Introduction
Acute myocardial infarction (AMI) is the most
important manifestation of ischemic heart disease and is one of the
leading causes of major morbidity and mortality in the modern
world. Recently, with the emergence of myocardial tissue
engineering, the delivery of ex vivo bone mesenchymal stem
cells (MSCs) to the infarcted heart has been successfully performed
(1–3).
MSCs are multipotent progenitor cells, which are
easy to purify and amplify (4–6). They
are able to differentiate into cardiomyocytes (CMs) or CM-like
cells (CLCs) in vivo and in vitro (7,8).
5-azacytidine is a classic inducer that enhances differentiation of
MSCs into CMs by random demethylation. However, it has been
demonstrated that 5-azacytidine is toxic and produces relatively
low differentiation ratios (9).
Transforming growth factor β1 (TGF-β1) is a
pleiotropic cytokine with numerous complex effects in cell and
tissue physiology. It is a multifunctional cytokine involved in the
differentiation, growth and survival of a variety of cells
(10). Salvianolic acid B (Sal-B) is
a water-soluble phenolic acid extracted from Salvia
miltiorrhiza, which is a Chinese herbal medicine used in the
treatment of various heart diseases for hundreds of years.
Therefore, its safety as an inducer of differentiation may be
comparatively higher than that of 5-azacytidine. In the present
study, a new system for cardiomyogenic differentiation of bone
marrow mesenchymal stromal cells (BMSCs) was established by using a
combination of TGF-β1 and Sal-B and electron microscopy,
immunofluorescence as well as reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis were used to elucidate
the biological effects and significance of TGF-β1 and Sal-B
regarding the differentiation of BMSCs into CMs or CLCs.
Materials and methods
Animals
A total of 10 male Sprague Dawley (SD) rats (weight,
35–45 g; age, 3 weeks) were obtained from HuaFuKang Bioscience Co.,
Inc. Beijing (certificate no. 11401300032331). They were kept in
plastic cages at a controlled temperature (18–21°C) and humidity
(55±5%) under a 12-h light/dark cycle. The animal experiments were
performed in accordance with protocols approved by the
Institutional Animal Care and Use Committee of Hebei North
University (Zhangjiakou, China).
Isolation and culture of BMSCs
Bone marrow was separated from the femur and tibial
bones of SD rats following sacrifice by cervical vertebra
dislocation. The marrows were collected and diluted with 5 ml
Iscove's modified Dulbecco's medium-low glucose (IMDM-LG; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), supplemented
with 15% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a
humidified atmosphere with 5% CO2. After 3 days,
non-adherent hematopoietic cells were discarded and the adherent
cells were washed twice with PBS. The culture medium was
replenished every 3 days. When the density of the cell colonies
reached ~90% confluence, the cells were detached with 0.25% trypsin
(Amresco, Solon, OH, USA) and transferred to fresh flasks at a
ratio of 1:2.
Flow cytometry analysis
Fourth generation BMSCs in the control group were
identified using flow cytometry (FACSAria™IISORP; BD
Biosciences, Franklin Lakes, NJ, USA). Stem cells at passage 4 were
harvested and suspended in IMDM-LG medium (Gibco; Thermo Fisher
Scientific, Inc.) containing 15% FBS (Gibco; Thermo Fisher
Scientific, Inc.) at a concentration of 1×106 cells/ml.
Following a brief centrifugation (7 min, 400 × g) at room
temperature (RT), cells resuspended in 100 µl of FACS buffer
(PBS/2% FCS) and blocked with 1 µl FC-block (cat. no. 553142, BD
Biosciences) for 10 min at RT. The following mouse anti-rat
monoclonal antibodies were used (BD Biosciences) to characterize
the BMSCs in the dark at 4°C for 30 min: Fluorescein
isothiocyabate- or PE-conjugated CD29 (1:10; cat. no. 562154), CD45
(1:20; cat. no. 561867) and CD90 (1:10; cat. no. 561404). Samples
were analyzed using a flow cytometer FACS Calibur (BD Biosciences)
operated using CellQuest Pro software (version 5.1; BD Biosciences)
and at least 20,000 events were collected per sample. Data were
analyzed using FlowJo software version 7.6 (Tree Star Inc.,
Ashland, OR, USA). Forward and side scatter profiles were obtained
from the same samples.
Induction and differentiation of
BMSCs
Previous studies by our group indicated that 5 ng/ml
TGF-β1 or 250 µg/l Sal-B may be a suitable concentration for
inducing BMSCs to differentiate into CMs (11,12).
Therefore, BMSCs at the second passage were co-incubated with
TGF-β1 (5 ng/ml) and Sal-B (250 µg/l), alone or in combination, for
72 h. Subsequently, the cells were washed three times with PBS and
the medium was replaced with complete medium without any induction
agent. BMSCs cultured without any inductive substance were used as
a control group. The medium was changed every 3 days for 4 weeks
and the cells were then prepared for the subsequent
experiments.
Immunofluorescence staining of
specific proteins of CMs
To evaluate cardiomyogenic differentiation of BMSCs
in each group, immunofluorescence staining of CM-specific proteins,
α-sarcomeric actin and cardiac troponin I (cTnI), was performed.
The cells were transferred to sterile glass cover slips coated with
0.01% poly-L-lysine (cat. no. P4707; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). After 4 days, they were fixed with 4%
formaldehyde for 15 min. Following blocking with 2% bovine serum
albumin (100 µg/ml; cat. no. A2058; Sigma-Aldrich) at room
temperature for 1 h, the cells were incubated with monoclonal
rabbit anti-α-sarcomeric actin primary antibody (1:50; cat. no.
ab-28052; Abcam, Cambridge, UK) and polyclonal goat anti-cTnI
primary antibody (1:50; cat. no. ab47003; Abcam) at 4°C for 24 h.
The cells were then stained with rhodamine-conjugated anti-rabbit
secondary antibody (1:100; cat. no. BA1001; Wuhan Boster Biological
Technology, Ltd., Wuhan, China) and fluorescein
isothiocyanate-conjugated anti-goat secondary antibody (1:100; cat.
no. sc-2348; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at
37°C for 60 min and washed three times with PBS. Negative controls
were also employed to offset the disturbance of the primary or
secondary antibody. Cells were observed and images were captured by
fluorescence microscopy (TCS-ST2; Leica Microsystems, Wetzlar,
Germany).
Transmission electron microscopy
(TEM)
After 4 weeks of differentiation, the cells were
harvested and fixed with 3% glutaraldehyde and 1% osmium tetroxide,
followed by embedding in epoxy resin. Ultra-thin sections were cut
horizontally and double-stained with uranyl acetate and lead
citrate. The cellular ultrastructure was observed using a
JEM-2000EX transmission electron microscope (Jeol Ltd, Tokyo,
Japan).
Analysis of cardiac
differentiation-specific gene expression by RT-qPCR
The transcription factors including GATA binding
protein 4 (GATA-4) and homeobox protein Nkx2.5 in each group were
assessed by RT-qPCR on day 7. Total RNA was extracted using an RNA
fast 200 kit (Fastagen, China) according to the manufacturer's
protocol. RNA was then reverse-transcribed into complementary
(c)DNA using an M-MLV RTase cDNA Synthesis kit (Invitrogen; Thermo
Fisher Scientific, Inc.). Changes in mRNA expression levels were
normalized to GAPDH levels. The cycle threshold (Cq) of each gene
was analyzed. The fold-change between the treatment groups and the
control group for target genes was calculated using the
2−∆∆Cq method (13).
Primers used for qPCR are listed in Table I.
 | Table I.Primers used for polymerase chain
reaction. |
Table I.
Primers used for polymerase chain
reaction.
| Gene | Primer sequence | Product length
(bp) |
|---|
| GATA-4 | Forward,
5′-GGCTATGTCCACCCCGCTCTG-3′ | 162 |
|
| Reverse,
5′-TGGCAGTTGGCACAGGAGAGG-3′ |
|
| Nkx2.5 | Forward,
5′-CCCCTGGATTTTGCATTCAC-3′ | 75 |
|
| Reverse,
5′-CGTGCGCAAGAACAAACG-3′ |
|
| GAPDH | Forward,
5′-GAAGGTGAAGGTCGGAGTC-3′ | 135 |
|
| Reverse,
5′-GAAGATGGTGATGGGATTTC-3′ |
|
Statistical analysis
Values are expressed as the mean ± standard
deviation. One-way analysis of variance was used to analyze the
differences in mRNA expression of the transcription factors
associated with cardiomyogenic differentiation of BMSCs. Other
comparisons were performed using the chi-square test. Statistical
values were calculated using SPSS software 17.0 (SPSS, Inc.,
Chicago, IL, USA). Differences were considered statistically
significant at P<0.05 with a 95% confidence interval.
Results
Morphological alterations of
BMSCs
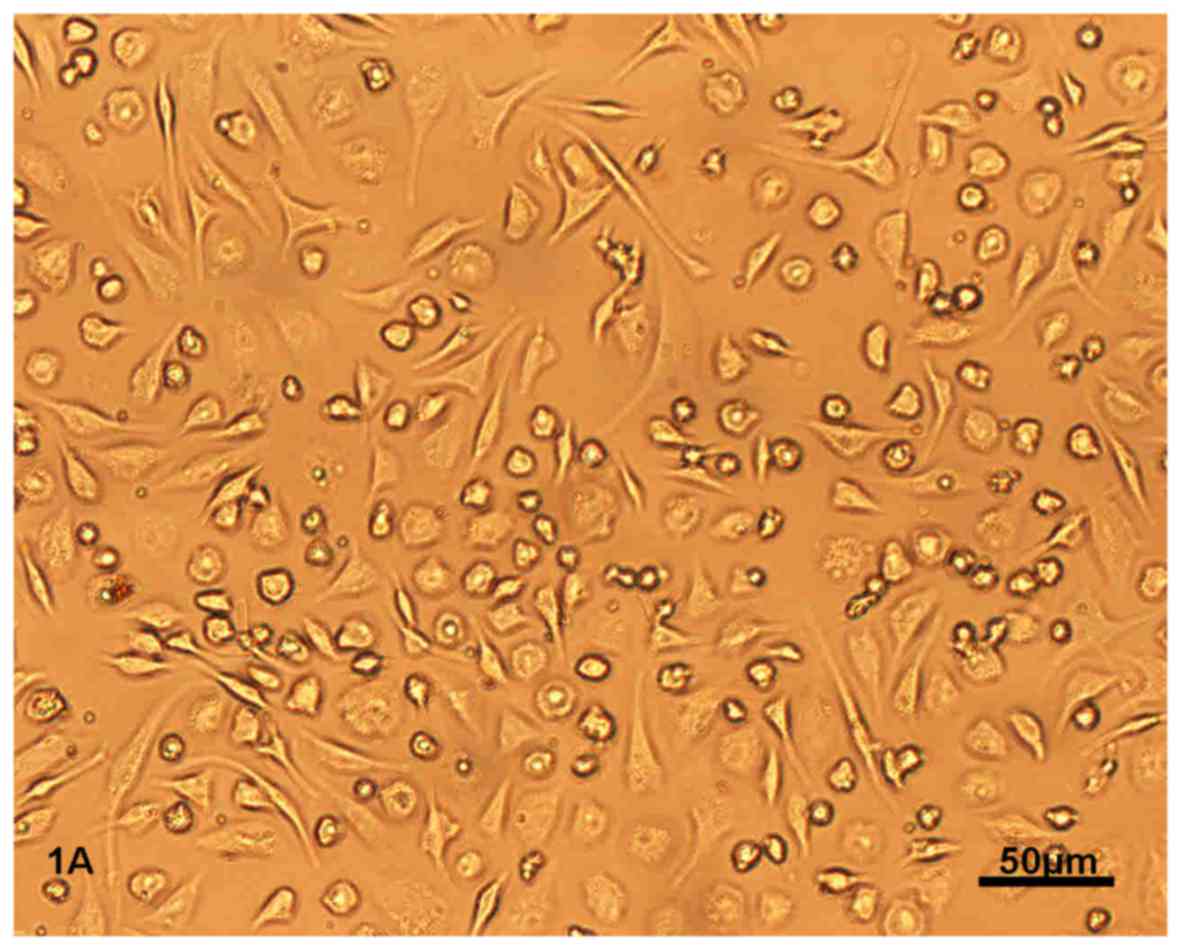
After 12 h of primary culture, BMSCs began to adhere
to the culture bottle. After 3 days, BMSCs appeared as circular or
short spindle-shaped cells with one nucleus. These cells began to
proliferate on day 7 and gradually grew to form small colonies
(Fig. 1A). After being subcultured,
the cells were either polygonal or long and spindle-shaped.
After the BMSCs at second passage were induced by
TGF-β1 and Sal-B, alone or in combination, for 72 h, the
morphological differentiation from BMSCs to CLCs was initiated. The
differentiated cells were spindle-shaped or branched, with one or
two round nuclei located in the center. On day 28, BMSCs in the
combined group had a fusiform shape, orientating with one accord
and were connected with adjoining cells forming myotube-like
structures (Fig. 1B, indicated by
black arrows). The morphology and architecture/myotubes of BMSCs in
the other treatment groups was similar to that in the combined
group, but the amount of cells was relatively low.
Flow cytometric analysis revealed that CD29 and CD90
(fiber-connecting receptors) were present, while CD45
(hematopoietic stem cell marker) was not present on the surface of
the fourth generation of BMSCs in the control group, which was
indicative of mesenchymal stem cells (Fig. 1C).
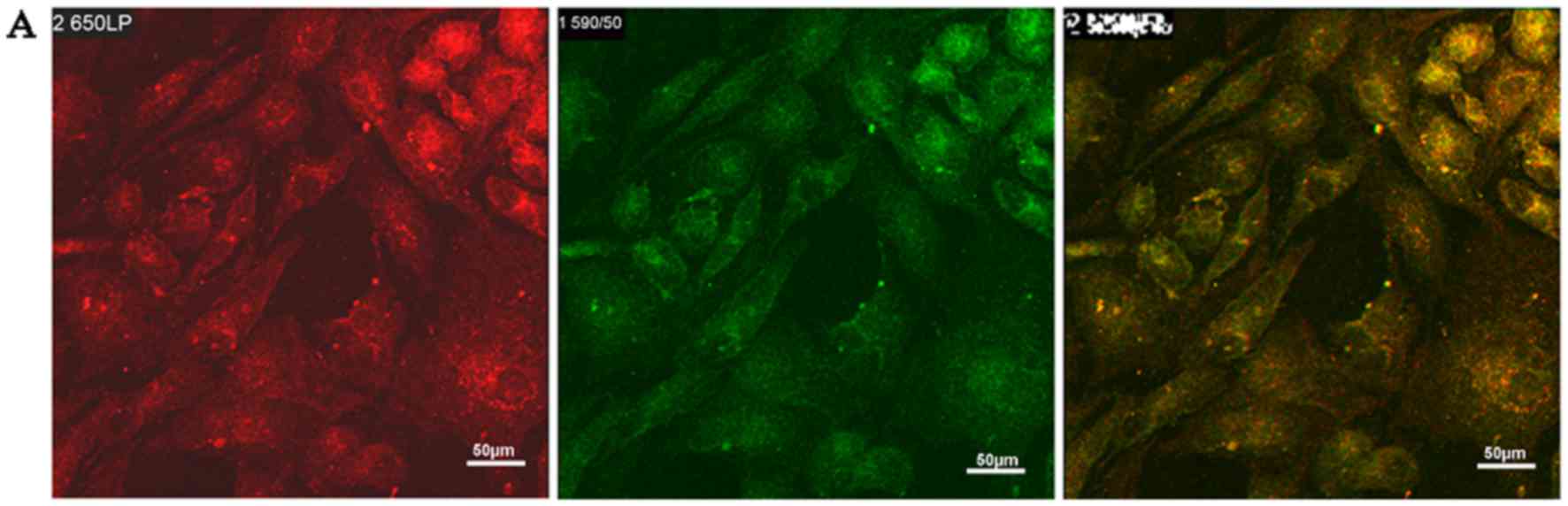
CM-specific protein expression during
BMSC differentiation
At week 4, most of the cells in the treatment groups
expressed α-sarcomeric actin (red) and cTnI protein (green)
(Fig. 2A). Quantitative analysis of
the fluorescence intensity further revealed that in the combined
group, the expression of α-sarcomeric actin and cTnI was
significantly higher than that in the control group (P<0.05;
Fig. 2B).
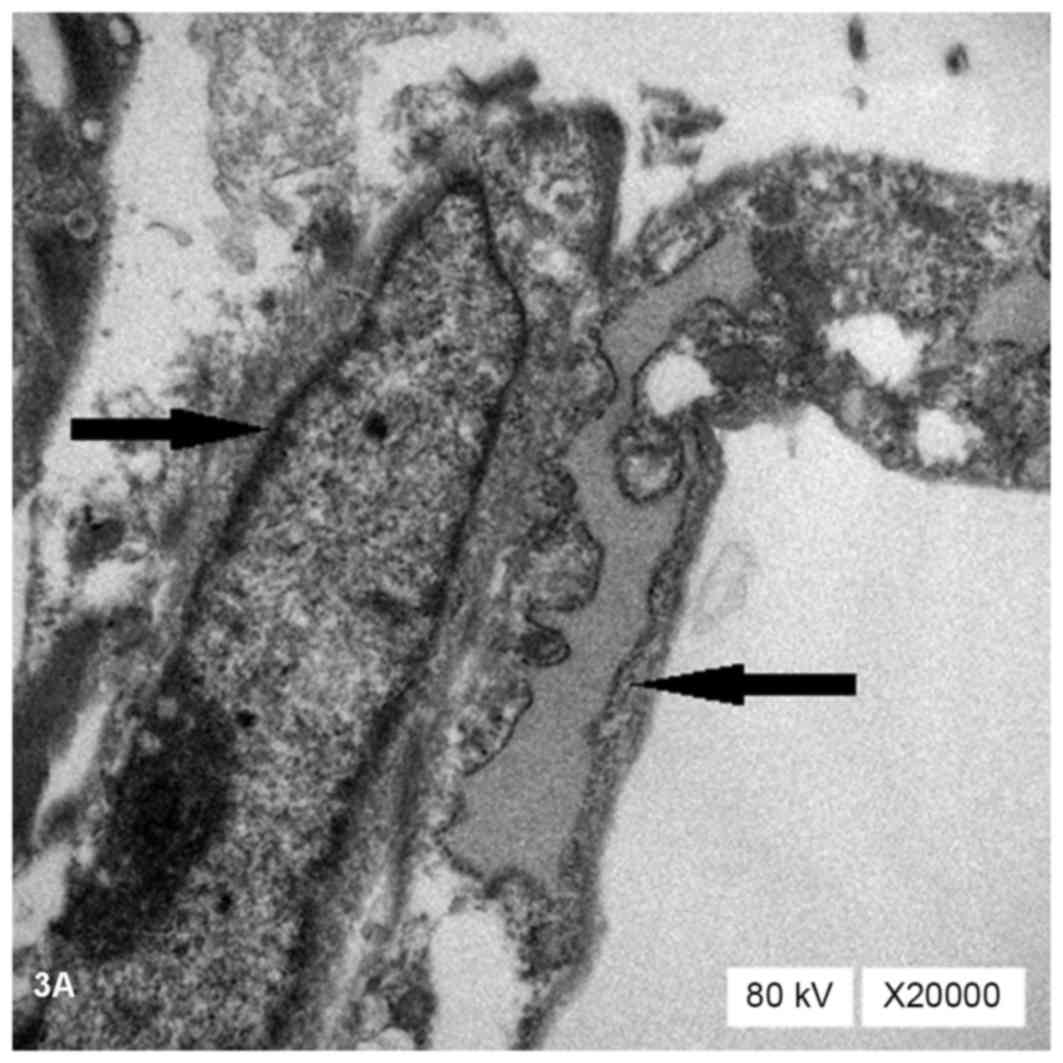
Ultrastructural characterization of
the differentiated cells
TEM observation revealed that the cells in the
treatment groups contained abundant organelles with oval nuclei
located in the center of the cells (→). These organelles contained
a large number of rough endoplasmic reticulum (←), mitochondria,
glycogen and ribosomes (Fig. 3A).
Myofilament was found to be parallel in the cytoplasm (←; Fig. 3B), suggesting characteristic
myofilament installation during differentiation. Ultrastructural
observation results in BMSCs of the combined group were more
typical than in those of the other treatment groups. By contrast,
no myofilaments were detected in the control group.
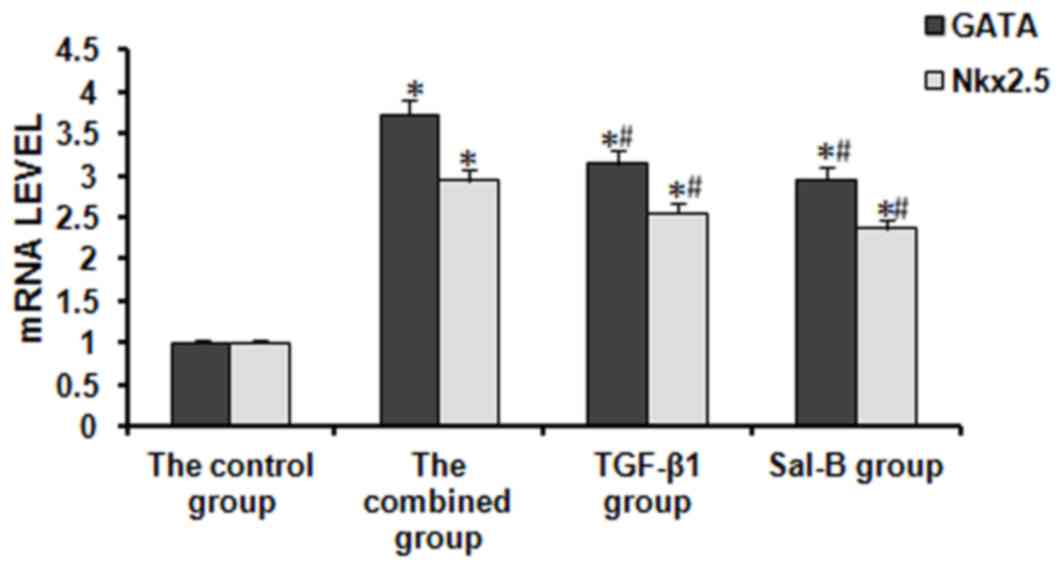
mRNA expression of transcription
factors during cardiac differentiation of BMSCs
The mRNA expression of GATA-4 and Nkx2.5 was
determined by RT-qPCR. A previous study showed that the expression
of GATA4 and NKx2.5 started to increase at the beginning of cardiac
differentiation, reached a plateau by day 7 of differentiation and
remained at high levels for up to 8 weeks (14). Therefore, the gene expression levels
were determined at day 7 of differentiation in the present study.
The expression of GATA4 and NKx2.5 mRNA in the treatment groups was
stable and significantly higher than that in the control group. For
GATA4 mRNA, the expression in the TGF-β1 group, the Sal-B group and
the combined group was 3.14-, 2.95- and 3.72-fold increased
compared with that in the control group, respectively. For NKx2.5
mRNA, it was 2.55-, 2.36- and 2.93-fold increased compared with
that in the control group, respectively (Fig. 4).
Discussion
The present study tested the hypotheses that i)
TGF-β1 and Sal-B, alone or in combination, enhance the
differentiation of BMSCs towards the cardiomyogenic phenotype and
that ii) TGF-β1 combined with Sal-B may achieve better effects than
each factor alone. The results indicated that the combination of
TGF-β1 and Sal-B effectively promotes cardiomyogenic
differentiation of BMSCs in vitro and they may represent a
therapeutic strategy for the treatment of ischemic heart
disease.
MSCs are non-specialized cells with the ability of
self-renewal and pluripotent differentiation potential. They are
easy to isolate from bone marrow, adapt to ex vivo expansion
and differentiate into various cell lineages in vitro and
in vivo without any ethical concerns or immunological
rejection (15). In vivo and
in vitro studies showed that the source of stem cells is
crucial for successful implantation (16). MSCs harvested from young rodents
demonstrated significantly increased cellular proliferation,
greater resistance to hypoxic conditions and improved
differentiation compared with MSCs obtained from older rodents.
Thus, in the present study, 3-week-old SD rats were selected in
order to achieve optimal cellular proliferation and
differentiation.
It has been demonstrated that MSCs are able to
differentiate into CMs in vivo and in vitro (17,18).
5-azacytidine is a classic inducer for MSCs to differentiate toward
CMs; however, it did not induce differentiation of BMSCs in the
expected cardiomyogenic manner (19). The percentage of MSCs that
differentiate toward CMs upon stimulation with 5-azacytidine is no
more than 30% (20,21). Furthermore, the high concentration of
5-azacytidine required for cardiomyogenic differentiation (10 mM)
produces toxicity and side effects (22). Therefore, it is required to identify
novel inducers to safely increase the differentiation rate of
MSCs.
TGF-β1 has an important role in AMI. A previous
study indicated an association between TGF-β1 polymorphisms and
risk of AMI in Iranian patients, suggesting that genetic
polymorphisms in TGF-β1 may be helpful for determining
susceptibility to AMI (23).
Furthermore, TGF-β1 is one of the most commonly used biological
agents for induction of cardiomyogenic differentiation of MSCs
(24). The cardioprotective effects
of MSCs include reduction of myocyte apoptosis and
TGF-β1-conditioned human MSC-laden patches were reported to reduce
myocyte apoptosis in the setting of AMI (25). A previous study by our group showed
that TGF-β1 may induce MSCs to acquire the cardiogenic phenotype
and that 5 ng/ml may be a suitable induction concentration
(11), which is in good agreement
with the experimental results by Li et al (26). Sal-B is a water-soluble phenolic acid
extracted from Salvia miltiorrhiza. It suppresses the
apoptotic effect of treatment with high glucose combined with
hypoxia in embryonic stem cell-derived CMs. In particular, Sal-B
inhibited the expression of hypoxia-inducible factor 1α and B-cell
lymphoma 2/adenovirus E1B 19 kDa protein-interacting protein 3 as
well as the levels of cleaved caspase 3, thereby suppressing
apoptosis (27). Lin et al
(28) detected autophagy and
apoptosis of myocardial cells in hearts of AMI rats by
immunofluorescence and terminal deoxynucleotidyl transferase dUTP
nick end labeling. They also examined the protein expression of
proteins associated with apoptosis, autophagy and angiogenesis by
western blot analysis, revealing that Sal B had a cardioprotective
effect on AMI and that Sal B may be a promising candidate for AMI
treatment. Since Sal B is a cardioprotective medicine, it is likely
to be safer as an inducer than 5-azacytidine. Based on this, the
present study established a novel system for cardiomyogenic
differentiation of BMSCs using a combination of TGF-β1 and
Sal-B.
To provide an unbiased comparison of the
differentiation potency of BMSCs towards the cardiomyogenic
phenotype using TGF-β1 and Sal-B, alone or in combination, in
vitro observations were contrasted by electron microscopy,
immunofluorescence and RT-qPCR. The myocardial markers α-sarcomeric
actin, cTnI, Nkx2.5 and GATA-4 were detected in the experiments.
cTnI has been demonstrated to be presented only in cardiac muscle
and is a proven diagnostic and risk stratification biomarker in
patients with acute coronary syndromes. Nkx2.5 and GATA-4 are
required for specification of the cardiac muscle phenotype
(29). Nkx2.5 regulates the
transcription of several cardiac genes, including α-sarcomeric
actin and GATA-4 (30). The present
study found that BMSCs treated with TGF-β1 and Sal-B, alone or in
combination, showed an increased expression of cardiac-specific
markers, including cTnI, GATA-4 and Nkx2.5, compared with that in
the control group. Furthermore, the expression of these
cardiac-specific markers in BMSCs of the combined group was
significantly higher than that in the other groups. The synergetic
effect between TGF-β1 and Sal-B may be achieved by reducing myocyte
apoptosis.
In conclusion, the results of the present study
indicated that the combination of TGF-β1 and Sal-B effectively
promotes cardiomyogenic differentiation of BMSCs in vitro
and that they may represent a therapeutic strategy for the
treatment of ischemic heart disease.
Acknowledgements
The present study was supported by grants from the
Natural Science Foundation of Hebei Province (no. H2014405005),
scientific research projects of the Chinese Medicine Administrative
Bureau of Hebei Province (no. 2014202), the Natural Science
Foundation of Hebei Province (no. H2015405017) and scientific
research projects of the Education Department of Hebei Province
(no. ZH2012001).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cutts J, Nikkhah M and Brafman DA:
Biomaterial approaches for stem cell-based myocardial tissue
engineering. Biomark Insights. 10 Suppl 1:S77–S90. 2015.
|
|
2
|
Jiang Q, Song P, Wang E, Li J, Hu S and
Zhang H: Remote ischemic postconditioning enhances cell retention
in the myocardium after intravenous administration of bone marrow
mesenchymal stromal cells. J Mol Cell Cardiol. 56:1–7. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen CH, Sereti KI, Wu BM and Ardehali R:
Translational aspects of cardiac cell therapy. J Cell Mol Med.
19:1757–1772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Williams AR and Hare JM: Mesenchymal stem
cells: Biology, patho-physiology, translational findings, and
therapeutic implications for cardiac disease. Circ Res.
109:923–940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stoltz JF, de Isla N, Li YP, Bensoussan D,
Zhang L, Huselstein C, Chen Y, Decot V, Magdalou J, Li N, et al:
Stem cells and regenerative medicine: Myth or reality of the 21th
century. Stem Cells Int. 2015:7347312015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matar AA and Chong JJ: Stem cell therapy
for cardiac dysfunction. Springerplus. 3:4402014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen H, Wang Y, Zhang Z, Yang J, Hu S and
Shen Z: Mesenchymal stem cells for cardiac regenerative therapy:
Optimization of cell differentiation strategy. Stem Cells Int.
2015:5247562015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galli D, Vitale M and Vaccarezza M: Bone
marrow-derived mesenchymal cell differentiation toward myogenic
lineages: Facts and perspectives. Biomed Res Int. 2014:7626952014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao Q, Guo M, Jiang X, Hu X, Wang Y and
Fan Y: A cocktail method for promoting cardiomyocyte
differentiation from bone marrow-derived mesenchymal stem cells.
Stem Cells Int. 2014:1620242014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kennard S, Liu H and Lilly B: Transforming
growth factor-beta (TGF-1) down-regulates Notch3 in fibroblasts to
promote smooth muscle gene expression. J Biol Chem. 283:1324–1333.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lv Y, Wang HP, Liu B, Wu ZG, Huo YL and
Gao CW: TGF-β1 induced bone marrow mesenchymal stem cells
differentiate into cardiomyocyte-like cells. Acta Anatomica Sinica.
44:49–54. 2013.
|
|
12
|
Lv Y, Wang HP and Wang HY, Huo YL and Wang
HY: Basic fibroblast growth factor and salvianolic acid B induce
bone marrowmesenchymal stem cells differentiating into
cardiomyocyte-like cells in vitro. Chin J Anatomy. 36:1026–1029.
2013.
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wen Z, Jie T, Deqin J, Lei Z, Jing Z and
Yuan C: Temporal expression of regulative genes in the process of
bone marrow mesenchymal stem cells differentiating into
cardiomyocytes in vitro. Zhonghua xin xue Guan Bing za zhi.
32:1004–1008. 2004.
|
|
15
|
Kobolak J, Dinnyes A, Memic A,
Khademhosseini A and Mobasheri A: Mesenchymal stem cells:
Identification, phenotypic characterization, biological properties
and potential for regenerative medicine through biomaterial
micro-engineering of their niche. Methods. 99:62–68. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nayan M, Paul A, Chen G, Chiu RC, Prakash
S and Shum-Tim D: Superior therapeutic potential of young bone
marrow mesenchymal stem cells by direct intramyocardial delivery in
aged recipients with acute myocardial infarction: In vitro and in
vivo investigation. J Tissue Eng. 2011:7412132011.PubMed/NCBI
|
|
17
|
Nartprayut K, U-Pratya Y, Kheolamai P,
Manochantr S, Chayosumrit M, Issaragrisil S and Supokawej A:
Cardiomyocyte differentiation of perinatally-derived mesenchymal
stem cells. Mol Med Rep. 7:1465–1469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li M and Ikehara S: Bone-marrow-derived
mesenchymal stem cells for organ repair. Stem Cells Int.
2013:1326422013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y, Song J, Liu W, Wan Y, Chen X and Hu
C: Growth and differentiation of rat bone marrow stromal cells:
Does 5-azacytidine trigger their cardiomyogenic differentiation?
Cardiovasc Res. 58:460–468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bae S, Shim SH, Park CW, Son HK, Lee HJ,
Son JY, Jeon C and Kim H: Combined omics analysis identifies
transmembrane 4 L6 family member 1 as a surface protein marker
specific to human mesenchymal stem cells. Stem Cells Dev.
20:197–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tomita S, Li RK, Weisel R, Mickle DA, Kim
EJ, Sakai T and Jia ZQ: Autologous transplantation of bone marrow
cells improves damaged heart function. Circulation. 100 19
Suppl:II247–II256. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Breccia M, Loglisci G, Salaroli A, Serrao
A, Petrucci L, Mancini M and Alimena G: 5-azacitidine efficacy and
safety in patients aged >65 years with myelodysplastic syndromes
outside clinical trials. Leuk Lymphoma. 53:1558–1560. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Najar RA, Ghaderian SM and Panah AS:
Association of transforming growth factor-β1 gene polymorphisms
with genetic susceptibility to acute myocardial infarction. Am J
Med Sci. 342:365–370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu J, Niu J, Li X, Wang X, Guo Z and Zhang
F: TGF-β1 induces senescence of bone marrow mesenchymal stem cells
via increase of mitochondrial ROS production. BMC Dev Biol.
14:212014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Godier-Furnémont AF, Tekabe Y, Kollaros M,
Eng G, Morales A, Vunjak-Novakovic G and Johnson LL: Noninvasive
imaging of myocyte apoptosis following application of a stem
cell-engineered delivery platform to acutely infarcted myocardium.
J Nucl Med. 54:977–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li TS, Hayashi M, Ito H, Furutani A,
Murata T, Matsuzaki M and Hamano K: Regeneration of infarcted
myocardium by intramyocardial implantation of ex vivo transforming
growth factor-beta-preprogrammed bone marrow stem cells.
Circulation. 111:2438–2445. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang CY, Chen SY, Fu RH, Huang YC, Chen
SY, Shyu WC, Lin SZ and Liu SP: Differentiation of embryonic stem
cells into cardiomyocytes used to investigate the cardioprotective
effect of salvianolic acid B through BNIP3 involved pathway. Cell
Transplant. 24:561–571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin C, Liu Z, Lu Y, Yao Y, Zhang Y, Ma Z,
Kuai M, Sun X, Sun S, Jing Y, et al: Cardioprotective effect of
Salvianolic acid B on acute myocardial infarction by promoting
autophagy and neovascularization and inhibiting apoptosis. J Pharm
Pharmacol. 68:941–952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Faustino RS, Behfar A, Perez-Terzic C and
Terzic A: Genomic chart guiding embryonic stem cell cardiopoiesis.
Genome Biol. 9:R62008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Riazi AM, Takeuchi JK, Hornberger LK,
Zaidi SH, Amini F, Coles J, Bruneau BG and Van Arsdell GS: NKX2-5
regulates the expression of beta-catenin and GATA4 in ventricular
myocytes. PLoS One. 4:e56982009. View Article : Google Scholar : PubMed/NCBI
|