Introduction
Osteoarthritis (OA) is a chronic degenerative joint
disorder that causes pain, stiffness, and limitation in the range
of joint motion (1). OA is a major
public health problem in the elderly population; OA of the knee, as
one of the most common forms of OA, affected up to 25 million
people in Japan in 2006 (1). OA is
caused by articular cartilage degeneration, which involves
fibrillation of the articular surface and a decrease in the size
and aggregation of proteoglycan (PG) (2–4). These
alterations are principally the result of aging-associated changes
in chondrocyte function, including a decrease in synthetic
activity, that decrease their ability to maintain the tissue
(4). The dietary supplement
D-glucosamine hydrochloride (GlcN) has previously been used as a
safe and effective treatment for the management of OA symptoms
(5). GlcN has been documented to
decrease chemical mediators in chondrocytes (6), induce cartilaginous matrix regeneration
in experimental OA and restore the articular surface at the injury
site by affecting PG and collagen production (7,8).
However, previous results suggest that GlcN alone or in combination
with other supplements does not effectively reduce pain in patients
with knee OA (9), though it has been
reported that a combination of GlcN and chondroitin sulfate was
effective when used as a treatment in a subgroup of patients with
moderate-to-severe knee pain (9). To
develop a more effective supplement for the treatment of OA, the
aim of the present study was to investigate a compound that may
have therapeutic effects in this disease.
Ajuga decumbens (AD), a naturally occurring
herb that has a history of use as a pain relief medicine in Japan
(10), was selected as the candidate
supplement for the treatment of OA. AD extract (ADE) has previously
been reported to have preventative effects against osteoporosis
(10), to decrease the number of
osteoclasts following subchondral bone damage, and to work
synergistically with GlcN to improve cartilaginous injury in a
rabbit OA model (11).
20-hydroxyecdysone, an active component of ADE, has also been
demonstrated to decrease the number of osteoclasts following
subchondral bone damage in cartilaginous injuries (11). Furthermore, 20-hydroxyecdysone has
beneficial effects on epiphyseal cartilage tissue and trabecular
bone in ovariectomized rats (12),
and ADE has been reported to induce subchondral bone regeneration
(11). However, the mechanism
underlying ADE-induced regeneration in the cartilage matrix is
unclear. Chondrocytes have been implicated as a critical component
that influence repair during cartilage degradation (13). Chondrocytes are present in hyaline
cartilage and develop from the highly regulated differentiation of
mesenchymal stem cells (MSCs), mesodermal-derived stem cells
present in a number of fetal and adult tissues (13). MSCs have recently been applied as a
treatment for OA in clinical trials due to their regeneration
potential and anti-inflammatory effects, and therapeutic effects of
chondrogenic-differentiated MSCs on OA were observed (13). Although transplanted native MSCs may
be problematic due to their multipotent differentiation activity,
the use of pre-differentiated MSCs may increase the speed of defect
healing (14). Furthermore, some
compounds, such as kartogenin have been reported to affect MSC
differentiation and exert therapeutic effects on joint injury
(15).
In the present study, a concentrate of the effective
fraction of ADE, termed extra ADE (EADE), was evaluated for its
therapeutic effect in a rabbit model of cartilage injury. In
addition, the potential effects of EADE on MSC-differentiation and
anti-inflammatory responses in chondrocytes were assessed to
elucidate the molecular mechanisms underlying cartilage
regeneration following injury.
Materials and methods
Preparation of ADE and EADE
The whole dried AD plant was refluxed with aqueous
ethanol and AD was extracted to produce ADE. EADE was obtained from
the extract using semi-polarity resins and the concentrated
fraction had >1 wt% 20-hydroxyecdysone. ADE and EADE were
purchased ready made from Matsuura Yakugyo Co., Ltd. (Aichi,
Japan).
Animal model
The animal model was established using a previously
published method (11,16). A total of 18 healthy Japanese Albino
female rabbits, 12 weeks old and weighing 2.0±0.5 kg were purchased
from Shimizu Laboratory Supplies Co., Ltd. (Kyoto, Japan) and
acclimated for 1 week in the laboratory environment. The animals
were housed at 25°C in 50–60% relative humidity, in a 12 h
light/dark cycle, with free access to RC4 food (Oriental Yeast Co.,
Ltd., Tokyo, Japan) and tap of water. The use of the animals and
the procedures followed were approved by the Animal Research
Committee of Tottori University (Tottori, Japan).
Experimental procedures
The analgesic xylazine hydrochloride
(Selactar®; Bayer Yakuhin, Ltd., Osaka, Japan), was
administered (10 mg/kg) as premedication. Following sedation,
induction of anesthesia was performed in an anesthetizing box with
a mixture of 5% isoflurane (Intervet; Merck KGaA, Darmstadt,
Germany) in oxygen. Anesthesia was maintained by inhalation of a
mixture of 3% isoflurane in oxygen using a mask. The fur at the
left knee joint was clipped and the area was disinfected with
chlorhexidine solution (Hibiscrub; Sumitomo Dainippon Pharma Co.,
Ltd., Osaka, Japan) and 70% alcohol. Approaching from the lateral
portion of the knee joint, an incision was made vertically from the
central part of the femur toward the tibial tuberosity. The
articular capsule was incised, and the patella of the stifle joint
was exposed completely by artificially dislocating the patella
toward the medial side. Three holes measuring 2 mm in diameter and
4 mm in depth were made using a hand drill (Micro-engine BL-F;
Osada Electric Co., Ltd., Tokyo, Japan) at the articular cartilage
of the medial trochlea (one hole) and the trochlear sulcus (two
holes) of the distal femur. The wound was rinsed with saline
solution and the articular capsule was sutured and closed with a
synthetic absorbent thread (3-0 PDSII; Johnson & Johnson, New
Brunswick, NJ, USA). The subcutaneous tissues and skin were sutured
with nylon (USP 3-0 suture; Suprylon, Vomel, Germany). During the
1-week period following surgery, the wound surface was disinfected
with povidone-iodine once daily, and 10 mg/kg oxytetracycline
(Pfizer, New York, NY, USA) was subcutaneously administered twice
daily to prevent infection.
ADE and EADE administration
Rabbits were divided into four groups as follows:
Control, ADE, low dosage EADE (low EADE) and high dosage EADE (high
EADE) (n=3 in each). ADE contained 0.04% 20-hydroxyecdysone and
EADE contained 1.38% 20-hydroxyecdysone as specified by the
suppliers. Rabbits were administered with the following: ADE group,
500 mg ADE/kg/day (0.2 mg/kg/day 20-hydroxyecdysone); low EADE
group, 50 mg EADE/kg/day (0.69 mg/kg/day 20-hydroxyecdysone); high
EADE group, 500 mg EADE/kg/day (6.9 mg/kg/day 20-hydroxyecdysone).
ADE and EADE were dissolved in tap water and each dosage was orally
administered every day for 3 weeks. The control group had free
access to tap water. At 3 weeks post-surgery, the rabbits were
euthanized by overdose (160 mg/kg; intravenous injection) of
pentobarbital (Sumitomo Dainippon Pharm Co., Ltd., Osaka, Japan).
The stifle joints were opened and observed macro- and
microscopically to assess the injured cartilage.
Assessment of macroscopic changes
For macroscopic analysis, the extent of restoration
within the surgical holes was scored as previously reported
(17). The restoration scoring was
as follows: <50% restored, 0 points; >50–50%, 1 point;
>60–80% restored, 2 points; >80% restored, 3 points. The
degree of restoration was scored separately for the trochlear
sulcus and the medial trochlear ridge in each rabbit to calculate a
group mean for each region. The mean representative of both areas
was then calculated. The scoring was performed by a
veterinarian.
Assessment of histological
changes
Histological assessment was performed on the femurs
of the rabbits in each group. The recovered left femur was fixed in
10% neutral buffered formaldehyde solution for 1 h at room
temperature. Following fixation, the stifle joint that had been
operated on was trimmed to a thickness of 5 mm and decalcified by
agitating in 5% formic acid solution at 25°C for 1 day. The tissue
was subsequently soaked in 5% sodium sulfate solution at 25°C for 1
day to neutralize and was subsequently washed at 25°C for ~10 h
under running water. The tissue was then embedded in paraffin and
cut into 5-µm slices using a microtome. Staining was performed
using hematoxylin and eosin (H&E), Safranin O and Alcian blue
methods. All methods were performed at 25°C for 10 min. Images of
restored areas, articular cartilage and the growth zone were
captured using an OpticLab H850 (Plustek, Tokyo, Japan) and
evaluated with ImageJ software version 1.49 (National Institutes of
Health, Bethesda, MD, USA). The depth of restoration in the
cartilaginous and subchondral bone matrices were measured based on
the H&E staining according to a previously published method
(11,16). With Safranin O staining, the red
pixels indicating the presence of PGs were counted, while
non-specific colored pixels were not included. With Alcian blue
staining, the indigo pixels indicating the presence of
glycosaminoglycans (GAGs) were also counted. The difference between
the restored substances at the injured sites in all groups was
recorded based on observation with a light microscope (BX51-FL;
Olympus Corporation, Tokyo, Japan). The proportion of the pixels
counted in the desired color from a total of 120,000 pixels (random
sampling of 20,000 pixels at six locations in each cartilaginous
matrix) was then calculated the number of pixels using an image
processing technique in ImageJ. The number of osteoclasts
(multinucleated) and osteoblasts (mononuclear) in the subchondral
bone were recorded in 10 random areas under a light microscope, and
the mean number of osteoclasts per 100 osteoblasts was
calculated.
Culture and cytochemical staining of
human MSCs (hMSCs)
hMSCs derived from umbilical cord matrix (hMSC-UC
cell line) were obtained from PromoCell GmbH (Heidelberg, Germany).
The cells were seeded at 1×105 cells/well in a 96-well
plate and cultured at 37°C in a humidified atmosphere containing 5%
CO2 for 3 weeks in different media. Negative control
cells were cultured in 0.2 ml mesenchymal stem cell growth medium
which was purchased from PromoCell GmbH. Positive control cells
were cultured in a complete chondrogenic differentiation medium
(PromoCell GmbH) and EADE cells were cultured in different
concentrations of EADE (1, 10 or 100 µg/ml) resolved in
chondrogenic differentiation medium. The media were changed twice
weekly. Following the culture period, histochemical analysis of
chondrogenic differentiation was assessed by PG accumulation, as
measured by staining of cell clusters with Alcian blue. Cells were
first rinsed with PBS three times and fixed with 100% methanol for
10 min at room temperature. Staining was accomplished by applying a
solution of 0.1% Alcian blue pH 2.5 (Nacalai Tesque, Kyoto, Japan)
to the cells for 18 h at 4°C. To quantify the intensity of the
staining, the stained culture plates were rinsed with 0.1 N HCl
twice, and each well was extracted with 6 M guanidine-HCl overnight
at room temperature. The optical densities of the cell spheroids
and extracted dye were measured at 630 nm with a microplate
reader.
Chondrocyte culture and measurement of
prostaglandin E2 (PGE2)
Human chondrocytes (HC) derived from normal human
femoral cartilage were obtained from Cell Applications, Inc. (Merck
KGaA). The cells were maintained in chondrocyte growth medium which
was purchased from Cell Applications, Inc. (Merck KGaA) at 37°C in
a humidified atmosphere containing 5% CO2. For treatment
with interleukin (IL)-1β (PeproTech, Inc., Rocky Hill, NJ, USA),
the cells were seeded at 1×104 cells/well in a 24-well
plate. Following overnight incubation at 37°C in a humidified
atmosphere containing 5% CO2, the growth medium was
changed and cells were stimulated with 1 ng/ml IL-1β in the
presence of 10 or 100 µg/ml of EADE prior to further incubation at
37°C in a humidified atmosphere containing 5% CO2 for 24
h. Supernatants were subsequently collected to measure the levels
of PGE2 using a PGE2 Parameter Assay kit
(R&D Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's protocol.
Statistical analysis
Data are expressed as the mean + standard deviation
of the mean. Two groups of data were analyzed using a Student's
t-test. Multiple groups of data were analyzed using one way
analysis of variance followed by a Dunnett's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference. Microsoft Excel 2007 (Microsoft Corporation, Redmond,
WA, USA) was used for statistical analyses using t-tests, and IBM
SPSS version 19.0 (IBM Corp., Armonk, NY, USA) was used for the
other tests.
Results
Effect of EADE in vivo
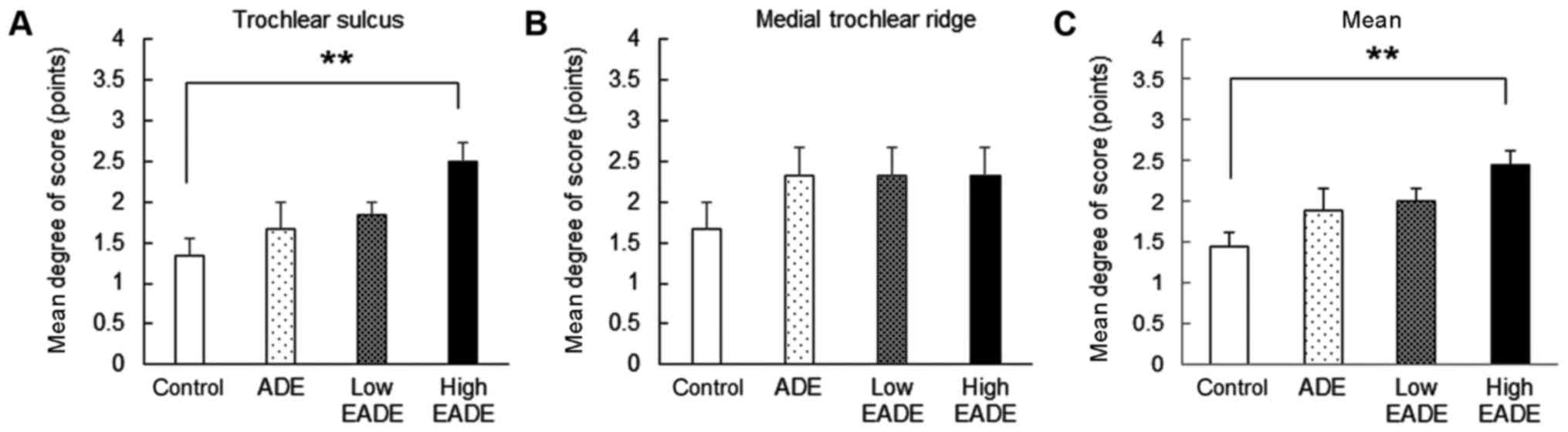
Macroscopic effects of EADE on
cartilage regeneration
In the control group, there was only a slight degree
of restoration at the trochlear sulcus and medial trochlear ridge
(Fig. 1). In the ADE and low and
high EADE groups, the degree of restoration was markedly increased
compared with the control group (Fig.
1). Notably, the degree of restoration in the trochlear sulcus
was significantly greater in the high EADE group compared with the
control group (P<0.01; Fig. 1A);
however, the degree of restoration in the medial trochlear ridge
did not differ significantly between the groups (Fig. 1B). Meanwhile, the mean restoration
score, obtained by pooling the data from the trochlear sulcus and
medial trochlear ridge, was significantly increased in the high
EADE group compared with the control group (P<0.01; Fig. 1C).
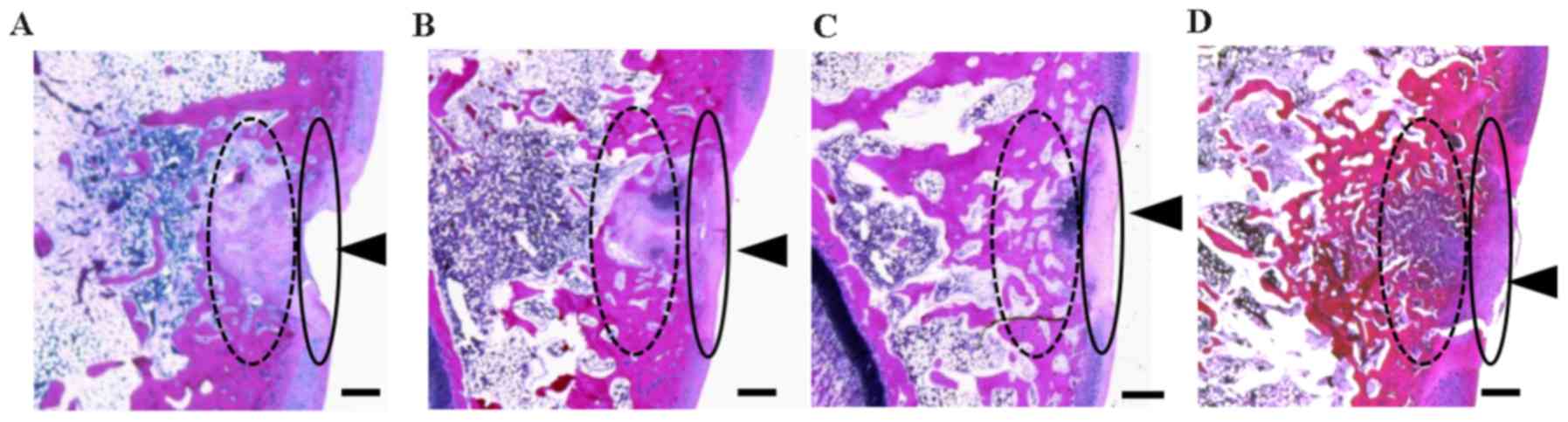
Effect of EADE on cartilage
regeneration assessed by histological H&E staining
Representative H&E staining images of the
trochlear sulcus at 3 weeks post-surgery are presented in Fig. 2. Tissue injury remained visible in
the control group (Fig. 2A). In
contrast, tissue regeneration was apparent in the cartilage and
subchondral matrices of the ADE, low EADE and high EADE groups,
with the defective areas filled with proliferating fibroblasts,
cartilaginous cells and subchondral bone matrix, and the surface of
the wound area covered with regenerated connective tissue (Fig. 2B-D). At a deeper level, the bone
trabecular was regenerated and filled with proliferating cells
(fibroblasts, cartilaginous cells and fibrous cartilage), giving it
an appearance similar to that of mature cartilaginous substrates in
the ADE, low EADE and high EADE groups. In the deeper zone, the
subchondral bone matrix was markedly regenerated in the ADE, low
EADE and high EADE groups (Fig.
2B-D). The cancellous bone structure was also partially
regenerated in these groups, particularly in the high EADE group
(Fig. 2D). Fig. 3 depicts the proportions of
restoration in the cartilage matrix (Fig. 3A-C) and subchondral bone matrix
(Fig. 3D-F). The proportion of
regenerated cartilage matrix in the trochlear sulcus was greater in
the ADE, low EADE and high EADE groups compared with the control
group (Fig. 3A). At the medial
trochlear ridge, the degree of restoration was markedly greater in
the high EADE group compared with the control group (Fig. 3B), and on pooling of the data, the
high EADE group exhibited a significantly greater combined mean
depth of the two areas compared with the control group (P<0.05;
Fig. 3C). The proportion of
regenerated subchondral bone matrix in the trochlear sulcus and the
medial trochlear ridge, as well as the mean combined depth, was
greater in the ADE, low EADE and high EADE groups compared with the
control group (Fig. 3D-F); however,
no significant differences were observed.
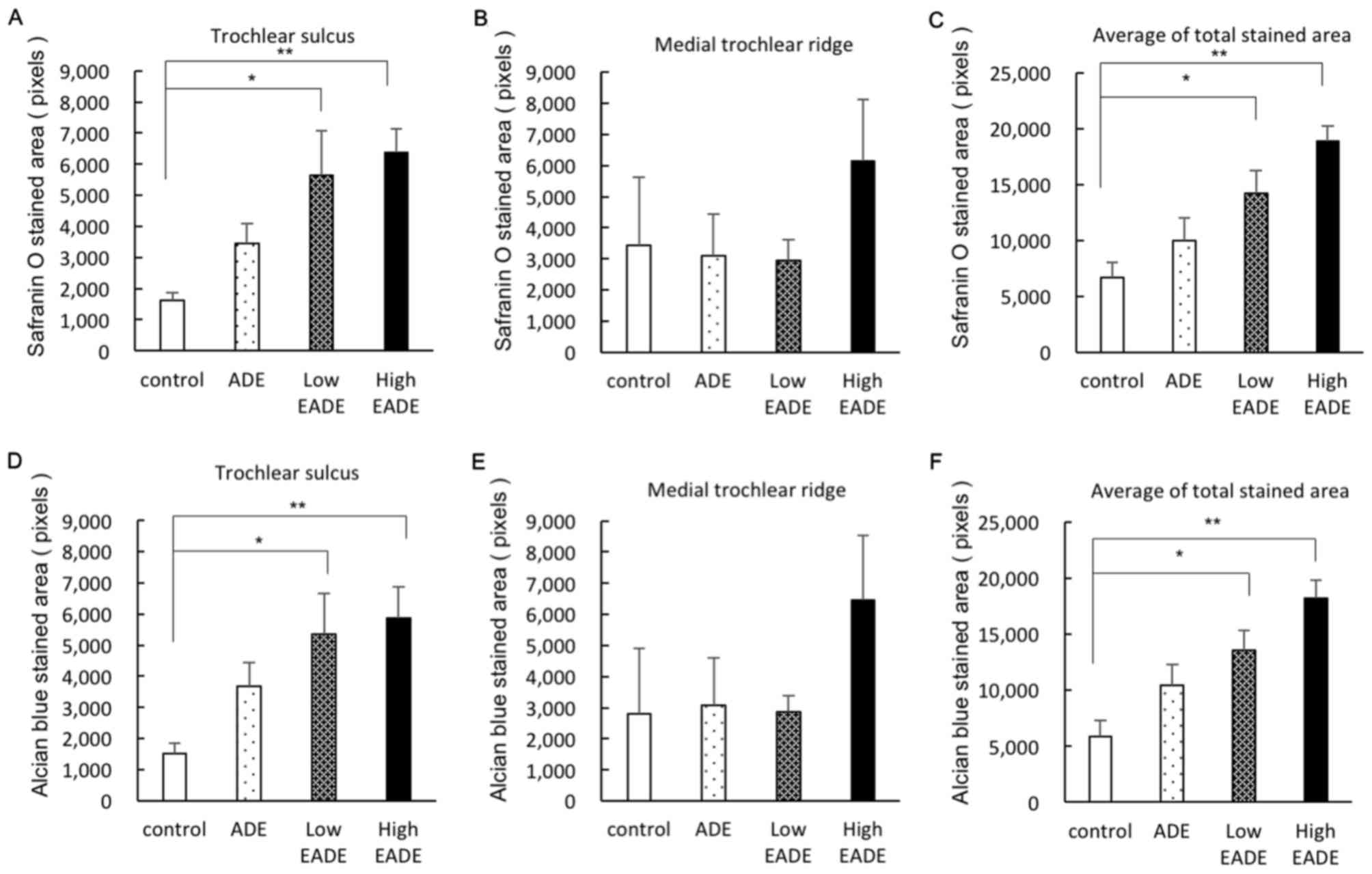
Effect of EADE on cartilage
regeneration assessed by histological Safranin O and Alcian blue
staining
To evaluate cartilaginous matrix regeneration,
histological staining with Safranin O and Alcian blue was
performed. Representative Safranin O and Alcian blue staining
images of the trochlear sulcus are presented in Fig. 4. On Safrinin O staining, PGs in the
cartilage matrix were stained dark red; a small degree of staining
of the cartilage matrix was observed in the control group, whereas
the cartilage matrix was stained more strongly in the ADE, low EADE
and high EADE groups as determined by image analysis (Fig. 4A). In tissues stained with Alcian
blue, the GAGs in the cartilage matrix were stained blue. The
stained areas and color strength were similar to what was observed
with Safranin O staining; stronger staining was observed in the
cartilage matrix of the trochlear sulcus in the ADE, low EADE and
high EADE groups compared with the control group (Fig. 4B). The image analysis of the
cartilage matrix following Safranin O and Alcian blue staining is
presented in Fig. 5. In the
trochlear sulcus, the regenerated areas were significantly
increased in the low and high EADE groups (P<0.05), with the two
staining methods resulting in similar results (Fig. 5A and D). In the medial trochlear
ridge, the strongest staining was observed in the high EADE group
(Fig. 5B and E). Meanwhile, the mean
of the total stained area was significantly higher in the low and
high EADE groups compared with the control group (P<0.05;
Fig. 5C and F), and collectively,
the results suggested that EADE increased the number of GAGs and
PGs in the cartilage matrix in a dose-dependent manner.
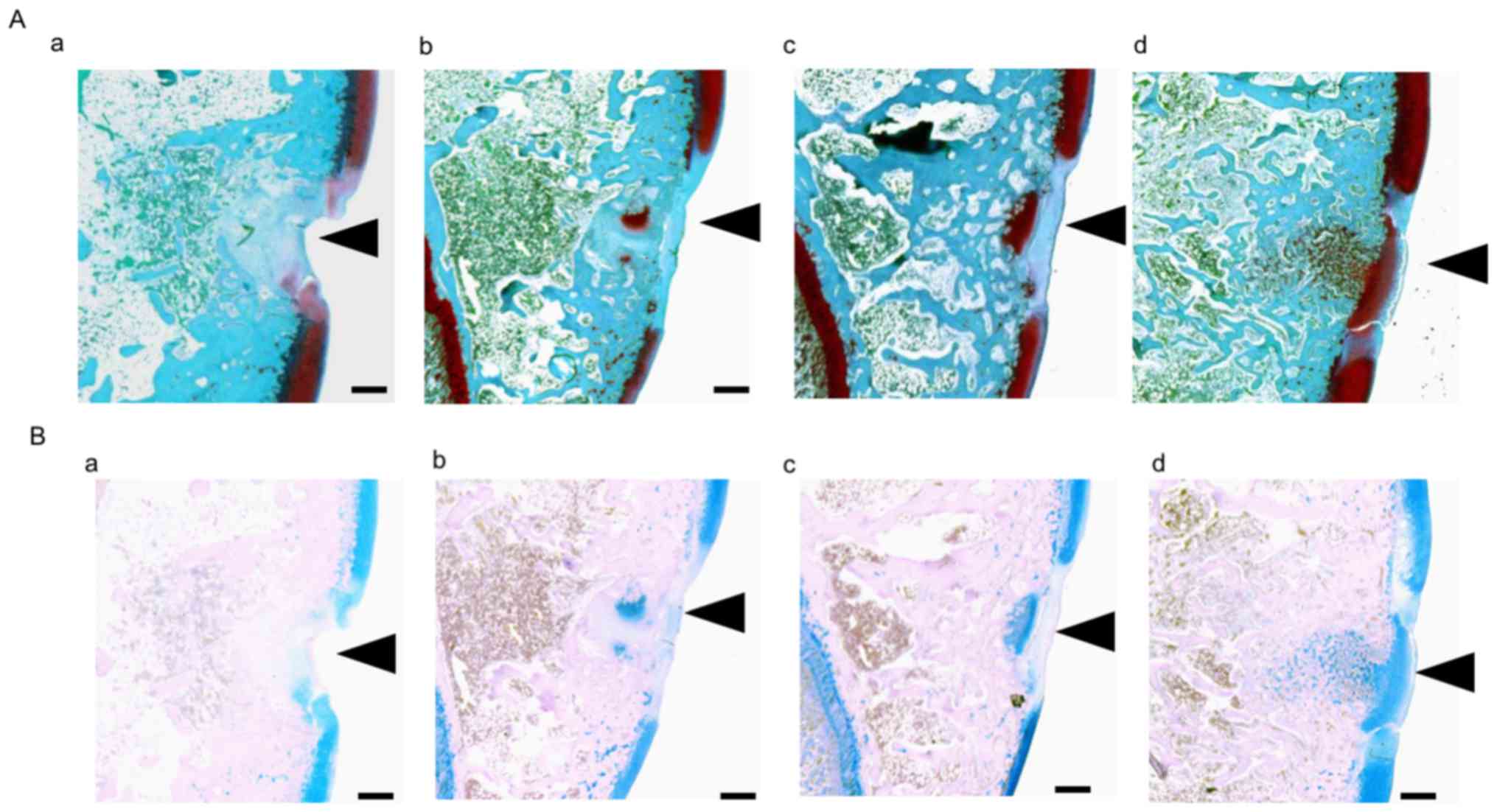 | Figure 4.(A) Safranin O and (B) Alcian blue
staining in the trochlear sulcus at 3 weeks post-surgery.
Proteoglycans in the cartilage matrix were stained with Safranin O
(red). Black arrowheads indicate damaged tissue areas. At the
trochlear sulcus, red staining was weak in the (Aa) control group,
whereas it was stronger in the (Ab) ADE, (Ac) low EADE and (Ad)
high EADE groups. The holes were filled with collagen fibers
(green) in the control, ADE, low EADE and high EADE groups. The
cartilage matrix was stained red to the greatest extent in the high
EADE group. The glycosaminoglycans in the cartilage matrix were
stained with Alcian blue (blue), which was of similar intensity to
the Safranin O staining. Compared with the (Ba) control group,
defects were filled with proteoglycan in the (Bb) ADE, (Bc) low
EADE and (Bd) high EADE groups. The cartilage matrix was stained
blue in the high EADE group. Scale bar, 500 µm. ADE, Ajuga
decumbens extract; EADA, extra ADE. |
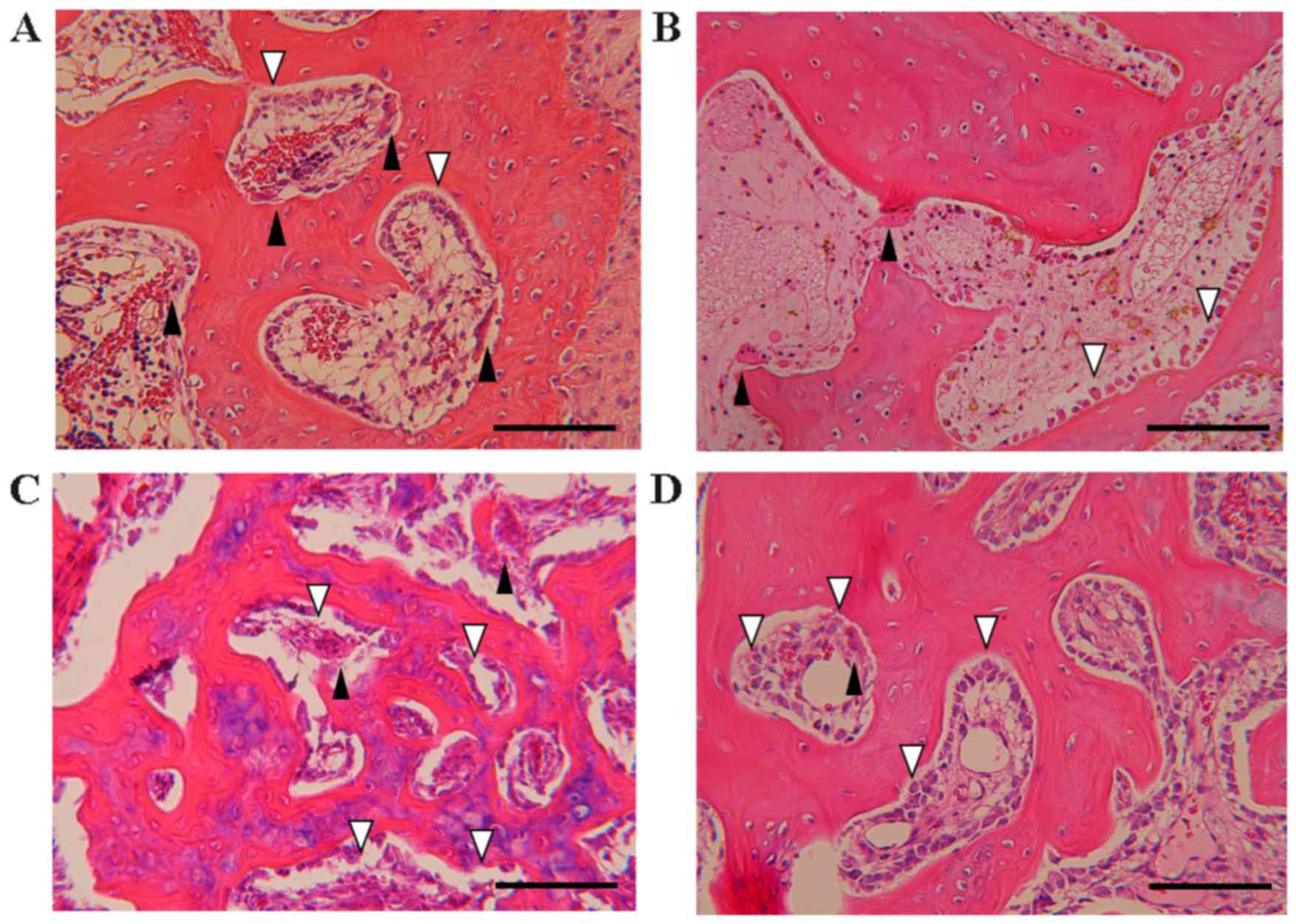
Effect of EADE on osteogenesis
assessed by histological H&E staining
To evaluate the effect of EADE on the balance of
osteogenesis, osteoclasts and osteoblasts were counted in the
subchondral bone following histological H&E staining (Fig. 6). In the control group, a marked
number of osteoclasts were present, while few osteoblasts were
observed (Fig. 6A). By contrast, in
the ADE group, fewer osteoclasts were observed (Fig. 6B), and in the low and high EADE
groups, the numbers of osteoblasts were increased (Fig. 6C and D). The mean number of
osteoclasts was significantly reduced in the high EADE group at the
trochlear sulcus compared with the control group (Fig. 7A; P<0.05); however, the mean
number of osteoclasts at the medial trochlear ridge did not differ
significantly between the groups (Fig.
7B). Similarly, across the trochlear sulcus and medial
trochlear ridge regions, the numbers of osteoclasts did not differ
significantly (Fig. 7C).
Subsequently, the mean number of osteoclasts per 100 osteoblasts in
each group was calculated. No significant differences in the number
of osteoclasts per 100 osteoblasts were observed between the groups
(Fig. 7D-F), though slight decreases
were observed in all treatment groups compared with the control
excluding for the ADE group at the trochlear sulcus. Collectively
these data suggest that EADE activates osteogenesis in subchondral
bone.
Effect of EADE in vitro
Effect of EADE on chondrogenic
differentiation in hMSCs
To determine whether chondrogenic differentiation is
associated with cartilage matrix regeneration, MSC differentiation
to chondrocytes following EADE treatment was assessed. The results
of cytochemical analysis by Alcian blue staining following 3 weeks
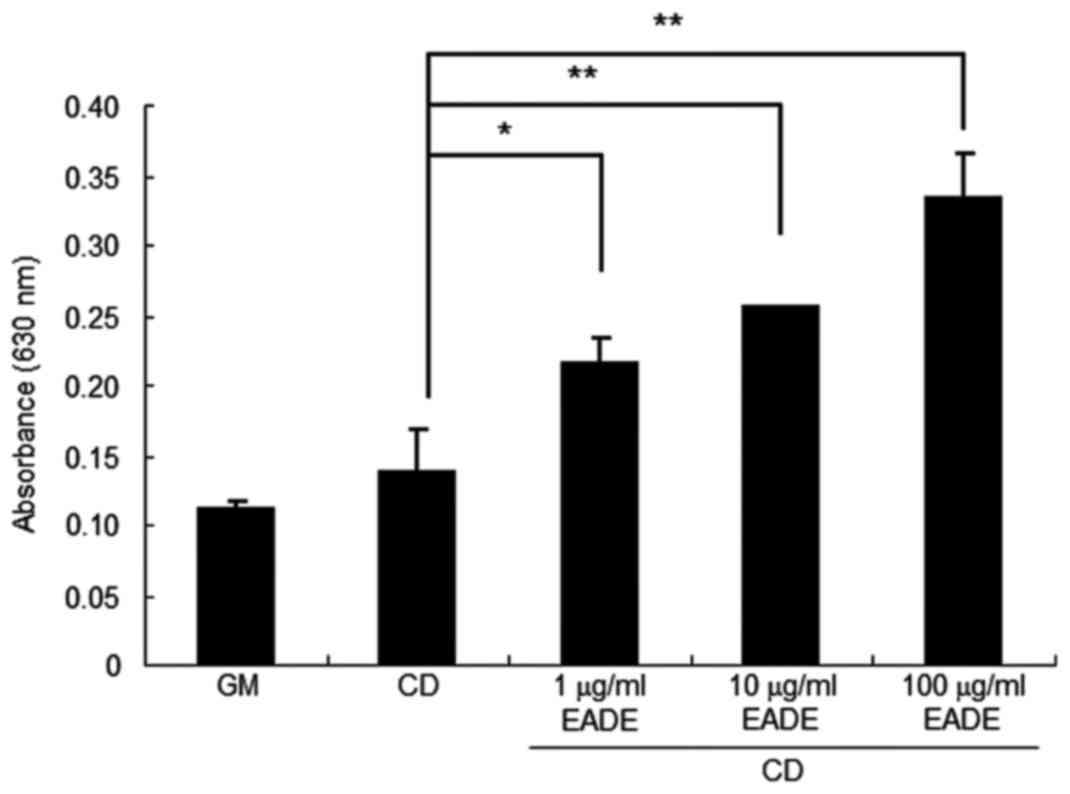
of hMSC culture are presented in Fig.
8. The hMSCs were cultured for 3 weeks with normal growth
medium or chondrogenic differentiation medium with or without EADE
(1–100 µg/ml). While undifferentiated MSCs have little
extracellular matrix, chondrogenic differentiation results in the
formation of cartilage with a typical extracellular matrix composed
of PG aggrecan and other glycosaminoglycans (15). PG aggrecan and other
glycosaminoglycans are therefore used as indicators of cartilage
formation (15), and were thus
detected by Alcian blue staining in the present study. The analysis
indicated that EADE treatment increased staining associated with
aggrecan and other glycosaminoglycans in cells cultured in
chondrogenic differentiation medium (P<0.05) in an apparent
dose-dependent manner (Fig. 8).
Effect of EADE on PGE2
production in chondrocytes
The repressive effect of EADE on PGE2
production was measured to evaluate its effect on osteogenesis. For
control cells cultured without IL-1β, a small amount of
PGE2 was observed in the cultured cells (Fig. 9). By contrast, cells cultured in the
presence of IL-1β (1 ng/ml) produced PGE2 at a markedly
higher level compared with the control cells (P<0.05). The
effect of EADE on IL-1β-induced PGE2 production was also
assessed. Co-culture with 10 or 100 µg/ml EADE significantly
blocked the stimulation of PGE2 production by IL-1β
(P<0.05) in an apparent dose-dependent manner (Fig. 9).
Discussion
In the present study, it was investigated whether
the concentrate of the effective fraction of ADE was effective in
the treatment of OA and cartilage regeneration. In a cartilage
injury model, the degree of restoration within surgical holes was
increased in an apparent dose-dependent manner by EADE. In
particular, the cartilage matrix was significantly regenerated in
the EADE groups compared with the control group and the
regeneration area was improved in the EADE treatment groups, most
notably in the high EADE group, as demonstrated by Safranin O and
Alcian blue staining. The data obtained from Safranin O and Alcian
blue staining were consistent and the extent of staining exhibited
an apparent correlation with the concentration of EADE, which
suggests that this compound increases the amount of GAGs and PGs in
cartilage matrix. In a previous study by our group, the number of
osteoclasts was significantly decreased following administration of
ADE or 20-hydroxyecdysone (11);
however, its influence on bone metabolism was unclear. Bone
metabolism is regulated by a balance between the functions of
osteoclast and osteoblast cells (17). ADE has previously been demonstrated
to inhibit osteoclast differentiation (10) and 20-hydroxyecdysone has also been
reported to have beneficial effects in epiphyseal cartilage and
trabecular bone in ovariectomized rats (12). In the present study, the mean number
of osteoclasts per 100 osteoblasts in the subchondral bone was
decreased to the greatest extent in the high ADE group compared
with the control group. These results suggest that EADE may
influence the number of osteoclasts as well as bone metabolism and
the regeneration of subchondral bone. It has been reported that
20-hydroxyecdysone stimulates MSC osteogenic differentiation
(18), and so as an active component
in EADE, it may stimulate MSCs and enhance osteogenesis in
subchondral bone.
When cartilage is injured, natural healing is slow
and typically results in the formation of nonfunctional
fibro-cartilage, while regeneration of hyaline cartilage rarely
occurs (7). However, the
administration of GlcN can regenerate hyaline cartilage (7). Experimental data have demonstrated that
the mechanism underlying this action is associated with
chondroblast activation (19).
Glucosamine promotes a chondrogenic phenotype in MSCs (20) and inhibits matrix metalloproteinase
(MMP)-13 expression and matrix degradation (20). MMPs and IL-1β have been demonstrated
to serve a role in the degradation of articular cartilage (21–23).
IL-1β is produced by mononuclear cells in the arthritic synovium
and chondrocytes (21) and enhances
the production of various chemical mediators, including
PGE2 (22), nitric oxide
(NO) and MMPs (23), in
chondrocytes. In the present study, the ability of EADE to induce
MSC differentiation and its anti-inflammatory effects in
chondrocytes were assessed in order to investigate the mechanisms
underlying the regeneration process following cartilage injury.
EADE was indicated to stimulate PG production and induce in
vitro chondrogenic differentiation of MSCs. Furthermore, EADE
(10–100 µg/ml) significantly attenuated IL-1β-induced
PGE2 production in chondrocytes. It has been reported
that 20-hydroxyecdysone suppresses IL-1β-induced catabolic gene
expression in cartilage (24) and
that ADE inhibits the expression of iNOS and NO production in
macrophages (10). It has also been
documented that PGE2 may induce receptor activator of
nuclear factor kappa-B ligand (RANKL) expression in osteoblasts and
directly enhance RANKL-induced osteoclastogenesis in precursors
(25). In addition,
20-hydroxyecdysone has been demonstrated to stimulate MSC
osteogenic differentiation (18),
suggesting that 20-hydroxyecdysone may suppress IL-1β-induced
inflammation and PGE2-induced RANKL expression. EADE may
therefore have an anti-inflammatory effect and suppress
osteoclastogenesis.
In conclusion, the present study investigated the
effect of EADE on the acceleration of healing in an experimental
model of cartilage injury. The cartilage matrix, along with the
subchondral matrix, was markedly regenerated in the low and high
EADE groups. Additionally, EADE stimulated PG production, induced
in vitro chondrogenic differentiation of MSCs, and
significantly attenuated IL-1β-induced PGE2 production
in chondrocytes. Thus, EADE not only exerted a chondroprotective
effect, but also influenced bone metabolism and stimulated
subchondral bone restoration. The results of the present study
suggest that EADE may be an effective treatment for cartilaginous
damage and have greater therapeutic effect than the currently used
therapeutic ADE. The present study only investigated the effect of
EADE on improving knee destruction in vivo and in
vitro. Further study is required to determine whether EADE may
improve the join function and QOL in patients with knee
injuries.
Acknowledgements
The authors would like to thank Professor Saburo
Minami (Tottori University, Tottori, Japan) for his valuable advice
and Mr. Shohei Hishikawa (Tottori University) for his
assistance.
References
|
1
|
Yoshimura N, Muraki S, Oka H, Mabuchi A,
En-Yo Y, Yoshida M, Saika A, Yoshida H, Suzuki T, Yamamoto S, et
al: Prevalence of knee osteoarthritis, lumbar spondylosis, and
osteoporosis in Japanese men and women: The research on
osteoarthritis/osteoporosis against disability study. J Bone Miner
Metab. 27:620–628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buckwalter JA, Martin J and Mankin HJ:
Synovial joint degeneration and the syndrome of osteoarthritis.
Instr Course Lect. 49:481–489. 2000.PubMed/NCBI
|
|
3
|
Buckwalter JA, Roughley PJ and Rosenberg
LC: Age-related changes in cartilage proteoglycans: Quantitative
electron microscopic studies. Microsc Res Tech. 28:398–408. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin JA and Buckwalter JA: Roles of
articular cartilage aging and chondrocyte senescence in the
pathogenesis of osteoarthritis. Iowa Orthop J. 21:1–7.
2001.PubMed/NCBI
|
|
5
|
Reginster JY, Deroisy R, Rovati LC, Lee
RL, Lejeune E, Bruyere O, Giacovelli G, Henrotin Y, Dacre JE and
Gossett C: Long-term effects of glucosamine sulphate on
osteoarthritis progression: A randomised, placebo-controlled
clinical trial. Lancet. 357:251–256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakamura H, Shibakawa A, Tanaka M, Kato T
and Nishioka K: Effects of glucosamine hydrochloride on the
production of prostaglandin E2, nitric oxide and metalloproteases
by chondrocytes and synoviocytes in osteoarthritis. Clin Exp
Rheumatol. 22:293–299. 2004.PubMed/NCBI
|
|
7
|
Tamai Y, Miyatake K, Okamoto Y, Takamori
Y, Sakamoto H and Minami S: Enhanced healing of cartilaginous
injuries by glucosamine hydrochloride. Carbohydr Polym. 48:369–378.
2002. View Article : Google Scholar
|
|
8
|
Naito K, Watari T, Furuhata A, Yomogida S,
Sakamoto K, Kurosawa H, Kaneko K and Nagaoka I: Evaluation of the
effect of glucosamine on an experimental rat osteoarthritis model.
Life Sci. 86:538–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clegg DO, Reda DJ, Harris CL, Klein MA,
O'Dell JR, Hooper MM, Bradley JD, Bingham CO III, Weisman MH,
Jackson CG, et al: Glucosamine, chondroitin sulfate, and the two in
combination for painful knee osteoarthritis. N Engl J Med.
354:795–808. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ono Y, Fukaya Y, Imai S and Yamakuni T:
Beneficial effects of Ajuga decumbens on osteoporosis and
arthritis. Biol Pharm Bull. 31:1199–1204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sawada Y, Sugimoto A, Fukuda K, Kurosawa
T, Ogawa M, Osaki T and Minami S: Oral administration of Ajuga
decumbens extract has a synergetic effect with glucomsaine on
cartilaginous injury in a rabbit osteoaruthritis model. J Chitin
Chitosan Sci. 2:191–196. 2014. View Article : Google Scholar
|
|
12
|
Kapur P, Wuttke W, Jarry H and
Seidlova-Wuttke D: Beneficial effects of beta-Ecdysone on the
joint, epiphyseal cartilage tissue and trabecular bone in
ovariectomized rats. Phytomedicine. 17:350–355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mardones R, Jofré CM and Minguell JJ: Cell
therapy and tissue engineering approaches for cartilage repair
and/or regeneration. Int J Stem Cells. 8:48–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ham O, Lee CY, Kim R, Lee J, Oh S, Lee MY,
Kim J, Hwang KC, Maeng LS and Chang W: Therapeutic potential of
differentiated mesenchymal stem cells for treatment of
osteoarthritis. Int J Mol Sci. 16:14961–14978. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johnson K, Zhu S, Tremblay MS, Payette JN,
Wang J, Bouchez LC, Meeusen S, Althage A, Cho CY, Wu X and Schultz
PG: A stem cell-based approach to cartilage repair. Science.
336:717–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Osaki T, Kitahara K, Okamoto Y, Imagawa T,
Tsuka T, Miki Y, Kawamoto H, Saimoto H and Minami S: Effect of
fucoidan extracted from mozuku on experimental cartilaginous tissue
injury. Mar Drugs. 10:2560–2570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rodan G: Introduction to bone biology.
Bone. 13 Suppl 1:S3–S6. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao L, Cai G and Shi X: Beta-ecdysterone
induces osteogenic differentiation in mouse mesenchymal stem cells
and relieves osteoporosis. Biol Pharm Bull. 31:2245–2249. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hashida M, Miyatake K, Okamoto Y, Fujita
K, Matsumoto T, Morimatsu F, Sakamoto K and Minami S: Synergistic
effects of D-glucosamine and collagen peptides on healing
experimental cartilage injury. Macromol Biosci. 3:596–603. 2003.
View Article : Google Scholar
|
|
20
|
Derfoul A, Miyoshi AD, Freeman DE and Tuan
RS: Glucosamine promotes chondrogenic phenotype in both
chondrocytes and mesenchymal stem cells and inhibits MMP-13
expression and matrix degradation. Osteoarthritis Cartilage.
15:646–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Farahat MN, Yanni G, Poston R and Panayi
GS: Cytokine expression in synovial membranes of patients with
rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 52:870–875.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Campbell IK, Piccoli DS and Hamilton JA:
Stimulation of human chondrocyte prostaglandin E2 production by
recombinant human interleukin-1 and tumour necrosis factor. Biochim
Biophys Acta. 1051:310–318. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tetlow LC, Adlam DJ and Woolley DE: Matrix
metalloproteinase and proinflammatory cytokine production by
chondrocytes of human osteoarthritic cartilage: Associations with
degenerative changes. Arthritis Rheum. 44:585–594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sheu SY, Ho SR, Sun JS, Chen CY and Ke CJ:
Arthropod steroid hormone (20-Hydroxyecdysone) suppresses
IL-1β-induced catabolic gene expression in cartilage. BMC
Complement Altern Med. 15:12015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kotake S, Yago T, Kawamoto M and Nanke Y:
Effects of NSAIDs on differentiation and function of human and
murine osteoclasts-crucial ‘human osteoclastology’. Pharmaceuticals
(Basel). 3:1394–1410. 2010. View Article : Google Scholar : PubMed/NCBI
|