Introduction
Colorectal cancer (CRC) is one of the leading causes
of cancer-associated mortality in malignant tumors of the digestive
tract worldwide (1). The rapid
malignant transformation of cells, combined with the development of
resistance in CRC tumors makes effective treatment difficult.
Previous studies have demonstrated that CRC tumors undergo
metabolic reprogramming to favor aerobic glycolysis, even following
exposure to normoxic conditions; a phenomenon known as the Warburg
effect (2,3). This effect is important, as it
contributes to cancer proliferation and survival (2,3).
Although this bio-energetic switch occurs at the expense of
efficient glucose utilization, metabolic intermediates from aerobic
glycolysis may be used as biosynthetic precursors for the
generation of nucleic acids, phospholipids, fatty acids,
cholesterol and porphyrins, which are essential to support the
rapid proliferation of cancer cells (4). Aerobic glycolysis also confers survival
benefits to CRC cells within the ischemic and acidic tumor
microenvironment, and may function to facilitate immune system
evasion and maintenance of the cancer stem cell state (5). Through aerobic glycolysis, glucose is
metabolized to lactate, which is subsequently released from cells
and leads to the development of an acidic extracellular
microenvironment. The CRC cell metabolic shift affects cell
signaling, histone acetylation and the production of reactive
oxygen species, which are considered to be hallmarks of cancer
(6). However, their precise
influence requires further clarification (6). Therefore, understanding the mechanisms
underlying the bio-energetic switch of CRC cells may be crucial for
the development of effective cancer treatments.
Succinate dehydrogenase (SDH) is a metabolic enzyme
located on the inner mitochondrial membrane. It is required for the
oxidation of succinate to fumarate in the citric acid cycle; an
essential stage of oxidative phosphorylation (7). It also facilitates the engagement of
electron transportation (7). SDHB is
the core catalytic subunit of the heterotetrameric complex of SDH.
Previous studies have demonstrated that SDHB loss-of-function leads
to SDH dysfunction, which alters the citric acid cycle and
facilitates the development of different cancers, including
bladder, neuroendrocrine neoplasms, renal cell carcinoma and
gastrointestinal stromal tumors (8–10). SDHB
dysfunction can also suppress the α-ketoglutarate-dependent
synthesis of histone and DNA demethylases, which may influence the
expression of tumor suppressor genes and oncogenes (7). Additionally, the accumulation of
succinate as a consequence of SDHB dysfunction contributes to the
stabilization of hypoxia-inducible factor (HIF) under normoxic
conditions (7). This allows HIF to
facilitate the anaerobic metabolism and angiogenesis of tumor
cells. Therefore, SDHB loss-of-function may be implicated in the
Warburg effect observed in CRC cells.
Adenosine monophosphate activated protein kinase
(AMPK) is an important regulator of cellular metabolism and has
been implicated in the development of cancer (11,12). The
phosphorylation activity of AMPK is primarily, but not exclusively,
regulated by the ratio of AMP/adenosine 5′-triphosphate (ATP)
(11,12). AMPK activity is also affected by
reactive oxygen species, nitrous oxide, hormones (including
adiponectin, leptin, thyroid hormone, ghrelin and cannabinoids) and
pharmacological agents (11).
Previous studies have demonstrated that signaling pathways mediated
by the LKB1-STE20-related kinase adaptor-MO25 complex and by
calcium/calmodulin-activated protein kinase kinases, serve to
control AMPK phosphorylation (11,12). In
addition, AMPK has been implicated in the regulation of the Warburg
effect. Li et al (13)
reported that growth arrest-specific 1 activates AMPK and inhibits
mechanistic target of rapamycin (mTOR)/p70S6K signaling in CRC,
effectively inhibiting the Warburg effect, cell proliferation and
invasion. Further results have demonstrated that the multi-tyrosine
kinase inhibitors of anti-angiogenics initiates a cellular switch
from glycolysis to mitochondrial respiration-dependent oxidative
phosphorylation in spontaneous breast and lung tumor models
(14). This metabolic switch is
mediated by the downregulation of HIF1α and Akt and the
upregulation of AMPK (14). When
AMPK function is inhibited by AMPK inhibitors, AMPK
small-interfering RNA (siRNA) or dominant negative AMPK, the
inhibitory effects of 2-deoxyglucose on aerobic glycolysis is
diminished (14). These studies
therefore provide evidence to suggest that AMPK serves an
inhibitory role in aerobic glycolysis.
Based on a previous report indicating a close
association between SDHB and AMPK (15), it was hypothesized that AMPK may
mediate the aerobic glycolysis stimulated by SDHB deficiency. The
present study investigated the association between SDHB and the
diverse hallmarks of CRC cells, and further explored the regulatory
effect of SDHB on AMPK activity. The conclusions drawn from the
present study may provide some insight regarding how the Warburg
effect is generated in CRC.
Materials and methods
Tissue samples
CRC and corresponding para-carcinoma tissues were
obtained from a total of 23 patients (age, 43–51 years old; 16
males and 7 females) who were diagnosed between January 2014 and
May 2016 and underwent surgical resection in The Third Xiangya
Hospital of Central South University (Changsha, China). A pair of
CRC and para-carcinoma tissues were obtained from each patient. All
these patients included in the study did not receive radiotherapy
and chemotherapy treatments prior to the surgery. All tissue
samples were assessed via histopathological evaluation, and stored
in liquid nitrogen until utilization. The present study was
reviewed and approved by the Ethics Committee of Xiangya School of
Medicine (Changsha, China) and all patients enrolled in the study
had signed legally effective informed consent forms.
Cell culture and treatments
The human malignant colonic carcinoma cell line,
HT-29, was obtained from the American Type Culture Collection
(Manassas, VA, USA). Cells were incubated with Eagle's medium
(Hyclone, Logan, UT, USA) containing 10% fetal bovine serum (FBS,
Hyclone) at 37°C and 5% CO2. A total of four
short-hairpin RNAs (shRNAs), including SDHB-homo-305 (shRNA1),
SDHB-homo-447 (shRNA2), SDHB-homo-498 (shRNA3) and SDHB-homo-779
(shRNA4) targeting SDHB, were purchased from Shanghai GenePharma
Co., Ltd. (Shanghai, China). Following growth to 60–80% confluence,
1 µmol/ml shRNAs and 1 µmol/ml scrambled siRNA (Shanghai GenePharma
Co., Ltd.) were transfected into HT-29 cells using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. Transfection was
performed for 12 h at 37°C. An Olympus FluoView 1000 confocal
microscope was used to determine transfection efficiency and a
western blot analysis was performed to detect the inhibitory
effects of shRNAs on SDHB expression. A clone of the human SDHB
gene open reading frame was inserted into the enhanced green
fluorescent protein plasmid-C1 vector (Shanghai GenePharma Co.,
Ltd.) to construct an overexpression vector containing SDHB.
Transfection of HT-29 cells with overexpression vectors or empty
vectors was achieved using Lipofectamine® 2000
(Invitrogen: Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. 5-aminoimidazole-4-carboxamide
1-β-ribofuranoside (AICAR; an AMPK activator) and Compound C (an
AMPK inhibitor) were purchased from Selleck Chemicals (Houston,
Texas, USA). Cells were treated with 0.1 mM AICAR and 20 um/l
Compound C for 12 h to activate and inhibit the activity of AMPK,
respectively.
Adenosine 5′-diphosphate (ADP)/ATP
ratio assay
Changes in the ADP/ATP ratio were evaluated using
the ApoSENSOR™ ADP/ATP ratio assay kit (BioVision Inc.,
Milpitas, CA, USA) according to the manufacturer's protocol. The
luciferase enzyme included in the assay was used to catalyze the
generation of light from ATP and luciferin. ADP levels were
measured by its conversion to ATP, which was detected using the
same reaction. In the present study, the cells (1×105
cells/well) were plated onto the 96-well plate. The reaction mix
(100 µl, provided by the assay kit) was added to each well of a
96-well plate. The reaction mix was incubated for 3 h at room
temperature. Following removal, Nucleotide Releasing Buffer (50 µl)
was added, and the plate was gently agitated for 5 min at room
temperature. Light was measured using a luminometer.
Lactic acid measurement
Intracellular lactic acid concentrations were
determined using a Lactic Acid assay kit (cat. No. K607-100;
BioVision Inc.), according to the manufacturer's protocol. The kit
provides a high-sensitive probe that generates fluorescence
(excitation/emission wavelength, 535/587 nm) following a reaction
with lactic acid. Fluorescence was detected with an Infinite F500
Microplate Reader (Tecan Group, Ltd., Männedorf, Switzerland).
Analysis of cell viability
Cell viability was evaluated using an MTT assay.
HT-29 cells (1×105 cells/well) were first seeded in
96-well plates with 100 µl complete medium. Immediately after the
transfection and/or treatments with AMPK inhibitor or activator, 20
µl (at 5 mg/ml) MTT reagent (Sigma-Aldrich, Merck KGaA, Darmstadt,
Germany) was added to each well at 37°C. The medium containing MTT
was removed following 4 h, and 150 µl dimethyl sulfoxide was
subsequently added to terminate the reaction. A microplate reader
was used to examine the absorbance of each well at 570 nm.
Flow cytometry analysis
HT-29 cells (1×106 cells/tube) were
stained with Annexin-V-fluorescein isothiocyanate (FITC)/propidium
iodide (PI) prior to flow cytometry analysis. The HT-29 cells were
washed twice with cold PBS, and incubated with Annexin V-FITC and
PI (Beyotime Institute of Biotechnology, Nanjin, China) at room
temperature for 15 min in the dark. The collected cells were
analyzed using BD FACSCanto™ II clinical software and a BD
FACSCanto II flow cytometer (BD Biosciences, Franklin lakes, NJ,
USA) to evaluate the apoptosis rate.
Wound healing assay
HT-29 cells (3×105 cells/well) were
cultured in 6-well plates at 37°C for ~48 h until 100% cell
confluence. Immediately after the transfection and/or treatments
with AMPK inhibitor or activator, a 1-ml pipette tip was used to
aggregate a ‘scratch-wound’ on the cell monolayer. The culture
medium was then replaced with FBS-free medium. Microscope images of
the cells were captured immediately following scratching and
following 24 h. The cell migration rate was calculated based on the
movement of cells from initial placement to the final distance
travelled following 24 h. Cell migration rate was calculated using
the following equation: (initial distance-final distance)/initial
distance ×100.
Cell invasion assay
HT-29 cells (2×105 cells) were seeded in
the upper portion of Transwell chambers (Corning Inc., Corning, NY,
USA). The upper chamber contained an 8-µm pore and inserts coated
with Matrigel (Sigma-Aldrich; Merck KGaA). Complete medium was
subsequently added to the lower chamber. Following culture for 24
h, non-invading cells on the upper surface of the membrane were
removed with a cotton-tipped swab, while the invading cells on the
surface of the lower chamber were stained with 0.1% crystal violet
at room temperature for 20 min. The invading cells were quantified
by counting 10 random fields of view at ×200 magnification using a
light microscope (E200; Nikon Corporation, Tokyo, Japan).
Western blot assay
The tissues and cells used in the present study were
lysed with radioimmunoprecipitation assay buffer (Beyotime
institute of Biotechnology, Haimen, China) containing phosphatase
inhibitors (okadaic acid; cat. no. ab120375; Abcam, Cambridge, UK).
Equal quantities (20 µg) of total protein were separated on 10–12%
SDS-PAGE gels and then transferred to nitrocellulose membranes.
Following blocking with 5% non-fat milk overnight at 4°C, membranes
were immunoblotted with primary antibodies overnight at 4°C. These
included the anti-SDHB antibody (1:500 dilution; cat. no. ab14714;
Abcam), the anti-AMPKα antibody (1:1,000 dilution; cat. no. 5831;
CST Biological Reagent, Co., Ltd., Shanghai, China), the
anti-phosphorylated (p)-AMPKα (Thr172) antibody (1:1,000 dilution;
cat. no. 2535, CST Biological Reagent, Co., Ltd.),
anti-p-acetyl-CoA carboxylase (ACC; 1:1,000 dilution; cat. no.
sc-271965; Santa Cruz Biotechnology, Inc., Dallas, TX USA), the
anti-p21 antibody (1:1,000 dilution; cat. no. ab109199; Abcam), the
anti-active caspase-3 antibody (1:1,000 dilution; cat. no.
ab109199; Abcam) and the anti-β-actin antibody (1:1,000 dilution;
cat. no. ab2302; Abcam). Membranes were washed with Tris-buffered
saline-Tween-20, and then incubated with horseradish
peroxidase-conjugated anti-IgG secondary antibodies (Sigma-Aldrich;
Merck KGaA) at 37°C for 1 h. Blots were visualized using a Beyotime
Enhanced Chemiluminescence Kit (Beyotime Institute of
Biotechnology) and Fuji medical X-ray film (Fujifilm, Tokyo,
Japan).
Tumor xenografts in nude mice
The animal experiments performed in the present
study were approved by the Ethics Committee for Animal Research of
The Southern Medical University (protocol number: 2011–020;
Changsha, China). A total of 9 male nude mice (age, 4–5 weeks;
weight, ~25 g) were purchased from the Central Animal Facility of
The Southern Medical University. All animals were maintained under
environmentally controlled conditions (12-h light/dark cycles,
20–23°C and 50% relative humidity) with access to food and water
ad libitum. Animals were divided into three groups: Control
group, OV group and OV+Act group (3 mice per group). In the OV
group, HT-29 cells (1×106 cells in 20 ml medium), were
transfected with SDHB overexpression vectors for 24 h. These cells
were subsequently injected into the left side on the back of each
mouse. For the OV+Act group, at 12 days following the cell
injection, 5 mg/kg (body weight) of AICAR was subcutaneously
injected once a day for 6 days. Control mice were injected with
HT-29 cells that were transfected with empty vectors. Subsequently,
control mice were injected with saline solution instead of AICAR.
The tumors were obtained and removed 20 days following subcutaneous
injection of AICAR. Tumor volume (V) was calculated as V=(length ×
width2)/2.
Statistical analysis
The results were analyzed using SPSS 12.0 software
(SPSS, Inc., Chicago, IL, USA). Significant differences were
calculated using a one-way analysis of variance followed by
Scheffe's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression profiles of SDHB and AMPK
in CRC tissues
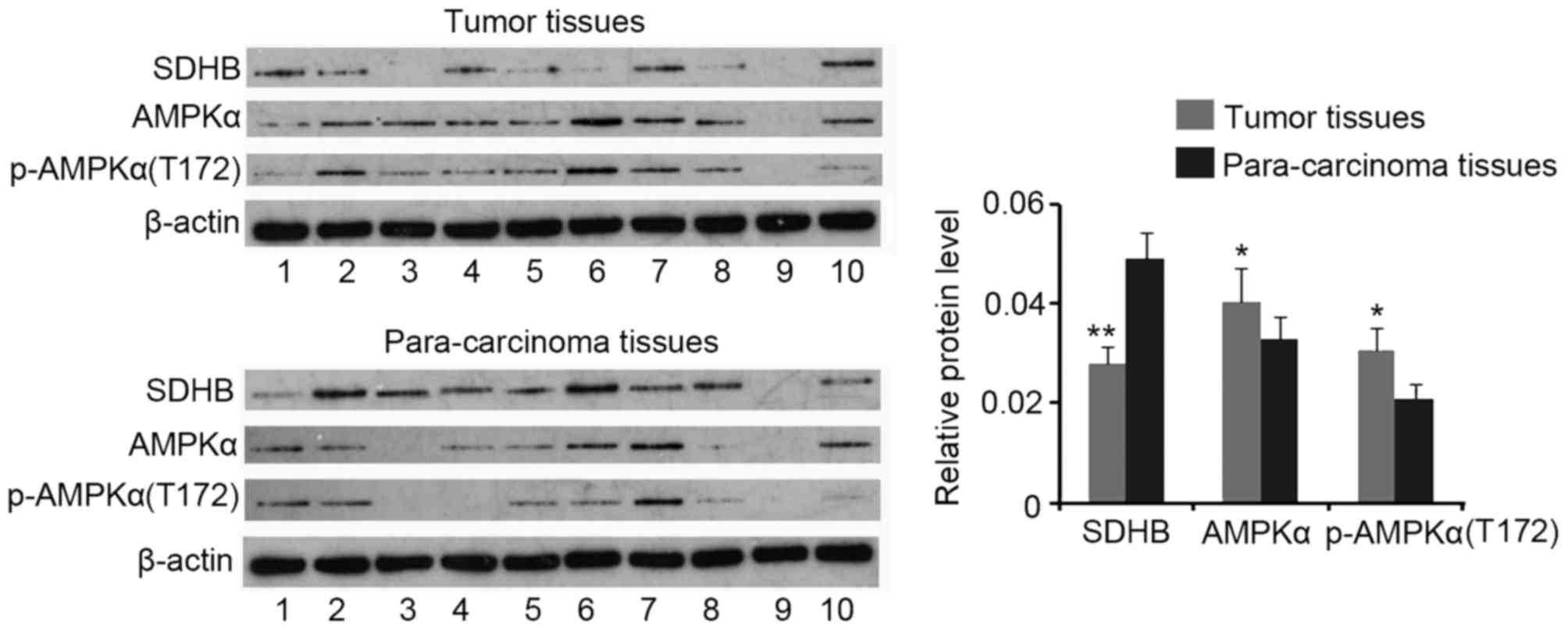
Western blotting analysis demonstrated a decrease in
SDHB protein expression in CRC tissues when compared with that of
para-carcinoma tissues (P<0.01), while AMPK and p-AMPK(Thr172)
expression were increased in CRC tissues (both P<0.05; Fig. 1). No significant differences in the
ratio of p-AMPK(Thr172)/AMPK were identified between CRC and
para-carcinoma tissues (data not shown).
SDHB regulates AMPK expression and
activity in CRC cells
SDHB in HT-29 CRC cells was overexpressed or
silenced in order to assess its regulatory effect on AMPK activity.
Compared with the control, cells transfected with the SDHB
expression vector exhibited an increase in SDHB protein expression
(P<0.01 vs. control; Fig. 2). A
total of four shRNAs targeting SDHB were transfected into HT-29
cells individually. SDHB protein levels were significantly
decreased following transfection with shRNA2 (P<0.01), shRNA3
(P<0.05) and shRNA4 (P<0.05; Fig.
2) when compared with controls. shRNA2 was selected for SDHB
knockdown in further experiments.
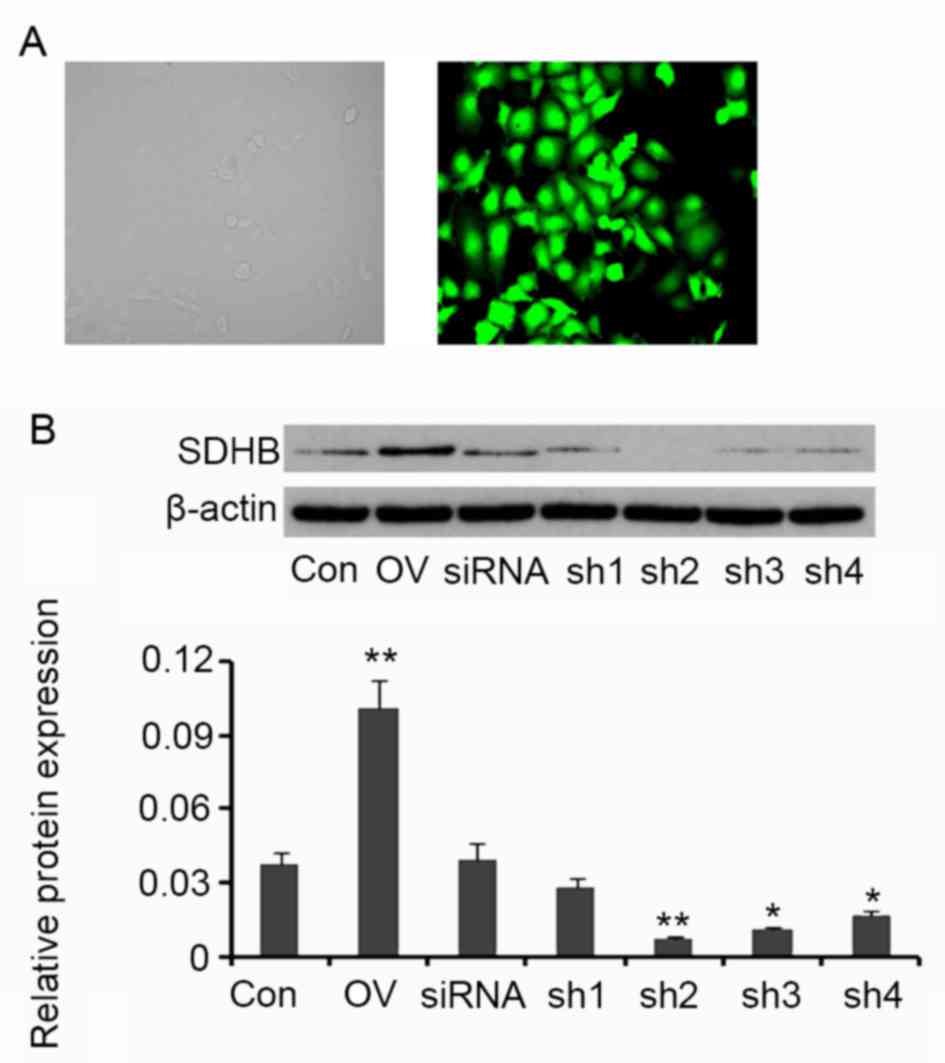 | Figure 2.Ectopic overexpression and knockdown
of SDHB in CRC HT-29 cells. Four shRNAs targeting SDHB and a
scrambled siRNA were transfected into HT-29 cells The human SDHB
open reading frame was inserted into an enhanced green fluorescent
protein plasmid-C1 vector to construct SDHB overexpression vectors.
(A) Fluorescent photographs indicate the transfection efficiency
(magnification, ×400). (B) Western blot analyses were performed to
detect SDHB protein expression in HT-29 cells. *P<0.05,
**P<0.01 vs. control group. SDHB, succinate dehydrogenase-B;
CRC, colorectal cancer; shRNA, short-hairpin RNA; Con, control; OV,
SDHB expression vector; sh1, SDHB-homo-305; sh2, SDHB-homo-447;
sh3, SDHB-homo-498; sh4, SDHB-homo-779. |
Western blotting analysis demonstrated that
upregulation of SDHB using the overexpression vector was associated
with reduced AMPK and p-AMPK(Thr172) protein levels, when compared
with the group that received no treatment (both P<0.05; Fig. 3). The subsequent addition of AICAR
increased the protein level of p-AMPK(Thr172) but did not affect
AMPK levels. HT-29 cells treated with AICAR alone exhibited an
increase in p-AMPK(Thr172) expression when compared with the
control (P<0.05). shRNA-mediated suppression of SDHB stimulated
the upregulation of AMPK and p-AMPK(Thr172) when compared with
controls (both P<0.05). Treatment of HT-29 cells with Compound C
alone resulted in a decrease in p-AMPK(Thr172) expression
(P<0.05) compared with control. The p-AMPK(Thr172)/AMPK ratio
was not affected by the ectopic over-expression of SDHB. The
addition of AICAR, alone or in combination with SDHB
overexpression, significantly elevated the p-AMPK(Thr172)/AMPK
ratio (P<0.05), indicating an increase in AMPK activity. SDHB
knockdown also increased the p-AMPK(Thr172)/AMPK ratio (P<0.05
vs. controls; Fig. 3). Cells treated
with Compound C demonstrated a significant reduction in the
p-AMPK(Thr172)/AMPK ratio, irrespective of SDHB knockdown
(P<0.05). ACC is a direct downstream target of AMPK; therefore,
the level of ACC phosphorylation is used to evaluate AMPK function
(16). The overexpression of SDHB or
the inhibition of AMPK activity resulted in a significant decrease
in p-ACC protein expression levels compared with the controls (both
P<0.05). Conversely, SDHB knockdown or an enhanced AMPK activity
demonstrated a significant increase in p-ACC levels (P<0.05 vs.
controls; Fig. 3).
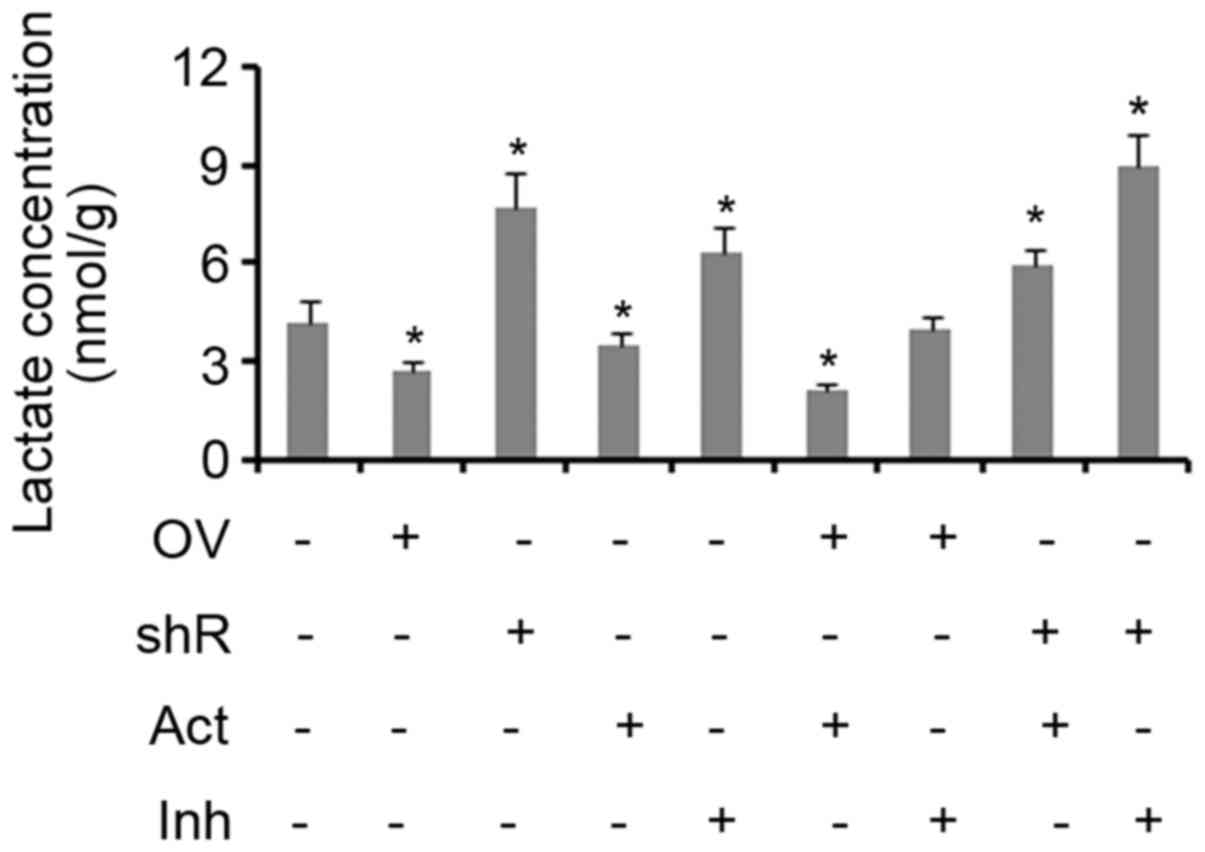
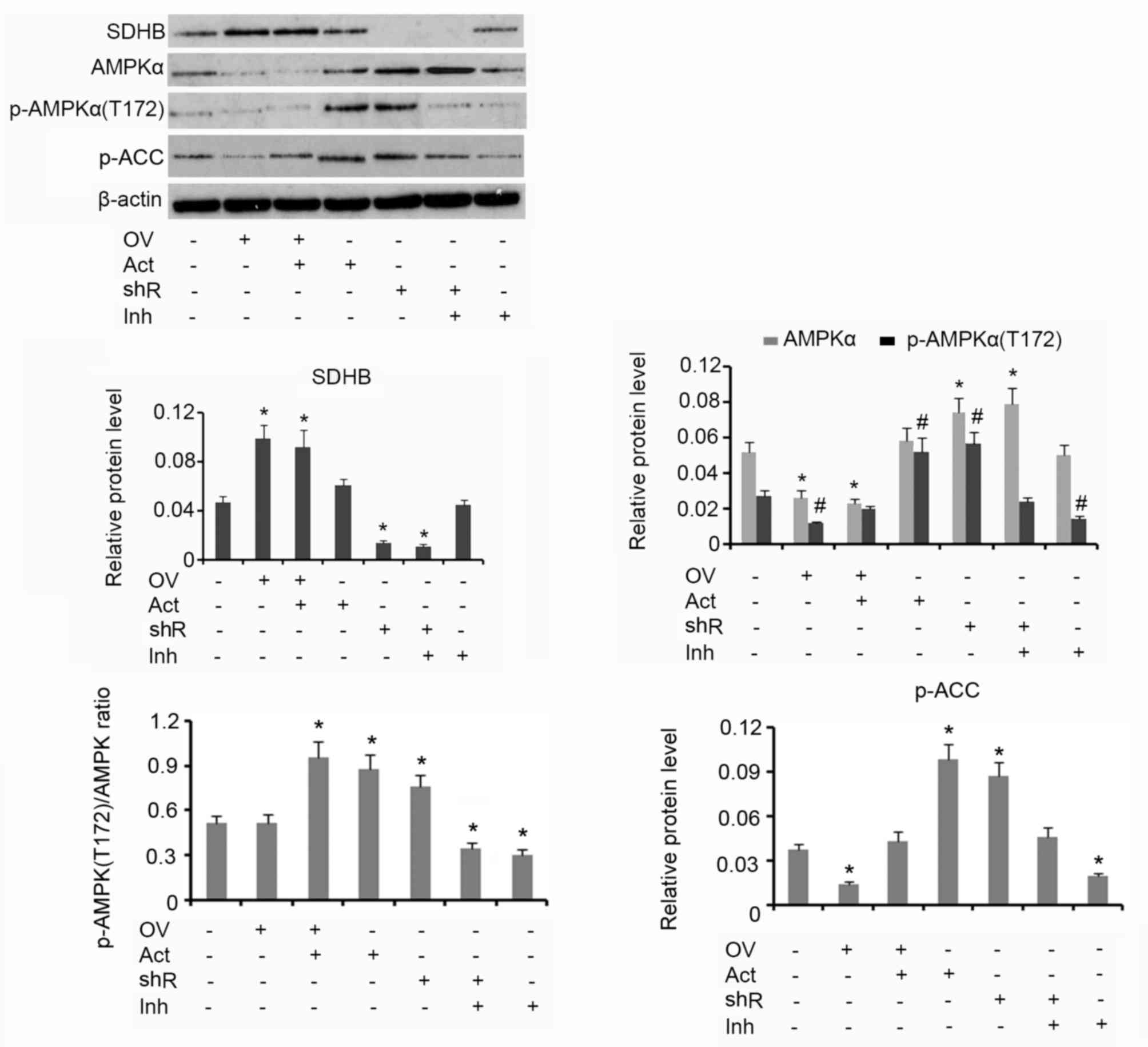 | Figure 3.Inhibitory effect of SDHB on AMPK in
CRC cells. SDHB was overexpressed or knocked down in HT-29 CRC
cells. A total of 0.1 mM AICAR (an AMPK activator) and 20 µm/l
Compound C (an AMPK inhibitor) were added to activate and inhibit
the activity of AMPK, respectively. Western blot analysis was
performed to detect the protein levels of SDHB, AMPK p-AMPK(Thr172)
and p-ACC in HT-29 cells. *P<0.05 and #P<0.05 vs.
the respective control group. SDHB, succinate dehydrogenase-B;
p-AMPK, phosphorylated adenosine monophosphate activated protein
kinase α; CRC, colorectal cancer; AICAR,
5-aminoimidazole-4-carboxamide ribonucleotide; OV, SDHB expression
vector; Act, AMPK activator; shR, shRNA targeting SDHB; Inh, AMPK
inhibitor; ACC, anti-p-acetyl-CoA carboxylase. |
Effect of SDHB and AMPK on aerobic
glycolysis in CRC cells
Lactic acid is produced as a by-product of aerobic
glycolysis, but not aerobic respiration. Therefore, the yield of
lactic acid can be used to evaluate the level of glycolysis
activity (17,18). The results of the present study
demonstrated that treatment with an AMPK activator inhibited lactic
acid production in cells when compared with controls (P<0.05;
Fig. 4). SDHB overexpression was
also identified to exert the same effect (P<0.05 vs. controls).
The suppression of SDHB and/or AMPK activity resulted in a
significant increase in the production of lactic acid in cells (all
P<0.05; Fig. 4). The combination
of SDHB overexpression and AMPK inhibition did not influence the
production of lactic acid. Treatment with an AMPK agonist did not
abrogate the effect of SDHB knockdown on lactic acid production, as
the levels remained significantly higher when compared with the
controls (P<0.05; Fig. 4).
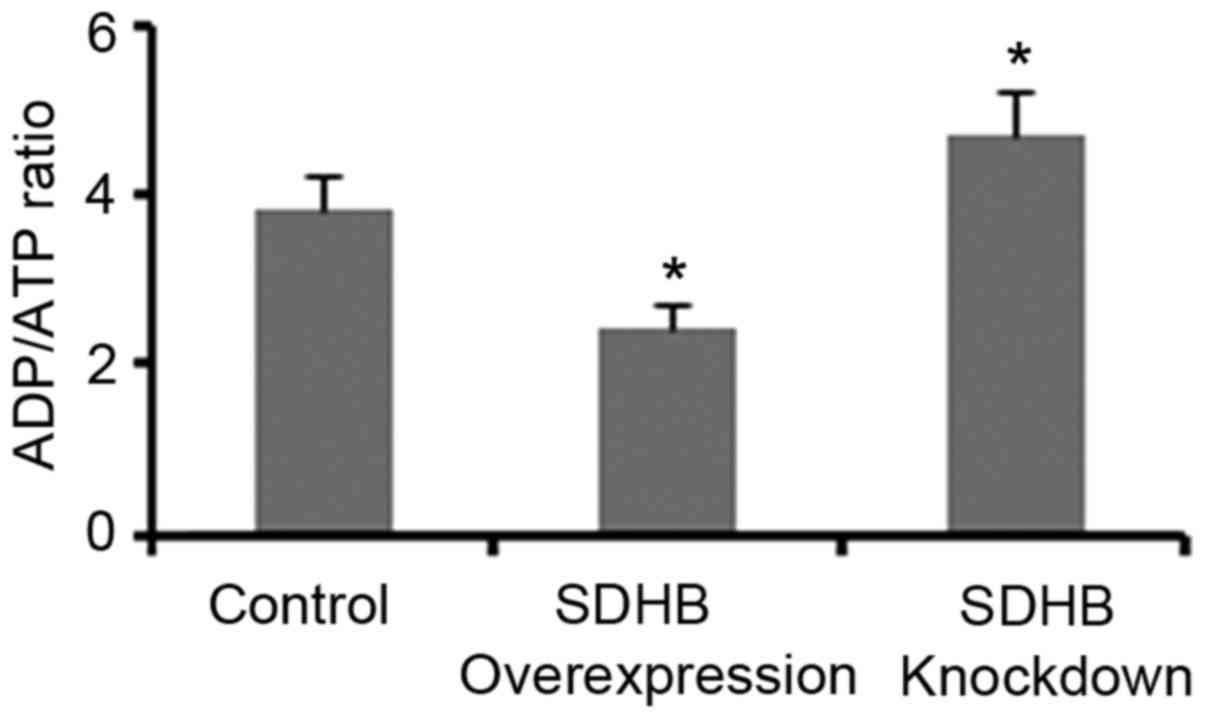
Regulation of the ADP/ATP ratio in CRC
cells by SDHB
The ADP/ATP ratio significantly influences AMPK
activity in cells (17,18). Therefore, the present study
investigated whether the regulation of AMPK activity via SDHB may
be dependent on the ADP/ATP ratio. As demonstrated in Fig. 5, SDHB overexpression and knockdown
resulted in a significant reduction and elevation of the ADP/ATP
ratio, respectively when compared with controls (both
P<0.05).
Regulation of the diverse hallmarks of
CRC cells through SDHB-mediated AMPK modulation
Results from the MTT assay demonstrated that
overexpression of SDHB in HT-29 cells is associated with a
significant decrease in cell viability (P<0.05 vs. controls;
Fig. 6A), and that the subsequent
addition of AICAR reverses this effect. SDHB silencing or the
addition of AICAR significantly increased the viability of HT-29
cells when compared with the control (both P<0.05). SDHB
knockdown in combination with Compound C treatment exerted no
effect on cell viability. However, treatment with Compound C alone
significantly inhibited cell viability when compared with controls
(P<0.05; Fig. 6A). The effect of
SDHB on the apoptosis rate of HT-29 cells using flow cytometry
analysis was then determined. The apoptosis rate was significantly
increased when HT-29 cells overexpressed SDHB compared with
controls (P<0.01; Fig. 6B).
Subsequent treatment with AICAR reversed the increase in apoptosis
rate; however, the rate remained significantly greater than that
observed in controls (P<0.05). AICAR also significantly
suppressed the apoptosis rate of HT-29 cells that were not
transfected with the SDHB expression vector (P<0.05 vs.
controls). SDHB silencing in HT-29 cells significantly reduced the
apoptosis rate (P<0.05); however co-treatment with Compound C
abrogated this effect. The addition of Compound C in HT-29 cells,
in which SDHB was not silenced, significantly enhanced the rate of
apoptosis (P<0.05 vs. controls; Fig.
6B).
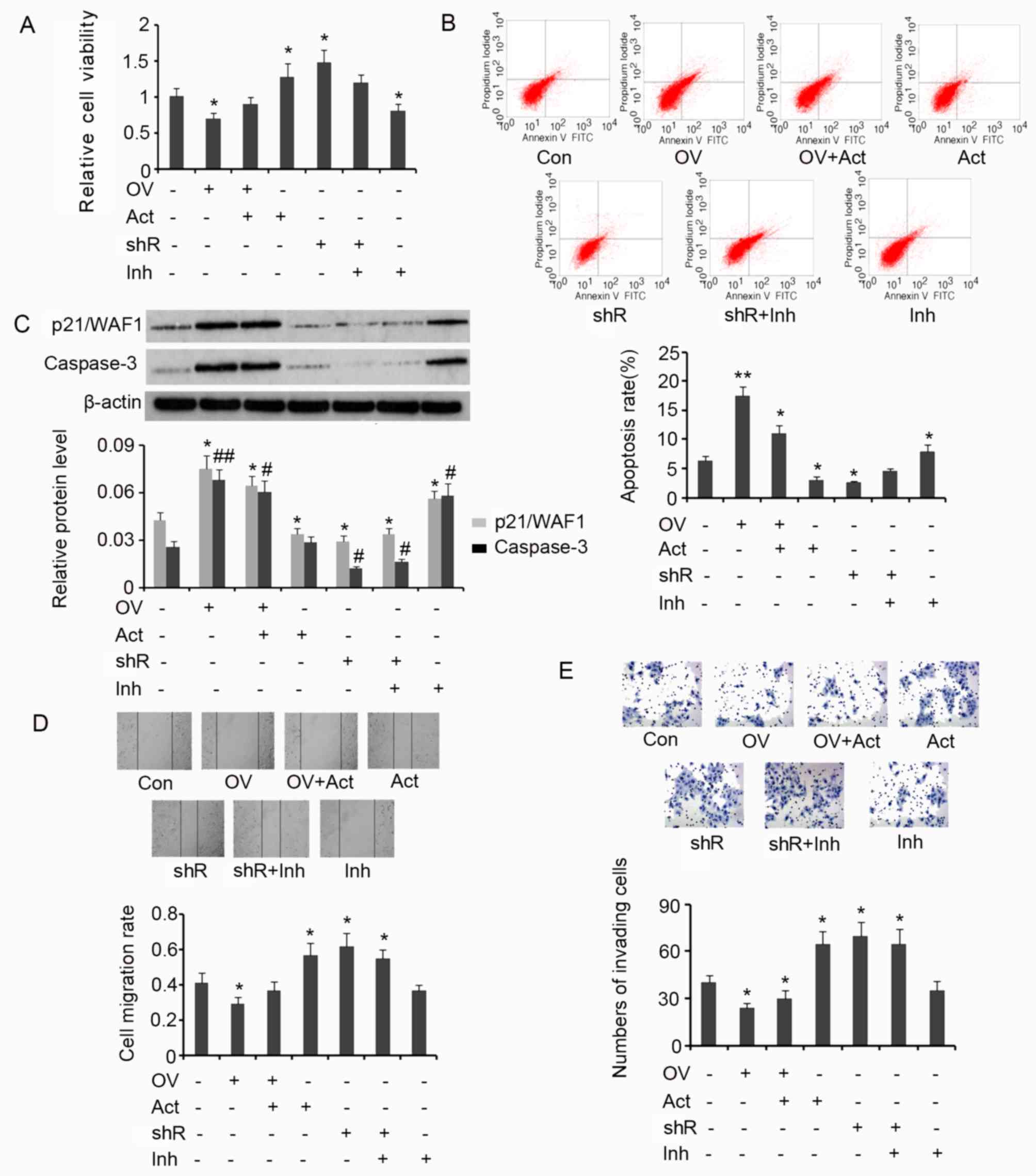 | Figure 6.SDHB-mediated regulation of the
diverse hallmarks of CRC cells through AMPK modulation. SDHB was
overexpressed or knocked down in CRC HT-29 cells. A total of 0.1 mM
AICAR (an AMPK activator) and 20 µm/l Compound C (an AMPK
inhibitor) were added to activate and inhibit the activity of AMPK,
respectively. Cell viability and apoptosis were evaluated using (A)
MTT and (B) flow cytometry analysis, respectively. (C) Western blot
analysis was performed to detect p21/WAF1 and caspase-3 expression.
Cell migration and invasion were evaluated using (D) wound healing
(magnification, ×200; Con indicated the 0 h time point) and (E)
invasion (magnification, ×400) assays. *P<0.05, **P<0.01,
#P<0.05 and ##P<0.01 vs. the respective
control group. SDHB, succinate dehydrogenase-B; CRC, colorectal
cancer; AMPK, adenosine monophosphate activated protein kinase;
AICAR, 5-aminoimidazole-4-carboxamide ribonucleotide; OV, SDHB
expression vector; Act, AMPK activator; shR, shRNA targeting SDHB;
Inh, AMPK inhibitor; FITC, Annexin-V-fluorescein
isothiocyanate. |
p21/WAF1 serves an inhibitory role in the regulation
of cell proliferation through the induction of cell cycle arrest
(19). A key step in the apoptosis
signaling pathway is the activation of caspase-3. This process is
dependent on the cleavage of the inactive form of caspase-3 to the
active form. Increased levels of activated caspase-3 are therefore
associated with the promotion of apoptosis (19). In the present study, the levels of
p21/WAF1 and activated caspase-3 in HT-29 cells were assessed to
investigate the effect of SDHB on CRC cell proliferation and
apoptosis. Induced expression of SDHB in HT-29 cells significantly
upregulated p21/WAF1 (P<0.05) and activated caspase-3
(P<0.01) expression when compared with controls (Fig. 6C). Despite reducing the effects of
SDHB overexpression on cell proliferation, the levels of p21/WAF1
and activated caspase-3 remained significantly higher following
AICAR treatment compared with controls (both P<0.05; Fig. 6C). Cells that were not subjected to
SDHB overexpression, demonstrated a significant reduction in
p21/WAF1 levels following treatment with AICAR (P<0.05 vs.
controls). However, the level of activated caspase-3 remained the
same. Silencing of SDHB expression with shRNA resulted in a
significant decrease in p21/WAF1 (P<0.05) and activated
caspase-3 (P<0.05) expression, regardless of additional
treatment with Compound C. Compound C treatment alone significantly
upregulated p21/WAF1 (P<0.05) and activated caspase-3 levels
(P<0.05) in HT-29 cells compared with controls (Fig. 6C).
HT-29 cell migration and invasion were then
evaluated by wound healing and cell invasion assays, respectively.
SDHB upregulation significantly attenuated cell migration
(P<0.05 vs. controls; Fig. 6D);
however, subsequent AICAR treatment reversed the observed
inhibitory effects. AICAR treatment or SDHB knockdown significantly
promoted cell migration (both P<0.05 vs. controls). Compound C
moderately attenuated the increase in migration of cells treated
with SDHB shRNA (Fig. 6D). Cell
invasion was also significantly attenuated by the upregulation of
SDHB (P<0.05 vs. controls; Fig.
6E). Subsequent AICAR treatment did not restore the
invasiveness of the cells. AICAR treatment and/or SDHB knockdown
instead promoted cell invasion (all P<0.05 vs. controls). The
subsequent addition of Compound C led to a moderate decrease in the
cell invasiveness following knockdown of SDHB (Fig. 6E).
SDHB inhibits CRC growth in nude mice
through AMPK downregulation
A tumor xenograft model involving nude mice was
developed to assess the inhibitory effect of SDHB on CRC growth
in vivo. Western blotting demonstrated that SDHB
upregulation in xenografted tumors was associated with a
significant decrease in AMPK and p-AMPK(Thr172) expression
(P<0.05 vs. controls; Fig. 7A).
The subsequent injection of AICAR restored protein levels to that
of the controls. Upregulation of SDHB significantly decreased the
volume of xenografted tumors (P<0.05 vs. controls), while the
injection of AICAR abrogated this effect (Fig. 7B).
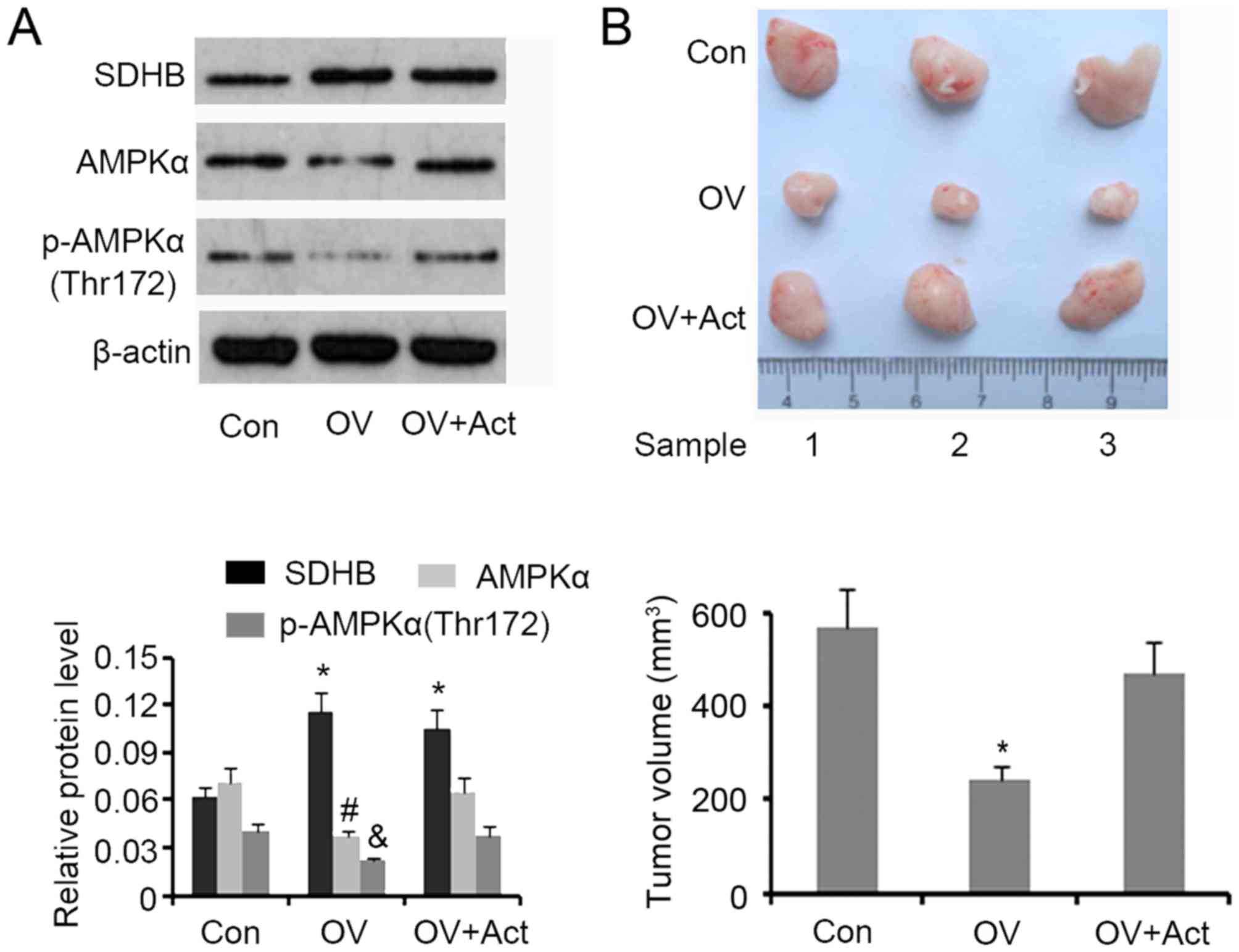 | Figure 7.Effect of SDHB on CRC growth in nude
mice and the role of AMPK. HT29 cells (1×106) were
transfected with SDHB overexpression vectors or empty vectors, and
were then injected into the left side on the back of each mouse (3
mice per group). At 12 days following injection, 5 mg/kg (body
weight) AICAR (an AMPK inhibitor) was subcutaneously injected once
a day for 6 days. The tumors were obtained and removed 20 days
following the injection of cells. (A) Western blotting was
performed to detect SDHB, AMPK and p-AMPK(Thr172) expression. (B)
Images of the xenografted tumors are presented and tumor volumes
were ascertained. *P<0.05, #P<0.05 and
&P<0.05 vs. the respective control group. SDHB,
succinate dehydrogenase-B; CRC, colorectal cancer; AMPK, adenosine
monophosphate activated protein kinase; AICAR,
5-aminoimidazole-4-carboxamide ribonucleotide; OV, SDHB expression
vector; Act, AMPK activator. |
Discussion
SDHB dysfunction and downregulation has been closely
associated with the development of cancer. Individuals with
germline mutations in the genes encoding SDHB are predisposed to
paraganglioma, pheochromocytoma, renal cell carcinoma and
gastrointestinal stromal tumors (8,9). In
patients with recurrent nasopharyngeal carcinoma, low SDHB
expression rates are associated with reduced overall survival
(20). A previous study involving
SDHB-transfected cells and cDNA microarray assays, demonstrated
that the majority of differentially expressed genes are associated
with cell cycle control and cell proliferation (21). The present study demonstrated that
SDHB was downregulated in CRC tissues when compared to
para-carcinoma tissues, indicating that SDHB may be involved in the
development of CRC. Cardaci et al (7) investigated the changes in metabolism
following SDHB ablation in immortalized mouse kidney cells. Through
metabolomics screening and isotope-labeling, it was established
that cellular loss of SDHB is sufficient to inhibit the
tricarboxylic acid (TCA) cycle and promote the Warburg-like
bioenergetic features of aerobic glycolysis in proliferating cells
(7). In addition, SDHB silencing
induced via siRNA promoted the occurrence of characteristic
features of the tumor phenotype, such as the shift from respiration
to glycolysis (22). Microarray
analysis identified >400 genes that are influenced by SDHB
silencing, and several of these genes were associated with the TCA
cycle and tumor development (22).
The results of these studies therefore suggest that a decrease in
SDHB in CRC tissues may facilitate aerobic glycolysis and cancer
progression.
AMPK, an established regulator of metabolic
pathways, serves an inhibitory role in aerobic glycolysis and
participates in the regulation of the diverse hallmarks of cancer
(13,14,23,24). The
present study identified that AMPK and p-AMPK(Thr172) were
increased in CRC tissues; however, the ratio of p-AMPK(Thr172)/AMPK
remained unchanged. The authors hypothesized that AMPK exerts an
inhibitory effect on aerobic glycolysis in CRC. In accordance with
this, in vitro experiments demonstrated that AMPK negatively
regulated lactic acid production in CRC cells, further indicating
that aerobic glycolysis was affected.
Further in vitro experiments demonstrated
that knockdown of SDHB increased AMPK and p-AMPK expression, while
overexpression of SDHB decreased the expression of AMPK alone. This
indicated that AMPK and p-AMPK(Thr172) protein levels may be
negatively regulated by SDHB. However, the regulatory effect of
SDHB on AMPK activity may be condition-dependent as SDHB knockdown
increased the expression of p-AMPK, whereas SDHB overexpression
demonstrated no effect. The present study demonstrated that the
level of p-ACC was negatively correlated with the level of SDHB.
This suggested that SDHB exerted a negative effect on AMPK, as ACC
is a direct downstream target (16).
It is well established that AMPKα function is influenced by AMP/ATP
and ADP/ATP ratios. AMPKα is activated in response to a shortage of
ATP in conditions of metabolic stress (11–13). As
SDH is an essential enzyme involved in the citric acid cycle, SDHB
dysfunction inhibits the production of ATP (25,26).
Previous studies have determined that a lower concentration of ATP
is present in tumor cells than in their normal cellular
counterparts (25,26). The present study demonstrated that
SDHB knockdown increased the ADP/ATP ratio, whereas ectopic
overexpression of SDHB was associated with a decreased ADP/ATP
ratio. These results indicate that SDHB negatively regulates the
ADP/ATP ratio in CRC cells, which may be important for the
SDHB-mediated regulation of AMPK expression and activity.
The results of the current study demonstrated that
oncogenic actions triggered by SDHB deficiency may be closely
associated with an increase in AMPK function. Suppression of AMPKα
activity inhibited the promoting effects of SDHB downregulation on
CRC proliferation, migration and invasion. Although SDHB
upregulation inhibited the proliferation and invasion of CRC cells
and decreased AMPK expression, the addition of AICAR reversed this
effect. This phenomenon was also observed in xenograft tumors in
nude mice. Therefore, downregulation of SDHB in CRC may be
dependent on AMPK activation. However, this is contradictory to the
inhibitory role of AMPK in aerobic glycolysis. The stimulatory
effects of SDHB deficiency on CRC proliferation and invasion may
therefore be associated with AMPK functions that exclude those
associated with the modulation of aerobic glycolysis. The
regulatory roles of AMPKα in cancer are diverse. In the majority of
cases, increased AMPKα activity is associated with a poor
prognosis, as AMPK provides cells with a selective advantage to
proliferate and survive by activating a number of signaling
molecules, including eukaryotic elongation factor 2 kinase, NUAK1/2
and microtubule affinity regulating kinases (23,24).
However, AMPKα may also function as a tumor suppressor through the
negative regulation of aerobic glycolysis, mTOR and HIF-1α
signaling pathways (12).
Collectively, AMPK exerts a duality of functions that are either
pro- or anti-tumorigenic, depending upon the context. Further
studies are required to investigate the mechanisms by which AMPK
mediates SDHB deficiency-induced proliferation and invasion of CRC
cells.
In conclusion, the present study demonstrated that
SDHB may function as a negative regulator of AMPKα in CRC. AMPKα
mediated SDHB deficiency-induced proliferation, migration and
invasion of CRC cells. It was also determined that the utilization
of aerobic glycolysis, that arises from the deficiency of SDHB, is
unlikely to be a result of AMPK function. Further studies are
warranted to elucidate the mechanism by which SDHB regulates
aerobic glycolysis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81402010).
References
|
1
|
Li M, Song LH, Yue GG, Lee JK, Zhao LM, Li
L, Zhou X, Tsui SK, Ng SS, Fung KP, et al: Bigelovin triggered
apoptosis in colorectal cancer in vitro and in vivo via
upregulating death receptor 5 and reactive oxidative species. Sci
Rep. 7:421762017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sprowl-Tanio S, Habowski AN, Pate KT,
McQuade MM, Wang K, Edwards RA, Grun F, Lyou Y and Waterman ML:
Lactate/pyruvate transporter MCT-1 is a direct Wnt target that
confers sensitivity to 3-bromopyruvate in colon cancer. Cancer
Metab. 4:202016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taniguchi K, Sugito N, Kumazaki M,
Shinohara H, Yamada N, Matsuhashi N, Futamura M, Ito Y, Otsuki Y,
Yoshida K, et al: Positive feedback of DDX6/c-Myc/PTB1 regulated by
miR-124 contributes to maintenance of the Warburg effect in colon
cancer cells. Biochim Biophys Acta. 1852:1971–1980. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parra-Bonilla G, Alvarez DF, Al-Mehdi AB,
Alexeyev M and Stevens T: Critical role for lactate dehydrogenase A
in aerobic glycolysis that sustains pulmonary microvascular
endothelial cell proliferation. Am J Physiol Lung Cell Mol Physiol.
299:513–522. 2010. View Article : Google Scholar
|
|
5
|
Lee N and Kim D: Cancer metabolism:
Fueling more than just growth. Mol Cells. 39:847–854. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gu Z, Xia J, Xu H, Frech I, Tricot G and
Zhan F: NEK2 promotes aerobic glycolysis in multiple myeloma
through regulating splicing of pyruvate kinase. J Hematol Oncol.
10:172017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cardaci S, Zheng L, MacKay G, van den
Broek NJ, MacKenzie ED, Nixon C, Stevenson D, Tumanov S, Bulusu V,
Kamphorst JJ, et al: Pyruvate carboxylation enables growth of
SDH-deficient cells by supporting aspartate biosynthesis. Nat Cell
Biol. 17:1317–1326. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saxena N, Maio N, Crooks DR, Ricketts CJ,
Yang Y, Wei MH, Fan TW, Lane AN, Sourbier C, Singh A, et al:
SDHB-deficient cancers: The role of mutations that impair iron
sulfur cluster delivery. J Natl Cancer Inst. 108:2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuroda N, Yorita K, Nagasaki M, Harada Y,
Ohe C, Jeruc J, Raspollini MR, Michal M, Hes O and Amin MB: Review
of succinate dehydrogenase-deficient renal cell carcinoma with
focus on clinical and pathobiological aspects. Pol J Pathol.
67:3–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mason EF, Sadow PM, Wagner AJ, Remillard
SP, Flood TA, Belanger EC, Hornick JL and Barletta JA:
Identification of succinate dehydrogenase-deficient bladder
paragangliomas. Am J Surg Pathol. 37:1612–1618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marín-Aguilar F, Pavillard LE, Giampieri
F, Bullón P and Cordero MD: Adenosine Monophosphate (AMP)-activated
protein kinase: A new target for nutraceutical compounds. Int J Mol
Sci. 18:pii: E2882017. View Article : Google Scholar
|
|
12
|
Ata R and Antonescu CN: Integrins and cell
metabolism: An intimate relationship impacting cancer. Int J Mol
Sci. 18:pii: E1892017. View Article : Google Scholar
|
|
13
|
Li Q, Qin Y, Wei P, Lian P, Li Y, Xu Y, Li
X, Li D and Cai S: Gas1 inhibits metastatic and metabolic
phenotypes in colorectal carcinoma. Mol Cancer Res. 14:830–840.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Navarro P, Bueno MJ, Zagorac I, Mondejar
T, Sanchez J, Mourón S, Muñoz J, Gómez-López G, Jimenez-Renard V,
Mulero F, et al: Targeting tumor mitochondrial metabolism overcomes
resistance to antiangiogenics. Cell Rep. 15:2705–2718. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen L, Liu T, Zhang S, Zhou J, Wang Y and
Di W: Succinate dehydrogenase subunit B inhibits the AMPK-HIF-1α
pathway in human ovarian cancer in vitro. J Ovarian Res. 7:1152014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang YY, Attané C, Milhas D, Dirat B,
Dauvillier S, Guerard A, Gilhodes J, Lazar I, Alet N, Laurent V, et
al: Mammary adipocytes stimulate breast cancer invasion through
metabolic remodeling of tumor cells. JCI Insight. 2:e874892017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Devic S: Warburg effect - a consequence or
the cause of carcinogenesis? J Cancer. 7:817–822. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang D, Fei Q, Li J, Zhang C and Sun Y,
Zhu C, Wang F and Sun Y: 2-deoxyglucose reverses the promoting
effect of insulin on colorectal cancer cells in vitro. PLoS One.
11:e01511152016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Festuccia C, Gravina GL, D'Alessandro AM,
Muzi P, Millimaggi D, Dolo V, Ricevuto E, Vicentini C and Bologna
M: Azacitidine improves antitumor effects of docetaxel and
cisplatin in aggressive prostate cancer models. Endocr Relat
Cancer. 16:401–413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai Z, Pan S, Chen C, Cao L, Li X, Chen X,
Su X and Lin S: Down-regulation of succinate dehydrogenase subunit
B and up-regulation of pyruvate dehydrogenase kinase 1 predicts
poor prognosis in recurrent nasopharyngeal carcinoma. Tumour Biol.
37:5145–5152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang D, Wang W, Xiang B, Li N, Huang S,
Zhou W, Sun Y, Wang X, Ma J, Li G, et al: Reduced succinate
dehydrogenase B expression is associated with growth and
de-differentiation of colorectal cancer cells. Tumour Biol.
34:2337–2347. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cervera AM, Apostolova N, Crespo FL, Mata
M and McCreath KJ: Cells silenced for SDHB expression display
characteristic features of the tumor phenotype. Cancer Res.
68:4058–4067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Monteverde T, Muthalagu N, Port J and
Murphy DJ: Evidence of cancer-promoting roles for AMPK and related
kinases. FEBS J. 282:4658–4671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lipovka Y and Konhilas JP: AMP-activated
protein kinase signalling in cancer and cardiac hypertrophy.
Cardiovasc Pharm Open Access. 4:pii: 1542015.
|
|
25
|
Moreira JD, Hamraz M, Abolhassani M, Bigan
E, Pérès S, Paulevé L, Nogueira ML, Steyaert JM and Schwartz L: The
Redox Status of Cancer Cells Supports Mechanisms behind the Warburg
Effect. Metabolites. 6:pii: E332016. View Article : Google Scholar
|
|
26
|
Lustgarten MS, Jang YC, Liu Y, Qi W, Qin
Y, Dahia PL, Shi Y, Bhattacharya A, Muller FL, Shimizu T, et al:
MnSOD deficiency results in elevated oxidative stress and decreased
mitochondrial function but does not lead to muscle atrophy during
aging. Aging Cell. 10:493–505. 2011. View Article : Google Scholar : PubMed/NCBI
|