryo, whereas it does not in the Ihh-null embryo; this suggests
that Ihh-induced osteoblast differentiation requires additional
effectors (27).
The results of the present study demonstrate that
the mRNA and protein expression of hypertrophic markers, including
COL10A, RANKL, Smad2 and Smad3, are upregulated in chondrocytes
following Ihh knockdown, whereas levels of OCN, OPG, PTHrP Runx2
and TGF-β are significantly reduced. Previous studies have reported
that multiple signaling pathways, including Wnt/β-catenin and
TGF-β/Smad pathways, are able to regulate chondrocyte hypertrophy
(28). The Smad2/3 pathway is
directly activated by TGF-β, which leads to inhibited hypertrophy
(29). TGF-β activation of Smad3
also inhibits Runx2 via epigenetic regulation (30). Glioma-associated oncogene-Krüppel
family members (Gli) 1 and 2, which are Ihh downstream
transcription factors, increase COL10A activity and Runx2 promotes
COL10A1 expression via interacting with Ihh (31). Furthermore, Gli1 and Gli2 act in a
complex with Runx2/Smad to induce chondrocyte differentiation
(30), suggesting that Ihh signaling
may be an important factor for early chondrocyte differentiation
and the maturation and calcification of chondrocytes.
In conclusion, knockdown of Ihh suppresses
chondrocyte growth and differentiation and this effect may be
associated with the TGF-β/Smad and OPG/RANKL signaling pathways.
These results suggest that chondrocyte-derived Ihh is essential for
maintaining the growth plate and that manipulating Ihh expression
or its signaling components may be a novel effective treatment for
achondroplasia and other skeletal diseases.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Hunan Province (grant no. 2016JJ3160) and the
National Natural Science Foundation of China (grant no.
81472145).
References
|
1
|
Wang T, Zhang X and Bikle DD: Osteogenic
differentiation of periosteal cells during fracture healing. J Cell
Physiol. 232:913–921. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Komori T: Cell death in chondrocytes,
osteoblasts, and osteocytes. Int J Mol Sci. 17:pii: E20452016.
View Article : Google Scholar
|
|
3
|
Xu J, Li Z, Hou Y and Fang W: Potential
mechanisms underlying the Runx2 induced osteogenesis of bone marrow
mesenchymal stem cells. Am J Transl Res. 7:2527–2535.
2015.PubMed/NCBI
|
|
4
|
Day TF and Yang Y: Wnt and hedgehog
signaling pathways in bone development. J Bone Joint Surg Am. 90
Suppl 1:S19–S24. 2008. View Article : Google Scholar
|
|
5
|
Cai H and Liu A: Spop promotes skeletal
development and homeostasis by positively regulating Ihh signaling.
Proc Natl Acad Sci USA. 113:pp. 14751–14756. 2016; View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Liang W, Ye H, Weng X, Liu F, Lin P
and Liu X: Overexpression of Indian hedgehog partially rescues
short stature homeobox 2-overexpression-associated congenital
dysplasia of the temporomandibular joint in mice. Mol Med Rep.
12:4157–4164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang J, Andre P, Ye L and Yang YZ: The
hedgehog signalling pathway in bone formation. Int J Oral Sci.
7:73–79. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou S, Xie Y, Tang J, Huang J, Huang Q,
Xu W, Wang Z, Luo F, Wang Q, Chen H, et al: FGFR3 deficiency causes
multiple chondroma-like lesions by upregulating hedgehog signaling.
PLoS Genet. 11:e10052142015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Amano K, Densmore M, Fan Y and Lanske B:
Ihh and PTH1R signaling in limb mesenchyme is required for proper
segmentation and subsequent formation and growth of digit bones.
Bone. 83:256–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bechtold TE, Saunders C, Decker RS, Um HB,
Cottingham N, Salhab I, Kurio N, Billings PC, Pacifici M, Nah HD
and Koyama E: Osteophyte formation and matrix mineralization in a
TMJ osteoarthritis mouse model are associated with ectopic hedgehog
signaling. Matrix Biol 52–54. 1–354. 2016.
|
|
11
|
Breidenbach AP, Aschbacher-Smith L, Lu Y,
Dyment NA, Liu CF, Liu H, Wylie C, Rao M, Shearn JT, Rowe DW, et
al: Ablating hedgehog signaling in tenocytes during development
impairs biomechanics and matrix organization of the adult murine
patellar tendon enthesis. J Orthop Res. 33:1142–1151. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Orimo H: The mechanism of mineralization
and the role of alkaline phosphatase in health and disease. J
Nippon Med Sch. 77:4–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fujisawa R and Tamura M: Acidic bone
matrix proteins and their roles in calcification. Front Biosci
(Landmark Ed). 17:1891–1903. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X, Macica CM, Nasiri A and Broadus
AE: Regulation of articular chondrocyte proliferation and
differentiation by indian hedgehog and parathyroid hormone-related
protein in mice. Arthritis Rheum. 58:3788–3797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maeda Y, Nakamura E, Nguyen MT, Suva LJ,
Swain FL, Razzaque MS, Mackem S and Lanske B: Indian hedgehog
produced by postnatal chondrocytes is essential for maintaining a
growth plate and trabecular bone. Proc Natl Acad Sci USA. 104:pp.
6382–6387. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xing W, Cheng S, Wergedal J and Mohan S:
Epiphyseal chondrocyte secondary ossification centers require
thyroid hormone activation of Indian hedgehog and osterix
signaling. J Bone Miner Res. 29:2262–2275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gualeni B, Facchini M, De Leonardis F,
Tenni R, Cetta G, Viola M, Passi A, Superti-Furga A, Forlino A and
Rossi A: Defective proteoglycan sulfation of the growth plate zones
causes reduced chondrocyte proliferation via an altered Indian
hedgehog signalling. Matrix Biol. 29:453–460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sugita D, Yayama T, Uchida K, Kokubo Y,
Nakajima H, Yamagishi A, Takeura N and Baba H: Indian hedgehog
signaling promotes chondrocyte differentiation in enchondral
ossification in human cervical ossification of the posterior
longitudinal ligament. Spine (Phila Pa 1976). 38:E1388–E1396. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kobayashi T, Chung UI, Schipani E,
Starbuck M, Karsenty G, Katagiri T, Goad DL, Lanske B and
Kronenberg HM: PTHrP and Indian hedgehog control differentiation of
growth plate chondrocytes at multiple steps. Development.
129:2977–2986. 2002.PubMed/NCBI
|
|
21
|
Zhou J, Wei X and Wei L: Indian hedgehog,
a critical modulator in osteoarthritis, could be a potential
therapeutic target for attenuating cartilage degeneration disease.
Connect Tissue Res. 55:257–261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mak KK, Kronenberg HM, Chuang PT, Mackem S
and Yang Y: Indian hedgehog signals independently of PTHrP to
promote chondrocyte hypertrophy. Development. 135:1947–1956. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brechbiel JL, Ng JM and Curran T: PTHrP
treatment fails to rescue bone defects caused by hedgehog pathway
inhibition in young mice. Toxicol Pathol. 39:478–485. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Minina E, Wenzel HM, Kreschel C, Karp S,
Gaffield W, McMahon AP and Vortkamp A: BMP and Ihh/PTHrP signaling
interact to coordinate chondrocyte proliferation and
differentiation. Development. 128:4523–4534. 2001.PubMed/NCBI
|
|
25
|
Kronenberg HM: PTHrP and skeletal
development. Ann N Y Acad Sci. 1068:1–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tu X, Joeng KS and Long F: Indian hedgehog
requires additional effectors besides Runx2 to induce osteoblast
differentiation. Dev Biol. 362:76–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Studer D, Millan C, Öztürk E,
Maniura-Weber K and Zenobi-Wong M: Molecular and biophysical
mechanisms regulating hypertrophic differentiation in chondrocytes
and mesenchymal stem cells. Eur Cell Mater. 24:118–135. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim KO, Sampson ER, Maynard RD, O'Keefe
RJ, Chen D, Drissi H, Rosier RN, Hilton MJ and Zuscik MJ: Ski
inhibits TGF-β/phospho-Smad3 signaling and accelerates hypertrophic
differentiation in chondrocytes. J Cell Biochem. 113:2156–2166.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang JS, Alliston T, Delston R and Derynck
R: Repression of Runx2 function by TGF-beta through recruitment of
class II histone deacetylases by Smad3. EMBO J. 24:2543–2555. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shimoyama A, Wada M, Ikeda F, Hata K,
Matsubara T, Nifuji A, Noda M, Amano K, Yamaguchi A, Nishimura R
and Yoneda T: Ihh/Gli2 signaling promotes osteoblast
differentiation by regulating Runx2 expression and function. Mol
Biol Cell. 18:2411–2418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Amano K, Densmore M, Nishimura R and
Lanske B: Indian hedgehog signaling regulates transcription and
expression of collagen type X via Runx2/Smads interactions. J Biol
Chem. 289:24898–24910. 2014. View Article : Google Scholar : PubMed/NCBI
|