Introduction
Lymphoma is a malignant tumor of the hematological
system that has high histological and genetic heterogeneity.
Lymphoma is classified into B-cell or T-cell lymphoma, depending on
the pathological type. Several approaches including radiotherapy,
chemotherapy, and hematopoietic stem cell transplantation have
shown some efficacy in treating T-cell lymphoma; however, the
prognosis and survival of patients with T-cell lymphoma remain poor
(1). RNA interference (RNAi) has
recently emerged as a potential strategy for targeted therapy, and
the antitumor effects of several RNAi strategies in tumor cells are
currently under exploration. However, few studies have investigated
the therapeutic effect of RNAi on T-cell lymphoma.
Notch1 signaling regulates self-renewal,
proliferation, differentiation, and apoptosis in T-cells (2,3). The
c-Myc protein, which is encoded by a gene that is a downstream
target of Notch1, regulates protein stabilization, promotes cell
division and accelerates cell entry to S phase from G0/G1 phase.
Previous studies showed that mutations in the Notch1 gene result in
sustained activation of Notch1 signaling, eventually leading to the
occurrence of T cell tumors in mice (4). In addition, c-Myc promotes cell
proliferation and inhibits apoptosis in many tumor cells (5–7).
Mutation of the Notch1 gene may lead to T-cell lymphoblastic
leukemia by directly increasing c-Myc expression. Furthermore, a
previous study showed that Notch1 and c-Myc cooperate in the
development of T-cell lymphoblastic leukemia in vivo, and
Notch1 may promote the growth of leukemic cells by maintaining
c-Myc mRNA levels (8). Other studies
found that the inhibition of Notch1 signaling with small molecule
inhibitors in mouse leukemic cells led to an increase apoptosis and
decrease of c-Myc expression (4).
Together these studies suggest that the Notch1/c-Myc signaling
pathway contributes to T-cell lymphoma oncogenesis. Previous
research has suggested that siRNA targeting Notch1 (siNotch1) could
significantly suppress proliferation and promote apoptosis in
lymphoma cells, and siNotch1 also exhibited anti-cancer activity in
non-small cell lung cancer and malignant melanoma via its effects
on the Notch1 signaling pathway (9–11).
Together these data suggest that siNotch1 may be an effective
anti-cancer agent in T-cell lymphoma.
Dihydroartemisinin (DHA), which is derived from the
traditional Chinese medical herb Artemisia annua L., is
widely used as an antimalarial drug (12) and has a cytotoxic effect on T-cell
lymphoma cells (13). Pure DHA can
block endothelial cell proliferation via effects on the ERK
signaling pathway (14).
Additionally, histone deacetylase inhibitors combined with DHA have
been shown to activate caspase-3 and enhance the anticancer effect
of the ERK signaling pathway in liver tumors (15). Co-treatment of DHA with gemcitabine
has been demonstrated to exhibit a therapeutic effect in a
pancreatic cancer model, via a proposed mechanism involving NF-κB
inactivation (16).
To improve treatment outcomes for T-cell lymphoma,
here we carried out research to explore the possible mechanisms of
combined DHA and siNotch1 therapy for T-cell lymphoma using Jurkat
cells, and we also explored the involvement of the Notch1/c-Myc
signaling pathway in mediating any antineoplastic effect of this
treatment. We hope that this study may provide the basis for a
novel, efficient, and safe treatment for T-cell lymphoma.
Materials and methods
Cell culture
Jurkat cells (a T-cell lymphoma cell line) were
purchased from the Chinese Academy of Sciences Cell Bank. Cells
were grown in RPMI-1640 medium (HyClone, Logan, UT, USA) in 10% FBS
(Gibco, Carlsbad, CA, USA), penicillin and streptomycin, and were
cultured at 37°C in 5% CO2 in a humidified incubator.
Jurkat cells in the logarithmic growth phase were used for all
experiments.
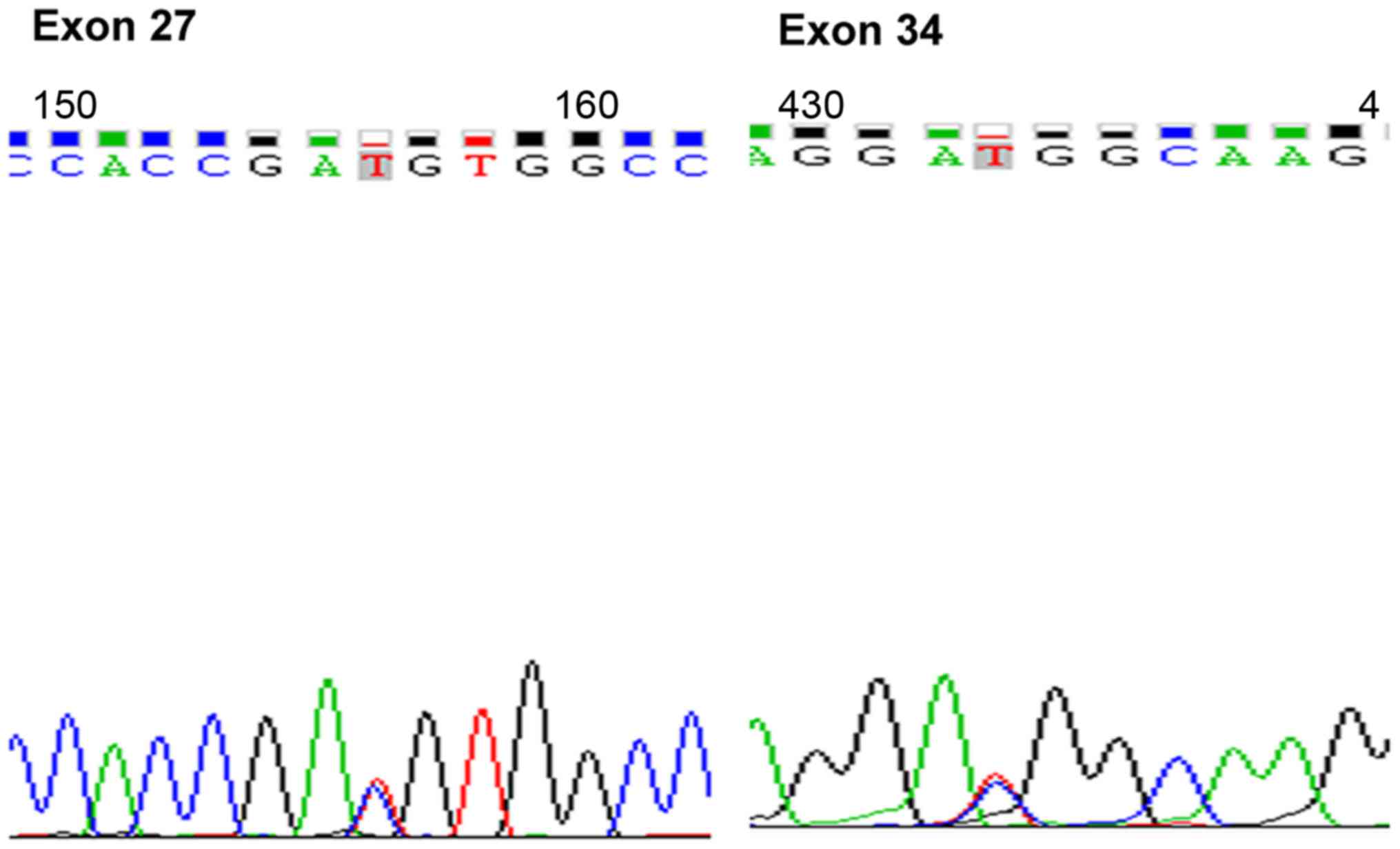
Detection of Notch1 DNA mutations
Jurkat cell DNA was isolated according to the
manufacturer's instructions (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Notch1 DNA sequence mutations in exons 27 and 34
(Fig. 1) were detected by MapBioo
(Shanghai, China).
Notch siRNA
The siNotch1 sequences (siRNA I and II) were
designed and synthesized by Genechem (Shanghai, China). Jurkat
cells were seeded in 6-well plates (2×105 cells/well in
1 ml culture medium) and divided into three groups: Then control
siRNA cells were transfected with control siRNA at multiplicity of
infection (MOI) = 80 (lentiviral vector titer 1×109;
sequence, TCTCCGAACGTGTCACGT), siRNA I-treated cells were
transfected with siRNA I (MOI = 80, lentiviral vector titer
3×108; sequence, CTGCCTGGACAAGATCAAT), and siRNA
II-treated cells were transfected with siRNA II (MOI = 80,
lentiviral vector titer 3×108; sequence,
TGCCAAATGCCTGCCAGAA). Last Fluorescence in transfected cells was
observed by laser scanning confocal microscope after 72 h.
Preliminary experiments indicated that siRNA I had a higher
transfection rate than siRNA II, so siRNA I was used for the
subsequent experiments. Since the lentiviral vector carries the
anti-puromycin gene, stably transfected Jurkat cells were selected
using puromycin (2 µg/ml).
Determination of optimal DHA
concentration
DHA (Chunyou Biological Technology Co., Ltd.,
Shanghai, China) was dissolved in dimethyl sulfoxide
(Sigma-Aldrich;. Merck KGaA, Darmstadt, Germany) to form an 8 mM
stock solution and stored at −20°C.
Jurkat cells (8×103 cells/well in 100 µl
medium) were seeded in 96-well plates, and various concentrations
of DHA (0, 2.5, 5, 10, 20, or 40 µM) were added. After 24, 48, or
72 h, 10 µl CCK-8 reagent was added and the cells were incubated
for 2 h. Cell viability was then assessed by CCK-8 assay (10 µl
reagent/well; Dojindo Molecular Technologies, Inc., Kyushu, Japan).
The optimal DHA treatment was determined as 20 µM for 24 h.
Cell viability assay
Five groups were included in the experiments:
untreated control cells, control siRNA cells, siRNA I-treated
cells, DHA-treated cells, and siRNA-DHA-treated cells. Jurkat cells
(8×103 cells/well in 100 µl medium) were seeded in
96-well plates. DHA (20 µM) was added, and the cells were cultured
for 24 h. Cell viability was subsequently assessed by CCK-8 assay.
A microplate absorbance reader (Tecan Group Ltd., Männedorf,
Switzerland) was used to measure absorbance at 450 nm. Each
experiment was repeated three times.
Cell apoptosis assay
Jurkat cells were seeded in 6-well plates
(2×105 cells/well in 1 ml culture medium). After 24 h,
cells were collected and washed once in phosphate-buffered saline
(PBS) and once in binding buffer, and then resuspended in binding
buffer. Fluorochrome-conjugated Annexin V (5 µl) was added, and the
cells were incubated for 15 min at room temperature. Next, 5 µl of
propidium iodide staining solution was added. Samples were analyzed
by flow cytometry (BD FACSCalibur; BD Biosciences, Franklin Lakes,
NJ, USA).
RT-qPCR
Total RNA was extracted using TRIzol reagent
(Takara, Tokyo, Japan) and reverse-transcribed using a Roche kit
(Roche Diagnostics GmbH, Mannheim, Germany) according to the
manufacturer's instructions. RNA quality and quantity were assessed
by spectrophotometry (Dynamica Scientific Ltd., Milton Keynes, UK).
Gene-specific primers were synthesized by Sangon Biotech (Shanghai,
China); sequences are provided in Table
I. The RT-qPCR program used was as follows: Preincubation at
95°C for 600 sec; 40 cycles of amplification, with initial
denaturation at 95°C for 10 sec, 60°C for 20 sec, and then 72°C for
20 sec; melting was performed at 95°C for 10 sec, 65°C for 60 sec
and 97°C for 1 sec. The 2−ΔΔCq method (17) was used to calculate relative
expression of Notch1, c-Myc, and caspase-3 mRNA.
 | Table I.Gene-specific primer sequences for
reverse transcription quantitative-polymerase chain reaction. |
Table I.
Gene-specific primer sequences for
reverse transcription quantitative-polymerase chain reaction.
| Gene | Sequence |
|---|
| GAPDH |
5′-GATGACCTTGCCCACAGCCT-3′ |
|
|
5′-ATCTCTGCCCCCTCTGCTGA-3′ |
| Notch1 |
5′-CCAGTTTGAATGGTCAATGC-3′ |
|
|
5′-AGAGGGTTGTATTGGTTCGG-3′ |
| c-Myc |
5′-CTACCCTCTCAACGACAGCA-3′ |
|
|
5′-AGAGCAGAGAATCCGAGGAC-3′ |
| Caspase-3 |
5′-TAAATGAATGGGCTGAGCTG-3′ |
|
|
5′-ATGGAGAAATGGGCTGTAGG-3′ |
Western blot analysis
Jurkat cells were collected and protein was
extracted using Radio Immunoprecipitation Assay and
phenylmethanesulfonyl fluoride (Beyotime, Shanghai, China). Protein
were analyzed by spectrophotometry. Equivalent amounts of protein
with adjusted concentration were separated by SDS-PAGE (10%) and
transferred to polyvinylidene difluoride membranes. Membranes were
blocked with 5% fat-free milk and incubated with primary antibodies
(1:1,000) for 2 h, followed by secondary antibody (1:2,000) (both
from Abcam, Cambridge, UK) for 1 h at room temperature. β-actin was
used as a loading control. An ECL chemiluminescence kit (Thermo
Fisher Scientific, Inc.) was used to visualize the signals. Images
were analyzed using imaging software (Vilber Lourmat,
Marne-la-Vallée, France).
Statistical analysis
All data were analyzed by one-way ANOVA using
Graphpad Prism 5.0. All results are expressed as mean ± standard
deviation (mean ± SD). The differences between groups were analyzed
using LSD-t. P<0.05 was considered to indicate a statistically
significant difference.
Results
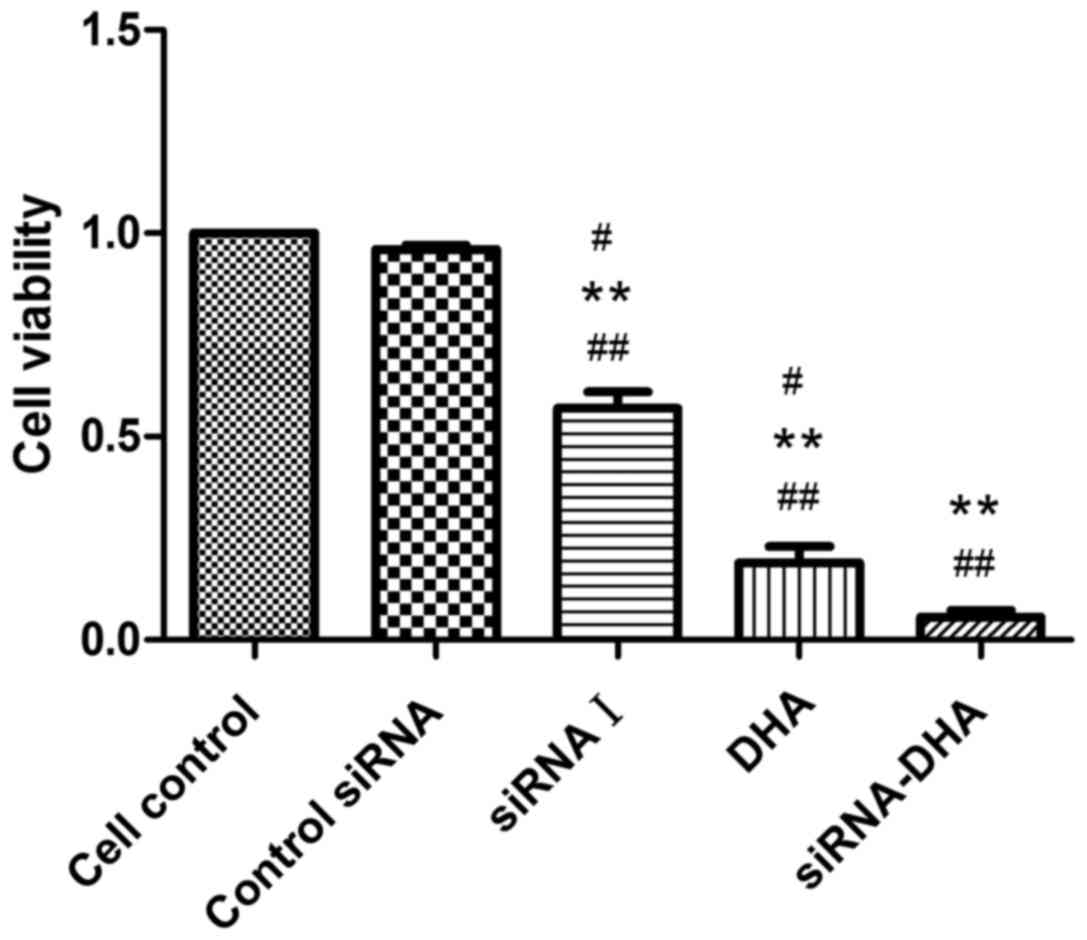
DHA combined with siNotch1 decreases
Jurkat cell viability
We first evaluated the viability of Jurkat cells in
response to Notch siRNA, DHA (20 µM) or the combined treatment for
24 h using CCK-8 assays (Fig. 2).
Compared with the viability of control siRNA cells (95.90%), the
cell viabilities for siRNA I-, DHA- and siRNA-DHA-treated cells
were 57.25, 19.12, and 5.66%, respectively. These results
demonstrate that DHA combined with siNotch1 showed enhanced
reduction of cell viability compared with compared with either
treatment alone.
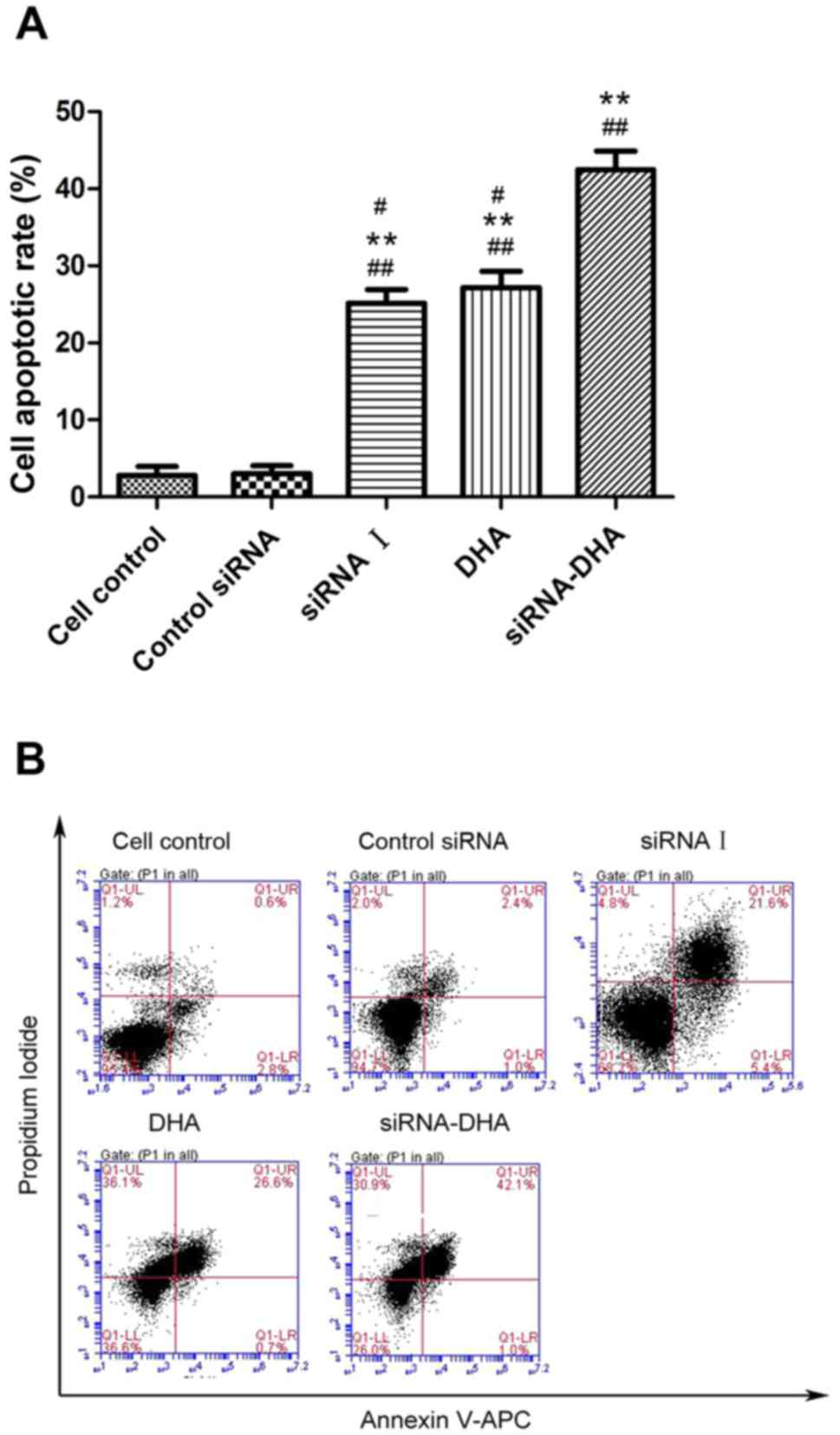
DHA combined with siNotch1 promotes
Jurkat cell apoptosis
DHA combined with siRNA treatment significantly
enhanced Jurkat cell apoptosis (Fig.
3). The Jurkat cell apoptosis rates for cell control, and
control siRNA cells, siRNA I-, DHA-, and siRNA-DHA-treated cells
were: 2.83, 3.00, 25.17, 27.17, and 42.47%, respectively. DHA
combined with siNotch1 promoted higher Jurkat cell apoptosis
(42.47%) compared with siNotch- or DHA-treated cells (P<0.01,
P<0.01). These results demonstrate that DHA combined with
siNotch1 promoted Jurkat cell apoptosis.
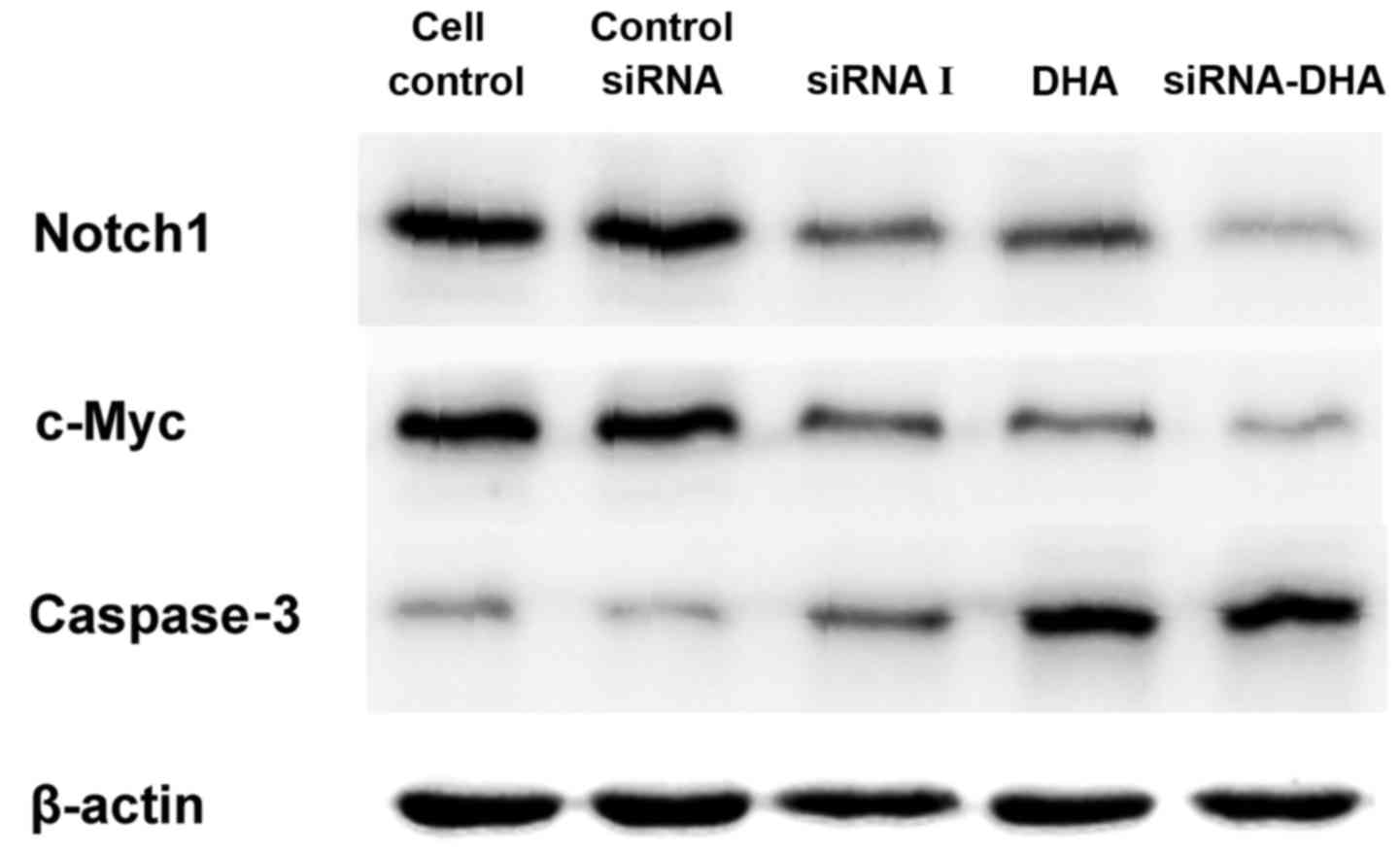
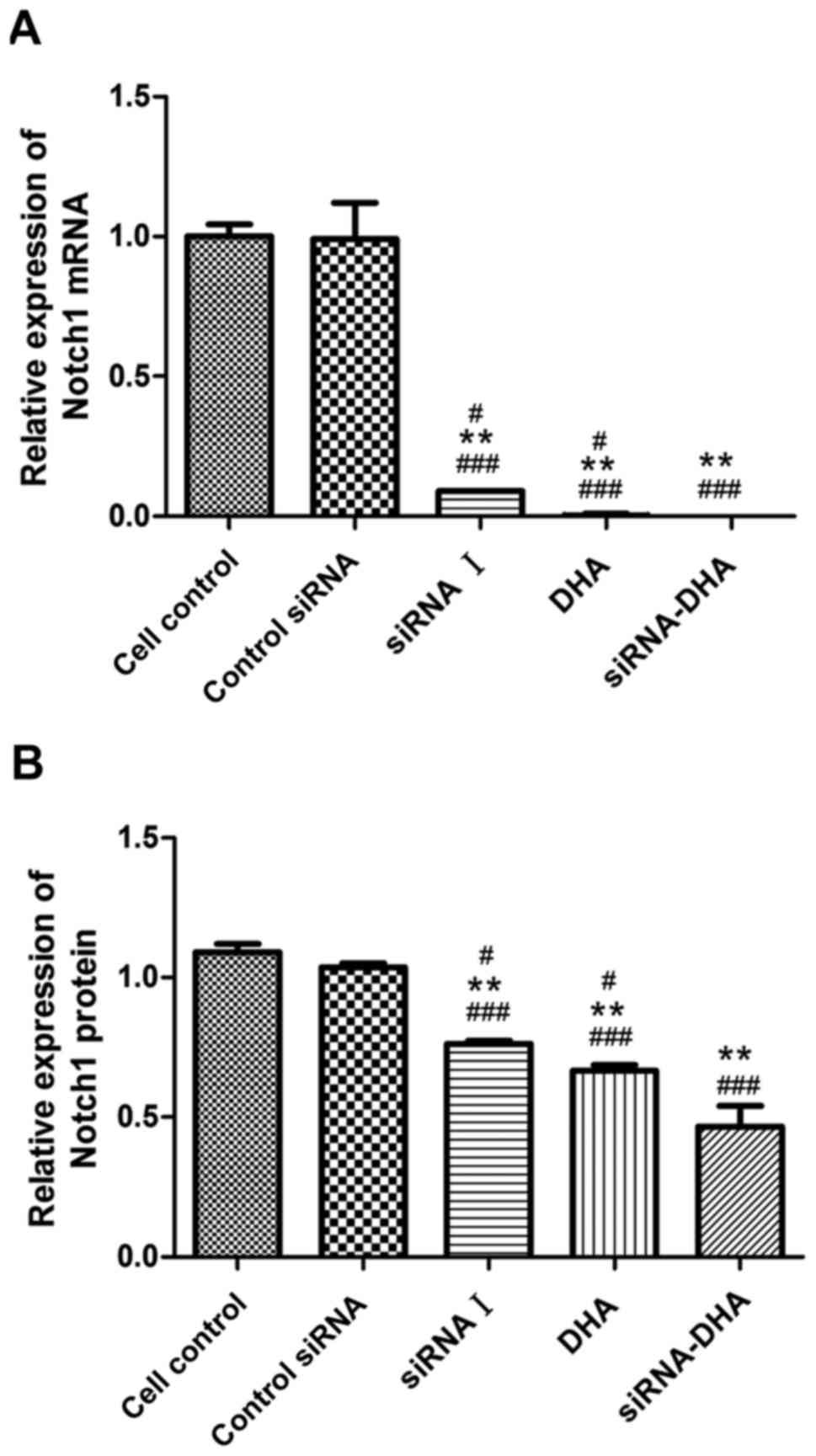
DHA combined with siNotch1 inhibits
Notch1 mRNA and protein expression in Jurkat cells
We evaluated Notch1 mRNA and protein levels in the
control siRNA cells, siRNA I-, DHA-, and siRNA-DHA-treated cells
groups (Figs. 4 and 5). Notch1 mRNA expression in cell control,
and in control siRNA cells, siRNA I-, DHA-, and siRNA-DHA-treated
cells were 1.00±0.04, 0.99±0.13, 0.58±0.09, 0.36±0.05, and
0.16±0.05, respectively (Fig. 5A).
Compared with cell control, control siRNA, siRNA I-, DHA-, and
siRNA-DHA treatment decreased expression of Notch1 mRNA by 1.12,
41.97, 63.86, and 84.23%, respectively. Significant differences
were found between the siRNA-DHA- and siRNA I-treated cells
(P<0.01), and between the siRNA-DHA- and DHA-treated cells
(P<0.05).
Fig. 5B shows the
relative expression of Notch1 protein. Notch1 protein expression in
cell control, and in control siRNA cells, siRNA I-, DHA-, and
siRNA-DHA-treated cells were 1.09±0.03, 1.04±0.01, 0.76±0.01,
0.67±0.02, and 0.47±0.07, respectively. Of all the treatment
conditions tested, the Notch1 protein level was reduced the most by
treatment with DHA combined with siNotch1.
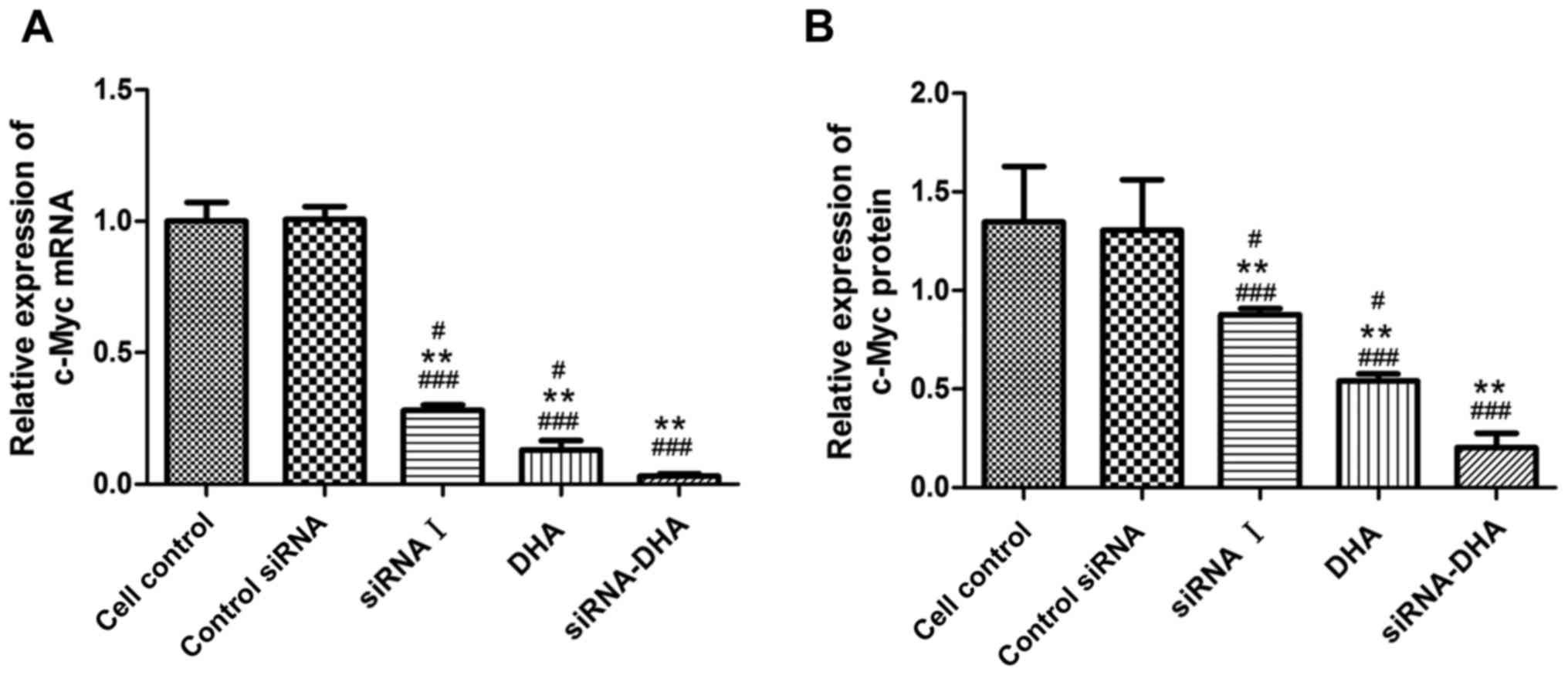
DHA combined with siNotch1 inhibits
c-Myc mRNA and protein expression in Jurkat cells
Expression of c-Myc mRNA and protein in Jurkat cells
treated with DHA combined with siNotch1 (Figs. 4 and 6). Treatment with siRNA-DHA, DHA and siRNA
I decreased expression of c-Myc mRNA by 96.86, 92.33, and 72.91%,
respectively, compared with expression in cell control (Fig. 6A).
c-Myc protein expression levels followed a similar
pattern to c-Myc mRNA expression levels. Treatment with siRNA-DHA,
siRNA I, and DHA decreased expression of c-Myc protein by 85.19,
34.81, and 60.12%, respectively, compared with expression in cell
control (P<0.01, P<0.05, P<0.05; Fig. 6B). Of all the treatment conditions
tested, c-Myc mRNA and protein expression were reduced the most by
treatment with DHA combined with siNotch1.
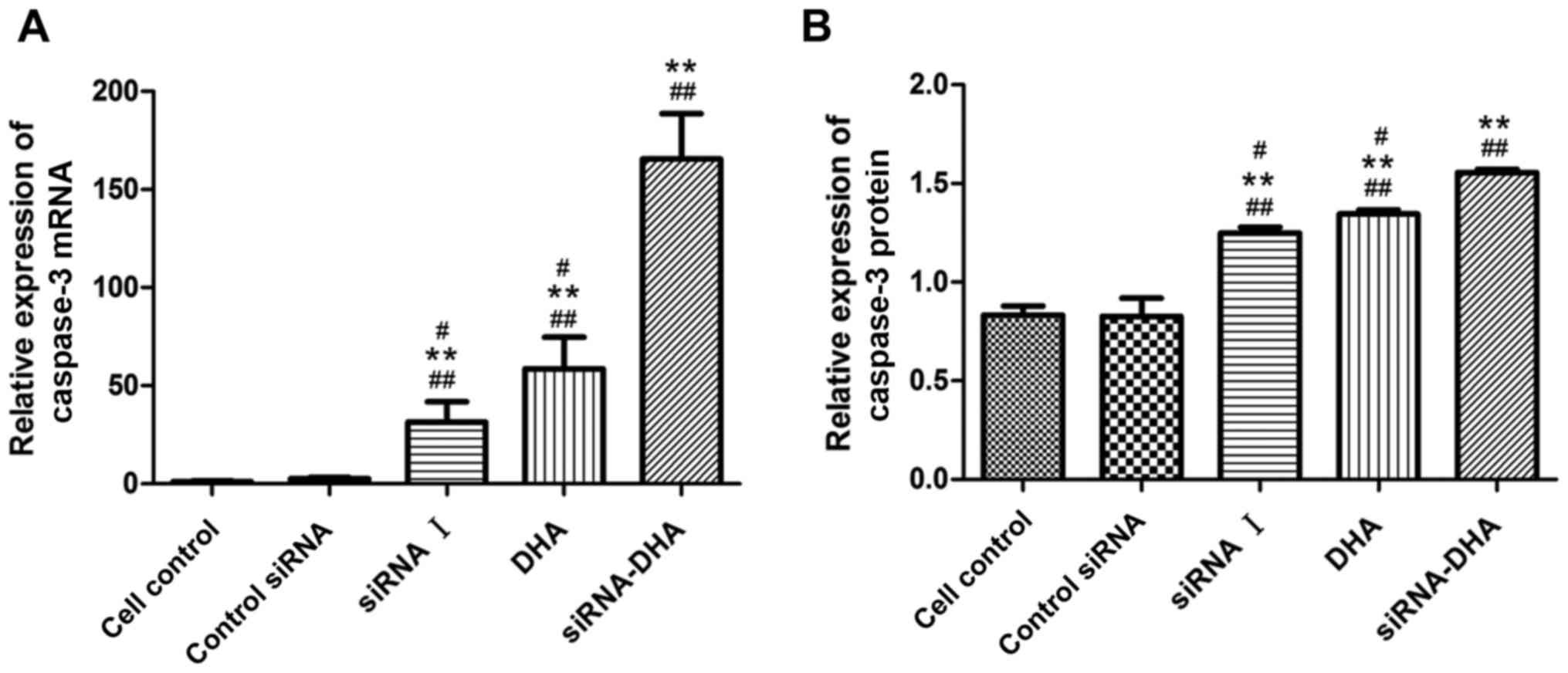
DHA combined with siNotch1 promotes
caspase-3 mRNA and protein expression in Jurkat cells
caspase-3 mRNA and protein expression were promoted
in Jurkat cells treated with DHA combined with siNotch1 (Figs. 4 and 7). Treatment with siRNA-DHA, DHA, and siRNA
I increased the expression of caspase3 mRNA by 99.36, 98.21, and
96.66%, respectively, compared with expression in cell control
(Fig. 7A). siRNA-DHA treatment
promoted caspase-3 mRNA expression more strongly than siRNA I or
DHA treatment alone (P<0.01, P<0.01).
siRNA-DHA, DHA, and siRNA I treatment increased
caspase-3 protein levels by 46.79, 38.52, and 33.60%, respectively,
compared with levels in cell control (Fig. 7B). There were significant differences
in caspase-3 protein levels between siRNA-DHA-treated cells and
DHA-treated or siRNA I-treated cells (P<0.01, P<0.01).
Treatment of Jurkat cells with combined DHA and siNotch1 promoted
caspase-3 protein expression to a greater extent than treatment
with either alone.
Discussion
Notch1 signaling is closely related to T-cell
lymphoma oncogenesis (18,19). Therefore, therapy targeting Notch1
signaling may be an effective approach for treating T-cell lymphoma
and we hypothesized that silencing Notch1 gene by RNAi technology
in T-cell lymphoma cells may be a useful therapeutic strategy. DHA
is an anti-tumor drug from traditional Chinese herbal medicine, and
thus DHA combined with siNotch1 may be more effective for lymphoma
therapy. The aim of the study was to determine whether the novel
combination treatment of DHA with siNotch1 was effective against
for T-cell lymphoma, and thus we explored the cytotoxic effects of
DHA and siNotch1 alone and combined in T-cell lymphoma cells.
Jurkat cells (a T-cell lymphoma cell line) were
sensitive to DHA alone in vitro (Fig. 2). Consistent with our results,
previous studies showed that DHA alone exhibits antitumor activity
in lung, breast, colon, and pancreatic cancer by suppressing
proliferation (20). We also
observed that Jurkat cells were sensitive to siNotch1 alone in
vitro (Fig. 2). Previous studies
also showed that targeted RNAi therapy can inhibit proliferation
and invasion of human gastric carcinoma cells and human prostate
cancer cells in vitro (21,22). Our
findings showed that DHA combined with siNotch1 reduced cell
proliferation (Fig. 2) and induced
cell apoptosis (Fig. 3) in Jurkat
cells. Together, the results of our study indicated that DHA
combined with siNotch1 was highly effective for Jurkat cells
compared with DHA or siNotch1 alone. We will further study the
underlying mechanisms of how DHA promote the function of siNotch
and inhibit proliferation in T-cell lymphoma cells.
The cytotoxic mechanisms of DHA have been
investigated in several studies, showing that dihydroarteminin
enhances cell apoptosis via its effects on the ERK signaling
pathway in endothelial cells (14),
inactivating NF-κB in pancreatic cancer (16) and by upregulation of Noxa by
promotion of FOXO3a expression in acute myeloid leukemia cells
(23). Additionally, DHA exerts an
antineoplastic effect in prostate cancer cells by increasing death
receptor 5 expression (24) and
induces NOXA-dependent mitochondrial cell apoptosis in melanoma
cells with upregulation of cellular oxidative stress (25), furthermore, DHA triggers cell cycle
arrest via effects on the AKT/GSK3β/cyclin D1 pathway in A549 lung
cancer cells (26). DHA could also
inhibit the Wnt/β-catenin signaling pathway in lung cancer, and the
expressions of key proteins including Wnt5-a/b, LRP6 and Dvl2 in
the Wnt/β-catenin signaling pathway were significantly decreased
(27). More importantly, DHA has
been shown to increase reactive oxygen species generation,
downregulate transferrin receptor mRNA expression and telomerase
activity, upregulate NOXA protein in T-cell lymphoma cells
(13,28). We intend to study the mechanisms
underlying the effects of DHA on NOXA signaling in T cell lymphoma
cells in future. Together these results showed that DHA is a
regulator of signaling pathways and gene expressions in cancer
cells. Taken together, these studies, including our study, showed
that DHA exhibits a therapeutic effect in cancer cells, including
T-cell lymphoma cells, through regulation of signaling pathways and
gene expressions.
We propose that inhibition of Notch1/c-Myc signaling
is one of the mechanisms by which DHA combined with siNotch1 exert
a marked antineoplastic effect on T-cell lymphoma cells: Τhe
relative expression of c-Myc mRNA and protein were markedly
decreased, and as a direct downstream target gene of Notch1, c-Myc
can be inhibited via the Notch1/c-Myc signaling pathway. Our
findings showed the silencing of Notch1 gene and/or DHA may
downregulate the expression level of c-Myc mRNA and protein in
T-cell lymphoma cells (Figs. 4 and
6). Sharma et al (8) also found that c-Myc levels clearly
correlate with Notch1 activity in acute T-cell leukemia, and c-Myc
could be induced in the presence of active Notch1 signaling. Active
Notch1 signaling induces the overexpression of c-Myc, and c-Myc may
activate many genes in cellular processes, including those involved
in promoting growth and proliferation. These data indicate that
Notch1 is necessary for T-cell lymphoma cell proliferation, so
silencing the Notch1 gene may exhibit a therapeutic effect in
T-cell lymphoma cell growth control via reducing the overexpression
of c-Myc.
Our results showed that DHA combined with siNotch1
could inhibit the Notch1/c-Myc pathway in T-cell lymphoma cells
in vitro. Some studies also showed that RNAi technology
exerts an antineoplastic effect on cancer cells (9,21,29–31).
Activation of Notch signaling promotes tumor progression by
regulation of downstream target genes such as c-Myc. DHA combined
with siRNA targeting Rac1 also induces apoptosis by inhibiting
NF-κB activity in colon tumor cells (28). These observations may partly explain
our results, showing that DHA combined with siNotch1 could inhibit
the Notch1/c-Myc pathway in T-cell lymphoma cells.
In this study, T-cell lymphoma cell proliferation
was suppressed and apoptosis was promoted following DHA combined
with siNotch1. Furthermore, expression levels of Notch1 and c-Myc
mRNA and protein were reduced in T-cell lymphoma cells by treatment
with DHA or siNotch1 alone or in combination, and caspase-3 mRNA
and protein expression levels were increased. We also intend to
study the impact of DHA on cleavaged caspase 3 in future. These
effects were greatly enhanced by combining the two treatments,
which suggested that the antitumor effect of DHA combined with
siNotch1 was superior to that of each agent alone. Our findings
suggest that the cytotoxic effect of the combined therapy was
achieved by inhibition of Notch1 and c-Myc mRNA and protein
expression, which suppressed the Notch1/c-Myc signaling
pathway.
Together these results suggest that the combined use
of DHA and siNotch1 therapy for T-cell lymphoma is useful. The
underlying mechanism of combined therapy is the effect on the
expression of Notch1/c-Myc signaling pathway components. Thus,
combining traditional Chinese medicine containing DHA with siRNA
targeting the Notch1/c-Myc signaling pathway may represent a novel
strategy for treating human T-cell lymphoma.
Acknowledgements
We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this
study. This study was supported by Youth Funding of Affiliated
Hospital, Qingdao University, Shandong, China (KZJ-34) and Science
and Technology plan of Traditional Chinese Medicine Development
Projects, Shandong, China (2017-197).
Glossary
Abbreviations
Abbreviations:
|
DHA
|
dihydroartemisinin
|
|
siNotch1
|
siRNA targeting Notch1
|
|
CCK-8
|
Cell Counting Kit-8
|
|
RT-PCR
|
reverse transcription PCR
|
|
RNAi
|
RNA interference
|
|
DMSO
|
dimethyl sulfoxide
|
|
PBS
|
phosphate-buffered saline
|
|
MOI
|
multiplicity of infection
|
References
|
1
|
Taylor GP and Matsuoka M: Natural history
of adult T-cell leukemia/lymphoma and approaches to therapy.
Oncogene. 24:6047–6057. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharma VM, Draheim KM and Kelliher MA: The
Notch1/c-Myc pathway in T cell leukemia. Cell Cycle. 6:927–930.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weng AP, Millholland JM, Yashiro-Ohtani Y,
Arcangeli ML, Lau A, Wai C, Del Bianco C, Rodriguez CG, Sai H,
Tobias J, et al: c-Myc is an important direct target of Notch1 in
T-cell acute lymphoblastic leukemia/lymphoma. Gene Dev.
20:2096–2109. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pear WS, Aster JC, Scott ML, Hasserjian
RP, Soffer B, Sklar J and Baltimore D: Exclusive development of T
cell neoplasms in mice transplanted with bone marrow expressing
activated Notch alleles. J Exp Med. 183:2283–2291. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klinakis A, Szabolcs M, Politi K, Kiaris
H, Artavanis-Tsakonas S and Efstratiadis A: Myc is a Notch1
transcriptional target and a requisite for Notch1-induced mammary
tumorigenesis in mice. Pro Natl Acad Sci USA. 103:9262–9267. 2006.
View Article : Google Scholar
|
|
6
|
Rizzo P, Osipo C, Foreman K, Golde T,
Osborne B and Miele L: Rational targeting of Notch signaling in
cancer. Oncogene. 27:5124–5131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roy M, Pear WS and Aster JC: The
multifaceted role of Notch in cancer. Curr Opin Genet Dev.
17:52–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sharma VM, Calvo JA, Draheim KM,
Cunningham LA, Hermance N, Beverly L, Krishnamoorthy V, Bhasin M,
Capobianco AJ and Kelliher MA: Notch1 contributes to mouse T-cell
leukemia by directly inducing the expression of c-Myc. Mol Cell
Biol. 26:8022–8031. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kong R, Feng J, Ma Y, Zhou B, Li S, Zhang
W, Jiang J, Zhang J, Qiao Z, Zhang T, et al: Silencing NACK by
siRNA inhibits tumorigenesis in non-small cell lung cancer via
targeting Notch1 signaling pathway. Oncol Rep. 35:2306–2314. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Wang J, Li C and Ke XY: Contribution
of PD-L1 to oncogenesis of lymphoma and its RNAi-based targeting
therapy. Leuk Lymphoma. 53:2015–2023. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Z, Qi Y, Lu C, Zhang J, Luo R and
Kang S: Small interfering RNA (siRNA)-mediated knockdown of Notch1
suppresses tumor growth and enhances the effect of IL-2
immunotherapy in malignant melanoma. J BUON. 20:1553–1564.
2015.PubMed/NCBI
|
|
12
|
Woo SH, Parker MH, Ploypradith P, Northrop
J and Posner GH: Direct conversion of pyranose anomeric OH→F→R in
the artemisinin family of antimalarial trioxanes. Tetrahedron Lett.
39:1533–1536. 1998. View Article : Google Scholar
|
|
13
|
Wang Q, Wu S, Zhao X, Zhao C, Zhao H and
Huo L: Mechanisms of Dihydroartemisinin and
Dihydroartemisinin/holotransferrin cytotoxicity in T-cell lymphoma
cells. PLoS One. 10:e01373312015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong F, Tian H, Yan S, Li L, Dong X, Wang
F, Li J, Li C, Cao Z, Liu X and Liu J: Dihydroartemisinin inhibits
endothelial cell proliferation through the suppression of the ERK
signaling pathway. Int J Mol Med. 35:1381–1387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang CZ, Pan Y, Cao Y, Lai PB, Liu L,
Chen GG and Yun J: Histone deacetylase inhibitors facilitate
dihydroartemisinin-induced apoptosis in liver cancer in vitro and
in vivo. PLoS One. 7:1726–1729. 2012.
|
|
16
|
Wang SJ, Gao Y, Chen H, Kong R, Jiang HC,
Pan SH, Xue DB, Bai XW and Sun B: Dihydroartemisinin inactivates
NF-kappa B and potentiates the anti-tumor effect of gemcitabine on
pancreatic cancer both in vitro and in vivo. Cancer Lett.
293:99–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamstrup MR, Biskup E, Gjerdrum LM,
Ralfkiaer E, Niazi O and Gniadecki R: The importance of Notch
signaling in peripheral T-cell lymphomas. Leuk lymph. 55:639–644.
2014. View Article : Google Scholar
|
|
19
|
Pear WS and Aster JC: T cell acute
lymphoblastic leukemia/lymphoma: A human cancer commonly associated
with aberrant NOTCH1 signaling. Curr Opin Hematol. 11:426–433.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu JJ, Meng LH, Shankavaram UT, Zhu CH,
Tong LJ, Chen G, Lin LP, Weinstein JN and Ding J:
Dihydroartemisinin accelerates c-MYC oncoprotein degradation and
induces apoptosis in c-MYC-overexpressing tumor cells. Biochem
Pharmaco. 80:22–30. 2010. View Article : Google Scholar
|
|
21
|
Sun HW, Wu C, Tan HY and Wang QS:
Combination DLL4 with Jagged1-siRNA can enhance inhibition of the
proliferation and invasiveness activity of human gastric carcinoma
by Notch1/VEGF pathway. Hepatogastroenterology. 59:924–929.
2012.PubMed/NCBI
|
|
22
|
Bin Hafeez B, Mustafa Adhami V, Asim M,
Siddiqui IA, Bhat KM, Zhong W, Saleem M, Din M, Setaluri V and
Mukhtar H: Targeted knockdown of Notch1 inhibits invasion of human
prostate cancer cells concomitant with inhibition of matrix
metalloproteinase-9 and urokinase plasminogen activator. Clin
Cancer Res. 15:452–459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao X, Zhong H, Wang R, Liu D, Waxman S,
Zhao L and Jing Y: Dihydroartemisinin and its derivative induce
apoptosis in acute myeloid leukemia through Noxa-mediated pathway
requiring iron and endoperoxide moiety. Oncotarget. 6:5582–5596.
2015.PubMed/NCBI
|
|
24
|
He Q, Shi JX, Shen XL, An J, Sun H, Wang
L, Hu YJ, Sun Q, Fu LC, Sheikh MS and Huang Y: Dihydroartemisinin
upregulates death receptor 5 expression and cooperates with TRAIL
to induce apoptosis in human prostate cancer cells. Cancer Biol
Ther. 9:819–824. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cabello CM, Lamore SD, Bair WB III, Qiao
S, Azimian S, Lesson JL and Wondrak GT: The redox antimalarial
dihydroartemisinin targets human metastatic melanoma cells but not
primary melanocytes with induction of NOXA-dependent apoptosis.
Invest New Drug. 30:1289–1301. 2012. View Article : Google Scholar
|
|
26
|
Liao K, Li J and Wang ZL:
Dihydroartemisinin inhibits cell proliferation via
AKT/GSK3β/cyclinD1 pathway and induces apoptosis in A549 lung
cancer cells. Int J Clin Exp Patho. 7:8684–8691. 2014.
|
|
27
|
Tong Y, Liu Y, Zheng H, Zheng L, Liu W, Wu
J, Ou R, Zhang G, Li F, Hu M, et al: Artemisinin and its
derivatives can significantly inhibit lung tumorigenesis and tumor
metastasis through Wnt/β-catenin signaling. Oncotarget.
7:31413–31428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Handrick R, Ontikatze T, Bauer KD, Freier
F, Rübel A, Dürig J, Belka C and Jendrossek V: Dihydroartemisinin
induces apoptosis by a Bak-dependent intrinsic pathway. Mol Cancer
Ther. 9:2497–2510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han P, Luan Y, Liu Y, Yu Z, Li J, Sun Z,
Chen G and Cui B: Small interfering RNA targeting Rac1 sensitizes
colon cancer to dihydroartemisinin-induced cell cycle arrest and
inhibited cell migration by suppressing NFκB activity. Mol Cell
Biochem. 379:171–180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim MJ, Park JS, Lee SJ, Jang J, Park JS,
Back SH, Bahn G, Park JH, Kang YM, Kim SH, et al: Notch1 targeting
siRNA delivery nanoparticles for rheumatoid arthritis therapy. J
Control Release. 216:140–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tezgel AÖ, Gonzalez-Perez G, Telfer JC,
Osborne BA, Minter LM and Tew GN: Novel protein transduction domain
mimics as nonviral delivery vectors for siRNA targeting NOTCH1 in
primary human T cells. Mol Ther. 21:201–209. 2013. View Article : Google Scholar : PubMed/NCBI
|