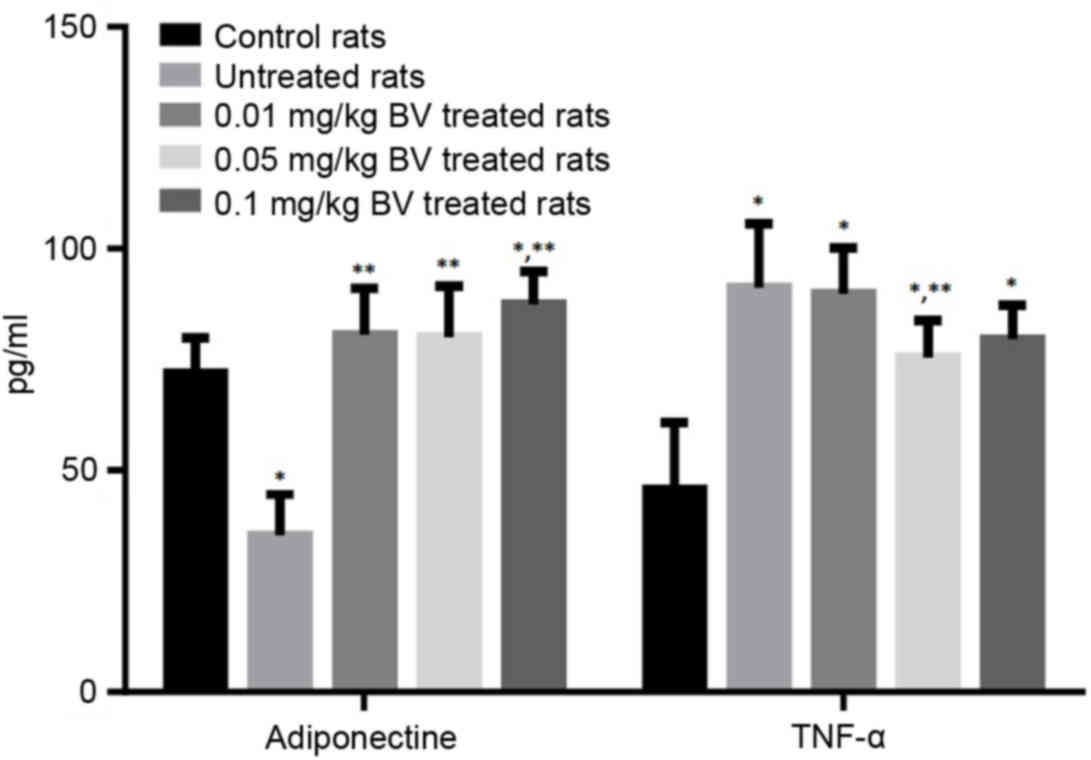
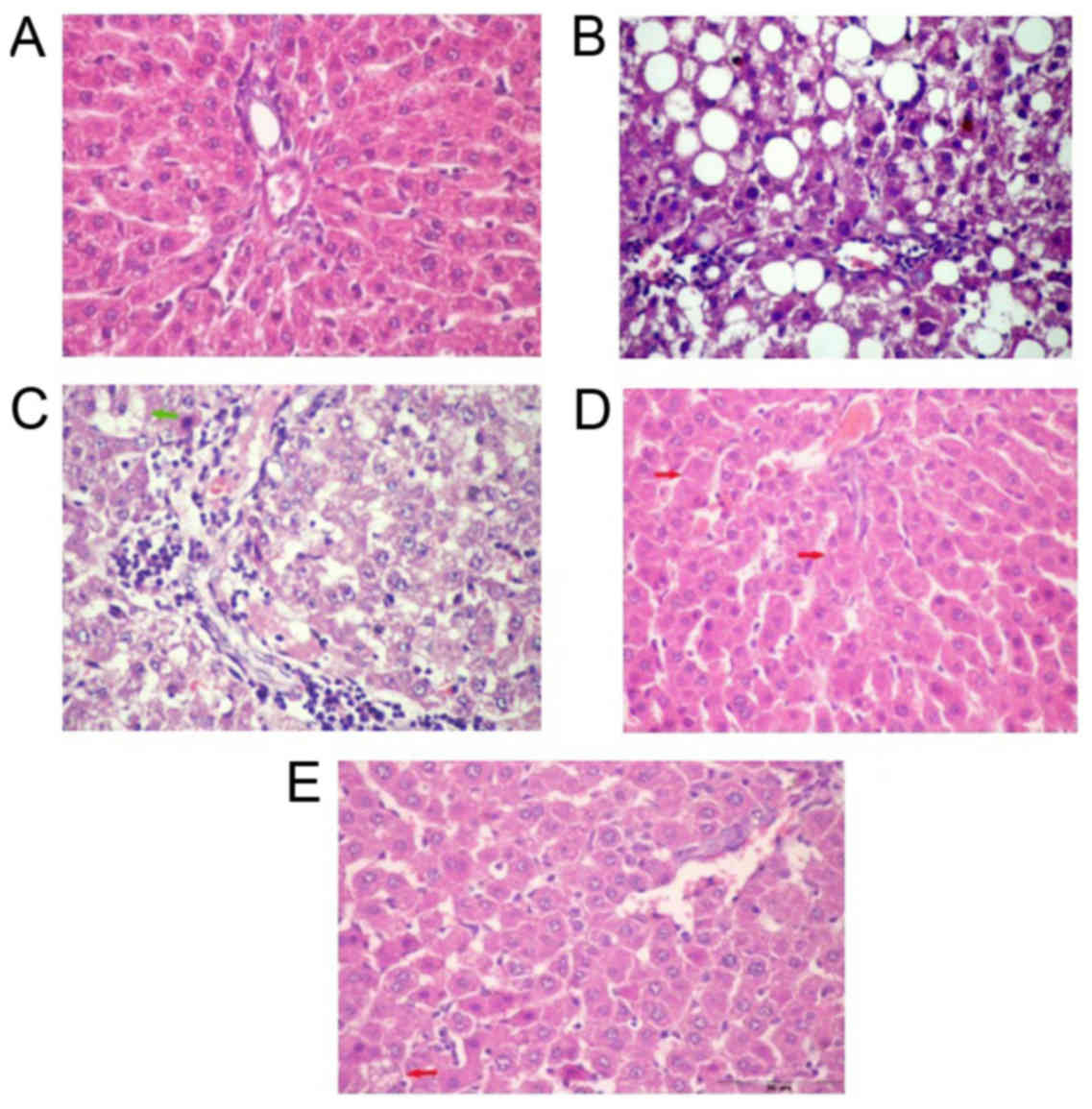
|
1
|
Neuschwander-Tetri BA and Caldwell SH:
Nonalcoholic steatohepatitis: Summary of an AASLD single topic
conference. Hepatology. 37:1202–1219. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Musso G, Cassader M, De Michieli F, Rosina
F, Orlandi F and Gambino R: Nonalcoholic steatohepatitis versus
steatosis: Adipose tissue insulin resistance and dysfunctional
response to fat ingestion predict liver injury and altered glucose
and lipoprotein metabolism. Hepatology. 56:933–942. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Halbleib M, Skurk T, de Luca C, von
Heimburg D and Hauner H: Tissue engineering of white adipose tissue
using hyaluronic acid-based scaffolds. I: In vitro differentiation
of human adipocyte precursor cells on scaffolds. Biomaterials.
24:3125–3132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Day CP and James OF: Steatohepatitis: A
tale of two ‘hits’? Gastroenterology. 114:842–845. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bugianesi E, Gastaldelli A, Vanni E,
Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E and
Rizzetto M: Insulin resistance in non-diabetic patients with
non-alcoholic fatty liver disease: Sites and mechanisms.
Diabetologia. 48:634–642. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hui JM, Hodge A, Farrell GC, Kench JG,
Kriketos A and George J: Beyond insulin resistance in NASH:
TNF-alpha or adiponectin? Hepatology. 40:46–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Houstis N, Rosen ED and Lander ES:
Reactive oxygen species have a casual role in multiple forms of
insulin resistance. Nature. 440:944–948. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Franco R, Schoneveld OJ, Pappa A and
Panayiotidis MI: The central role of glutathione in the
pathophysiology of human diseases. Arch Physiol Biochem.
113:234–258. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kwak MK, Wakabayashi N, Itoh K, Motohashi
H, Yamamoto M and Kensler TW: Modulation of gene expression by
cancer chemopreventive dithiolethiones through the Keap1-Nrf2
pathway. Identification of novel gene clusters for cell survival. J
Biol Chem. 278:8135–8145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yates MS, Tran QT, Dolan PM, Osburn WO,
Shin S, McCulloch CC, Silkworth JB, Taguchi K, Yamamoto M, Williams
CR, et al: Genetic versus chemoprotective activation of Nrf2
signaling: Overlapping yet distinct gene expression profiles
between Keap1 knockout and triterpenoid-treated mice.
Carcinogenesis. 30:1024–1031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sugimoto H, Okada K, Shoda J, Warabi E,
Ishige K, Ueda T, Taguchi K, Yanagawa T, Nakahara A, Hyodo I, et
al: Deletion of nuclear factor-E2-related factor-2 leads to rapid
onset and progression of nutritional steatohepatitis in mice. Am J
Physiol Gastrointest Liver Physiol. 298:G283–G294. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang YK, Yeager RL, Tanaka Y and Klaassen
CD: Enhanced expression of Nrf2 in mice attenuates the fatty liver
produced by a methionine- and choline-deficient diet. Toxicol Appl
Pharmacol. 245:326–334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jang MH, Shin MC, Lim S, Han SM, Park HJ,
Shin I, Lee JS, Kim KA, Kim EH and Kim CJ: Bee venom induces
apoptosis and inhibits expression of cyclooxygenase-2 mRNA in human
lung cancer cell line NCI-H1299. J Pharmacol Sci. 91:95–104. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong SJ, Rim GS, Yang HI, Yin CS, Koh HG,
Jang MH, Kim CJ, Choe BK and Chung JH: Bee venom induces apoptosis
through caspase-3 activation in synovial fibroblasts of patients
with rheumatoid arthritis. Toxicon. 46:39–45. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sene-Fiorese M, Duarte FO, Scarmagnani FR,
Cheik NC, Manzoni MS, Nonaka KO, Rossi EA, de Oliveira Duarte AC
and Dâmaso AR: Efficiency of intermittent exercise on adiposity and
fatty liver in rats fed with high fat diet. Obesity (Silver
Spring). 16:2217–2222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lopes-Virella MF, Stone PG and Colwell JA:
Serum high density lipoprotein in diabetic patients. Diabetologia.
13:285–291. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Friedewald WT, Levy RI and Fredrickson DS:
Estimation of the concentration of low-density lipoprotein
cholesterol in plasma, without use of the preparative
ultracentrifuge. Clin Chem. 18:499–502. 1972.PubMed/NCBI
|
|
18
|
Caumo A, Perseghin G, Brunani A and Luzi
L: New insights on the simultaneous assessment of insulin
sensitivity and beta-cell function with the HOMA2 method. Diabetes
Care. 29:2733–2734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bligh EG and Dyer WJ: A rapid method of
total lipid extraction and purification. Can J Biochem Physiol.
37:911–917. 1959. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muthusamy VR, Kannan S, Sadhaasivam K,
Gounder SS, Davidson CJ, Boeheme C, Hoidal JR, Wang L and
Rajasekaran NS: Acute exercise stress activates Nrf2/ARE signaling
and promotes antioxidant mechanisms in the myocardium. Free Radic
Biol Med. 52:366–376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Draper HH and Hadley M: Malondialdehyde
determination as index of lipid peroxidation. Methods Enzymol.
186:421–431. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Griffith OW: Determination of glutathione
and glutathione disulfide using glutathione reductase and
2-vinylpyridine. Anal Biochem. 106:207–212. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith IK, Vierheller TL and Thorne CA:
Assay of glutathione reductase in crude tissue homogenates using
5,5′-dithiobis(2-nitrobenzoic acid). Anal Biochem. 175:408–413.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Habig WH, Pabst MJ and Jakoby WB:
Glutathione S-transferase AA from rat liver. Arch Biochem Biophys.
175:710–716. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Flohé L and Günzler WA: Assays of
glutathione peroxidase. Methods Enzymol. 105:114–121. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tangvarasittichai S: Oxidative stress,
insulin resistance, dyslipidemia and type 2 diabetes mellitus.
World J Diabetes. 6:456–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Di Chiara T, Argano C, Corrao S, Scaglione
R and Licata G: Hypoadiponectinemia: A link between visceral
obesity and metabolic syndrome. J Nutr Metab. 2012:1752452012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tilg H and Moschen AR: Adipocytokines:
Mediators linking adipose tissue, inflammation and immunity. Nat
Rev Immunol. 6:772–783. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tilg H and Hotamisligil GS: Nonalcoholic
fatty liver disease: Cytokine-adipokine interplay and regulation of
insulin resistance. Gastroenterology. 131:934–945. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kono H, Rusyn I, Yin M, Gäbele E,
Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal
BH, et al: NADPH oxidase-derived free radicals are key oxidants in
alcohol-induced liver disease. J Clin Invest. 106:867–872. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weltman MD, Farrell GC, Hall P,
Ingelman-Sundberg M and Liddle C: Hepatic cytochrome P450 2E1 is
increased in patients with nonalcoholic steatohepatitis.
Hepatology. 27:128–133. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shoelson SE, Herrero L and Naaz A:
Obesity, inflammation, and insulin resistance. Gastroenterology.
132:2169–2180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee JY, Kang SS, Kim JH, Bae CS and Choi
SH: Inhibitory effect of whole bee venom in adjuvant-induced
arthritis. In Vivo. 19:801–805. 2005.PubMed/NCBI
|
|
35
|
Mousavi SM, Imani S, Haghighi S, Mousavi
SE and Karimi A: Effect of Iranian honey bee (Apis
mellifera) venom on blood glucose and insulin in diabetic rats.
J Arthropod Borne Dis. 6:136–143. 2012.PubMed/NCBI
|
|
36
|
Behroozi J, Divsalar A and Saboury AA:
Honey bee venom decreases the complications of diabetes by
preventing hemoglobin glycation. J Molec Liquids. 199:371–375.
2014. View Article : Google Scholar
|
|
37
|
Yang EJ, Jiang JH, Lee SM, Yang SC, Hwang
HS, Lee MS and Choi SM: Bee venom attenuates neuroinflammatory
events and extends survival in amyotrophic lateral sclerosis
models. J Neuroinflammation. 7:692010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kwak MK, Wakabayashi N, Itoh K, Motohashi
H, Yamamoto M and Kensler TW: Modulation of gene expression by
cancer chemopreventive dithiolethiones through the Keap1-Nrf2
pathway. Identification of novel gene clusters for cell survival. J
Biol Chem. 278:8135–8145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mo C, Wang L, Zhang J, Numazawa S, Tang H,
Tang X, Han X, Li J, Yang M, Wang Z, et al: The crosstalk between
Nrf2 and AMPK signal pathways is important for the
anti-inflammatory effect of berberine in LPS-stimulated macrophages
and endotoxin-shocked mice. Antioxid Redox Signal. 20:574–588.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Joo MS, Kim WD, Lee KY, Kim JH, Koo JH and
Kim SG: AMPK facilitates nuclear accumulation of Nrf2 by
phosphorylating at serine 550. Mol Cell Biol. 36:1931–1942. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hwang DS, Kim SK and Bae H: Therapeutic
effects of bee venom on immunological and neurological diseases
review. Toxins (Basel). 7:2413–2421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ram SKM, Jayapal N, Nanaiah P, Aswal GS,
Ramnarayan BK and Taher SM: The therapeutic benefits of bee venom.
Int J Curr Microbiol App Sci. 3:377–381. 2014.
|