Introduction
Chronic obstructive pulmonary disease (COPD) is one
of the most common chronic illnesses and is now the third leading
cause of death worldwide (1). COPD
is characterized by progressive airflow limitation and loss of lung
function. Four primary mechanisms, including oxidative stress,
inflammation, protease-antiprotease imbalance and apoptosis, have
been implicated in the pathophysiological process of COPD (2,3). The
diaphragm is the principal muscle for breathing. In COPD patients,
the diaphragm undergoes fiber type transformation, which results in
diaphragmatic atrophy, injury and apoptosis (4). Morphological and functional changes of
the diaphragm in COPD are complex, and the underlying mechanisms
have remained to be fully elucidated. Myostatin, also known as
growth differentiation factor 8 (GDF-8), is a negative regulator of
skeletal muscle growth and is overexpressed in muscle wasting
diseases. Myostatin knockout mice displayed an increase in muscle
mass and myostatin transgenic mice developed cachexia characterized
by extensive muscle loss (5). Under
physiological conditions, myostatin mainly exists in the inactive
form, and is activated and upregulated by hypoxia, acidosis and
glucocorticoids (6,7). These risk factors for inducing
myostatin expression are present in patients with COPD,
particularly at moderate and severe disease stages. There is
evidence that myostatin protein expression is significantly
increased in COPD patients. However, whether myostatin is
associated with diaphragmatic apoptosis and atrophy has remained
elusive. In the present study, an experimental rat model of COPD
induced by cigarette smoke exposure was generated to observe
myostatin expression, diaphragmatic apoptosis and the correlation
between them.
Materials and methods
Animals
Male Sprague Dawley rats (age, 8 weeks; weight,
200–250 g) were purchased from SJA Laboratory Animal Co., Ltd.
(Changsha, China). The rats were housed in mesh cages with food and
water supply under a 12-h light/dark cycle for at least 5 days
prior to the experiments. All procedures performed were approved by
the Institutional Animal Ethics Committee and conformed to the
guide for the care and use of experimental animals of Hunan
Provincial People's Hospital (Changsha, China).
Cigarette smoke exposure
Cigarette smoke was generated from Furong Cigarettes
(China Tobacco Hunan Industrial Co., Ltd, Changsha, China). The
animals were randomly divided into two groups as follows: Rats
undergoing sham exposure (control group) and rats undergoing
cigarette smoke exposure (COPD group). Rats were exposed to
mainstream smoke of cigarettes at a concentration of 300 mg
particulate matter/m3 for 20 min for four times per day
over 5 months (8).
Measurement of lung function
Lung function was assessed as previously described
(9). In brief, the rats were
anaesthetized with 10% chloral hydrate (3 ml/kg, corresponding to
300 mg/kg). The trachea was opened with an inverted T-shaped
incision between the 2nd and 3rd cartilage ring, rapidly intubated
and the animal was placed into an HX200 respiratory-flow transducer
(Xinhangxinye Co., Ltd., Beijing, China) for measuring the forced
expiratory volume at 0.3 sec and forced vital capacity
(FEV0.3/FVC) and the peak expiratory flow (PEF). A total
of 6 ml air was injected at the end of the exhalation, which
induced a passive deep inspiration, and the lung function was
recorded.
Transmission electron microscopy (TEM)
examination of diaphragms
Diaphragms were collected, weighed and fixed with
2.5% paraformaldehyde, and then refixed with 1% osmium tetroxide,
washed with PBS, dehydrated with a graded ethanol series and pure
acetone, paraffin-embedded, sectioned (50 nm), uranium-lead double
stained (2% uranyl acetate, saturated aqueous lead citrate) and
observed under a H-7500 electron microscope (Hitachi, Tokyo,
Japan).
Histopathological examination of the
lung
The lung tissues were fixed in 4% paraformaldehyde
for 12 h, dehydrated using a graded ethanol series, and then placed
in xylene for 2 h, followed by paraffin embedding overnight.
Sections (4 µm) were prepared and mounted on slides, and the
samples were stained with H&E for 10 min at room temperature
and examined under an Olympus BX71 microscope (Olympus, Tokyo,
Japan).
Mean linear intercept (MLI) and mean alveolar number
(MAN) were examined as previously described (10). The MLI was used to estimate the
average diameter of a single alveolus by using the formula MLI =
total length/alveolar septal number. The intercepts of the alveolar
septal number were counted at the intersection point of the two
lines, and the total length of all of the lines combined divided by
the number of intercepts provided the mean linear intercept for the
region studied. The MAN was an indicator for the density of the
alveoli, which was determined as the number of alveolar per square
area in the field.
Assessment of diaphragmatic
apoptosis
Diaphragmatic apoptosis was identified using the
in situ terminal deoxynucleotidyl transferase deoxyuridine
triphosphate nick end labeling (TUNEL) assay (KeyGen biotech,
Nanjing, China) following the manufacturer's instructions. The
diaphragmatic apoptotic index was the percentage of TUNEL-positive
cells among the total cell population. Two slices of each sample
were taken to acquire data from five different fields of view at
high-power magnification (×400). At least 400 cells were
examined.
Determination of myostatin
protein
Western blot analysis was performed as previously
described (11). In brief, the
diaphragms were rinsed with ice-cold PBS, harvested with
radioimmunoprecipitation assay buffer (Applygen, Beijing, China)
and quantified using a bicinchoninic acid protein assay
(WellBiology, Changsha, China). Total protein (30 µg/lane) was
separated by 12% SDS-PAGE and transferred to polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked in 5% bovine serum albumin (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) for 1 h at room temperature and
probed with antibody against myostatin (cat no. 19142-1-AP) and
β-actin (cat no. 20536-1-AP; 1:1,000; Proteintech, Chicago, IL,
USA) at 4°C overnight, followed by incubation with a secondary
antibody (cat no. SA00001-2; 1:5,000; Proteintech, Chicago, IL,
USA) conjugated to horseradish peroxidase at room temperature for 1
h. Immunoreactivity was detected by enhanced chemiluminescent agent
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's instructions. The protein expression levels were
quantitatively analyzed and normalized against the β-actin loading
control.
Statistical analysis
Values are expressed as the mean ± standard
deviation and analyzed using SPSS 20.0 (IBM Corp., Armonk, NY,
USA). The two independent samples t-test was used for analysis of
differences between the two groups. Pearson's linear correlation
analysis was employed to identify any possible correlation between
parameters. P<0.05 was considered to indicate a statistically
significant difference.
Results
COPD is associated with changes in
diaphragm weight and lung function
The diaphragm mass, FEV0.3/FVC and PEF
were determined at the end of the challenge procedure and the
results are displayed in Table I.
Compared with that in the control group, the diaphragm mass in the
COPD group was significantly decreased (1.726±0.073 vs. 1.311±0.156
g; P<0.05). The average values for FEV0.3/FVC and FEP
were 83.5±4.9% and 40.2±3.7 ml/sec in the control group, and were
significantly decreased to 66.2±4.1% and 24.8±2.2 ml/sec in the
COPD group, respectively (P<0.05).
 | Table I.Effects of COPD on the diaphragm mass,
FEV0.3/FVC and FEP of rats. |
Table I.
Effects of COPD on the diaphragm mass,
FEV0.3/FVC and FEP of rats.
| Group | n | Diaphragm mass
(g) | FEV0.3/FVC
(%) | FEP (ml/sec) |
|---|
| Control | 8 |
1.726±0.073 |
83.5±4.9 |
40.2±3.7 |
| COPD | 8 |
1.311±0.156a |
66.2±4.1a |
24.8±2.2a |
COPD is associated with changes of
diaphragm ultrastructure and pulmonary histopathology
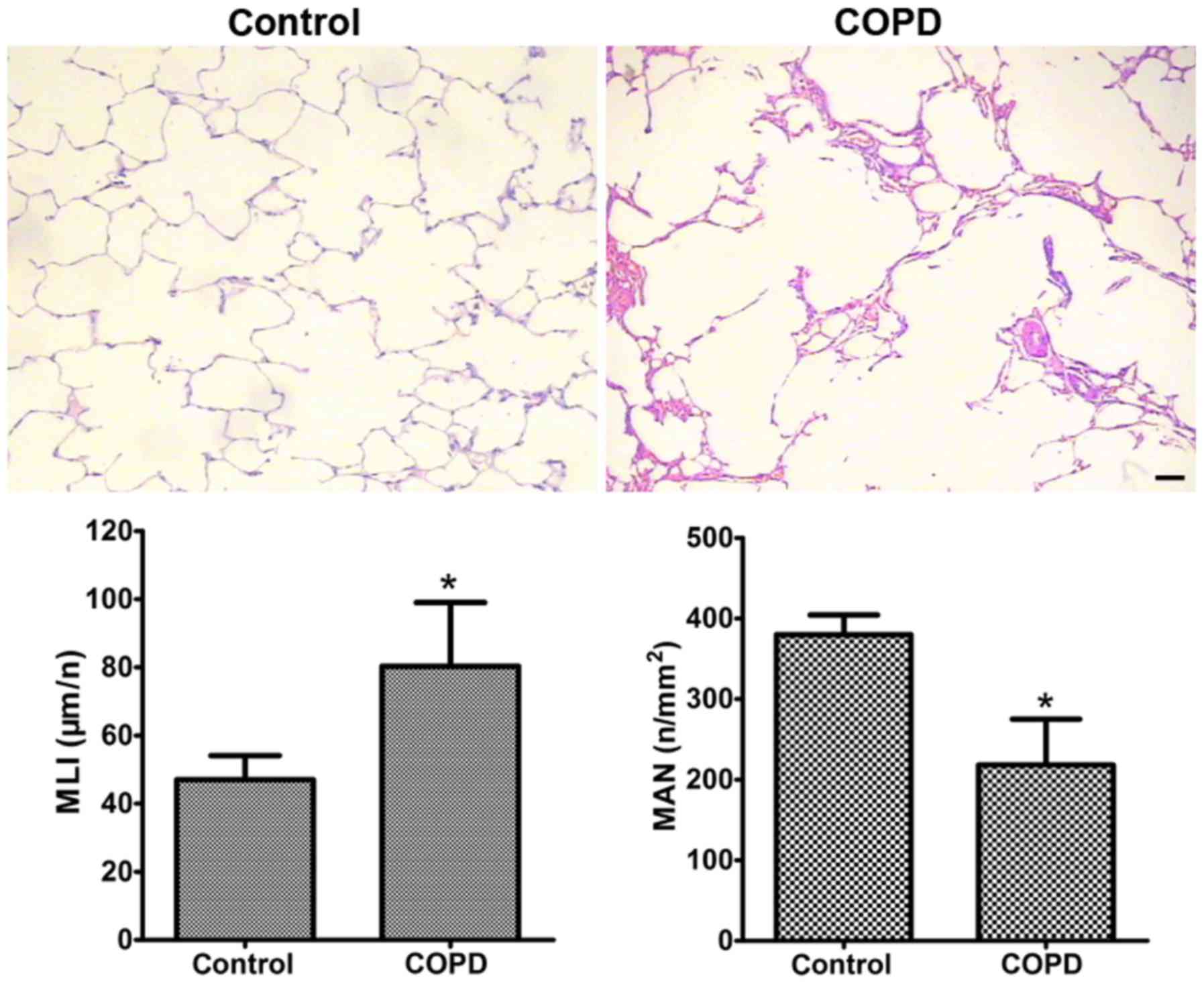
The lung tissues of COPD rats exhibited significant
histopathological changes under light microscopy. Compared with
those in the control group, the alveolar spaces were larger and the
alveolar walls were thinner in the COPD group. Certain alveoli were
broken and had fused into bullae. In addition, infiltration of
inflammatory cells into the mucosa, detachment of epithelial cells,
submucosal gland hyperplasia and hypertrophy, as well as disorders
and detachment of cilia were also observed in the COPD group
(Fig. 1). The average MLI in the
COPD group (80.3±18.7 mm) was ~70% higher than that in the control
group (47.0±7.1 mm; P<0.05; Fig.
1). The average MAN in the COPD group
(218.0±57.2/mm2) was significantly lower than that in
the control rats (379.8±24.4/mm2; P<0.05; Fig. 1). TEM indicated that compared with
control rats, COPD rats exhibited obvious ultrastructural changes
of the diaphragm, including muscle fiber atrophy, sarcomere
arrangement anomaly, myofilament breakage and dissolution,
mitochondrial swelling and vacuolization, cristae lysis,
karyopyknosis and nuclear chromatin aggregation and margination, as
well as nuclear membrane thickening, shrinkage and irregularities
(Fig. 2).
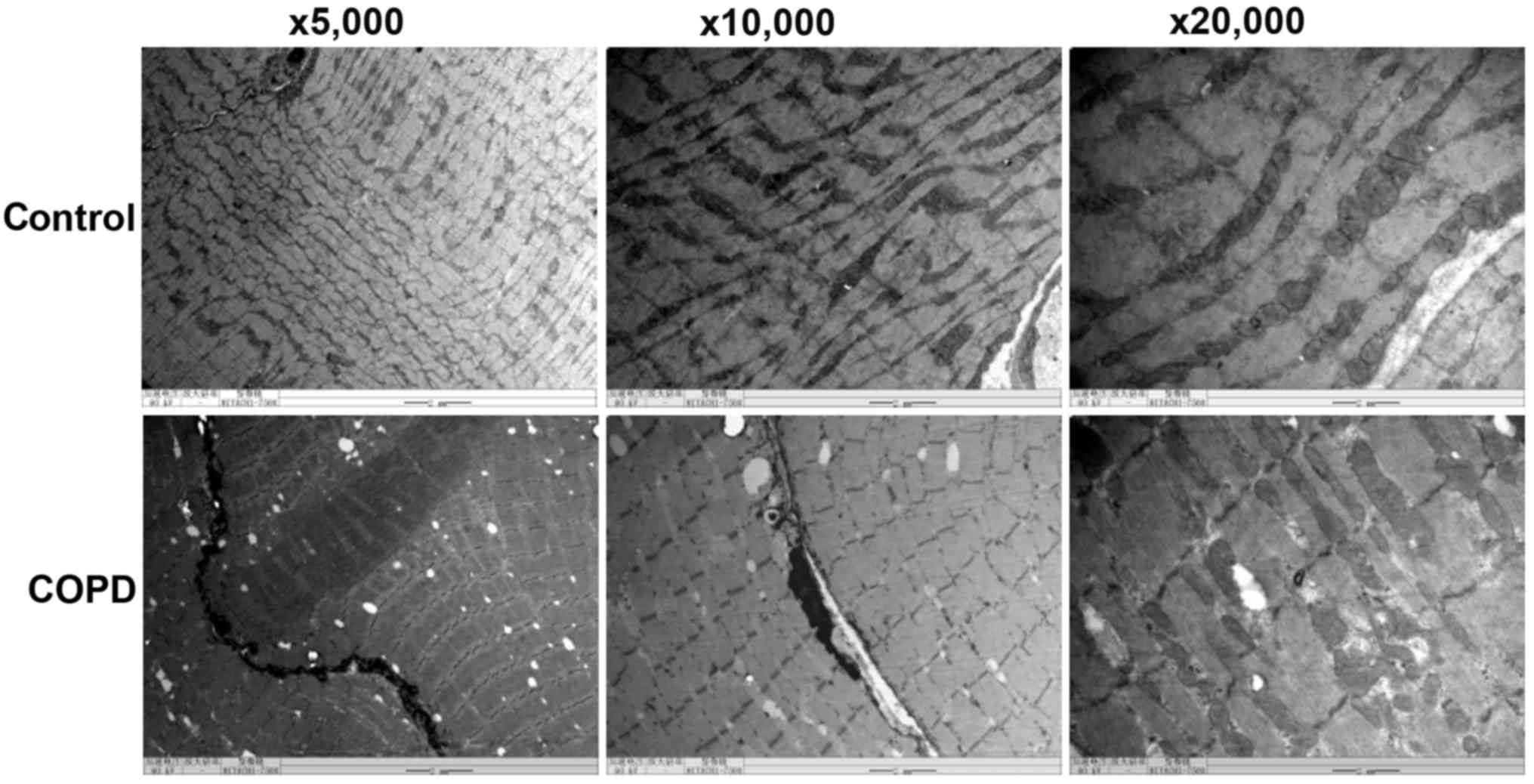 | Figure 2.Effects of COPD on diaphragm
ultrastructure of rats. COPD induced significant ultrastructural
changes in the diaphragm, including muscle fiber atrophy, anomalies
in sarcomere arrangement, myofilament breaking and dissolution,
mitochondrial swelling and vacuolization, cristae lysis,
karyopyknosis and nuclear chromatin aggregation and margination, as
well as nuclear membrane thickening, shrinkage and irregularities
(magnification, ×5,000, ×10,000 or ×20,000). COPD, chronic
obstructive pulmonary disease. |
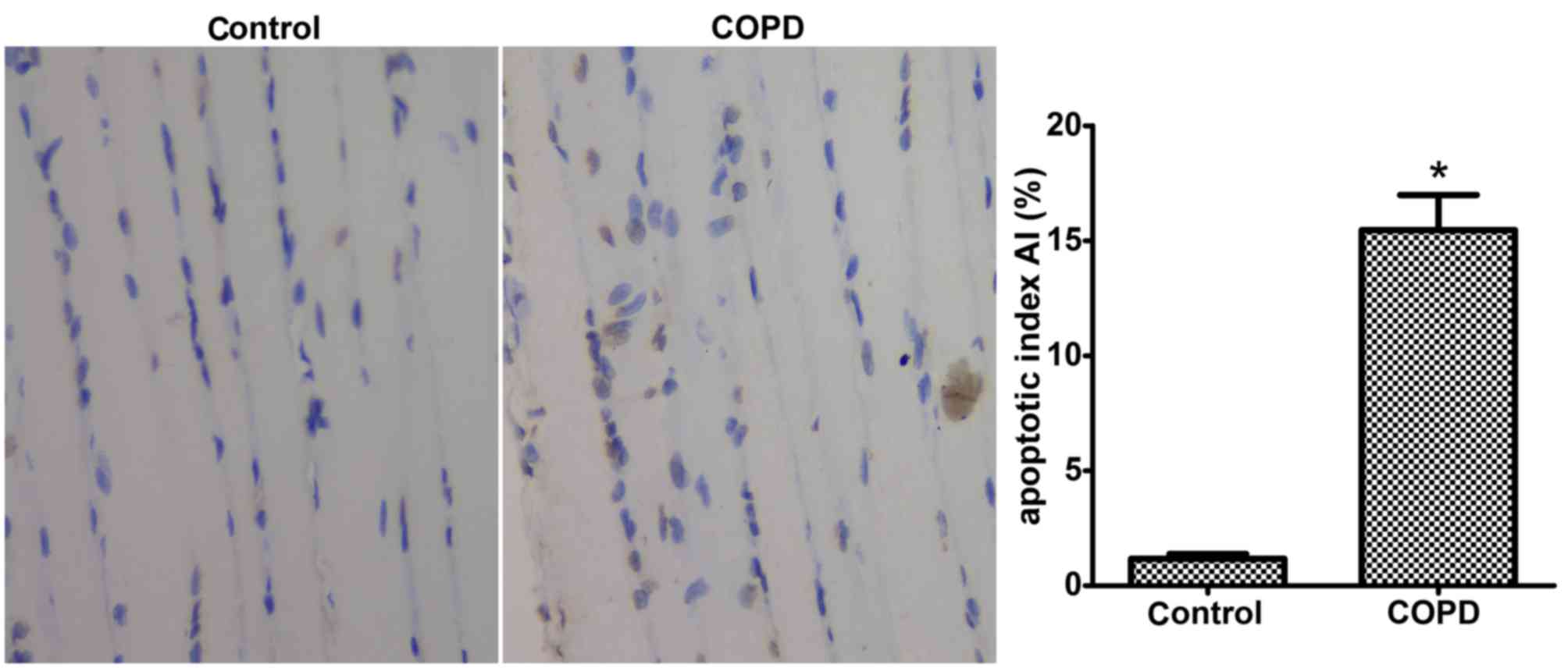
COPD induces diaphragmatic
apoptosis
TUNEL-positive diaphragm cells were identified as
brown bodies by light microscopic analysis. The results indicated
that the amount of TUNEL-positive cells was significantly greater
in the diaphragms of COPD rats compared with that in control rats.
The apoptotic index was 15.46±1.53% in the COPD group, which was
significantly higher than that in the control group (1.17±0.21%;
P<0.05; Fig. 3).
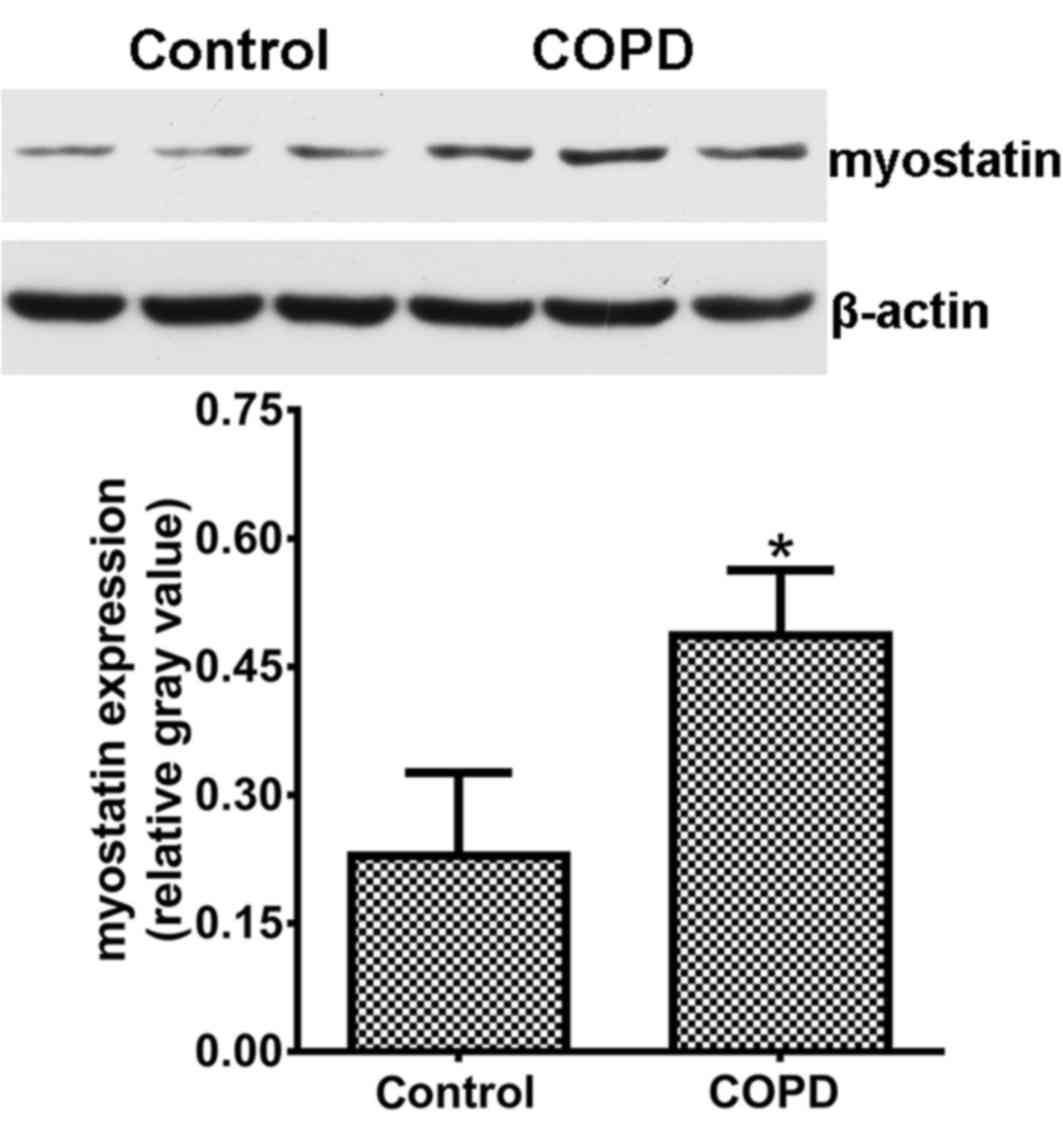
Myostatin expression is upregulated in
the diaphragms of COPD rats
Myostatin expression in the diaphragms was assessed
by western blot analysis and the results are displayed in Fig. 4. The expression levels of myostatin
in the diaphragms of COPD rats were obviously increased as compared
with those in the control animals (P<0.05).
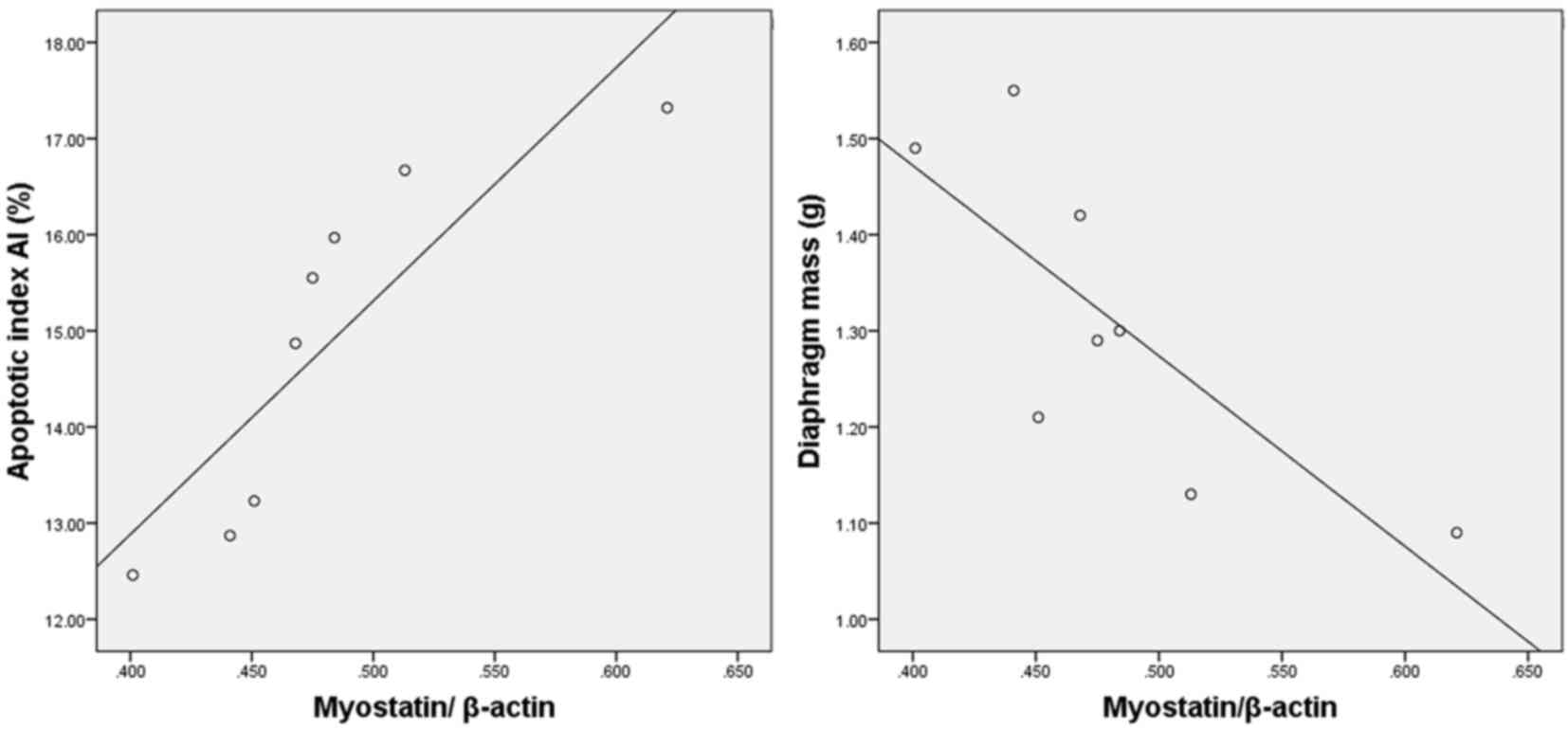
Myostatin expression is correlated
with diaphragmatic degeneration in COPD rats
A positive correlation was identified between the
levels of myostatin and the diaphragmatic apoptotic index (r=0.865;
P<0.01). In addition, a negative correlation was identified
between the levels of myostatin and the diaphragm mass (r=−0.777;
P<0.05; Fig. 5).
Discussion
COPD is characterized by progressive and incomplete
reversible progressive airflow limitation, which remains a
challenge for clinicians and represents a global health burden.
Smoking is one of the most important risk factors for COPD, as
harmful gases or particles in cigarette smoke may induce abnormal
pulmonary inflammation (12). In the
present study, an animal model of COPD was established through a
modified protocol comprising a 5-month challenge of the rats
through exposure to cigarette smoke (13). The presence of airflow obstruction is
key in the diagnosis of COPD, and the present results revealed that
this rat model was associated with a significant decrease in
FEV0.3/FVC and FEP, which are critical parameters of lung injury in
obstructive lung disease (14). In
addition, the histopathological changes of lungs and diaphragms of
COPD rats were similar to those seen in humans with COPD. In
support of the pathological results, the MLI and MAN in the COPD
model group were measured for quantitative analysis of lung
structure and function (15). The
results indicated enlargement of air spaces with a significant
decrease in the alveolar number in the COPD rats as compared with
those in the control, which provided direct evidence of lung
injury. MLI and MAN are accurate and efficient indicators to
reflect alveolar airspace size and lung architecture, and these
parameters reasonably demonstrated that cigarette smoke exposure
was an efficient way to establish a COPD model in rats.
Respiratory muscle dysfunction and particularly
diaphragm dysfunction may induce respiratory failure, which was
reported to be the leading cause of death in patients with COPD
(16). Assessment of the
pathogenesis of COPD has led to the recognition of apoptosis as an
important factor in clinical COPD and experimental models thereof
(17). Apoptosis may be triggered
through three major pathways, one of which involves death receptor
ligation, another is based on the release of cytochrome c
from mitochondria, and the third is the endoplasmic reticulum
pathway (18). Skeletal muscle cell
apoptosis may be responsible for muscle atrophy, which induces
weight loss and weakness in patients with COPD and seriously
affects their quality of life. As the pivotal respiratory muscle,
diaphragmatic apoptosis has important roles in the pathogenesis of
COPD. The present results indicated a significant loss of diaphragm
mass in COPD rats, which indicated diaphragmatic atrophy.
Furthermore, the apoptotic index was significantly increased in the
diaphragms of COPD rats. The present results therefore indicated
diaphragmatic apoptosis in COPD, which is consistent with those of
previous studies (18,19). However, the exact mechanisms of
diaphragmatic apoptosis and their role in the pathophysiological
processes of COPD remain to be fully elucidated. The Fas/Fas ligand
pathway was reported to participate in the regulation of
diaphragmatic apoptosis (20), and
diaphragmatic fiber type transformation (21). Diaphragms of COPD patients generated
a lower specific force, and Type I fibers generated a lower
specific force than Type II fibers (22). Fiber type transformations in COPD
decreased diaphragmatic force generation and finally resulted in
respiratory failure (23).
Preventing diaphragmatic apoptosis and reducing fiber type
transformation may be promising approaches for preventing and
curing respiratory failure in COPD.
Myostatin, also known as GDF-8, is a member of the
TGF-β superfamily that is highly expressed in skeletal muscle
(24), functions as a negative
regulator of skeletal muscle growth and is overexpressed in muscle
atrophy and wasting diseases, including bedrest and disuse atrophy,
anorexia nervosa and cancer-associated cachexia. Myostatin
expression limits the size of muscles during development (25). Myostatin-null mice exhibited muscle
hypertrophy, and myostatin transgenic mice displayed muscle atrophy
and cachexia. Myostatin protein remains inactive until it is
cleaved by a protease. Activated myostatin binds to the activin
type II receptor, recruits activin receptor-like kinase 4 and then
initiates cell signaling cascades in the muscle, which include the
SMAD and mitogen-activated protein kinase families (26). Furthermore, to cause muscle
hypertrophy, AKT kinase may be inhibited by myostatin through
partially suppressing protein synthesis (27). In the present study, myostatin
protein expression was assessed by western blot analysis,
indicating that diaphragmatic myostatin in rats with COPD was
significantly overexpressed compared with that in the control rats,
which was consistent with the results of previous studies (6,28).
After diaphragm mass, apoptosis and myostatin
expression were assessed in COPD rats, their correlation was then
assessed in the present study. A negative correlation between
myostatin expression and diaphragm mass, and a positive correlation
between myostatin expression and apoptosis was identified in the
diaphragms of COPD rats. However, the underlying mechanisms of the
link between these parameters remains to be fully elucidated and
requires to be further investigated. It may be speculated that
myostatin overexpression in the diaphragm promotes diaphragmatic
apoptosis and atrophy, leading to diaphragm weakness and
respiratory muscle dysfunction, and finally aggravates the
progression of COPD. Type II respiratory failure and persistent
CO2 retention are present in the late stage of COPD, are
closely associated with respiratory muscle dysfunction and are the
major cause of COPD-associated death (29). Inhibition of myostatin in the early
stage of COPD may reduce diaphragmatic apoptosis and improve
respiratory muscle function, which may provide a promising strategy
for the prevention and treatment of COPD.
In conclusion, a rat model of COPD was established
using chronic cigarette smoke exposure. A positive correlation was
identified between myostatin expression and diaphragmatic
apoptosis. Myostatin may promote diaphragmatic apoptosis and
atrophy, cause respiratory muscle dysfunction and contribute to the
pathogenesis of COPD. However, the present study had certain
limitations. No method to interfere with the protein expression of
myostatin was employed to then observe its potential effect to
reduce the severity of COPD and diaphragmatic apoptosis. A future
study by our group will assess whether myostatin regulates
apoptosis in COPD. Identification of the role of myostatin in COPD
may provide novel potential targets for the prevention or cure of
COPD.
Acknowledgements
This study was financially supported by a Program of
the Hunan Provincial Department of Science and Technology (grant
no. 2010SK3071).
References
|
1
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the Global
Burden of Disease Study 2010. LANCET. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barnes PJ: Cellular and molecular
mechanisms of chronic obstructive pulmonary disease. Clin Chest
Med. 35:71–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hillas G, Nikolakopoulou S, Hussain S and
Vassilakopoulos T: Antioxidants and mucolytics in COPD management:
When (if ever) and in whom? Curr Drug Targets. 14:225–234. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ottenheijm CA, Heunks LM and Dekhuijzen
RP: Diaphragm adaptations in patients with COPD. Respir Res.
9:122008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Elkasrawy MN and Hamrick MW: Myostatin
(GDF-8) as a key factor linking muscle mass and bone structure. J
Musculoskelet Neuronal Interact. 10:56–63. 2010.PubMed/NCBI
|
|
6
|
Hayot M, Rodriguez J, Vernus B, Carnac G,
Jean E, Allen D, Goret L, Obert P, Candau R and Bonnieu A:
Myostatin up-regulation is associated with the skeletal muscle
response to hypoxic stimuli. Mol Cell Endocrinol. 332:38–47. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma K, Mallidis C, Bhasin S, Mahabadi V,
Artaza J, Gonzalez-Cadavid N, Arias J and Salehian B:
Glucocorticoid-induced skeletal muscle atrophy is associated with
upregulation of myostatin gene expression. Am J Physiol Endocrinol
Metab. 285:E363–E371. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vlahos R and Bozinovski S: Recent advances
in pre-clinical mouse models of COPD. Clin Sci (Lond). 126:253–265.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Jiang X, Zhang L, Wang L, Li Z and
Sun W: Simvastatin mitigates functional and structural impairment
of lung and right ventricle in a rat model of cigarette
smoke-induced COPD. Int J Clin Exp Pathol. 7:8553–8562.
2014.PubMed/NCBI
|
|
10
|
Wang Y, Jiang X, Zhang L, Wang L, Li Z and
Sun W: Simvastatin mitigates functional and structural impairment
of lung and right ventricle in a rat model of cigarette
smoke-induced COPD. Int J Clin Exp Pathol. 7:8553–8562.
2014.PubMed/NCBI
|
|
11
|
Jiang Y, Gao M, Wang W, Lang Y, Tong Z,
Wang K, Zhang H, Chen G, Liu M, Yao Y and Xiao X: Sinomenine
hydrochloride protects against polymicrobial sepsis via autophagy.
Int J Mol Sci. 16:2559–2573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Margaritopoulos GA, Vasarmidi E, Jacob J,
Wells AU and Antoniou KM: Smoking and interstitial lung diseases.
Eur Respir Rev. 24:428–435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fricker M, Deane A and Hansbro PM: Animal
models of chronic obstructive pulmonary disease. Expert Opin Drug
Discov. 9:629–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morris ZQ, Coz A and Starosta D: An
isolated reduction of the FEV3/FVC ratio is an indicator of mild
lung injury. Chest. 144:1117–1123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Andersen MP, Parham AR, Waldrep JC,
McKenzie WN and Dhand R: Alveolar fractal box dimension inversely
correlates with mean linear intercept in mice with elastase-induced
emphysema. Int J Chron Obstruct Pulmon Dis. 7:235–243. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wouters EF: Local and systemic
inflammation in chronic obstructive pulmonary disease. Proc Am
Thorac Soc. 2:pp. 26–33. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Plataki M, Tzortzaki E, Rytila P,
Demosthenes M, Koutsopoulos A and Siafakas NM: Apoptotic mechanisms
in the pathogenesis of COPD. Int J Chron Obstruct Pulmon Dis.
1:161–171. 2006.PubMed/NCBI
|
|
18
|
Degens H, Swisher AK, Heijdra YF, Siu PM,
Dekhuijzen PN and Alway SE: Apoptosis and Id2 expression in
diaphragm and soleus muscle from the emphysematous hamster. Am J
Physiol Regul Integr Comp Physiol. 293:R135–R144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barreiro E, Ferrer D, Sanchez F, Minguella
J, Marin-Corral J, Martinez-Llorens J, Lloreta J and Gea J:
Inflammatory cells and apoptosis in respiratory and limb muscles of
patients with COPD. J Appl Physiol (1985). 111:808–817. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tan Y, Sun L, Gao J and Ouyang X:
Apoptosis of diaphragmatic muscle cell in Chronic obstructive
pulmonary disease. J Clin Pulm Med,. 15:1580–1582. 2010.
|
|
21
|
Ji L, Sun L, Tan Y and Gu W: Adaptations
of diaphragmatic muscle fibres and apoptosis of diaphragmatic
muscle cell in rats with chronic obstructive pulmonary disease.
Acta U Med Nanjing(Natural Science). 32:194–198. 2012.
|
|
22
|
Levine S, Nguyen T, Kaiser LR, Rubinstein
NA, Maislin G, Gregory C, Rome LC, Dudley GA, Sieck GC and Shrager
JB: Human diaphragm remodeling associated with chronic obstructive
pulmonary disease: Clinical implications. Am J Respir Crit Care
Med. 168:706–713. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sauleda RJ: Clinical consequences of
muscle dysfunction in chronic obstructive pulmonary disease. Nutr
Hosp. 21(Suppl 3): 69–75. 2006.(In Spanish). PubMed/NCBI
|
|
24
|
McPherron AC, Lawler AM and Lee SJ:
Regulation of skeletal muscle mass in mice by a new TGF-beta
superfamily member. Nature. 387:83–90. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Amthor H, Huang R, McKinnell I, Christ B,
Kambadur R, Sharma M and Patel K: The regulation and action of
myostatin as a negative regulator of muscle development during
avian embryogenesis. Dev Biol. 251:241–257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang W, Chen Y, Zhang Y, Wang X, Yang N
and Zhu D: Extracellular signal-regulated kinase 1/2
mitogen-activated protein kinase pathway is involved in
myostatin-regulated differentiation repression. Cancer Res.
66:1320–1326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sartori R, Gregorevic P and Sandri M:
TGFbeta and BMP signaling in skeletal muscle: potential
significance for muscle-related disease. Trends Endocrinol Metab.
25:464–471. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Testelmans D, Crul T, Maes K, Agten A,
Crombach M, Decramer M and Gayan-Ramirez G: Atrophy and hypertrophy
signalling in the diaphragm of patients with COPD. Eur Respir J.
35:549–556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gea J, Agusti A and Roca J:
Pathophysiology of muscle dysfunction in COPD. J Appl Physiol
(1985). 114:1222–1234. 2013. View Article : Google Scholar : PubMed/NCBI
|