Introduction
Prion disease, also known as transmissible
spongiform encephalopathy (TSE), is a lethal neurodegenerative
disease that affects both humans and livestock, and is
characterized by amyloidosis of the brain tissue (1). Abnormal prion protein (PrP) deposition
is the main component of amyloidosis, and large quantities of glial
cells have been observed around PrP deposition sites (2), which results in progressive neuronal
degeneration and neuronal vacuolation (3). Human prion diseases include Kuru,
Creutzfeldt-Jakob disease (CJD), Gerstmann-Sträussler-Scheinker
syndrome (GSS) and fatal familial insomnia (FFI). The most
prevalent human prion disease is CJD. It is reported that 85–90% of
CJD cases occur sporadically and affect 1–1.5 people per million
annually (4). Glial cells are
important in providing support, nutrition, protection and repair
for the survival and vital movement of neurons. These cells present
various immunocompetencies and constitute the initial protection of
the central nervous system (CNS) against the invasion of pathogens.
Cytokines are the key regulators of innate and adaptive immunity
(5). Among CNS infectious diseases,
tissue-infiltrating immunocytes, CNS-associated macrophages,
microglial cells and astrocytes are the sources of cytokines in
CNS-specific inflammation (6).
Microglial cells are the main source of pivotal proinflammatory
factors and immune regulatory cytokines in vivo and in
vitro (7). In addition, the
levels of the proinflammatory cytokine interleukin (IL)-6 and the
chemokine IL-8 have been demonstrated to significantly increase in
the cerebrospinal fluid of patients with Creutzfeldt-Jakob disease
(CJD) (8,9).
The mechanisms of neuronal loss during prion disease
are not fully understood. Previous studies have shown that glial
activation precedes neuronal loss (10), and that cytokines secreted by
activated microglia are important in neurodegeneration and neuronal
loss (11). In our previous study,
IL-8 was secreted from microglial cells treated with PrP in
vitro (12). In the present
study, microglial cells were treated with PrP to investigate the
source and possible pathways of IL-6 and IL-8 in prion disease.
Materials and methods
Ethics statement
The present study was performed in strict accordance
with the recommendations of the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health.
Furthermore, the protocols were approved by the Institutional
Animal Care and Use Committee of Inner Mongolia Medical University
(Hohhot, China; permit no. YKD2013163). All surgical procedures
were performed under sodium pentobarbital anesthesia (1% sodium
pentobarbital, 40 mg/kg, intraperitoneal injection; Shanghai
Westang Bio-Tech Co., Ltd., Shanghai, China) with all efforts made
to minimize animal suffering.
Materials and animals
A total of 10 healthy newborn (1-day-old) Wistar
rats (weight, 5.3±0.3 g) were acquired from the Experimental Animal
Center of Jilin University (Jilin, China). ELISA kits for the
determination of IL-6 and IL-8 levels were purchased from Shanghai
Westang Bio-Tech Co., Ltd. (cat. nos. F01310 and F15880). TRIzol
reagent was purchased from Thermo Fisher Scientific, Inc. (Waltham,
MA, USA; cat. no. 15596-026). One Step RNA polymerase chain
reaction (PCR) kit and DL2000 DNA marker were purchased from Takara
Bio, Inc. (Otsu, Japan; cat. nos. RR024A and 3427A). In addition,
the primers were synthesized by Invitrogen (Thermo Fisher
Scientific, Inc., Shanghai, China), while PrP105-132 peptides,
MG132 and cyclosporin A (CsA) were obtained from Shanghai Bootech
Bioscience & Technology Co., Ltd. (Shanghai, China), EMD
Millipore (Billerica, MA, USA; cat. no. 474790) and Huadong
Medicine Co., Ltd. (Hangzhou, China; cat. no. 59865-13-3),
respectively. Anti-CD68 antibody was purchased from Thermo Fisher
Scientific, Inc. (cat. no. MS-397-R7). Streptavidin-peroxidase (SP)
immunohistochemistry kit and DAB color developing reagent kit were
purchased from Fuzhou Maxim Bioscience & Technology Co., Ltd.
(Fuzhou, China; cat. nos. KIT-9710 and DAB-0031).
Treatment of PrP peptides
PrP105-132 (KTN LKH VAG AAA AGA VVG GLG GYM LGSA)
was synthesized using the solid-phase method (13). Small quantities of peptides (0.5 mg)
were transferred to an Eppendorf tube, and then dissolved using
diluted acetic acid. Subsequently, the peptides were further
diluted by adding distilled water (1:1), and the pH was neutralized
using diluted acetic acid.
Nerve glial cell culture
The crania of 10 newborn Wistar rats were opened
under sterile conditions. The brain tissues containing cortex and
medulla were dissected and placed in a dish with D-Hanks solution
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany). Blood was
removed by repeated washing with D-Hanks solution. Next, the
meninges and blood vessels on the surface of the brain tissue were
also removed, and the brain tissue was washed once or twice with
D-Hanks solution. The brain tissue was then cut into cubes of ~1–3
mm3, and 40 times volume of trypsin (Sigma-Aldrich) was
added according to the tissue mass. The mixture was repeatedly
pipetted at 37°C for 5–10 min until it became cloudy. Subsequently,
the digestion was terminated by adding complete medium, consisting
of high-glucose DMEM/F12 (1:1; Gibco; Thermo Fisher Scientific,
Inc.), supplemented with 10% fetal bovine serum (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA). The single-cell
suspension was transferred into a sterile Eppendorf tube, and the
cells were centrifuged at 111.8 × g, 4°C for 10 min. The
supernatant was discarded, and the cells were resuspended by adding
complete medium. Cells were then seeded into six 50-ml cell culture
flasks at a density of 1.0–1.2×105 cells/ml and cultured
in an incubator at 37°C until further use.
Separation, purification and passage
of microglial cells
Microglial cells were separated from the nerve glial
cell solution, purified and passaged as described previously
(14). Briefly, the complete culture
medium in the culture of glial cells (1.0–1.2×105
cells/ml per 50-ml cell culture flask) was replaced on days 3 and
12 of culture. A solution of trypsin-ethylenediaminetetraacetic
acid (EDTA) was prepared by mixing 0.25% trypsin and 0.02% EDTA at
a ratio of 1:1, and was further diluted using D-Hanks solution at a
ratio of 3:1. At 24 h after refreshment of the medium for the
second time, the cells were treated with a diluted trypsin-EDTA
solution at 37°C for 40 min. The microglial cells were separated
from the adherent astrocytes by shaking the flask, and these cells
were transferred into a new cell culture flask. After 24 h, the
medium was replaced. Finally, these cells were passaged using
trypsin at a confluence of 100%, and purified microglial cells were
obtained.
Identification of cultured cells
The obtained microglial cells were seeded onto a
coverslip and washed with 0.9% saline three times for 5 min. Next,
these cells were fixed by 4% paraformaldehyde for 40 min, and
further washed with 0.01 M phosphate-buffered solution (pH 7.3)
three times for 5 min. The coverslip was then stored at 4°C.
Immunocytochemical identification of the microglial cells was
performed by staining with anti-CD68 antibody (1:50) at 4°C for 24
h and the SP immunohistochemistry kit, following the manufacturers'
protocols. Five fields of high magnification were selected
randomly, and the number of CD68-positive microglial cells in these
fields was counted under an optical microscope.
PrP105-132 treatment of microglial
cells and sample collection
The cultured microglial cells were divided into four
groups as follows: i) Control, ii) PrP, iii) PrP+MG132 and iv)
PrP+CsA groups. In these groups, MG132 served as a specific
inhibitor of nuclear factor (NF)-κB, and CsA as a specific
inhibitor of nuclear factor of activated T cells (NFAT). Six
repeated wells were established for each group, and
1×106 cells were seeded into each well. The microglial
cells were cultured with complete medium only in the control group.
In the PrP group, the cells were treated with 80 µM PrP105-132. In
the PrP+MG132 and PrP+CsA groups, the cells were treated with 80 µM
PrP105-132 for 24 h, followed by treatment with 3 µmol/l MG132 and
1.0 µg/ml CsA, respectively. After 48 h of culture, the cells were
centrifuged at 1,000 × g, 4°C for 10 min. The supernatant and cells
were collected and stored at 20°C until further use to detect the
IL-6 and IL-8 protein levels or the NF-κB and NFAT mRNA
expression.
Detection of IL-6 and IL-8 levels
The levels of IL-6 and IL-8 in the various groups
were detected using the aforementioned ELISA kits in accordance
with the kit manufacturer's instructions.
Detection of NF-κB and NFAT at the
mRNA level
The mRNA expression of NF-κB and NFAT was detected
using reverse transcription (RT)-PCR. Briefly, total RNA was
extracted from the treated microglial cell samples using an RNA
extraction kit. The target gene in the RNA was then amplified by
RT-PCR using the One Step RNA PCR kit. The PCR primers for NF-κB
(accession no. NM002502), NFAT (accession no. NM001107425) and
β-actin (accession no. BC063166) were designed using Primer Premier
6.0 (Premier Biosoft, Palo Alto, CA, USA) according to their
accession number. The primer sequences were as follows: β-actin
(147 bp), 5′-GTCAGGTCATCACTATCGGCAAT-3′ (sense) and
5′-AGAGGTCTTTACGGATGTCAACGT-3′ (antisense); NF-κB (378 bp),
5′-ATGCGTTTCCGTTACAAGTGCGAGG-3′ (sense) and
5′-GACCGCATTCAAGTCATAGTCCCCG-3′ (antisense); NFAT (365 bp),
5′-GACCTGGAATCGCCCAAGTCCCTGT-3′ (sense) and
5′-GTTACTTACCCCCACGGCTGAGGAG-3′ (antisense). PCR was conducted
using the CFX96 Touch Real-Time PCR Detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), with a reaction mixture
consisting of 2.5 µl 10X One Step RNA PCR Buffer, 5 µl
MgCl2 (25 mM), 5 µl dNTP Mixture (10 mM), 0.5 µl
AMV-Optimized Taq (5 U/µl), 1 µl forward and reverse primer (100
pM), 2 µl cDNA (5 ng/µl) and 10.5 µl dH2O. The PCR
cycling conditions were as follows: 95°C for 15 min, followed by 30
cycles of 94°C for 30 sec and 55°C for 30 sec, and a final
extension step at 72°C for 1.5 min. Following PCR assay, the
products were analyzed using 1.5% TAE (40 mmol/l Tris-HAc, 1 mmol/l
EDTA) agarose gel electrophoresis.and the densities of the
electrophoretic bands were analyzed using a gel imaging analysis
system (BOT-860SR; Beijing Zhongyiboteng Technology Co., Ltd.,
Beijing, China). The relative expression of each gene was obtained
using the following formula: Relative expression of gene = (density
of the gene band) / (density of β-actin band).
Statistical analysis
Results are expressed as mean ± standard deviation.
One-way analysis of variance was used to compare groups. P<0.05
was considered to indicate a statistically significant difference.
The statistical analysis was conducted using SPSS 13.0 software
(IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Observation of cultured microglial
cells in vitro
First-generation microglial cells were obtained,
which floated in the medium, and presented rotundity and strong
refractivity. The cells were passaged and further adhered to the
culture dish, presenting numerous short and bent protuberances
after 5–10 days of culture. The cell bodies of the
PrP105-132-treated microglial cells were enlarged and presented
round, rod and amoeba-like morphologies. In addition, the
protuberances were shortened and eventually disappeared following
treatment with PrP. However, no significant morphological changes
were observed after these cells were treated with MG132 or CsA
(data not shown).
Purity of cultured microglial
cells
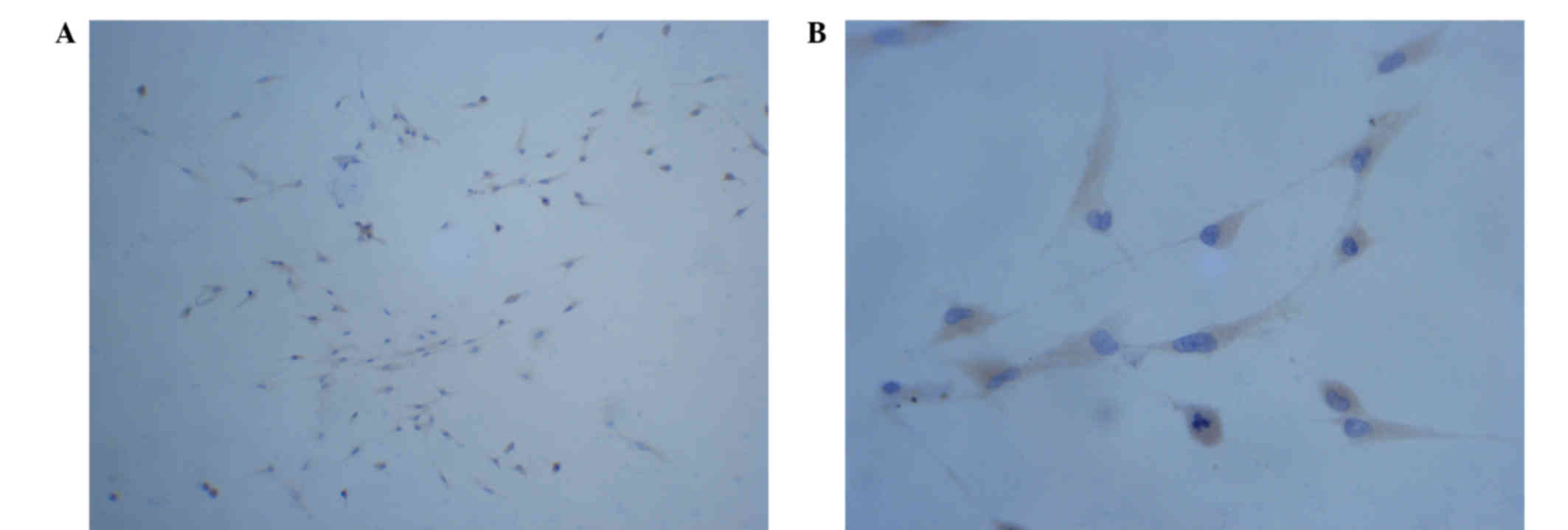
The microglial cells were detected by
immunohistochemistry using a CD68 monoclonal antibody. As shown in
Fig. 1, large quantities of round
cells were confirmed by the observation of strong positive
staining, and some cells exhibited short protuberances. The
percentage of cells that were positively stained (microglial cells)
was >95% (Fig. 1).
IL-6 and IL-8 levels
ELISA kits were used to determine the protein levels
of IL-6 and IL-6 in the various groups. The levels of IL-6 and IL-8
in the supernatant of the PrP group were significantly higher
compared with those in the control group (P<0.001; Tables I and II). Furthermore, IL-6 and IL-8 levels in
the PrP+MG132 group were markedly lower compared with those in the
PrP group (P<0.001). However, the IL-6 protein level was
markedly decreased in the PrP+CsA group compared with the PrP group
(P=0.024; Table I), while that of
IL-8 did not show a significant reduction (P=0.180; Table II). In addition, the IL-6 and IL-8
levels in the PrP+MG132 group were significantly lower than those
in the PrP+CsA group (P<0.001; Tables
I and II).
 | Table I.Secretion of IL-6 from microglial
cells in vitro. |
Table I.
Secretion of IL-6 from microglial
cells in vitro.
| Group | No. of wells | Maximum value
(pg/ml) | Minimum value
(pg/ml) | Concentration (mean ±
SD) |
|---|
| Control | 6 |
73.56 |
65.25 |
68.09±3.04 |
| PrP | 6 | 169.48 | 142.13 |
157.79±9.69a |
| PrP+MG132 | 6 |
71.30 |
68.71 |
70.13±1.04b |
| PrP+CsA | 6 | 145.97 | 134.52 |
138.55±3.99b,c |
 | Table II.Secretion of IL-8 from microglial
cells in vitro. |
Table II.
Secretion of IL-8 from microglial
cells in vitro.
| Group | No. of wells | Maximum value
(pg/ml) | Minimum value
(pg/ml) | Concentration (mean ±
SD) |
|---|
| Control | 6 | 21.15 | 17.26 |
19.44±1.40 |
| PrP | 6 | 39.66 | 35.05 |
37.94±1.69a |
| PrP+MG132 | 6 | 30.49 | 22.99 |
27.07±2.74b |
| PrP+CsA | 6 | 40.65 | 32.77 |
35.78±3.22c |
mRNA expression levels of NF-κB and
NFAT
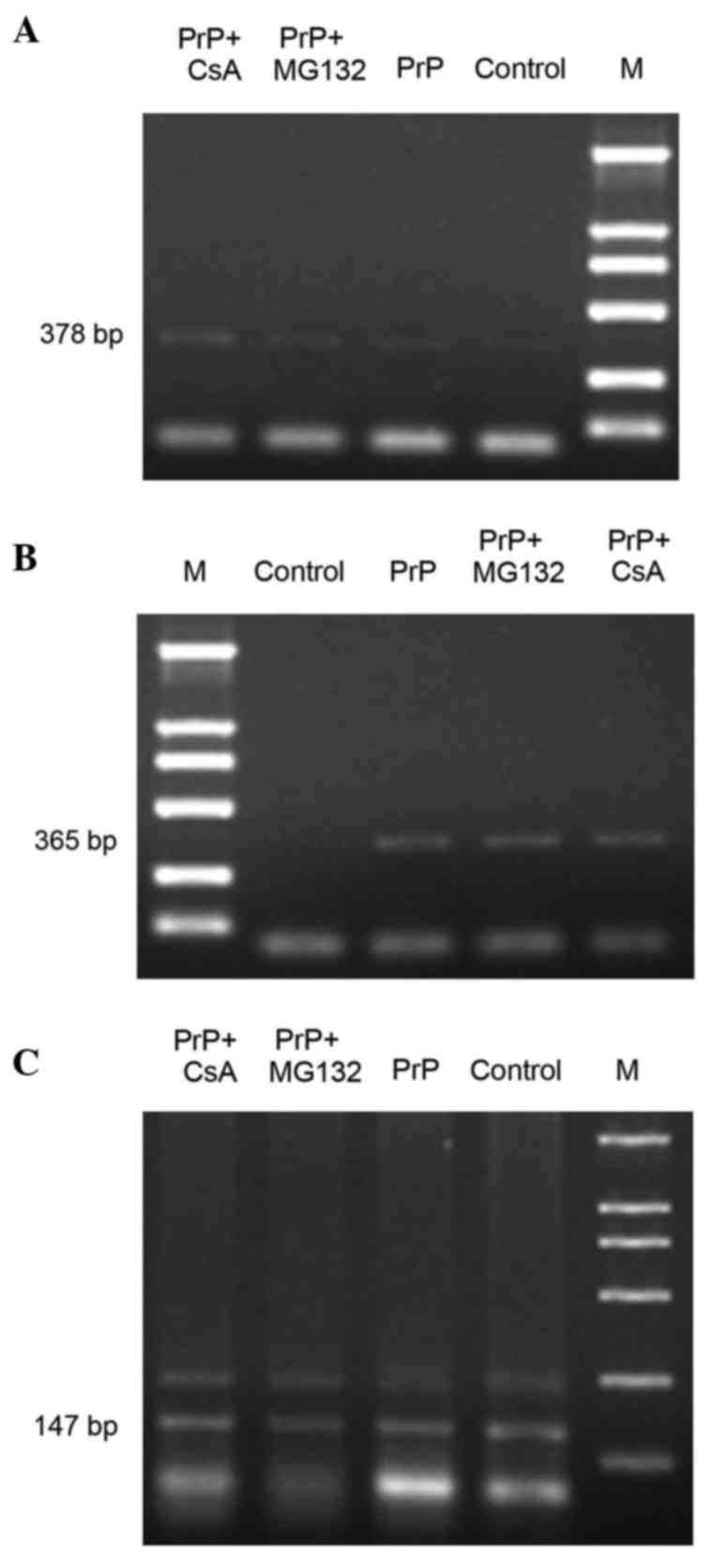
As shown in Table
III and Fig. 2, the mRNA
expression of NF-κB in the PrP group was significantly higher
compared with that in the control group (P<0.001). However, the
mRNA expression of NF-κB was significantly decreased after these
cells were treated with MG132 (P<0.001). Furthermore, the mRNA
expression of NFAT in the PrP group was significantly higher
compared with that in the control group (P<0.001). However, the
mRNA expression of NFAT significantly decreased following the
treatment of these cells with CsA (P<0.001).
 | Table III.Expression of NF-κB and NFAT at the
mRNA level. |
Table III.
Expression of NF-κB and NFAT at the
mRNA level.
| Group | NF-κB expression
(mean ± SD) | NFAT expression (mean
± SD) |
|---|
| Control |
0.6323±0.0414 |
0.6476±0.0168 |
| PrP |
1.0221±0.0184a |
0.9727±0.0122a |
| PrP+MG132 |
0.8334±0.0232b |
1.0058±0.0308 |
| PrP+CsA |
0.9549±0.0365 |
0.7934±0.0461b |
Discussion
Prion disease, also known as TSE, is a lethal
neurodegenerative illness that affects human beings and livestock.
Previous evidence indicates that prion disease is mainly caused by
the transformation of normal to abnormal PrP (PrPC to
PrPSc) (15).
PrPC and PrPSc present the same amino acid
sequence but have different spatial configurations. In the brain
tissue of scrapie-infected mice, the activation of microglial
cells, the inflammatory mediator IL-1β, and the prostaglandins
E2 (PGE2) and PGF2α were
demonstrated to be associated with the accumulation of PrP in the
brain (16). In addition, the
distribution of activated microglial cells was consistent with the
distributions of PrPSc and dead neurons (17,18).
These observations indicate that the activation of microglial cells
serves an important role in the neuropathological changes of
PrP-mediated scrapie infection (19). In the present study, microglial cells
were activated by treatment with PrP, as demonstrated by the cell
morphological changes. Furthermore, the possible sources and
pathways of IL-6 and IL-8 in prion disease were explored.
The PrP105-132 peptide (KTN LKH VAG AAA AGA VVG GLG
GYM LGSA) is the transmembrane region of PrPC, and it is
a key position that mediates the transformation of PrPC
to PrPSc (20). In
addition, the PrP105-132 peptide is the common structure of all
abnormal PrP isoforms and exhibits different secondary structures
under different conditions, including ion strength, pH value and
solute composition (21).
Furthermore, PrP105-132 shares certain common characteristics with
the entire PrPSc structure, and it is able to form
amyloid fibrils with proteinase K resistance (21). In the present study, the cell bodies
of PrP105-132-treated microglial cells were enlarged, with round,
rod and amoeba-like morphologies. The protuberances on these cells
were shortened and eventually disappeared following treatment with
PrP. In addition, the results indicated increased secretion of IL-6
and IL-8 following treatment with PrP105-132. Thus, the role of
PrP105-132 in the activation of microglial cells was further
clarified.
The activation of microglial cells is a
neuropathological characteristic of prion disease, and previous
histological analyses have indicated that the activation of
microglial cells in the CNS is associated with the accumulation of
abnormal PrP in prion disease (22).
In addition, PrP induced an inflammatory reaction mediated by
microglial cells, leading to a deficiency of neurons (23). Therefore, the synthesis and
participation of various cytokines are required for an inflammatory
reaction mediated by microglial cells. It has previously been
demonstrated that PrP promotes the expression of cyclooxygenase-2
and the synthesis of IL-1β and PGE2 in microglial cells
(23,24). In the present study, IL-6 and IL-8
levels in the supernatant increased following the treatment of
microglial cells with PrP, confirming that microglial cells are one
of the sources of IL-6 and IL-8 in prion disease.
The expression of NF-κB has been reported to be
elevated in brain microglial cells of CJD patients, and the 20S
proteasome was observed on the cell membranes of neurons and glial
cells of pathologically changed brain tissue (25). These previous findings indicate that
the proteasome system is involved in the pathogenesis of prion
disease. In addition, the mRNA expression of NF-κB increased after
microglial cells were treated with PrP; simultaneously, the IL-6
and IL-8 levels increased in the supernatant. By contrast, the mRNA
expression of NF-κB and the IL-6 and IL-8 levels in the supernatant
fluid decreased compared with that in the PrP group after cells
were treated with MG132, a specific inhibitor of NF-κB. Therefore,
the association between the activation of NF-κB and the secretion
of IL-6 and IL-8 in microglial cells was further confirmed.
However, the mechanism of NF-κB activation induced
by PrP remains largely unknown. The possible mechanisms are
hypothesized as follows: i) PrP-activated protein kinases and
protein phosphatases act directly on microglial cells, and IκB, an
NF-κB inhibitory protein, is further degraded by the proteasome;
subsequently, the activated NF-κB is released. ii) PrP activates
microglial cells, and these activated cells release IL-1 and tumor
necrosis factor (TNF)-α (16,26),
which then promote the degradation of IκB, activating NF-κB. iii)
Activated NF-κB promotes the expression of IL-1, TNF-α and IL-6,
and these cytokines reversely activate NF-κB, resulting in a
positive feedback loop (27).
In the present study, the mRNA expression of NFAT,
as well as the IL-6 and IL-8 levels in the supernatant, increased
after microglial cells were treated with PrP in vitro. This
result suggests that PrP can activate NFAT in microglial cells.
After these cells were treated with CsA, the mRNA expression of
NFAT decreased, and the IL-6 level rather than the IL-8 level in
the supernatant decreased. These observations indicate that the
secretion of IL-6, but not that of IL-8, may be promoted through
the NFAT pathway in microglial cells.
In conclusion, PrP treated microglial cells secreted
IL 6 and IL 8, and the secretion of IL 6 was associated with the
activation of NF-κB and NFAT pathways. In addition, the secretion
of IL 8 was mainly dependent on the NF-κB pathway. These results
will provide an experimental basis for further studies on the
pathogenesis of prion disease.
Acknowledgements
The present study was supported by Inner Mongolia
Autonomous Region Science Foundation (grant no. 2013MS1130).
References
|
1
|
Bastian FO, Sanders DE, Forbes WA, Hagius
SD, Walker JV, Henk WG, Enright FM and Elzer PH: Spiroplasma spp.
from transmissible spongiform encephalopathy brains or ticks induce
spongiform encephalopathy in ruminants. J Med Microbiol.
56:1235–1242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iwasaki Y, Iijima M, Kimura S, Yoshida M,
Hashizume Y, Yamada M, Kitamoto T and Sobue G: Autopsy case of
sporadic Creutzfeldt-Jakob disease presenting with signs suggestive
of brainstem and spinal cord involvement. Neuropathology.
26:550–556. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prusiner SB: Prions. Proc Natl Acad Sci
USA. 95:pp. 13363–13383. 1998; View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mead S, Stumpf MP, Whitfield J, Beck JA,
Poulter M, Campbell T, Uphill JB, Goldstein D, Alpers M, Fisher EM
and Collinge J: Balancing selection at the prion protein gene
consistent with prehistoric kurulike epidemics. Science.
300:640–643. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iwata K, Chiang CY and Cherkas P: Glial
cells in orofacial pathological pain mechanismsEncyclopedia of
Pain. Schmidt RF and Gebhart GF: 10. 2nd. Springer; Berlin: pp.
1374–1378. 2013, View Article : Google Scholar
|
|
6
|
Zindler E and Zipp F: Neuronal injury in
chronic CNS inflammation. Best Pract Res Clin Anaesthesiol.
24:551–562. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakamichi K, Kitani H, Takayama-Ito M,
Morimoto K, Kurane I and Saijo M: Celastrol suppresses
morphological and transcriptional responses in microglial cells
upon stimulation with double-stranded RNA. Int J Neurosci.
120:252–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang YT, Jiang XM and Lin SH: The change
of cytokine IL-6 in the cerebrospinal fluid of patients with
Creutzfeldt-Jakob disease. Zhong Guo Hao Nian Xue Za Zhi.
29:187–188. 2009.(In Chinese).
|
|
9
|
Yang YT, Jiang XM and Lin SH: The change
of cytokine IL-8 in the cerebrospinal fluid of patients with
Creutzfeldt-Jakob disease. Zhong Guo Ren Shou Gong Huan Bing Xue
Bao. 25:1083–1084. 2009.(In Chinese).
|
|
10
|
Moresco RM, Messa C, Tagliavini F,
Panzacchi A, Giovagnoli MR, Matarrese M, Pietra L, Bertoldo A,
Rizzo G, Giaccone G, et al: Creutzfeldt-Jakob disease: Activated
microglia detected in vivo by molecular imaging. Neuropathol Appl
Neurobiol. 30:415–416. 2004.
|
|
11
|
Peyrin JM, Lasmézas CI, Haïk S, Tagliavini
F, Salmona M, Williams A, Richie D, Deslys JP and Dormont D:
Microglial cells respond to amyloidogenic PrP peptide by the
production of inflammatory cytokines. NeuroReport. 10:723–729.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang YT and Fu Q: Effect of PrP105-132 on
secretion of IL-8 from microglial cells in vitro. Zhong Guo Ren
Shou Gong Huan Bing Xue Bao. 31:1046–1049. 2015.(In Chinese).
|
|
13
|
Raibaut L, Mahdi OE and Melnyk O: Solid
phase protein chemical synthesis. Top Curr Chem. 363:103–154. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giulian D and Baker TJ: Characterization
of ameboid microglia isolated from developing mammalian brain. J
Neurosci. 6:2163–2178. 1986.PubMed/NCBI
|
|
15
|
Tatzelt J and Schätzl HM: Molecular basis
of cerebral neurodegeneration in prion diseases. FEBS J.
274:606–611. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Williams AE, van Dam AM, Man-A-Hing WK,
Berkenbosch F, Eikelenboom P and Fraser H: Cytokines,
prostaglandins and lipocortin-1 are present in the brains of
scrapie-infected mice. Brain Res. 654:200–206. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dheen ST, Kaur C and Ling EA: Microglial
activation and its implications in the brain diseases. Curr Med
Chem. 14:1189–1197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garção P, Oliveira CR and Agostinho P:
Comparative study of microglia activation induced by amyloid-beta
and prion peptides: Role in neurodegeneration. J Neurosci Res.
84:182–193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song PJ, Barc C, Arlicot N, Guilloteau D,
Bernard S, Sarradin P, Chalon S, Garreau L, Kung HF, Lantier F and
Vergote J: Evaluation of prion deposits and microglial activation
in scrapie-infected mice using molecular imaging probes. Mol
Imaging Biol. 12:576–582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thellung S, Florio T, Corsaro A, Arena S,
Merlino M, Salmona M, Tagliavini F, Bugiani O, Forloni G and
Schettini G: Intracellular mechanisms mediating the neuronal death
and astrogliosis induced by the prion protein fragment 106–126. Int
J Dev Neurosci. 18:481–492. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Veerhuis R, Hoozemans JJ, Janssen I,
Boshuizen RS, Langeveld JP and Eikelenboom P: Adult human microglia
secrete cytokines when exposed to neurotoxic prion protein peptide:
No intermediary role for prostaglandin E2. Brain Res. 925:195–203.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyazono M, Iwaki T, Kitamoto T, Kaneko Y,
Doh-ura K and Tateishi J: A comparative immunohistochemical study
of Kuru and senile plaques with a special reference to glial
reactions at various stages of amyloid plaque formation. Am J
Pathol. 139:589–598. 1991.PubMed/NCBI
|
|
23
|
Fonseca AC, Romão L, Amaral RF, Assad Kahn
S, Lobo D, Martins S, Marcondes de Souza J, Moura-Neto V and Lima
FR: Microglial stress inducible protein 1 promotes proliferation
and migration in human glioblastoma cells. Neuroscience.
200:130–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walsh DT, Perry VH and Minghetti L:
Cyclooxygenase-2 is highly expressed in microglial-like cells in a
murine model of prion disease. Glia. 29:392–396. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adori C, Kovács GG, Low P, Molnár K,
Gorbea C, Fellinger E, Budka H, Mayer RJ and László L: The
ubiquitin-proteasome system in Creutzfeldt-Jakob and Alzheimer
disease: Intracellular redistribution of components correlates with
neuronal vulnerability. Neurobiol Dis. 19:427–435. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu ZY, Baker CA and Manuelidis L: New
molecular markers of early and progressive CJD brain infection. J
Cell Biochem. 93:644–652. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ledoux AC and Perkins ND: NF-κB and the
cell cycle. Biochem Soc Trans. 42:76–81. 2014. View Article : Google Scholar : PubMed/NCBI
|