Introduction
Major advances in the management of cancer have been
achieved since cancer chemotherapy, as we know it today, began to
take shape with the discovery of the antitumoral activity of
alkylating agents (1), certain
hormones (2) and the advent of
antimetabolites of DNA building blocks (3). In addition, aberrant histone
methylation associated with gene mutation, translocation or
overexpression may often lead to initiation of a disease process
leading to cancer (3,4). In recent years, some specific molecule
inhibitors of such histone modifying enzymes that correct their
abnormal methylation are being used as novel therapeutics for these
pathologies (4). In the 1960s a
clinical study investigating combination therapy, brought about
major advances, leading to the demonstration that complete
remission of certain neoplasia could be induced with available
anticancer drugs (5). At the same
time, novel agents, including anthracycline antibiotics, the
Vinca alkaloids, the platinum complexes and hormone
antagonists, provided additional powerful tools (5–7). The
advances in cancer chemotherapy were also greatly aided by progress
made in diagnostic procedures, by the advent of combined modalities
of treatment and by the development of improved criteria for
regimen design and result assessment (8–10). As a
consequence of these advances, it is now possible to induce
complete tumor regression in patients with different types of
neoplasia and to obtain disease-free survival lasting 10 years or
longer in a notable percentage of them (11–14).
Despite this progress, major difficulties remain to
be overcome before cancer therapeutics may become generally
successful in curative management (15–17).
This is particularly observed in the case of the common solid
tumors. These difficulties may be mainly attributed to the lack of
agents acting uniquely and specifically on tumors, or at least
having sufficiently marked selectivity of antitumor action, and
resistance phenomenon (16). Over
the past two decades, new vistas have been opening up in cancer
therapeutics, consequent to progress made in the understanding of
the molecular biology of the cancer cell, interactions between
tumor cells, host regulatory mechanisms, and mechanisms responsible
for different forms of resistance (18–21). The
present study presents the functional effect of Congerine
(AntiGan), a mucosal galectin produced in the epidermis and
esophagus of the Conger conger, that provides
immune-chemical fortification properties through its agglutinating
and opsonizing activity (22). It
has been demonstrated that Congerine may reach and function in the
intestinal lumen since it presents marked resistance against
digestion by gastric and enteric enzymes (22,23). In
humans and mice, galectin-4 is known to be expressed in the
alimentary canal from the tongue to the large intestine (24), and probably functions intracellularly
in the defense of the colonic mucosal surfaces. In the present
work, different concentrations of AntiGan, a lipofishin extract
obtained from C. conger, was used to evaluate its preventive
and/or therapeutic effects on colorectal adenocarcinoma cell
cultures (HL60, HS 274.T, HS 313.T, H2126, WM 115, HS 281T, Caco-2,
HT-29 and SW-480) and in an animal model of colon inflammation
induced by dextran sulphate sodium (DSS). In light of its
anti-inflammatory effect on colonic mucosal surfaces, its
anti-tumor potential on apoptotic activation processes, the easy
cell/tissue isolation method, and the fact that lyophilization of
this bioactive product (E-Congerine) conserves their original
properties, AntiGan is a promising candidate for preclinical and
clinical applications in anticancer immunotherapy.
Materials and methods
Cell lines and cell culture
All cell lines were purchased from the American Type
Culture Collection (Manassas, MA, USA). Human colorectal
adenocarcinoma cell line (SW-480) was maintained in Leibovitz-15
medium with L-glutamine, supplemented with 10% heat-inactivated
fetal bovine serum (FBS) (both from PAA; GE Healthcare Life
Sciences, Little Chalfont, UK) and sodium bicarbonate (1.5 g/l)
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and
incubated at 37°C in 5% CO2. HL-60 (promyelocytic
leukemia) cell line was grown in RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% heat-inactivated FBS, 100
IU penicillin, 100 µg/ml streptomycin and 2 mM L-glutamine. HS
274.T (breast adenocarcinoma) and HS 313.T (lymphoma) cell lines
were grown in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10%
heat-inactivated FBS, 100 IU penicillin, 100 µg/ml streptomycin and
2 mM L-glutamine. H2126 hypotriploid cell line from a metastatic
site, pleural effusion adenocarcinoma, was grown in DMEM/F12
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 5%
heat-inactivated FBS, 100 IU penicillin, 100 µg/ml streptomycin, G5
supplement (Gibco; Thermo Fisher Scientific, Inc.) and 2 mM
L-glutamine. Caco-2 colorectal adenocarcinoma cell line was grown
in Eagle's minimum essential medium (EMEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 20% heat-inactivated FBS, 100
IU penicillin, 100 µg/ml streptomycin and 2 mM L-glutamine. HT-29
colorectal adenocarcinoma cell line was grown in McCoy's 5A
modified medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% heat-inactivated FBS, 100 IU penicillin, 100
µg/ml streptomycin and 2 mM L-glutamine. WM 115 (melanoma) cell
line was grown in EMEM, supplemented with 10% heat-inactivated FBS,
100 IU penicillin, 100 µg/ml streptomycin and 2 mM L-glutamine. HS
281.T (breast adenocarcinoma) cell line was grown in DMEM
supplemented with 10% heat-inactivated FBS, 100 IU penicillin, 100
µg/ml streptomycin and 2 mM L-glutamine. Cell stocks were
maintained in liquid nitrogen.
MTT reduction assay
Cell proliferation was determined using MTT assay.
Cell lines (1×105 cells/ml) were incubated in 96-well
plates with different doses of AntiGan (10, 25 and 50 µl/well) at
37°C for 24 h (Ebiotec, Bergondo, Spain). A total of 10 µl MTT (10
mg/ml) was added to each well and incubated further at 37°C for 4
h. After incubation, MTT-formazan precipitate was dissolved in 100
µl dimethyl sulfoxide and absorbance was recorded at 570 nm in an
ELISA plate reader. The antiproliferative effect of AntiGan was
investigated in Caco-2, HT-29 and SW-480 cells, by treating them
with 10, 25 and 50 µl/ml AntiGan or culture medium. Data were
presented as the percentage of cytotoxicity of treated vs.
untreated cells.
DNA ladder assay
DNA ladder assay was conducted as per a standard
method. This method prevents the contamination of entire genomic
DNA with fragmented DNA. Briefly, following treatment with AntiGan,
cells were harvested (centrifugation at 27,000 × g at 4°C for 30
min), washed twice with cold PBS and lysed for 30 min at 4°C in
lysis buffer (50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 0.2% Triton X-100)
using zirconium beads (cat. no. 11079107zx; BioSpec, Bartlesville,
USA) and an automatic cell lyser. Following centrifugation at
15,000 × g at 4°C for 20 min, the supernatants were treated with
protease inhibitor cocktail (cat. no. P8465-5ML; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) and 0.5% SDS for 1 h at 37°C. DNA
was extracted twice with phenol and precipitated with 150 mM NaCl
and two volumes of ethanol at −20°C. DNA precipitate was washed
twice with cold 70% ethanol, dissolved in TE buffer (cat. no.
93283-100ML) and treated for 1 h with RNase (cat. no. 10109134001)
(both from Sigma-Aldrich; Merck KGaA) at 37°C. Finally, DNA
precipitates were stained with propidium iodide at room temperature
(RT) for 10 min, electrophoresed on 2% agarose gel and visualized
in an automatic gel documentation system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
A cytometric bead array was performed to assess the
levels of interleukin (IL)-6 (cat. no. SCU0001; 1:100), IL-10 (cat.
no. I17002; 1:100), IL-17 (cat. no. SRP3080; 1:100), IL-1β (cat.
no. SRP6169; 1:100), interferon (IFN)-γ (cat. no. I17001; 1:100)
and tumor necrosis factor (TNF)-α (cat. no. T6674; 1:100) in the
samples (all from Sigma-Aldrich; Merck KGaA). Each capture bead had
a distinct fluorescein isothiocyanate (FITC) conjugate and was
coated with a capture antibody specific for each soluble protein.
The detection reagent was a mixture of phycoerythrin
(PE)-conjugated antibodies (cat. no. MABF925; 1:200; Sigma-Aldrich;
Merck KGaA), which provided a fluorescent signal in proportion to
the amount of bound analytes. When the capture and detection beads
were incubated with the samples and controls (beads without
conjugated antibodies), sandwich complexes (capture bead + analyte
+ detection reagent) were formed. These complexes were subsequently
measured using flow cytometry to identify particles with
fluorescence characteristics of both the bead and the detector.
The bead population was resolved in two fluorescence
channels of a FACStar flow cytometer (Becton Dickinson; BD
Biosciences, Franklin Lakes, NJ, USA) equipped with a 5 W argon ion
laser emitting 488 nm at 0.2 W. Green fluorescence (FITC) was
collected through a 530/30 nm filter and red fluorescence (PE)
through a 585/42 nm filter. Beads with different positions were
combined in the assay to create a six-plex assay. The intensity of
PE fluorescence of each sandwich complex revealed the concentration
of each cytokine. Data were collected in four-parameters with
linear amplification for forward light scatter and logarithmic
amplification for side light scatter, green and red fluorescence.
Data was processed using Consort 30 software (Consort BVBA,
Turnhout, Belgium).
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Single step phenol-chloroform-isoamyl alcohol
extraction was used to isolate the total cellular RNA by lysis in a
guanidinium isothiocyanate buffer (cat. no. G9277-100G;
Sigma-Aldrich; Merck KGaA), as previously described (25,26). RNA
was converted into cDNA by reverse transcription using a Maxima
Reverse Transcriptase kit (cat. no. EP0741), using reverse
transcriptase M-MLV and RiboLock RNase inhibitor agents (all from
Applied Biosystems; Thermo Fisher Scientific, Inc.). The primer
sequences used are detailed in Table
I. The concentration of cDNA was measured using a
NanoDrop™ spectrophotometer (Thermo Fisher Scientific,
Inc.) and the samples were preserved at −80°C. PCR was performed as
follows: 40 cycles of denaturation at 95°C for 5 sec and
annealing-extension at 60°C for 5 sec. 1.5% agarose gel
electrophoresis was used to analyze the resulting PCR products. The
primers were synthesized by Bio Basic, Inc. (Markham, ON, Canada).
RT-PCR was performed using a ABI Model 7500 Sequence Detector
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with a
SYBR-Green Real-Time PCR kit (Takara Bio, Inc., Otsu, Japan).
Specific primers and their sequences are indicated in Table I. The molecular mechanisms
responsible for the AntiGan activity in the cell lines used were
tested by the gene expression of the following apoptotic-related
genes: p53, p21, Bcl-2-associated X protein (Bax) and Bcl-2.
 | Table I.Oligonucleotides used in reverse
transcription-polymerase chain reaction. |
Table I.
Oligonucleotides used in reverse
transcription-polymerase chain reaction.
| Gene | Direction | Sequence
(5′-3′) |
|---|
| p53 | Sense |
AAAACTTACCAAGGCAACTA |
|
| Antisense |
TGAAATATTCTCCATCGAGT |
| p21 | Sense |
CATGTCCGATCCTGGTGATG |
|
| Antisense |
AGTGCAAGACAGCGACAAGG |
| Bcl-2 | Sense |
TGCACCTGACGCCCTTCAC |
|
| Antisense |
AGACAGCCAGGAGAAATCAAACAG |
| Bcl-2-associated X
protein | Sense |
ACCAAGAAGCTGAGCGAGTGTC |
|
| Antisense |
ACAAAGATGGTCACGGTCTGCC |
Animals
A total of 56 specific pathogen-free Swiss CD1
female mice (7 weeks old and 20–25 g; Santiago de Compostela's
University Animal Breeding Core, Santiago de Compostela, Spain)
were maintained (2 or 3 mice/cage) in isolator plastic cages with
shavings under standard laboratory conditions (sterilizable diet;
50% humidity; 23–24°C temperature; and 12-h light/dark cycle). All
mice were quarantined 3 weeks after arrival and then randomized by
body weight into experimental and control groups. All mice were
permitted free access to a commercial diet and treatment or normal
drinking tap water in individual bottles. All procedures conformed
to the guidelines established by the European Communities Council
Directive of 24 November 1986 (86/609/EEC) and by the Spanish Royal
Decree 1201/2005 for animal experimentation, and were approved by
the Ethical Committee of EuroEspes Biotechnology (Coruna, Spain;
EE-12/223).
Study design
The present experimental study was designed to
induce colitis-associated dysplasia and/or tumor hallmarks by
administering DSS (Sigma-Aldrich; Merck KGaA) to mice and feeding
them a diet with different AntiGan dilutions. In the present study,
the calculation of sample size was performed by power analysis
(software G Power; gpower.hhu.de/en.html, version 3192) (23), which takes into account the effect
size (difference between the mean of two groups), standard
deviation (variability within the sample), type 1 error
[significance level of 5% (P=0.05)], power of the study
(probability of finding an effect, kept at 80%), direction of
effect (two-tailed test), statistical tests (analysis of variance)
and expected attrition (mortality of animals).
Experimental mice were separated into two control
groups (D and E, n=10/group) and three treatment groups (A-C,
n=12/group). AntiGan extract (E-Congerine 10423®) was
integrated in the diet as pellet biscuits and elaborated in our
laboratory by adding different % of powder treatment ingredients
(AntiGan) using diet wheat as the main flour, adding 10% (w/w)
Milli Q-purified water for pelleting and then drying the pellets at
34°C overnight. In the first 2 weeks, diet containing different
AntiGan concentrations (2.5% in group A, 5% in group B, 10% in
group C and 5% in group D) was administered to mice in groups A, B,
C and D. In the third week, tap water containing 20 g/l (2%)
synthetic DSS (mol mass, 5,000; D4911) was administered to mice in
groups A, B, C and E. Control groups D and E were treated only with
AntiGan or DSS, respectively. At the end of the experiment (sixth
week), mice were sacrificed (15 weeks of age) and different
colorectal segments and blood samples were collected for specific
immunohistochemistry and staining analysis. The overall health of
the mice before and after treatment was assessed and normal
parameters were observed.
Tissue preparation
Mice were deeply anesthetized by inhalation of
diethyl ether (cat. no. 100921; Sigma-Aldrich; Merck KGaA) and
blood samples were extracted. The location, storage and use of
diethyl ether was approved by the Ethical Committee of EuroEspes
Biotechnology (EE-12/223), Bureau Veritas Certification (ISO
9001:2008), the European Quality Assurance in Lab (UNE 166002:2006)
and the Human Health Agency of Spain (U:75/2013). Subsequently,
mice were intracardially perfused with saline buffer and then fixed
by 4% paraformaldehyde (30 min at RT) in 0.1 M phosphate buffer (pH
7.4). Euthanasia was performed under anesthesia by exsanguination
and cervical dislocation. Confirmation of euthanasia was performed
by decapitation. The entire colorectum (from ileocecal junction to
the anal verge) was removed, measured, examined macroscopically
(Olympus BX50; magnification, ×4), washed with saline buffer and
immediately fixed by immersion in the same fixative for 48 h at RT.
Part of the colon was divided into three equal segments (proximal,
middle and distal), portions were determined under a dissecting
stereo light microscope at ×20 magnification (Leica M125; Leica
Microsystems, Inc., Buffalo Grove, IL, USA). Intestine portions
were cryoprotected with 30% sucrose in 0.1 M phosphate buffer,
embedded in OCT compound (Tissue Tek; Sakura Finetek USA, Inc.,
Torrance, CA, USA), and frozen with liquid nitrogen-cooled
isopentane. Parallel series of transverse sections (14–16 µm) were
obtained on a cryostat (Starlet 2212; Bright Instruments, Ltd.,
Luton, UK) and mounted on Superfrost Plus (cat. no. 4951PLUS4;
Menzel-Gläser®; Thermo Fisher Scientific, Inc.)
slides.
Histological staining and
immunohistochemistry
Prior to staining sections were fixed with 4%
paraformaldehyde (Sigma-Aldrich; Merck KGaA) in a phosphate buffer
for 48–72 h at RT. Routine histological examination was performed
on 14-µm-thick hematoxylin and eosin (H&E)-stained sections
(hematoxylin 60% at RT for 5 min and eosine 50% at RT for 1 min;
MEDITE GmbH, Burgdorf, Germany) where different morphological
alterations that occured during colorectal carcinogenesis (such as
crypt abscess, mucosal dysplasia, adenomas and adenocarcinomas)
(8), were identified by light
microscopy (magnification, ×20) and diagnosed according to a
previously published study (27). To
detect the expression of colorectal histopathological markers,
including β-catenin, Bcl-2 and cyclooxygenase (Cox)-2,
immunohistochemical techniques were used. The sections (FSC 22
frozen section media; Leica Microsystems, Ltd., Milton Keynes, UK)
of 14 µm were pretreated with H2O2 to
eliminate endogenous peroxidase, rinsed twice for 10 min each in
PBS at pH 7.4, and then treated with nonspecific binding blocking
solution [0.1 M PBS containing 0.2% Tween-20 and 15% normal goat
serum (Dako; Agilent Technologies, Inc., Santa Clara, CA, USA)] for
1 h at RT. Following this, the sections were incubated with primary
rabbit polyclonal antibodies that were affinity-purified from
rabbit antiserum by affinity-chromatography using mouse
epitope-specific immunogen, including anti-β-catenin, anti-Bcl-2
and anti-Cox-2 (cat. nos. BS3603, BS3736 and BS1017, respectively;
dilution, 1:200; Bioworld Technology, Inc., St. Louis Park, MN,
USA) overnight at RT. The sections were then washed in PBS (two
10-min rinses), incubated with goat anti-rabbit immunoglobulin G
serum biotinylated (cat. no. E-0432; dilution, 1:100; Dako; Agilent
Technologies, Inc.) for 1 h at RT, washed with PBS (two 10-min
rinses), treated with a Vectastain ABC kit (Vector Laboratories,
Inc., Burlingame, CA, USA) for 1 h at RT, and finally washed again
in PBS (two 10-min rinses). As a negative control, omission of the
primary, secondary or tertiary antibodies was used and no
immunostaining was observed. At the last step, the immunoreaction
was developed with 0.005% diaminobenzidine (Sigma-Aldrich-Aldrich;
Merck KGaA) and 0.003% H2O2. All dilutions
were made in PBS containing 0.2% Tween-20, and incubations were
made in a humid chamber at RT. Finally, the sections were
dehydrated, mounted and cover-slipped.
Antibody characterization and
specificity
According to the technical information supplied by
the manufacturer (Bioworld Technology, Inc.), the primary
antibodies used were raised against denatured mouse epitopes from
rabbit antiserum and they were affinity-purified by chromatography
using epitope-specific immunogen with purity >95% (by SDS-PAGE).
Its specificity has been assessed by western blotting; it
recognizes a single protein band of 86–90 kD (β-catenin), 26–28 kD
(Bcl-2) and 74–76 kD (Cox-2). Additionally, antibodies have wide
species cross-reactivity and were used for demonstrating their
expression in mice, rats and humans.
In the present study, lesions were classified as
positive for β-catenin/Bcl-2/Cox-2 if cytoplasmic/nuclear staining
was detected. Two different observers individually and
independently evaluated the experimental group slides in a
double-blind manner and achieved a high level of concordance.
Imaging
The sections were photographed with an Olympus
microscope (BX50) equipped with a color digital camera (DP10;
magnification, ×20; both from Olympus Corporation, Tokyo, Japan).
The photographs were converted to grayscale and adjusted for
brightness and contrast with Corel Draw and the image size was
adjusted with Corel Photo Paint version 11 (Corel Corporation,
Ottawa, ON, Canada).
Statistical analysis
The statistical parameters of the results obtained
were performed using SPSS v.11.0 (SPSS, Inc., Chicago, IL, USA).
Differences between treated groups were compared with
Kruskal-Wallis test followed by Mann-Whitney U test for
nonparametric data, while normally distributed data was tested
using the one-way analysis of variance. Bonferroni's correction was
used to avoid false positives while Games-Howell was used to
compare combinations of treatment groups. Data was presented as the
mean ± standard error of the mean. P<0.05 was considered to
indicate a statistically significant difference.
Results
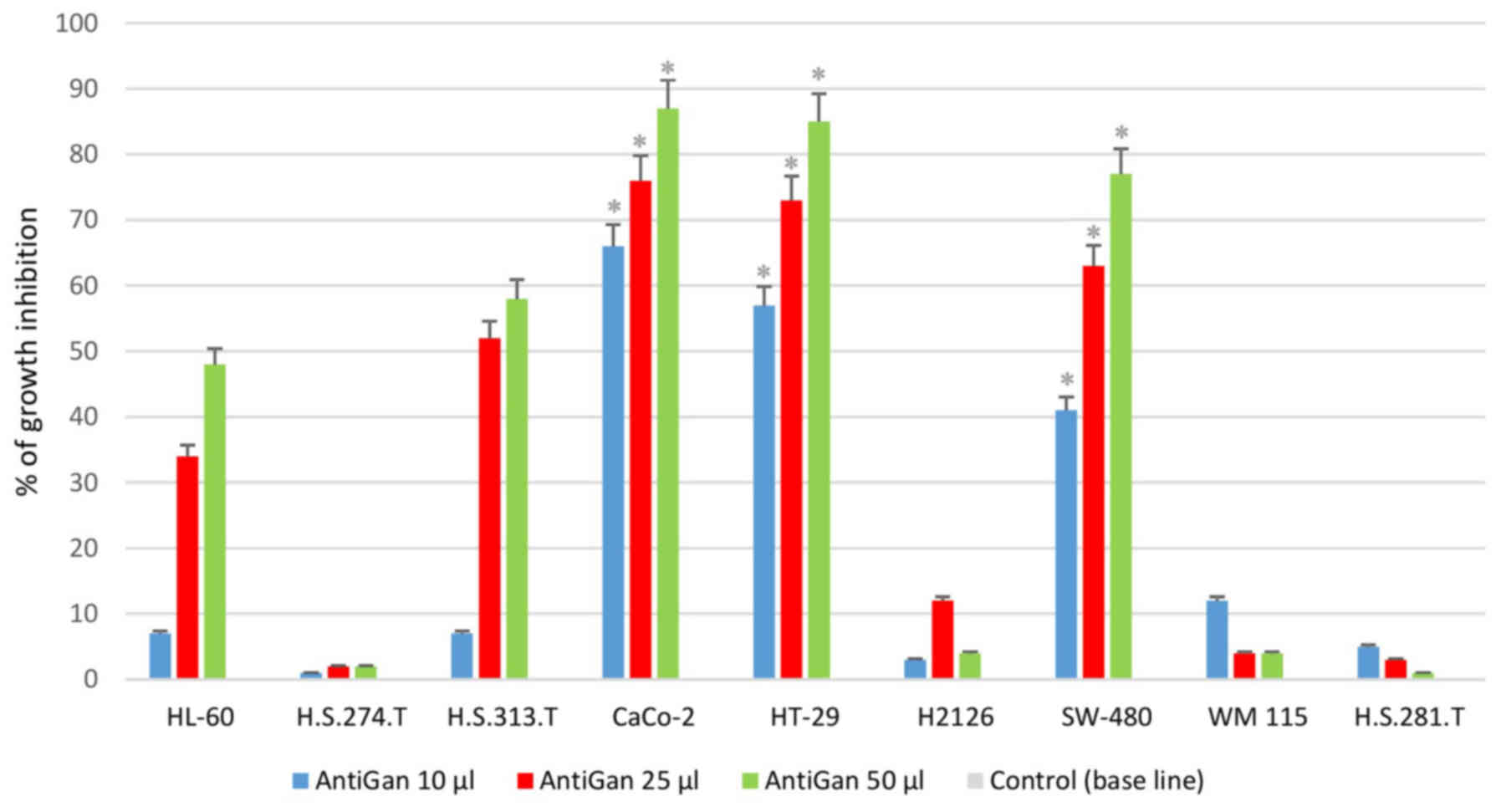
AntiGan treatment inhibits growth in
tumor cell lines
In order to analyze the possible antineoplastic
effect of AntiGan in different tumor cell lines, its effect on cell
growth was assessed by MTT assay, measuring the live cell
metabolism rates based on the enzyme activity levels of
mitochondrial dehydrogenase. Results demonstrated that AntiGan
induced cell growth inhibition in a dose-dependent manner in the
majority of cell lines used (Fig.
1). The cytotoxicity detected was not restricted to a specific
tumor cell line, as five different cell lines were sensitive to the
effects of AntiGan. The highest level of growth inhibition was
observed in Caco-2 (66, 75.8 and 88.1% growth inhibition for 10, 25
and 50 µl/ml AntiGan, respectively), HT-29 (56, 73 and 86% growth
inhibition for 10, 25 and 50 µl/ml AntiGan, respectively) and
SW-480 (38.5, 61.6 and 78.6% growth inhibition for 10, 25 and 50
µl/ml AntiGan, respectively) cells; while HL-60 (5.8, 33.5 and
47.1% growth inhibition for 10, 25 and 50 µl/ml AntiGan,
respectively) and HS 313.T (5.3, 50.3 and 59.6% growth inhibition
for 10, 25 and 50 µl/ml AntiGan, respectively) cells demonstrated a
slight, cytotoxic effect with respect to untreated cells. At the
lowest concentration of AntiGan (10 µl/ml) a significant difference
between controls (untreated) and treated cells was observed in
Caco-2, HT-29 and SW480 cell lines. No significant cytotoxic
effects were observed in the HS 274.T, H2126, WM 115 and HS281.T
tumor cell lines.
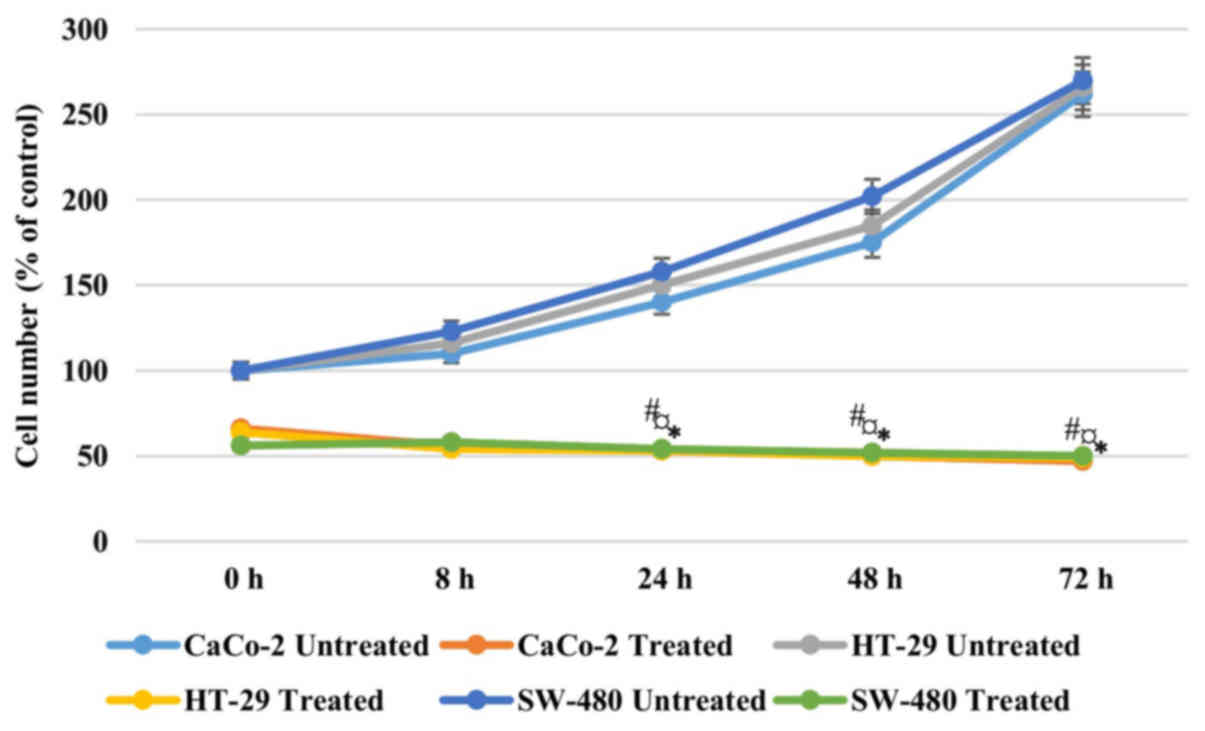
In order to address if the antiproliferative effect
of AntiGan was reversible, Caco-2, HT-29 and SW-480 cells were
treated with 25 µl/ml AntiGan or culture medium. After 48 h of
incubation with AntiGan, the cell density was reduced by >50% in
all cell lines. Once AntiGan was removed from the cell culture, the
cell density showed a small decrease, while in the absence of
treatment, as expected, cell density increased with time (Fig. 2). These data indicate that the
AntiGan effect was not reversible.
AntiGan induces apoptosis in tumor
cell lines
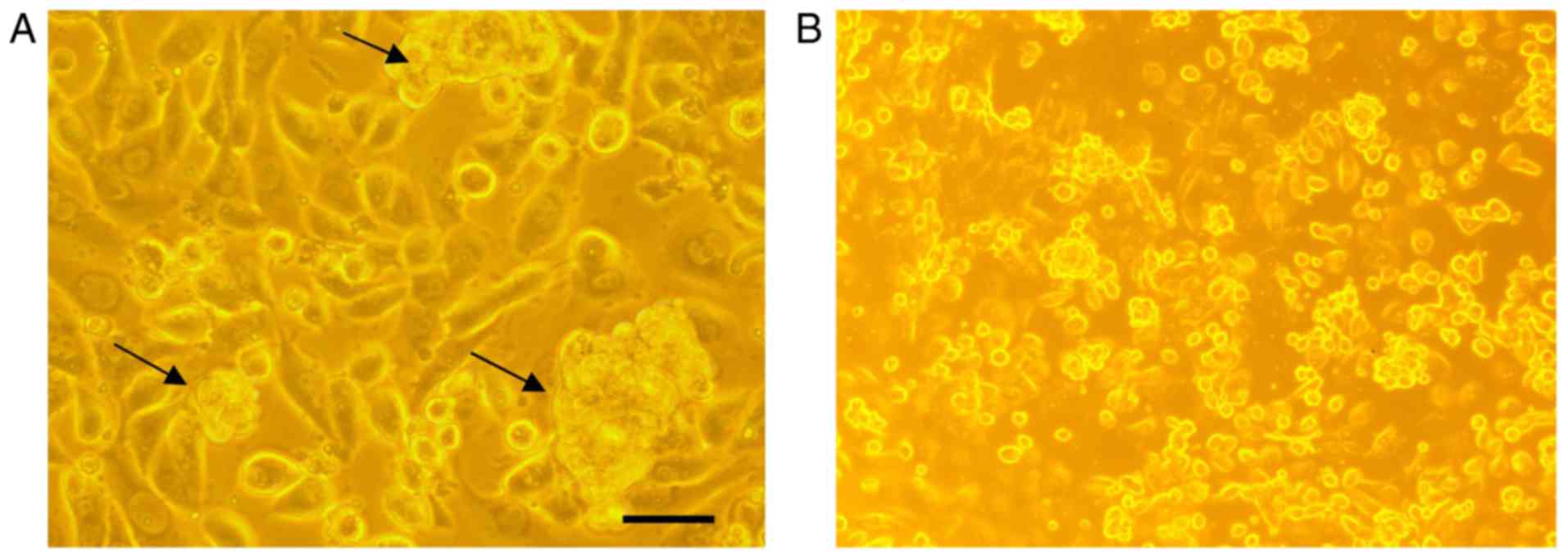
In order to analyze if there are any cytotoxic
effects of AntiGan, HL-60, HS 274.T, HS 313.T, H2126, Caco-2, WM
115, HT-29, SW-480 and HS 281T human tumor cell lines were treated
with 25 µl/ml AntiGan. Microscopic analysis of SW-480 cells
revealed that numerous cells exhibited condensation (arrows) and
cleavage of their nuclei, characteristic of an apoptotic process
(Fig. 3A). These apoptotic hallmarks
were not observed in control (untreated) cells (Fig. 3B). The results obtained, expressed as
absorbances (570 nm), indicated that treatment with AntiGan induced
apoptosis in the HL-60 cell line. DNA prepared from HL-60, HS 313T,
Caco-2, SW-480 and HT-29 cell lines treated with AntiGan
demonstrated oligonucleosomal ladder fragmentation on agarose gel
electrophoresis (data not shown). No signs of apoptosis were
observed in HS 274.T, H2126, WM 115 and HS 281T cells (data not
shown).
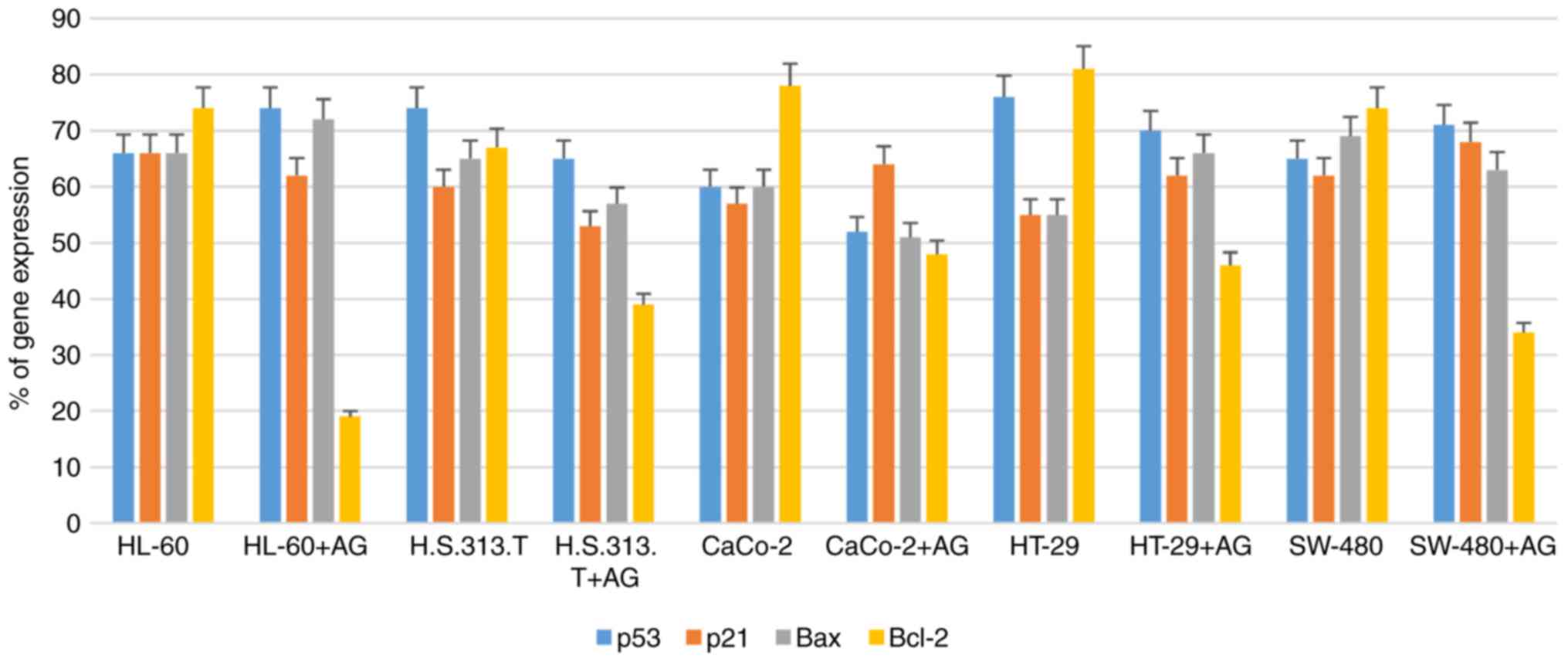
AntiGan treatment downregulates Bcl-2
gene expression
In order to study the molecular mechanisms of
AntiGan that induced apoptosis in HL-60, HS 313T, Caco-2, HT-29 and
SW-480 cell lines, the gene expression patterns of some
apoptotic-associated genes, including p53, p21, Bax and Bcl-2, were
analyzed following treatment with AntiGan (10, 25 and 50 µl/ml) by
RT-PCR. AntiGan treatment (10 µl/ml) triggered downregulation of
Bcl-2 gene expression in all cell lines, with no significant effect
on the expression levels of the other genes, resulting in the
relative increase of Bax/Bcl-2 ratio (Fig. 4). AntiGan treatment at 25 and 50
µl/ml did not have a significant effect on the expression level of
the genes that were investigated (data not shown).
Pathological and inflammatory
findings
Experimental mice treated with 2% DSS and lower or
absent concentrations of AntiGan (groups A and E) exhibited bloody
stools from the third week of experimentation, while no such
inflammatory effects were noticed in any other group. A macroscopic
evaluation (data not shown) recognized some gross inflammatory
polypoids in groups E (6/6; 100%) and A (5/6; 83.3%), with the
majority of them located in the middle and distal portions of the
colorectal segment, while only few of them were identified in group
D (1/6; 16.6%). Notably, no mice in group C (DSS/10% AntiGan)
demonstrated these ulcerative hallmarks, similar to that observed
in the control mice (group D; 5% AntiGan). This macroscopic
evaluation profile was corroborated by microscopic analysis of the
histological colorectal morphology, with special identification of
inflammatory hallmarks on the colonic mucosa and submucosa, mainly
represented by dysplastic epithelium and ulcerative alterations.
The histological observation of hematoxylin and eosin-stained
sections (Fig. 5) revealed some
ulcers that represent a severe inflammation hallmark within the
submucosa and mucosal areas. Additionally, there were also some
moderate crypt hyperplasia (epithelium with abnormal thickness),
and epithelial dysplasia, which is the alteration of the normal
differentiation process of epithelial cells that may progress to
invasive colorectal carcinoma (17).
However, the presence of these inflammatory alterations and their
severity differed among the experimental treatment groups. Mice in
group E, only treated with DSS, demonstrated high severity levels
of these colorectal inflammatory hallmarks, which was in contrast
to the moderate and mild lesion levels observed in mice treated
with increased concentrations of AntiGan (groups A and B,
respectively). These characteristic colitic alterations were mild
or absent in the colorectal samples of mice treated with high
concentrations of AntiGan (group C). Mice treated only with AntiGan
(control group D; 5% AntiGan), demonstrated no adverse effects or
histological alterations at microscopic examination (Fig. 5).
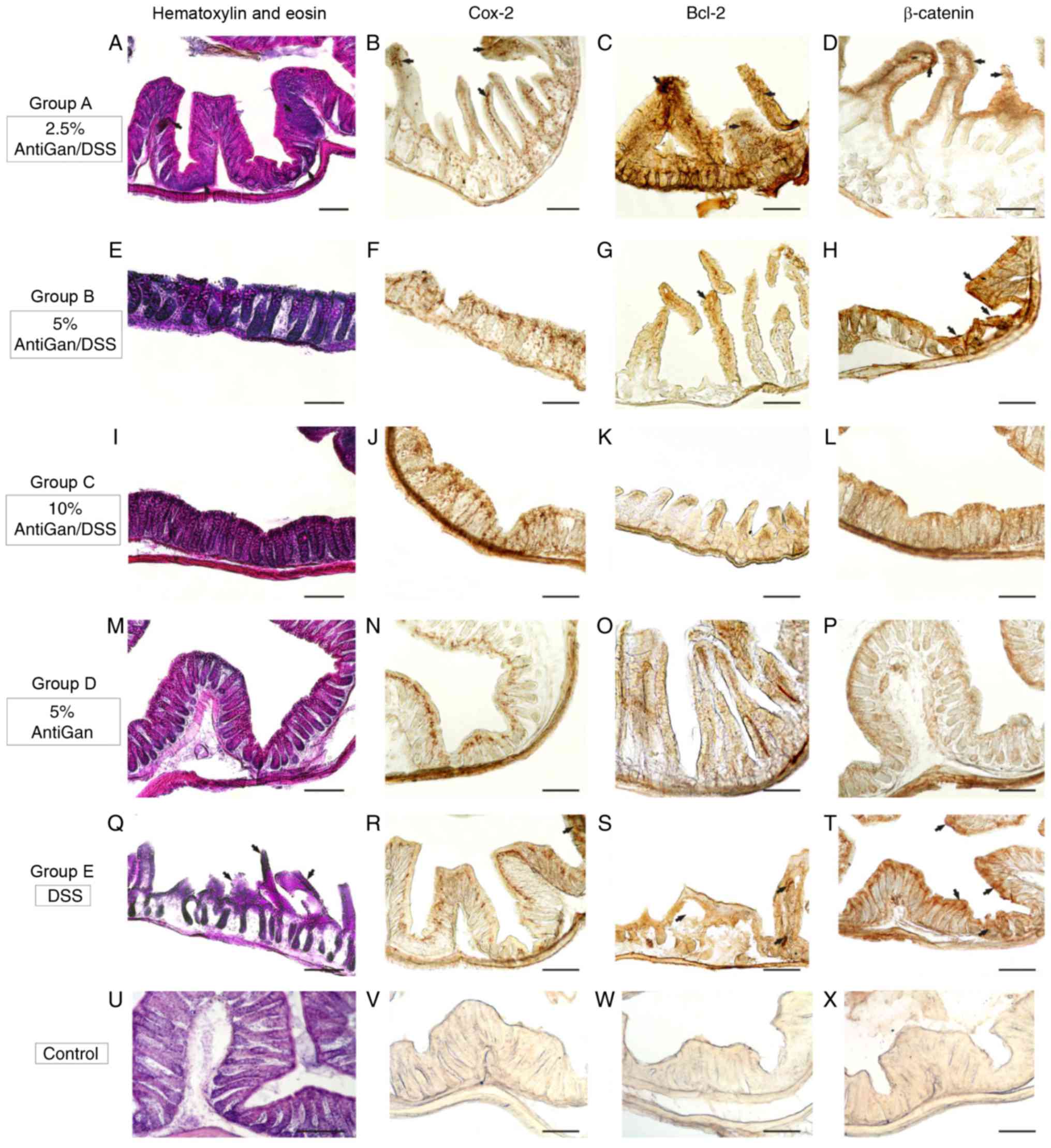 | Figure 5.Photomicrographs of different
portions of colon from experimental mice showing histopathological
lesions identified by hematoxylin and eosin staining, and
immunohistochemical detection. (A-H) Details of transverse sections
at the middle and distal colorectal levels of mice in groups A and
B (arrows), demonstrating high-moderate immunoreactivity to
β-catenin, Bcl-2, and Cox-2. (I-L) Colorectal sections of mice in
group C demonstrated a normal colonic epithelial organization with
well-differentiated cryptal cells. (M-P) Details of colorectal
sections of mice in group D, to highlight the strong immunoreactive
staining of the cell markers. (Q-T) A high density of
immunoreactive cells (arrows) were observed in mice in group E and
these were gathered at specific pathological lesions, demonstrating
a severe-grade of dysplasia. (U-X) Negative control sections. Scale
bar, 100 µm. DSS, dextran sulphate sodium; Bcl-2, B-cell lymphoma
2; Cox-2, cyclooxygenase-2. |
Immunohistochemistry of β-catenin,
Bcl-2 and Cox-2
Immunohistological markers with high specific
affinity against colorectal cell epitopes demonstrated notable
expression levels of β-catenin (cellular adhesion regulator), Bcl-2
(apoptotic regulator) and Cox-2 in colorectal lesions in mice. By
using β-catenin polyclonal antibody, the endogenous expression
levels of β-catenin protein within the cytoplasm of affected
colorectal cell layers were observed. As expected, high
immunoreactive expression levels of β-catenin were detected in the
proximal and middle colon segments (Fig.
5T) in mice treated with DSS, similar to the moderate
immunoreactivity patterns observed in the colorectal segments
(Fig. 5D and H) of mice treated with
low concentrations of AntiGan (DSS/2.5–5% AntiGan). These β-catenin
immunoreactive cells were mainly observed in the internal cryptal
layers, being characterized histologically as dysplastic cryptal
cells and adenocarcinoma cells (Fig. 5H
and T). However, cryptal cells of the colorectal segments of
mice treated with high AntiGan concentrations (group C)
demonstrated a weak or absent β-catenin immunoreactivity (Fig. 5L), similar to the control (Fig. 5X), as compared with the β-catenin
cellular basal expression of group D (Fig. 5P). A similar immunostaining pattern
was obtained when detecting reactivity levels of Bcl-2, an
anti-apoptotic oncoprotein, in the colorectal portions of groups A
(Fig. 5C), B (Fig. 5G) and E (Fig. 5S). Bcl-2 immunoreactivity was
specifically intense in the adenocarcinoma and cryptal cells of
colorectal portions of mice treated with DSS. Intense Bcl-2
staining patterns were mild or completely absent in colorectal
section of mice groups C, D and control, respectively (Fig. 5K, O and W, respectively). A strong
staining pattern of Cox-2 in immunoreactive cryptal cells was
observed in the colorectal dysplastic epithelium of mice treated
with DSS (Fig. 5R) and lower
concentrations of AntiGan (Fig. 5B),
being particularly intense in group E. Immunoreactive staining
signal of Cox-2 was mild or absent in colorectal portions of mice
from groups B, C, D and control (Fig.
5F, J, N and V, respectively).
DSS induces the production of
inflammatory cytokines
In order to address the potential mechanisms that
may trigger colitis generated by continuous treatment with DSS and
the possible beneficial effect of AntiGan in the diet, a
proinflammatory cytokine analysis was performed by cytokine
zirconium beads arrays. DSS treatment in mice significantly
increased the production of the proinflammatory cytokine interferon
(IFN)-γ compared with the levels in the negative control group.
There were no significant changes in the production levels of other
cytokines, including interleukin (IL)-1β, tumor necrosis factor
(TNF)-α, IL-6, IL-10 and IL-17, in both treated and untreated mice
(Table II).
 | Table II.Production of pro-inflammatory
cytokines in mice with dextran sulphate sodium-induced colitis
treated with AntiGan. |
Table II.
Production of pro-inflammatory
cytokines in mice with dextran sulphate sodium-induced colitis
treated with AntiGan.
|
| Pro-inflammatory
cytokines, pg/ml |
|
|
|---|
|
|
|
|
|
|---|
| Treatment | IL-1β | IL-6 | IL-10 | IL-17 | Interferon-γ | Tumor necrosis
factor-α |
|---|
| Negative
control | 8.5 | 11.7 | 10.6 | 12.3 | 13.2 | 11.3 |
| AntiGan |
|
|
|
|
|
|
| 2.5 µl/ml | 9.2 | 12.5 | 11.5 | 12.6 | 12.6 | 16.1 |
| 5 µl/ml | 10.3 | 11.2 | 13.5 | 12.6 | 28.7 | 11.1 |
| 10 µl/ml | 17.1 | 13.6 | 12.8 | 11.3 | 71.7a | 12.6 |
| Positive
control | 55.7 | 17.7 | 19.3 | 12.6 | 99.3 | 77.5 |
Discussion
In order to understand and treat human diseases,
natural components have been discovered and used for thousands of
years by all civilizations and cultures in the world. Previous
studies estimated that ~25% of the drugs prescribed worldwide are
derived from plant excipients and 60% of antitumor/anti-infectious
drugs already on the market or under clinical trial are of natural
origin (27–34). Thus, plant extracts derived from
Taxol, curcumin, phenolic acids and flavonoids are reported to
inhibit tumor cell growth (27–34).
It is known that there are at least 120 chemical
substances that are useful as antineoplastic drugs, such as
Paclitaxel (Taxol, genus Taxus), and are isolated from
plants; although research data regarding the role of natural
compounds in the treatment of different types of tumors is required
(27–34). An increasing number of
epidemiological studies assessing dietary intake of natural
compounds, based on biological and healthy effects, have provided
data supporting an inverse correlation between reduced risk and
bioactive effect of the tested compounds (27–34).
Although AntiGan has demonstrated significant antineoplastic
activities, in vitro and in vivo, against different
tumor cell lines, the molecular mechanism underlying this effect
has not been sufficiently studied. In the present investigation,
AntiGan demonstrated selective cytotoxicity in vitro for
different human tumor cell lines (pro-myelocitic and colon cancer
cell lines) when compared to untreated cells. The unusual ability
that AntiGan has demonstrated to effectively kill several types of
tumor cells without significant cytotoxicity to normal control
cells suggests that AntiGan may be a potential chemotherapeutic
agent. The present data indicated that AntiGan induces apoptosis in
specific cell lines, in a dose-dependent manner, probably generated
by three main cellular metabolic events, including the alteration
of the mitochondrial transmembrane potential, and the activation of
caspase-3 and caspase-8 pathways (35–43). The
results obtained in the present study indicate that AntiGan
inhibits the process of tumor cell proliferation by inducing cell
death through the activation of cytotoxic mechanisms.
Apoptosis is a genetically- and
epigenetically-directed process of cell self-destruction that is
marked by the fragmentation of nuclear DNA, resulting in programmed
cell death; however, when halted, it may lead to uncontrolled cell
growth and tumor formation (32–35).
Apoptosis may be triggered in a cell through either the extrinsic
or the intrinsic pathway: i) Positive stimulation of the
transmembrane death receptor (Fas receptor and caspases); and ii)
active release of intracellular apoptotic signal factors (32–39).
Positive stimulation involves ligands related to TNF, while the
induction of apoptosis by the release of signal factors involves
the mitochondria (35). It is known
that most types of tumor cell mechanisms are triggered by the
activity of dysfunctional apoptotic enzymes and by the balance
disturbance between apoptotic and proliferation processes (36). Two types of apoptotic-related genes
(Bcl-2 family) regulate these intracellular mechanisms: Apoptotic
repressor (Bcl-2) and apoptotic promoter (Bax). Research has
suggested that encoded proteins combined with Bcl-2 may resist the
action of repressing apoptosis, but also trigger a positive
regulatory action based on high Bcl-2/Bax ratios (37). All tumor cell lines used in the
present study have been induced by numerous chemical agents (AT101,
Brassinin, Erastin and MI-AF) to induce apoptosis through different
pathways, including p53-dependent or Bcl-2 family-related
pathways.
In order to address the molecular mechanism of
apoptosis mediated by different concentrations of AntiGan, the
present study investigated the expression of apoptotic-related
genes, including p53, p21, Bax and the Bcl-2 family by RT-PCR. The
data obtained suggested that apoptosis occurred in HL-60, HS 313T,
Caco-2, HT-29 and SW-480 cell lines treated with 10 µl/ml of
AntiGan, where a dose-dependent downregulation of Bcl-2 gene
expression was observed. The expression levels of Bcl-2 in the
other cell lines were not markedly altered. These results also
indicated that the relative increase of apoptotic Bax/Bcl-2 ratio
was associated with AntiGan-induced apoptosis in different human
cell lines. It is possible that AntiGan, through an appropriate
signal, induces a conformational change in Bax that modifies the
mitochondrial membrane, inducing the release of cytochrome c from
the mitochondrial membrane into the cytosol. Taken together, the
results of the in vitro studies demonstrated that AntiGan
exhibited an apoptotic-inducing effect in different human tumor
cell lines. Although further research is required to elucidate the
specific mechanisms by which AntiGan induces apoptosis in some
tumor cell lines, the present results indicate that AntiGan may be
a useful chemotherapeutic compound for patients with different
types of tumors.
In order to address the effect of AntiGan on colonic
inflammation in vivo, mice were exposed to DSS in drinking
water to induce ulcerative colitis. After 6 weeks of experimental
treatment, the analytical results performed in this model of colon
carcinogenesis demonstrated that AntiGan promoted a slight
reduction of colorectal histopathological hallmarks at lower doses
(2.5% AntiGan) and a moderate to almost complete reduction at
higher doses (5–10% AntiGan). In addition, histological samples of
different colon segments indicated that AntiGan has no adverse
effects on the morphological organization of the structured colonic
cell types, as demonstrated when analyzing mice treated with
AntiGan alone in the diet. Therefore, these promising results
indicate that AntiGan may be an important chemopreventive agent
against colon carcinogenesis.
In the present experimental study, colorectal
lesions were induced by a specific chemo-ulcerative agent to
evaluate the effects of a possible antitumor agent on chronic
ulcerative colitis in mice. The continuous administration of DSS as
a chemical inductor agent of ulcerative lesions in murine models
has been extensively reported (27,38–43), and
such studies are crucial to improve our knowledge of the complex
interactions between the social environment, genetics of each
population, and epithelial barrier dysfunction in human-related
inflammatory bowel disease (IBD) (44–46). In
the present study, as reported in the experimental results of the
murine model of acute colon injury, the administration of DSS in
drinking water resulted in colonic epithelial damage accompanied by
a strong inflammatory response. The results obtained by analyzing
the dose-response effect of AntiGan throughout the different
experimental groups indicated that the optimal dose-response was
the 10% AntiGan concentration in diet, where no histological
alterations or notable lesions were detected. The present study
also indicated a clear association between AntiGan dose and the
ulcerative effects observed in the colon, where progressive
severity on colorectal lesions was observed with lower doses of
AntiGan. These results reinforce the assumption that AntiGan may
act as a chemopreventive agent against carcinogenesis without
interfering with the normal epithelial histoarchitecture of the
colon (45,46).
In the present study, a significant increase in the
expression level of IFN-γ was observed in DSS-induced ulcerative
colitis. When translated into humans, it is clear that one of most
significant clues to understand ulcerative colitis (UC) and
associated pathologies is the alteration of various cytokines that
have been extensively reported in patients with IBD (47). Another parameter of active IBD is the
imbalance between regulatory T cells (Th3) and effector T cells
(Th1 and Th2). It is known that Crohn's disease is closely related
to the Th1 T-cell cytokine profile (IFN-γ, TNF-α and IL-12), while
UC is related to a modified Th2-type response cytokine profile
(IL-10 and IL-15) (38). Recent
findings have indicated that the IL-23/IL-17 axis may be part of
the effector T cell immunological response, which is involved in
IBD development (45). These
conclusions suggest that increased levels of IL-23 and IL-17
expression are characteristic biomarkers of patients with active
IBD.
Further research is required before the specific
mechanism of action of AntiGan may be fully elucidated for the
prevention and treatment of colitis. The present study demonstrated
that AntiGan displays a preventive effect against premalignant
tissue damage in a murine model of colitis, inhibiting the
development of colorectal ulcers and, consequently, progressive
tumors. AntiGan has been indicated to be a promising agent for
chemoprevention of colon carcinogenesis.
Acknowledgements
The authors of the present study declare that
AntiGan is an extract produced by EuroEspes Biotechnology (Corunna,
Spain), which the authors Valter Lombardi, Iván Carrera and Ramón
Cacabelos are affiliated with as scientific researchers.
References
|
1
|
Eckhardt S: Recent progress in the
development of anticancer agents. Curr Med Chem Anticancer Agents.
2:419–439. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Medina D, Sivaraman L, Hilsenbeck SG,
Conneely O, Ginger M, Rosen J and Omalle BW: Mechanisms of hormonal
prevention of breast cancer. Ann N Y Acad Sci. 952:23–35. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muller E, Gasparutto D and Cadet J:
Chemical synthesis and biochemical properties of oligonucleotides
that contain the
(5′S,5S,6S)-5′,6-cyclo-5-hydroxy-5,6-dihydro-2′-deoxyuridine DNA
lesion. Chembiochem. 3:534–542. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song Y, Wu F and Wu J: Targeting histone
methylation for cancer therapy: Enzymes, inhibitors, biological
activity and perspectives. J Hematol Oncol. 9:492016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gregory RK, Hill ME, Moore J, A'Hern RP,
Johnston SR, Blake P, Shephard J, Barton D and Gore ME: Combining
platinum, paclitaxel and anthracycline in patients with advanced
gynaecological malignancy. Eur J Cancer. 36:503–507. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spielmann M, Llombart A, Zelek L, Sverdlin
R, Rixe O and Le Cesne A: Docetaxel-cisplatin combination (DC)
chemotherapy in patients with anthracycline-resistant advanced
breast cancer. Ann Oncol. 10:1457–1460. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jelliffe AM: Vinblastine in the treatment
of Hodgkin's disease. Br J Cancer. 23:44–48. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lech G, Słotwiński R, Słodkowski M and
Krasnodębski IW: Colorectal cancer tumour markers and biomarkers:
Recent therapeutic advances. World J Gastroenterol. 22:1745–1755.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spiegelberg D, Stenberg J, Haylock AK and
Nestor M: A real-time in vitro assay as a potential predictor of in
vivo tumor imaging properties. Nucl Med Biol. 43:12–18. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wolff AC: Systemic therapy. Curr Opin
Oncol. 11:468–474. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gobbo OL, Sjaastad K, Radomski MW, Volkov
Y and Prina-Mello A: Magnetic nanoparticles in cancer theranostics.
Theranostics. 5:1249–1263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Campos-Lobato LF, Geisler DP, da Luz
Moreira A, Stocchi L, Dietz D and Kalady MF: Neoadjuvant therapy
for rectal cancer: The impact of longer interval between
chemoradiation and surgery. J Gastrointest Surg. 15:444–450. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bechis SK, Carroll PR and Cooperberg MR:
Impact of age at diagnosis on prostate cancer treatment and
survival. J Clin Oncol. 29:235–241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krug U, Röllig C, Koschmieder A, Heinecke
A, Sauerland MC, Schaich M, Thiede C, Kramer M, Braess J,
Spiekermann K, et al: Complete remission and early death after
intensive chemotherapy in patients aged 60 years or older with
acute myeloid leukaemia: A web-based application for prediction of
outcomes. Lancet. 376:2000–2008. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berz D and Wanebo H: Targeting the growth
factors and angiogenesis pathways: Small molecules in solid tumors.
J Surg Oncol. 103:574–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jeong JK, Moon MH, Seo JS, Seol JW, Lee YJ
and Park SY: Sulforaphane blocks hypoxia-mediated resistance to
TRAIL-induced tumor cell death. Mol Med Rep. 4:325–330.
2011.PubMed/NCBI
|
|
17
|
Villanueva A and Llovet JM: Targeted
therapies for hepatocellular carcinoma. Gastroenterology.
140:1410–1426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kline J and Gajewski TF: Clinical
development of mAbs to block the PD1 pathway as an immunotherapy
for cancer. Curr Opin Investig Drugs. 11:1354–1359. 2010.PubMed/NCBI
|
|
19
|
Goldman A: Tailoring combinatorial cancer
therapies to target the origins of adaptive resistance. Mol Cell
Oncol. 3:e10305342015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brett-Morris A, Mislmani M and Welford SM:
SAT1 and glioblastoma multiforme: Disarming the resistance. Mol
Cell Oncol. 2:e9833932015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bustany S, Bourgeais J, Tchakarska G, Body
S, Hérault O, Gouilleux F and Sola B: Cyclin D1 unbalances the
redox status controlling cell adhesion, migration, and drug
resistance in myeloma cells. Oncotarget. 7:45214–45224. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakamura O, Inaga Y, Suzuki S, Tsutsui S,
Muramoto K, Kamiya H and Watanabe T: Possible immune functions of
congerin, a mucosal galectin, in the intestinal lumen of Japanese
conger eel. Fish Shellfish Immunol. 23:683–692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Faul F, Erdfelder E, Lang AG and Buchner
A: G*Power 3: A flexible statistical power analysis program for the
social, behavioral, and biomedical sciences. Behav Res Methods.
39:175–191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huflejt ME and Leffler H: Galectin-4 in
normal tissues and cancer. Glycoconj J. 20:247–255. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yalon M, Tuval-Kochen L, Castel D, Moshe
I, Mazal I, Cohen O, Avivi C, Rosenblatt K, Aviel-Ronen S, Schiby
G, et al: Overcoming resistance of cancer cells to PARP-1
inhibitors with three different drug combinations. PLoS One.
11:e01557112016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Perše M and Cerar A: Morphological and
molecular alterations in 1,2 dimethylhydrazine and azoxymethane
induced colon carcinogenesis in rats. J Biomed Biotechnol.
2011:4739642011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lombardi VR, Etcheverría I, Carrera I,
Cacabelos R and Chacón AR: Prevention of chronic experimental
colitis induced by dextran sulphate sodium (DSS) in mice treated
with FR91. J Biomed Biotechnol. 2012:8261782012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barbuti AM and Chen ZS: Paclitaxel through
the ages of anticancer therapy: Exploring its role in
chemoresistance and radiation therapy. Cancers (Basel).
7:2360–2371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shindikar A, Singh A, Nobre M and
Kirolikar S: Curcumin and resveratrol as promising natural remedies
with nanomedicine approach for the effective treatment of triple
negative breast cancer. J Oncol. 2016:97507852016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Orfali GD, Duarte AC, Bonadio V, Martinez
NP, de Araújo ME, Priviero FB, Carvalho PO and Priolli DG: Review
of anticancer mechanisms of isoquercitin. World J Clin Oncol.
7:189–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lall RK, Adhami VM and Mukhtar H: Dietary
flavonoid fisetin for cancer prevention and treatment. Mol Nutr
Food Res. 60:1396–1405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ediriweera MK, Tennekoon KH, Samarakoon
SR, Thabrew I and Dilip DE Silva E: A study of the potential
anticancer activity of Mangifera zeylanica bark: Evaluation of
cytotoxic and apoptotic effects of the hexane extract and
bioassay-guided fractionation to identify phytochemical
constituents. Oncol Lett. 11:1335–1344. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martínez-Pérez C, Ward C, Turnbull AK,
Mullen P, Cook G, Meehan J, Jarman EJ, Thomson PI, Campbell CJ,
McPhail D, et al: Antitumour activity of the novel flavonoid
Oncamex in preclinical breast cancer models. Br J Cancer.
114:905–916. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Czyżewska U, Siemionow K, Zaręba I and
Miltyk W: Proapoptotic activity of propolis and their components on
human tongue squamous cell carcinoma cell line (CAL-27). PLoS One.
11:e01570912016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Spilioti E, Jaakkola M, Tolonen T,
Lipponen M, Virtanen V, Chinou I, Kassi E, Karabournioti S and
Moutsatsou P: Phenolic acid composition, antiatherogenic and
anticancer potential of honeys derived from various regions in
Greece. PLoS One. 9:e948602014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Giovannini C and Masella R: Role of
polyphenols in cell death control. Nutr Neurosci. 15:134–149. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fu J, Dang Z, Deng Y and Lu G: Regulation
of c-Myc and Bcl-2 induced apoptosis of human bronchial epithelial
cells by zinc oxide nanoparticles. J Biomed Nanotechnol. 8:669–675.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cho JY, Chang HJ, Lee SK, Kim HJ, Hwang JK
and Chun HS: Amelioration of dextran sulfate sodium-induced colitis
in mice by oral administration of beta-caryophyllene, a
sesquiterpene. Life Sci. 80:932–939. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Araki Y, Mukaisyo K, Sugihara H, Fujiyama
Y and Hattori T: Increased apoptosis and decreased proliferation of
colonic epithelium in dextran sulfate sodium-induced colitis in
mice. Oncol Rep. 24:869–874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang TC, Tsai SS, Liu LF, Liu YL, Liu HJ
and Chuang KP: Effect of Arctium lappa L. in the dextran sulfate
sodium colitis mouse model. World J Gastroenterol. 16:4193–4199.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Choi SY, Hur SJ, An CS, Jeon YH, Jeoung
YJ, Bak JP and Lim BO: Anti-inflammatory effects of inonotus
obliquus in colitis induced by dextran sodium sulfate. J Biomed
Biotechnol. 2010:9435162010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Whittem CG, Williams AD and Williams CS:
Murine colitis modeling using dextran sulfate sodium (DSS). J Vis
Exp. 19:pii: 16522010.
|
|
43
|
Slattery ML, Curtin KP, Edwards SL and
Schaffer DM: Plant foods, fiber, and rectal cancer. Am J Clin Nutr.
79:274–281. 2004.PubMed/NCBI
|
|
44
|
Okayasu I, Hana K, Nemoto N, Yoshida T,
Saegusa M, Yokota-Nakatsuma A, Song SY and Iwata M: Vitamin A
inhibits development of dextran sulfate sodium-induced colitis and
colon cancer in a mouse model. Biomed Res Int. 2016:48748092016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Miner-Williams WM and Moughan PJ:
Intestinal barrier dysfunction: Implications for chronic
inflammatory conditions of the bowel. Nutr Res Rev. 29:40–59. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang JG, Wang DF, Lv BJ and Si JM: A novel
mouse model for colitis-associated colon carcinogenesis induced by
1,2-dimethylhydrazine and dextran sulfate sodium. World J
Gastroenterol. 10:2958–2962. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu Z, Feng BS, Yang SB, Chen X, Su J and
Yang PC: Interleukin (IL)-23 suppresses IL-10 in inflammatory bowel
disease. J Biol Chem. 287:3591–3597. 2012. View Article : Google Scholar : PubMed/NCBI
|