Introduction
Osteoporosis (OP) is a systemic disease
characterized by the reduction of bone matrix and mineral,
resulting in the destruction and rupture of trabecular bone and the
increase of fragility of bone, thus reducing biomechanical
properties (1). Along with increase
in age, the absorption of calcium by the body decreases with the
reduction of osteoblast production, which leads to the gradual
increase of bone loss, finally resulting in the occurrence of OP
(2). The prevention and treatment of
OP include the adequate intake of vitamin D and calcium, proper
exercise and other lifestyle interventions (3). Melatonin is a hormone mainly secreted
by the pineal gland, which has antioxidant, scavenging free
radicals, ameliorating sleep, antitumor, regulating immune response
of the body and other biological functions (4,5). Studies
have indicated that (6,7) melatonin can mediate the secretion of
thyroxine and calcitonin in the body, thereby regulating calcium
metabolism in vivo. In recent years, melatonin has been
applied by some scholars in the prevention and treatment of OP
(8). However, there is no report on
melatonin combined with calcium carbonate to prevent and treat OP.
Hence, by adopting melatonin combined with calcium carbonate in
senile female rats, this study aimed to observe the effects of the
combination on bone density, bone mineral and oxidative stress, so
as to provide the basis for clinical prevention and treatment of
OP.
Materials and methods
Materials
Experimental animals
Ten female specific pathogen-free (SPF)
Sprague-Dawley (SD) rats aged 3 months, weighing 180–250 g, were
selected. Forty female SPF SD rats aged 15 months were enrolled,
which displayed osteoporosis via comparison with rats aged 3 months
through determination by dual-energy X-ray absorptiometry. Fifty
experimental animals were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd. (license no. SCXK 2012–0002; Shanghai, China). The
rats were kept in cage with controlled temperature and light cycles
(24°C and 12/12 light cycles) and free access to food and water.
The humidity was 40%. The study was approved by the Ethics
Committee of Dezhou People's Hospital (Dezhou, China).
Experimental reagents
Calcium carbonate, melatonin (Sigma-Aldrich, San
Francisco, CA, USA), and kits of superoxide dismutase (SOD),
malondialdehyde (MDA) and glutathione peroxidase (GSH-Px) (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) were
prepared.
Experimental instruments
Dual-energy X-ray absorptiometry (Hologic,
Marlborough, MA, USA); fully automatic biochemistry analyzer
(Mindray Biomedical Electronics Co., Ltd., Shenzhen, China);
continuous-wavelength multifunctional microplate reader (Tecan,
Salzburg, Austria).
Methods
Grouping and medication of
experimental animals
Forty senile female SPF rats were adaptively fed for
one week, followed by being randomly divided into four groups with
10 animals in each group, namely model group (group OP), melatonin
group (group M), calcium carbonate group (group Ca) and melatonin
combined with calcium carbonate group (group M+Ca), while 10 rats
aged 3 months were set as the control group (group NC). All rats
were reared in an SPF system, fed with ordinary feedstuff, and ate
freely during the experiment. Rats in group M were treated with 40
mg/kg melatonin via intragastric administration once a day; rats in
group Ca received 20 mg/kg calcium carbonate via intragastric
administration once a day; rats in group M+Ca were treated with 40
mg/kg melatonin combined with 20 mg/kg calcium carbonate via
intragastric administration once a day; rats in groups NC and OP
were given intragastric administration of normal saline every day
for consecutive 12 weeks.
Measurement of bone density
After the end of the last intragastric
administration, rats were treated with fasting for solids not
liquids for 8 h. The rats were weighed, followed by intraperitoneal
injection of 50 mg/kg 2% pentobarbital sodium for anesthesia. The
four limbs of rats were fixed, which were placed at supine position
under the probe of dual energy X-ray absorptiometry; it should keep
a vertical state of vertebra and horizontality of bilateral femur.
The bone density of lumbar vertebras, L4-L6, and left and right
femurs was measured by dual-energy X-ray absorptiometry.
Detection of serum indexes
After the measurement of bone density, the blood was
extracted from abdominal aorta and centrifuged at 2,030 × g at 4°C
for 10 min. The serum was separated and cryopreserved at −80°C.
Serum calcium and phosphorus were detected by fully automatic
biochemical analyzer. Serum SOD, MDA and GSH-Px were treated by
sequential sample loading according to the instructions for the
detection. The content of SOD in each group was measured at 550 nm
by continuous-wavelength multifunctional enzyme analyzer. The
content of MDA in each group was determined at 532 nm, and the
content of GSH-Px in each group was detected at 412 nm.
Determination of bone mineral
level
The left femur of rats was isolated, and the
attached soft tissue was removed. Femur was roasted in the oven at
110°C for 48 h to the constant weight. The bone dry weight was
weighed by an analytical balance, and the ratio of bone dry weight
to body mass was calculated. Then, the femur was incinerated in a
chamber-type electric resistance furnace at 700°C for 6 h. The ash
weight was weighted by an analytical balance, and the ratios of
bone ash weight to body mass and bone ash weight to bone dry weight
(%) were calculated.
Statistical analysis
Experimental results were expressed as mean ± SD.
The data were statistically analyzed by SPSS 20.0 software (IBM
Corp., Armonk, NY, USA). The independent-samples t-test was used
for comparisons between two groups, and one-way analysis of
variance (ANOVA) was utilized for comparisons among multiple groups
and Least Significant Difference was used for post hoc. P<0.05
was considered to indicate a statistically significant
difference.
Results
Changes of bone density of rats in
each group
Compared with that in group NC, bone density of
lumbar vertebra and bilateral femur was distinctly reduced in rats
of groups OP, M and Ca (P<0.05 or P<0.01); compared with that
in group OP, bone density of lumbar vertebra and bilateral femur
was remarkably increased in rats of groups M, Ca and M+Ca
(P<0.05); compared with that in groups M and Ca, bone density of
lumbar vertebra and bilateral femur was obviously elevated in rats
of group M+Ca (P<0.05); there was no significant difference in
comparison of bone density between groups M+Ca and NC (P>0.05).
The results indicated that melatonin and calcium carbonate could
significantly ameliorate bone density in rats with osteoporosis,
and the combination displayed more remarkable effects (Table I).
 | Table I.Comparisons of bone density of lumbar
vertebra and bilateral femur in rats among groups. |
Table I.
Comparisons of bone density of lumbar
vertebra and bilateral femur in rats among groups.
| Groups | No. | Lumbar vertebra | Left femur | Right femur |
|---|
| NC | 10 | 0.252±0.014 | 0.248±0.017 | 0.249±0.017 |
| OP | 10 |
0.177±0.021b |
0.172±0.016b |
0.174±0.018b |
| M | 10 |
0.210±0.018a,c |
0.212±0.021a,c |
0.215±0.015a,c |
| Ca | 10 |
0.221±0.018a,c |
0.219±0.016a,c |
0.220±0.022a,c |
| M+Ca | 10 |
0.248±0.020def |
0.243±0.018def |
0.245±0.019def |
Bone mineral metabolism in rats of
each group
Compared with those in group NC, bone dry weight,
the ratio of bone dry weight to body mass, bone ash weight and the
ratios of bone ash weight to body mass and bone ash weight to bone
dry weight of rats in groups OP, M and Ca were significantly
reduced (P<0.05 or P<0.01); compared with those in group OP,
these indexes of rats in groups M, Ca and M+Ca were obviously
increased (P<0.05 or P<0.01); compared with those in groups M
and Ca, these indexes of rats in group M+Ca were remarkably
elevated (P<0.05); the comparisons in these indexes between
groups M+Ca and NC had no distinct differences (P>0.05). The
results revealed that melatonin and calcium carbonate could
significantly reduce bone mineral loss in rats with osteoporosis,
and the combination displayed more remarkable effects (Tables II and III).
 | Table II.Comparisons of bone dry weight in rats
among groups. |
Table II.
Comparisons of bone dry weight in rats
among groups.
| Groups | No. | Body mass (g) | Bone dry weight
(mg) | Bone dry weight/body
mass (g/kg) |
|---|
| NC | 10 | 332.35±18.71 | 580.16±23.68 | 1.75±0.09 |
| OP | 10 | 328.91±17.22 |
497.47±25.10b |
1.51±0.06b |
| M | 10 | 330.64±12.16 |
532.04±27.82a,c |
1.61±0.09a,c |
| Ca | 10 | 334.11±14.40 |
538.48±20.98a,c |
1.62±0.10a,c |
| M+Ca | 10 | 331.52±17.84 |
571.60±22.73def |
1.72±0.13cdef |
 | Table III.Comparisons of bone ash weight in
rats among groups. |
Table III.
Comparisons of bone ash weight in
rats among groups.
| Groups | No. | Body mass (g) | Bone ash weight
(mg) | Bone ash
weight/body mass (g/kg) | Bone ash
weight/bone dry weight (%) |
|---|
| NC | 10 | 332.35±18.71 | 382.87±20.09 | 1.15±0.06 | 65.99±1.13 |
| OP | 10 | 328.91±17.22 |
290.04±18.53b |
0.88±0.08b |
58.30±0.94b |
| M | 10 | 330.64±12.16 |
331.61±16.78b,c |
1.00±0.05a,c |
62.33±0.81a,c |
| Ca | 10 | 334.11±14.40 |
335.29±19.24a,c |
1.01±0.05a,c |
62.27±0.90a,c |
| M+Ca | 10 | 331.52±17.84 |
374.83±18.33def |
1.13±0.04def |
65.58±0.77def |
Changes of serum calcium and
phosphorus levels in rats of each group
Compared with that in group NC, serum calcium level
was significantly reduced in rats of groups OP, M and Ca (P<0.05
or P<0.01); compared with that in group OP, serum calcium level
was obviously increased in rats of groups M, Ca and M+Ca
(P<0.05); compared with that in groups M and Ca, serum calcium
level was remarkably elevated in rats of group M+Ca (P<0.05);
the comparison in this level between groups M+Ca and NC had no
distinct difference (P>0.05). There was no evident difference in
comparison of serum phosphorus level in rats among groups
(P>0.05). The results revealed that melatonin and calcium
carbonate could significantly increase serum calcium level in rats
with osteoporosis, and the combination displayed more remarkable
effects (Table IV).
 | Table IV.Comparisons of calcium and phosphorus
levels in rats among groups. |
Table IV.
Comparisons of calcium and phosphorus
levels in rats among groups.
| Groups | No. | Calcium
(mmol/l) | Phosphorus
(mmol/l) |
|---|
| NC | 10 | 2.68±0.15 | 1.55±0.13 |
| OP | 10 |
1.93±0.20b | 1.52±0.17 |
| M | 10 |
2.27±0.18a,c | 1.56±0.21 |
| Ca | 10 |
2.36±0.21a,d | 1.53±0.19 |
| M+Ca | 10 |
2.59±0.17def | 1.55±0.17 |
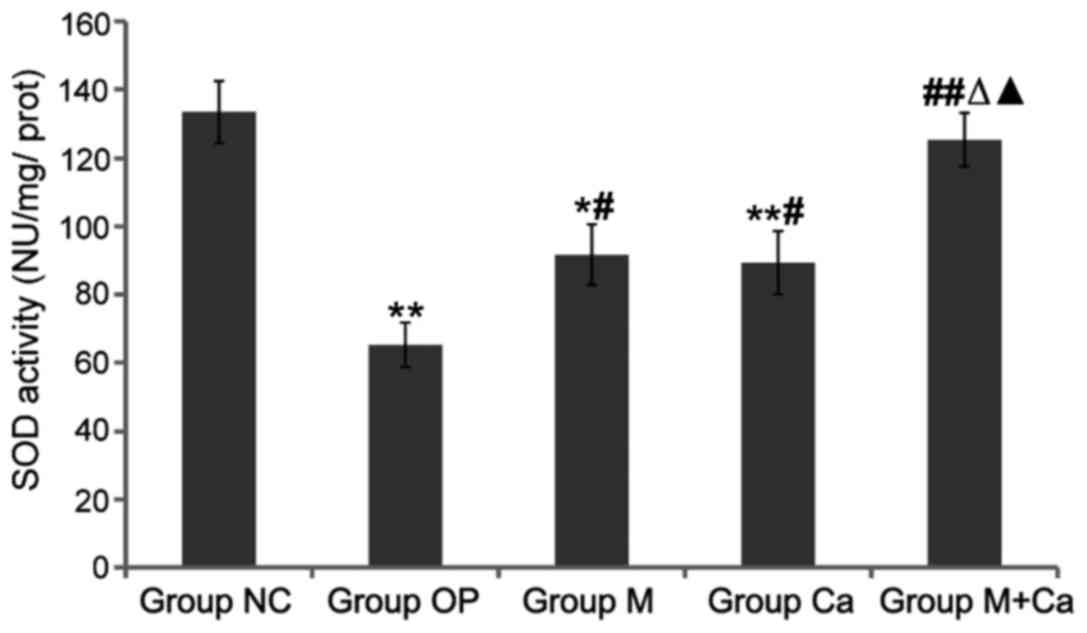
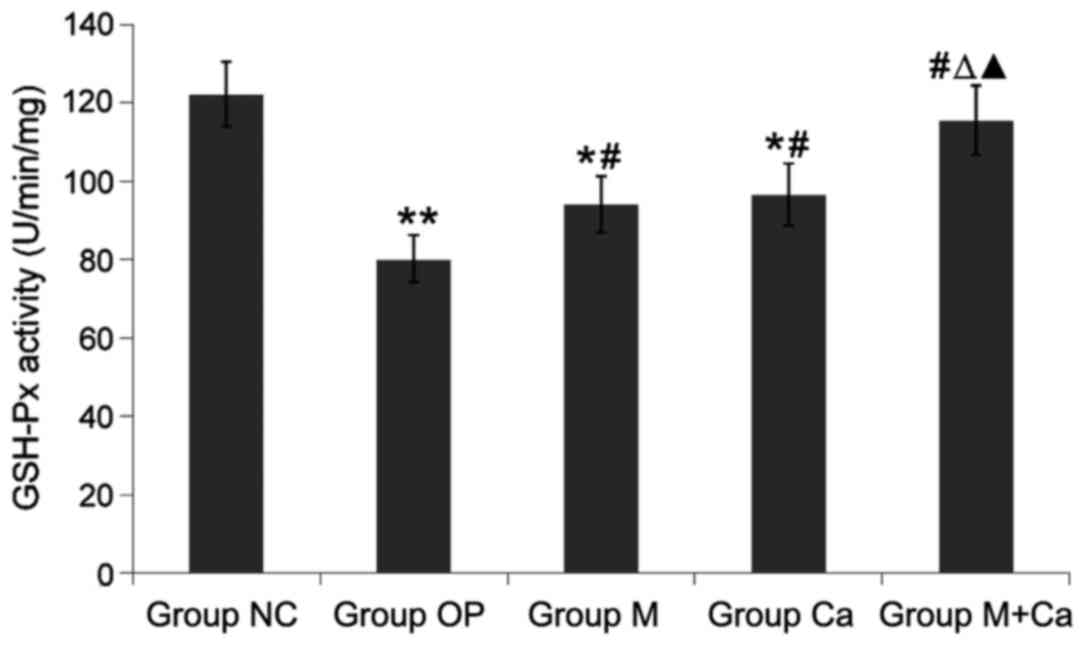
Changes of serum SOD, GSH-Px activity
and MDA content in rats of each group
Compared with those in group NC, serum SOD and
GSH-Px activities in rats of groups OP, M and Ca were significantly
reduced (P<0.05 or P<0.01), and MDA content was obviously
increased (P<0.05 or P<0.01); compared with those in group
OP, serum SOD and GSH-Px activities in rats of groups M, Ca and
M+Ca were obviously increased (P<0.05 or P<0.01), and MDA
content was obviously decreased (P<0.05 or P<0.01); compared
with those in groups M and Ca, serum SOD and GSH-Px activities in
rats of group M+Ca were remarkably elevated (P<0.05), and MDA
content was distinctly reduced (P<0.05); the comparison in these
levels between groups M+Ca and NC had no distinct differences
(P>0.05). The results revealed that melatonin and calcium
carbonate could significantly increase serum SOD and GSH-Px
activities and reduce MDA content, and the combination displayed
more remarkable effects (Figs.
1–3).
Discussion
OP is mainly characterized by degenerative changes
of bone tissue microstructure, osteopenia and increased bone
fragility thus remarkably increasing fracture risk (9). OP is prevalent in elderly people, and
age is a major risk factor of OP (10,11). In
recent years, as China entered the time of the aged, the incidence
of OP has increased year by year, and its fracture and other
complications seriously affect people's lives. The study on OP has
attracted wide attention of scholars in China and elesewhere.
Calcium is one of the important trace elements in minerals of the
body, and the main pathological change of OP is bone mineral loss
(12). Therein, calcium deficiency
is an important cause of OP (2).
Thus, calcium supplementation is the most effective and safe method
in the prevention and treatment of OP (13). Appropriate supplementation of calcium
can enhance bone calcium levels and increase bone density, thereby
significantly ameliorating bone formation (14). Adequate intake of calcium and vitamin
D can prevent bone loss in postmenopausal women, and is able to
maintain bone density of femoral neck and lumbar vertebras
(15), thus reducing the incidence
of fractures (16). The measurement
of serum calcium and phosphorus can indirectly reflect bone
metabolism in the body (17). This
study revealed severely decreased bone density, bone mineral loss
and reduced serum calcium level in elderly OP rats. The supplement
of exogenous calcium carbonate can significantly increase bone
density, elevate serum calcium level and reduce bone mineral loss
in OP rats.
Melatonin is an indole hormone mainly secreted by
pineal gland; the secretion is rhythmic, which is regulated by
photoperiod and suprachiasmatic nucleus (18). The synthesis and secretion of
melatonin is increased in the dark, and the amount of secretion at
night is 5–10 times than that of in the daytime (19). When an organism enters old age, the
circadian rhythm of melatonin gradually becomes gentle and even
disappears (20). Melatonin has a
wide range of biological functions, and its mechanisms are
different (21). In terms of bone
metabolism, melatonin can inhibit the proliferation and
differentiation of osteoclasts, advance the proliferation and
differentiation of osteoblasts and promote the mineralization of
bone matrix through a variety of signaling pathways (22,23).
Cell experiments have shown that melatonin can stimulate the
proliferation of bone cells and osteoblasts in a dose-dependent
manner (24). Moreover, it can
upregulate the expression of runt-related transcription factor 2
(RUNX2) and inhibit the expression of peroxisome
proliferator-activated receptor (PPAR-γ), thus promoting the
differentiation of bone marrow mesenchymal stem cells into
osteoblasts (25). Animal
experiments revealed that melatonin can significantly increase bone
density and trabecular bone volume in male mice, but distinctly
reduce the size and number of osteoclasts (26). This study indicated that supplement
of exogenous melatonin can significantly increase the bone density
in OP rats, elevate serum calcium level and reduce bone mineral
loss, which has a synergistic effect with calcium carbonate, and
the combination displayed more remarkable effects.
The bone toxicity caused by reactive oxygen species
plays an important role in the occurrence of OP (27). Excessive bone absorption in the
organism increases the production of reactive oxygen species, which
leads to the destruction of osteoclasts and osteoblasts, resulting
in the reduction of bone mass. With the increase of age, the level
of free radicals in the body is gradually increased, while smoking,
drinking and other unhealthy lifestyles can induce oxidative stress
and elevate the level of free radicals, thus increasing bone
absorption (28). Animal experiments
revealed that the level of antioxidant stress in the body decreases
with the reduction of melatonin levels. It can be seen that
maintaining normal melatonin levels can reduce the damage of free
radicals (29). This study indicated
that melatonin and calcium carbonate can increase the activities of
SOD and GSH-Px and reduce the production of MDA in OP rats, thereby
exerting the effect of antioxidative stress; they have a
synergistic effect, and the combination displayed more remarkable
effects.
In conclusion, the study reveals severely decreased
bone density, bone mineral loss, reduced serum calcium level and
oxidative stress in OP rats, but the supplement of exogenous
melatonin and calcium carbonate can significantly improve
antioxidative stress ability, increase bone density, elevate serum
calcium level and reduce bone mineral loss, thus preventing and
treating osteoporosis. These two substances have a synergistic
effect, and the combination displays more remarkable effects, which
provides an experimental basis for the clinical prevention and
treatment of osteoporosis. The related molecular biological
mechanisms need to be further investigated.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Raisz LG: Pathogenesis of osteoporosis:
concepts, conflicts, and prospects. J Clin Invest. 115:3318–3325.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reid IR, Ames RW, Evans MC, Gamble GD and
Sharpe SJ: Effect of calcium supplementation on bone loss in
postmenopausal women. N Engl J Med. 328:460–464. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shin S and Joung H: A dairy and fruit
dietary pattern is associated with a reduced likelihood of
osteoporosis in Korean postmenopausal women. Br J Nutr.
110:1926–1933. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zawawi MS, Dharmapatni AA, Cantley MD,
McHugh KP, Haynes DR and Crotti TN: Regulation of ITAM adaptor
molecules and their receptors by inhibition of calcineurin-NFAT
signalling during late stage osteoclast differentiation. Biochem
Biophys Res Commun. 427:404–409. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macchi MM and Bruce JN: Human pineal
physiology and functional significance of melatonin. Front
Neuroendocrinol. 25:177–195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Csaba G and Baráth P: The effect of
pinealectomy on the parafollicular cells of the rat thyroid gland.
Acta Anat (Basel). 88:137–146. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Csaba G and Bókay J: The effect of
melatonin and corpus pineale extract on serum electrolytes in the
rat. Acta Biol Acad Sci Hung. 28:143–144. 1977.PubMed/NCBI
|
|
8
|
Witt-Enderby PA, Radio NM, Doctor JS and
Davis VL: Therapeutic treatments potentially mediated by melatonin
receptors: Potential clinical uses in the prevention of
osteoporosis, cancer and as an adjuvant therapy. J Pineal Res.
41:297–305. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mundy G, Garrett R, Harris S, Chan J, Chen
D, Rossini G, Boyce B, Zhao M and Gutierrez G: Stimulation of bone
formation in vitro and in rodents by statins. Science.
286:1946–1949. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barrios C, Broström LA, Stark A and
Walheim G: Healing complications after internal fixation of
trochanteric hip fractures: The prognostic value of osteoporosis. J
Orthop Trauma. 7:438–442. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim WY, Han CH, Park JI and Kim JY:
Failure of intertrochanteric fracture fixation with a dynamic hip
screw in relation to pre-operative fracture stability and
osteoporosis. Int Orthop. 25:360–362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bidwell JP, Alvarez MB, Hood M Jr and
Childress P: Functional impairment of bone formation in the
pathogenesis of osteoporosis: The bone marrow regenerative
competence. Curr Osteoporos Rep. 11:117–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shuid AN, Mohamad S, Mohamed N, Fadzilah
FM, Mokhtar SA, Abdullah S, Othman F, Suhaimi F, Muhammad N and
Soelaiman IN: Effects of calcium supplements on fracture healing in
a rat osteoporotic model. J Orthop Res. 28:1651–1656. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Orwoll ES, McClung MR, Oviatt SK, Recker
RR and Weigel RM: Histomorphometric effects of calcium or calcium
plus 25-hydroxyvitamin D3 therapy in senile osteoporosis. J Bone
Miner Res. 4:81–88. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cooper L, Clifton-Bligh PB, Nery ML,
Figtree G, Twigg S, Hibbert E and Robinson BG: Vitamin D
supplementation and bone mineral density in early postmenopausal
women. Am J Clin Nutr. 77:1324–1329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chapuy MC, Arlot ME, Delmas PD and Meunier
PJ: Effect of calcium and cholecalciferol treatment for three years
on hip fractures in elderly women. BMJ. 308:1081–1082. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Botolin S, Faugere MC, Malluche H, Orth M,
Meyer R and McCabe LR: Increased bone adiposity and peroxisomal
proliferator-activated receptor-gamma2 expression in type I
diabetic mice. Endocrinology. 146:3622–3631. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zawilska JB, Skene DJ and Arendt J:
Physiology and pharmacology of melatonin in relation to biological
rhythms. Pharmacol Rep. 61:383–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeitzer JM, Duffy JF, Lockley SW, Dijk DJ
and Czeisler CA: Plasma melatonin rhythms in young and older humans
during sleep, sleep deprivation, and wake. Sleep. 30:1437–1443.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Toffol E, Kalleinen N, Haukka J, Vakkuri
O, Partonen T and Polo-Kantola P: Melatonin in perimenopausal and
postmenopausal women: Associations with mood, sleep, climacteric
symptoms, and quality of life. Menopause. 21:493–500. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Huang F and He HW: Melatonin
effects on hard tissues: Bone and tooth. Int J Mol Sci.
14:10063–10074. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Halıcı M, Öner M, Güney A, Canöz Ö, Narin
F and Halıcı C: Melatonin promotes fracture healing in the rat
model. Eklem Hastalik Cerrahisi. 21:172–177. 2010.PubMed/NCBI
|
|
23
|
Satomura K, Tobiume S, Tokuyama R,
Yamasaki Y, Kudoh K, Maeda E and Nagayama M: Melatonin at
pharmacological doses enhances human osteoblastic differentiation
in vitro and promotes mouse cortical bone formation in vivo. J
Pineal Res. 42:231–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakade O, Koyama H, Ariji H, Yajima A and
Kaku T: Melatonin stimulates proliferation and type I collagen
synthesis in human bone cells in vitro. J Pineal Res. 27:106–110.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Su P, Xu C, Chen C, Liang A, Du
K, Peng Y and Huang D: Melatonin inhibits adipogenesis and enhances
osteogenesis of human mesenchymal stem cells by suppressing PPARγ
expression and enhancing Runx2 expression. J Pineal Res.
49:364–372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koyama H, Nakade O, Takada Y, Kaku T and
Lau KH: Melatonin at pharmacologic doses increases bone mass by
suppressing resorption through down-regulation of the
RANKL-mediated osteoclast formation and activation. J Bone Miner
Res. 17:1219–1229. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park BG, Yoo CI, Kim HT, Kwon CH and Kim
YK: Role of mitogen-activated protein kinases in hydrogen
peroxide-induced cell death in osteoblastic cells. Toxicology.
215:115–125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee NK, Choi YG, Baik JY, Han SY, Jeong
DW, Bae YS, Kim N and Lee SY: A crucial role for reactive oxygen
species in RANKL-induced osteoclast differentiation. Blood.
106:852–859. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Galano A, Tan DX and Reiter RJ: Melatonin
as a natural ally against oxidative stress: A physicochemical
examination. J Pineal Res. 51:1–16. 2011. View Article : Google Scholar : PubMed/NCBI
|