Introduction
Chronic renal failure is a systemic urological
condition caused by a variety of chronic diseases and progressive
deterioration leading to kidney damage that represents a global
public health problem (1). In recent
years, chronic renal failure has had a significantly increasing
trend and studies have highlighted the role of type II diabetes in
the initiation and progression of chronic kidney disease (2,3). In
addition, patients with cancers frequently presented with acute
renal failure and membranoproliferative glomerulonephritis after
radiotherapy and chemotherapy (4).
Studies have suggested that chronic systemic inflammation
contributes to the progression of chronic renal failure and is
associated with complications, including heart disease,
cardiovascular disease and metabolic disorders (5,6).
Evidence has indicated that type II diabetes-induced inflammatory
responses promote the initiation and development of chronic renal
failure (7,8). Therefore, exploring efficient methods
to solve chronic renal failure induced by type II diabetes is
urgently required for patients in the clinic.
Inflammation is one of the most common
characteristics of in patients with Type II diabetes (9), and is associated with the dysfunction
of urinary albumin excretion, endothelial function and cellular
metabolism (10). Therefore,
hypertension is also common in patients with type-II diabetes and
its onset is frequently discovered in clinical investigations
(11–13). A previous study has reported that
inhibition of the renin-angiotensin system exerted potent effects
in decreasing blood pressure by reducing vascular inflammation
(14). However, long-term medication
of anti-hypertensive drug leads to decline of renal function and
even causes chronic renal failure in patients with type II diabetes
(15). Chronic renal failure is a
syndrome associated with serious metabolic disorders caused by a
variety of chronic kidney diseases (16). The major conditions associated with
its pathogenesis are glomerulonephritis, interstitial nephritis,
high blood pressure, diabetes and obstructed kidney disease
(17). With the rapid increase of
diabetes patients worldwide, diabetes-associated chronic renal
failure has recently demonstrated an increasing trend in clinical
investigations (18,19).
Resveratrol is a multifunctional compound that has
been reported to provide beneficial outcomes for patients with
type-II diabetes due to the prevention of oxidative stress and
apoptosis (20–22). Resveratrol is a naturally existing
polyphenol, which has provided therapeutic effects in the treatment
of diabetes and gestational diabetes mellitus (GDM) in most of the
available previous studies (23,24). In
addition, resveratrol has been found to be beneficial in the
treatment of human diseases, such as cancer, neurodegenerative
diseases, cerebrovascular conditions, type II diabetes mellitus and
GDM (25,26). Furthermore, high glucose-induced
cardiomyocyte apoptosis and oxidative stress have been proved to be
associated with the adenosine monophosphate-activated protein
kinase (AMPK) signaling pathway (27). However, the effects of resveratrol on
chronic renal failure remained to be fully elucidated. The present
study hypothesized that improvement of chronic renal failure after
resveratrol treatment may be regulated through the AMPK
pathway.
In the present study, the influence of resveratrol
on chronic renal failure induced by type II diabetes was
investigated. The activation of oxidative stress, apoptosis and
inflammatory factors was analyzed in the renal cells from
experimental mice with chronic renal failure induced by type II
diabetes after treatment with resveratrol. The results demonstrated
that resveratrol regulated inflammatory responses and improved
morphological changes and immunocytes in renal tissues through the
AMPK signaling pathway. These findings suggested that resveratrol
targeting the mitochondrial AMPK signaling may be a promising
strategy to improve chronic renal failure induced by type II
diabetes.
Materials and methods
Ethics statement
This pre-clinical study was performed according to
the recommendations in the Guide for the Care and Use of Laboratory
Animals of Fenyang College Shanxi Medical University (Fenyang,
China). All experimental protocols on animals were in accordance
with the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health and approved by the Ethics Committee
of Fenyang College Shanxi Medical University. All surgeries and
euthanasia were performed in a manner of causing minimal
suffering.
Animals and study design
A total of 60 C57BL/KsJ db/+ (db/+) mice with type
II diabetes (age, 6–8 weeks) were purchased from Charles River
Laboratories (Sulzfeld, Germany). All mice were housed in a
temperature-controlled room (25±1°C) with a 12-h light/dark cycle.
The mice received insulin once a day for a 365-day observation. The
concentration of creatinine in the serum was analyzed to identify
those mice in which chronic renal failure was induced by type II
diabetes. The mice with chronic renal failure induced by type II
diabetes were divided into three groups (n=20 in each) and received
resveratrol (10 mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), angiotensin-converting enzyme inhibitor (ACEI)
aldosterone (10 mg/kg, Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) or PBS by intravenous injection, and 20 healthy
C57BL/KsJ+/+ (wild-type) mice (6–8 weeks old; Beijing University,
Beijing, China) were used as controls. The treatments were
continued for 8 weeks at one-day intervals. The renal cells were
isolated from experimental mice for further analysis on day 60.
Glucose and insulin tolerance
tests
Mice with chronic renal failure induced by type II
diabetes were fasted 6 h and injected intraperitoneally with
glucose at a dose of 2 g/kg for the glucose tolerance test. Blood
glucose concentrations were analyzed using an ACCU-CHEK Advantage
glucometer (Roche Diagnostics, Basel, Switzerland). The results of
the glucose tolerance tests were recorded at baseline and after
glucose injection (120 min). For the insulin tolerance tests, mice
with GDM insulin were injected with insulin intraperitoneally at
0.75 U/kg body weight. The blood glucose concentration was measured
at baseline and 30 min after GDM mice received resveratrol,
aldosterone or PBS.
Physical activity analysis by indirect
calorimetry
Indirect calorimetric analysis was used to evaluate
the physical activity of mice with chronic renal failure induced by
type II diabetes by using the Comprehensive Laboratory Animal
Monitoring System (Oxymax/CLAMS; Columbus Instruments Corp.,
Columbus, OH, USA). On day 60 after treatment with resveratrol,
ACEI or PBS, physical activities of mice were monitored every 30
min for 24 h to analyze the therapeutic effects of the treatments
on chronic renal failure induced by type II diabetes. The
respiratory exchange ratio, volume of oxygen (VO2),
VCO2, heat production and physical activity were
measured according to the manufacturers' instructions.
Glucose-6-phosphatase activity
Glucose-6-phosphatase activity of mice with chronic
renal failure induced by type II diabetes was analyzed as
previously described (28). Total
protein in the blood of mice with chronic renal failure induced by
type II diabetes was analyzed by the Bradford method.
Glucose-6-phosphatase activity was normalized by subtracting
nonspecific phosphatase activity determined by
para-nitrophenylphosphate (p-NPPSt, Sigma-Aldrich; Merck KGaA).
Analysis of apoptosis
Renal sections from experimental mice were prepared
for apoptotic analysis. Renal cells were incubated with terminal
deoxynucleotidyl transferase deoxyuridine triphosphate nick end
labeling (TUNEL) stain (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) for 60 h according to the protocol of previous
study (29). The apoptotic renal
cells were analyzed by fluorescence microscopy as described in a
previous study (30).
ELISA
ELISA kits (Huiying, Shanghai, China) were used to
determine interleukin (IL)-6 (HY1738), IL-10 (HY1798), IL-17
(HY2013), IL-1β (HY0028), vascular endothelial growth factor (VEGF,
HY3014) and tumor necrosis factor (TNF)-α (HY62203) levels in the
sera of the mice. The procedures were performed according to the
manufacturer's instructions. The final results were recorded at 450
nm on an ELISA plate reader.
Cell viability assay
Renal cells from experimental mice
(1.0×103) were seeded in 96-well plates in MEM with 10%
FBS in a final volume of 100 µl/well at 37°C. Following 24 h, cell
viability was evaluated using the MTS assay (CellTiter
96® AQueous One Solution Cell Proliferation Assay;
Promega Corporation) according to the manufacturer's instructions.
Cells morphology was observed using a confocal microscope (Carl
Zeiss, Zen 2010) (magnification, ×40). The final absorbance was
recorded using a microplate reader (BMG Labtech Ltd., Aylesbury,
UK) at a wavelength of 450 nm. Cells viability was determined by
the absorbance of cells at 450 nm.
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR)
Total mRNA was isolated from renal cells by using an
RNA Easy Mini Extract kit (Sigma-Aldrich; Merck KGaA). RNA was
reversed transcribed using a PrimeScript RT Master Mix kit (Takara
Bio, Inc., Otsu, Japan). The expression of caspase-3, −8 and −9 as
well as apoptotic protease activating factor 1 (Apaf-1) in renal
cells was determined by using an RT-qPCR kit (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions with β-actin expression as an endogenous control. All
procedures were performed according to the manufacturer's
instructions. All of the primers were from Invitrogen (Thermo
Fisher Scientific, Inc.) and their sequences are listed in Table I. Relative mRNA expression levels
were determined by using the 2−ΔΔCq method (31). The final results were presented as
the fold of β-actin.
 | Table I.Primer sequences of
inflammation-associated cytokines used for polymerase chain
reaction in the present study. |
Table I.
Primer sequences of
inflammation-associated cytokines used for polymerase chain
reaction in the present study.
| Target gene | Forward primer | Reverse primer |
|---|
| Caspase-3 |
5′-GTCCCACT-GTCTGTCTCA-3′ |
5′-GAATGTCATCTCCGCTCTG-3′ |
| Caspase-8 |
5′-AGACATAACCCAACTCCG-3′ |
5′-TCATCAGGCACTCCTTTC-3′ |
| Caspase-9 |
5′-AATCCTGCTTGGGTATCAGG-3′ |
5′-GAGACCCAGTCTCAGGGAAA-3′ |
| Apaf-1 |
5′-ACTTGTCGGCCCTGCGCATC-3′ |
5′-GGGGCGAACGACTAAGCGGG-3′ |
| β-actin |
5′-GTGGGCGCCCAGGCACCA-3′ |
5′-CTCCTTAATGTCACGCACGATTT-3′ |
Western blot analysis
Renal tissues were isolated from mice with chronic
renal failure induced by type II diabetes and homogenized in 1X
radioimmunoprecipitation assay buffer on day 60. Subsequently,
western blotting was performed to analyze the expression of analyte
proteins. Protein concentrations were examined using a BCA protein
assay, and protein samples (40 µg) were loaded and separated using
15% SDS-PAGE. Protein were subsequently blotted on a nitrocellulose
membrane and hybridized using primary antibodies against AMPK
(1:2,000; 9839), phosphorylated AMPK (1:2,000; 4186), and β-actin
(1:500; 3700; Cell Signaling Technologies, Inc., Danvers, MA, USA)
were added for 12 h at 4°C after blocking with 5% skimmed milk for
1 h at 37°C, and membranes were then incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG mAb (1:5,000; PV-6001;
ZSGB-BIO, Beijing, China) for 24 h at 4°C. The blots were
visualized by using a chemiluminescence detection system (32209;
Invitrogen; Thermo Fisher Scientific, Inc.). Densitometric
quantification of the immunoblot data was performed by using the
software of Quantity-One (version 3.2; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Immunohistochemical staining
Immunohistochemical staining was performed by an
avidin-biotin-peroxidase technique. Paraffin-embedded tissue
sections were prepared and epitope retrieval was performed for
further analysis. The paraffin sections were incubated with
hydrogen peroxide (3%) for 15 min at 37°C and subsequently blocked
with 5% skimmed milk for 15 min at 37°C. Finally, the sections were
incubated in caspase-3 (1:2,000; 9662; Cell Signaling Technologies,
Inc.) at 4°C for 12 h. All sections were washed 3 times and
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:2,000; 12768; Cell Signaling Technologies, Inc.) for
1 h at 37°C. A total of 6 randomly selected fields of view were
observed under the fluorescence microscope (ECLIPSE TE300; Nikon
Corporation; Tokyo, Japan). Tissue sections were also stained with
4-hydroxy-2-nonenal (HNE; Cell Signaling Technologies, Inc.) for 2
h at 37°C and measured using ELISA with commercially available kits
(MBS027502; MyBioSource; San Diego, CA, USA).
Oxidative stress and anti-oxidant
enzyme levels
Renal tissues and cells were isolated from
experimental mice and homogenized in buffer containing 1 mM EDTA,
0.5 mM phenylmethane sulfonyl fluoride, 100 mM Tris-HCl (pH 7.4)
and 0.05% Triton X-100 with sonication. The homogenates were
centrifuged at 8,000 × g for 10 min at 4°C and the supernatants
were used to measure the total thiol, reduced glutathione (GSH),
lipid peroxidation and superoxide dismutase (SOD) activity. Total
thiol, GSH, lipid peroxidation and superoxide dismutase and
reactive oxygen species (ROS) activity in tissue homogenates was
evaluated according to a previous study (32). All of the spectrophotometric data
were recorded by a Spectramax spectrophotometer (Molecular Devices,
Inc., Sunnyvale, CA, USA).
Statistical analysis
Values are expressed as the mean ± standard
deviation from three independent experiments. All data were
analyzed using SPSS Statistics 19.0 (IBM Corp., Armonk, NY, USA).
Statistical significance of differences between groups was analyzed
using a two-tailed Student's t-test. Unpaired data were analyzed by
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Resveratrol improves chronic renal
failure induced by type II diabetes by regulation of the
inflammatory response
The inflammatory response is crucial for renal
failure induced by type II diabetes. Therefore, the present study
first analyzed the inflammatory factors in the renal cells from the
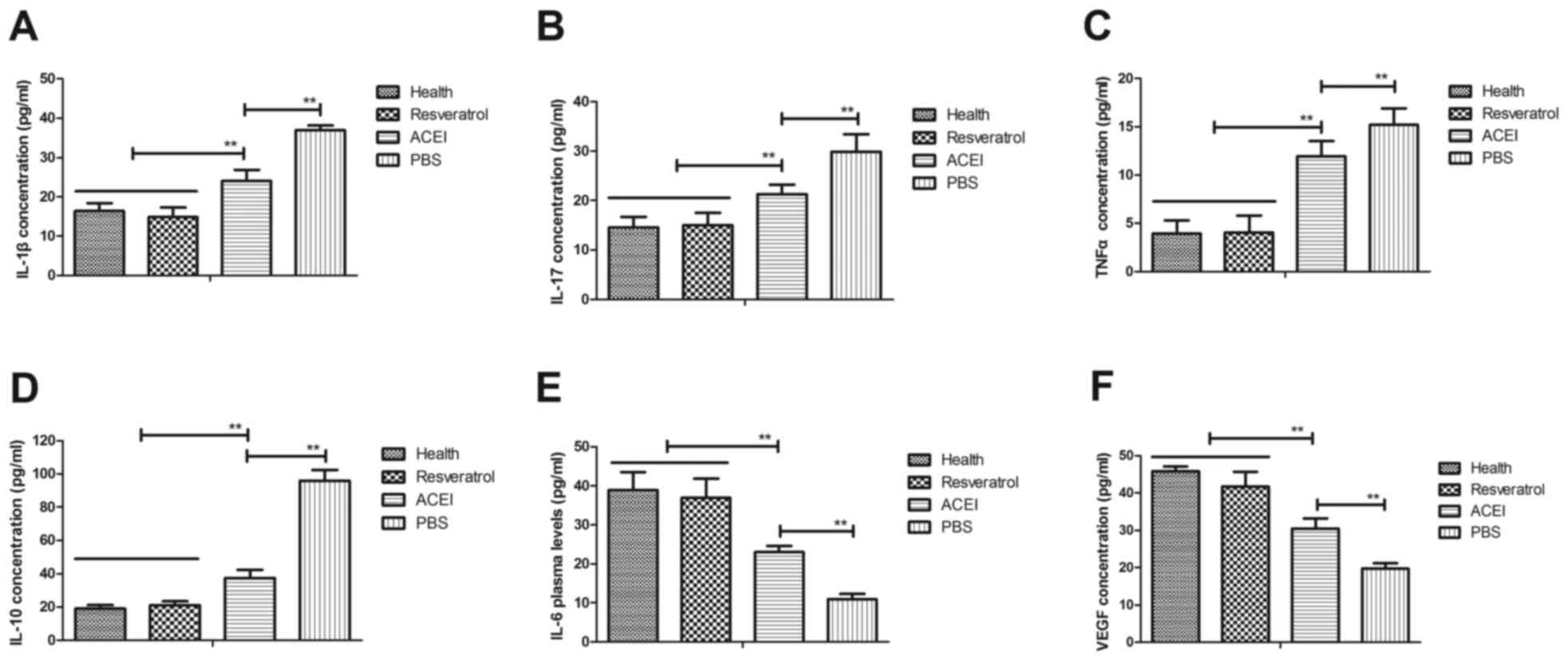
experimental mice. It was observed that inflammatory factors IL-1β,
IL-17, IL-10 and TNF-α in the serum in mice with chronic renal
failure induced by type II diabetes were significantly increased
compared with those in healthy mice, while IL-6 and VEGF were
decreased (Fig. 1). However, in
comparison with those in the PBS group, the plasma concentrations
of IL-1β, IL-17, IL-10 and TNF-α were significantly decreased after
resveratrol treatment. Furthermore, resveratrol treatment led to
increased IL-6 and VEGF levels compared with those in the PBS
group. ACEI treatment also significantly decreased IL-1β, IL-17,
IL-10 and TNF-α and increased IL-6 and VEGF levels compared with
those in the PBS group, but the effect was significantly lower than
that of resveratrol. These results indicated that resveratrol
regulates inflammatory factors, which may be beneficial for mice
with chronic renal failure induced by type II diabetes.
Resveratrol improves glucose
metabolism and insulin tolerance in mice with chronic renal failure
induced by type II diabetes
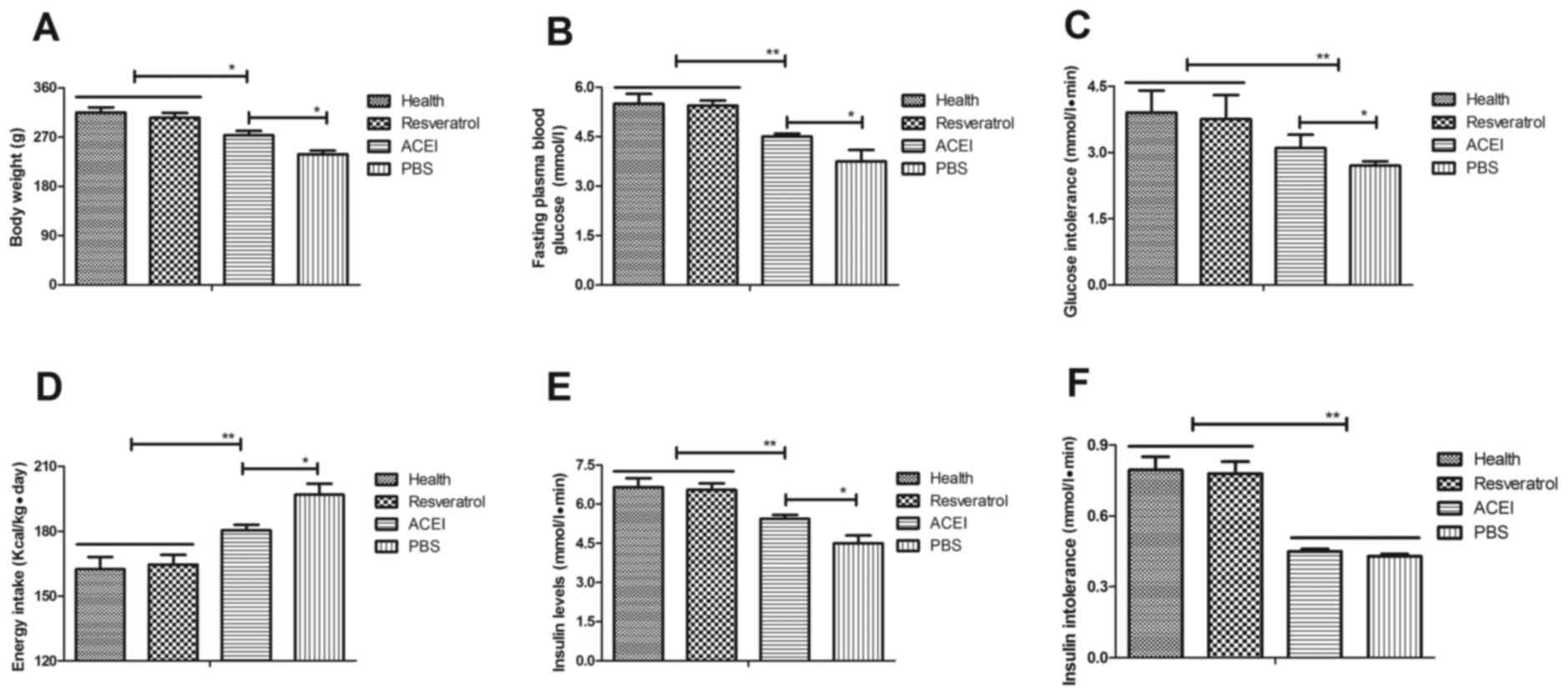
To investigate the efficacy of resveratrol on
chronic renal failure induced by type II diabetes, its effect on
glucose metabolism and insulin tolerance in the experimental mice
was assessed. First, changes in the body weight of mice with
chronic renal failure induced by type II diabetes were analyzed.
The results revealed that in the PBS group, the body weight was
significantly decreased compared with that in the Control group,
which was inhibited by resveratrol and, to a significantly lesser
extent, by ACEI (Fig. 2A). In
addition, resveratrol significantly improved the fasting blood
glucose concentration in mice with chronic renal failure induced by
type II diabetes (Fig. 2B).
Resveratrol treatment also significantly alleviated glucose
intolerance after the initial glucose injection compared with that
in the PBS and ACEI groups (Fig.
2C). Furthermore, resveratrol treatment decreased food intake
of mice with chronic renal failure induced by type II diabetes
compared with that in the ACEI and PBS groups (Fig. 2D). Of note, insulin levels in mice
treated with resveratrol were significantly higher than those in
the ACEI and PBS-treated groups (Fig.
2E). Furthermore, the insulin tolerance test indicated that
resveratrol treatment significantly improved insulin tolerance in
mice with chronic renal failure induced by type II diabetes
(Fig. 2F). These results suggested
that resveratrol improves body weight, fasting blood glucose
concentration and insulin tolerance in mice with chronic renal
failure induced by type II diabetes.
Resveratrol reduces renal apoptosis in
mice with chronic renal failure induced by type II diabetes
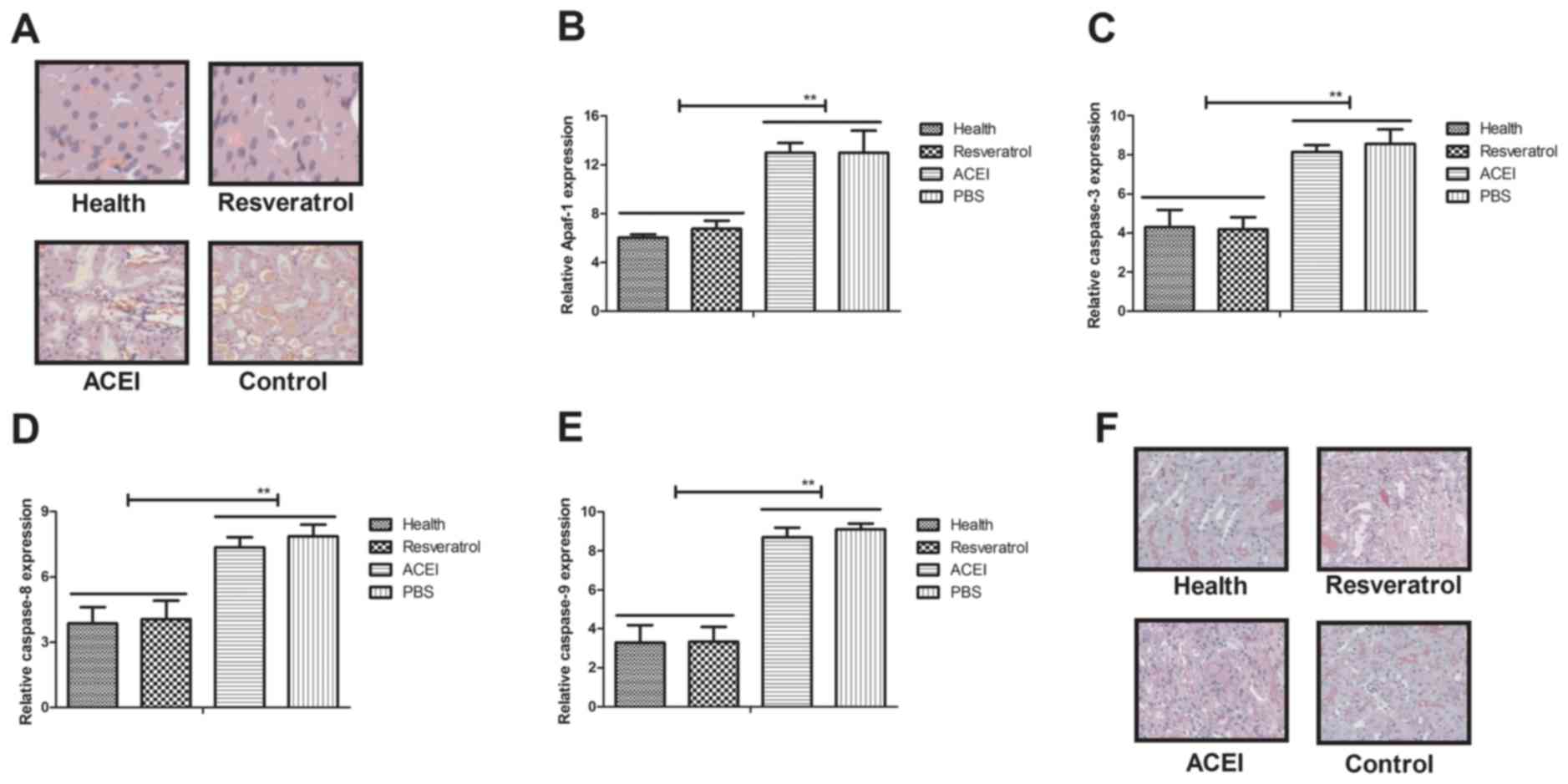
Apoptosis has an important role in mammals with
chronic renal failure induced by type II diabetes (33). In the present study, the inhibitory
effects of resveratrol on renal cell apoptosis in experimental mice
with chronic renal failure were assessed. A TUNEL staining assay
revealed that apoptotic renal cells were markedly decreased in mice
treated with resveratrol compared with those in the PBS- and
ACEI-treated groups (Fig. 3A).
Furthermore, resveratrol treatment markedly inhibited the
expression of apoptosis-associated genes (Apaf-1, caspase-3,
caspase-8 and caspase-9) in renal cells with chronic renal failure
(Fig. 3B-E). Immunohistochemical
analysis demonstrated that resveratrol treatment significantly
decreased caspase-3 expression, which confirmed the protective
effect of resveratrol on renal cells from experimental mice
(Fig. 3F). Collectively, these
results suggested that resveratrol treatment inhibited apoptosis of
renal cells by inactivation of caspase-3 in mice with chronic renal
failure induced by type II diabetes.
Resveratrol alters the expression and
phosphorylation of AMPK levels in rats with chronic renal failure
induced by type II diabetes
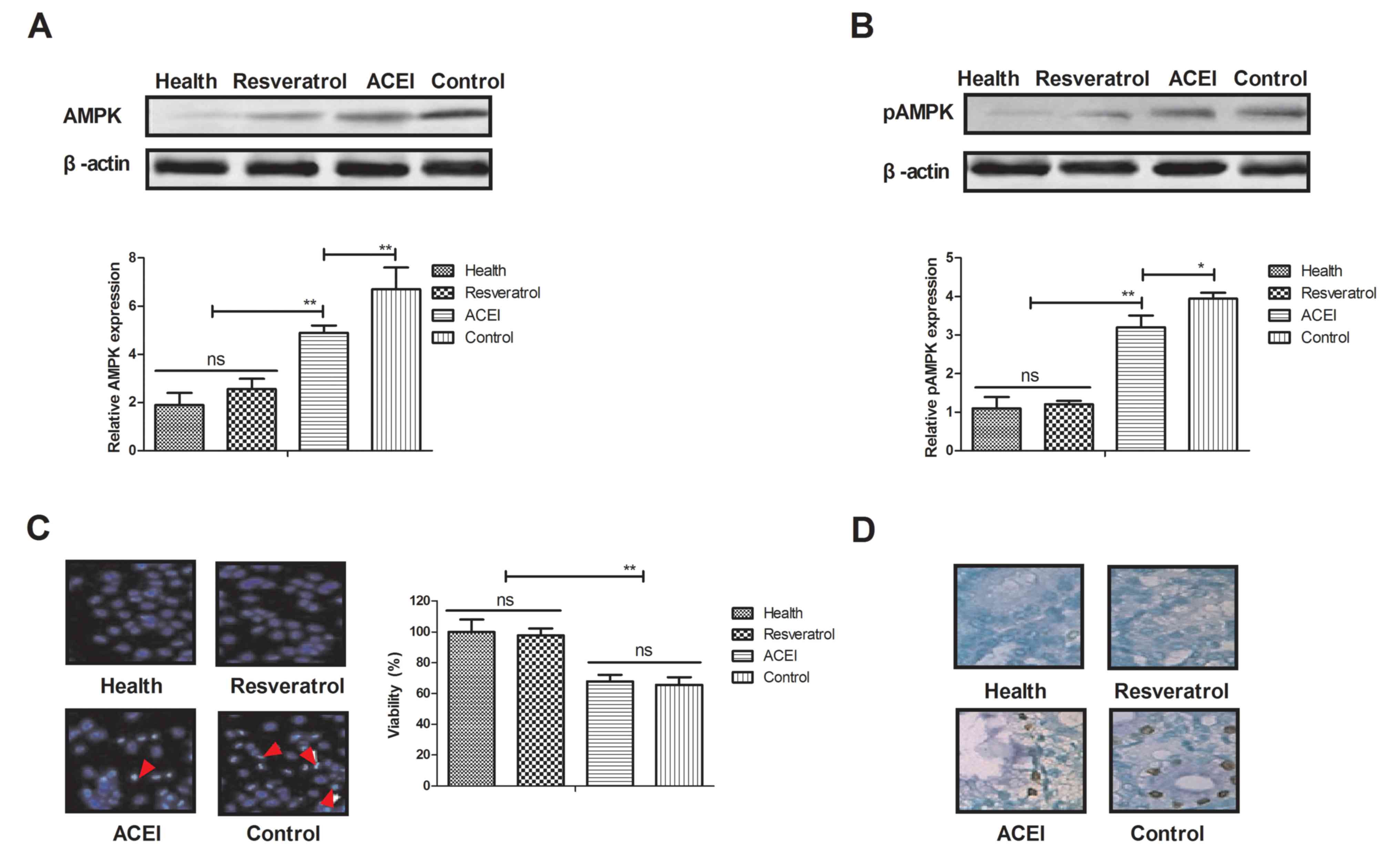
As AMPK is likely to be a target of resveratrol, via
which it exerts its pharmacodynamic function, the AMPK signaling
pathway in renal cells from experimental mice was examined. The
expression and phosphorylation levels of AMPK were decreased after
treatment with resveratrol (Fig. 4A and
B). In addition, morphological observation revealed that
resveratrol treatment improved the viability of renal cells
determined by cells viability (Fig.
4C). Furthermore, immunostaining demonstrated that immunocyte
numbers were decreased in renal cells of resveratrol-treated mice
compared with those in the ACEI- and PBS-treated groups (Fig. 4D). AMPK activation was also decreased
in renal cells of experimental mice after resveratrol treatment
compared with that in the ACEI- and PBS-treated groups (Fig. 4E). In addition, glucose-6-phosphate
activity was significantly increased in the serum of experimental
mice after treatment with resveratrol (Fig. 4F). These findings suggested that
decreased AMPK activation by resveratrol contributes to the
enzymatic capacity for glucose production in mice with chronic
renal failure induced by type II diabetes, which may proceed via
the AMPK-mediated signaling pathway.
Resveratrol decreases oxidative stress
and anti-oxidant status in mice with chronic renal failure induced
by type II diabetes
Oxidative stress contributes to type II
diabetes-induced chronic renal failure, leading to increased
apoptosis of renal cells in experimental mice. Hence, the present
study investigated the oxidative stress and anti-oxidant status in
renal cells after treatment with resveratrol. As presented in
Fig. 5A, total thiol levels were
decreased in renal cells after resveratrol treatment. Lipid
peroxidation in renal cells of resveratrol-treated mice was
increased compared with that in ACEI- and PBS-treated experimental
mice (Fig. 5B). In addition,
resveratrol treatment increased SOD activity and ROS levels in
renal cells from experimental compared with that in ACEI- and
PBS-treated experimental mice (Fig. 5C
and D). In addition, increases of GSH in renal cells of
experimental mice were found to be included in the benefits of
resveratrol treatment (Fig. 5E).
Furthermore, immunohistochemical detection of 4-hydroxy-2-nonenal
(HNE) revealed that resveratrol treatment decreased the intense
staining of HNE in renal cells compared with that in the controls,
which may also indicate the enhancement of the anti-oxidant status
(Fig. 5F). These results indicated
that resveratrol treatment decreased oxidative stress and improved
the anti-oxidant status in renal cells from mice with chronic renal
failure induced by type II diabetes.
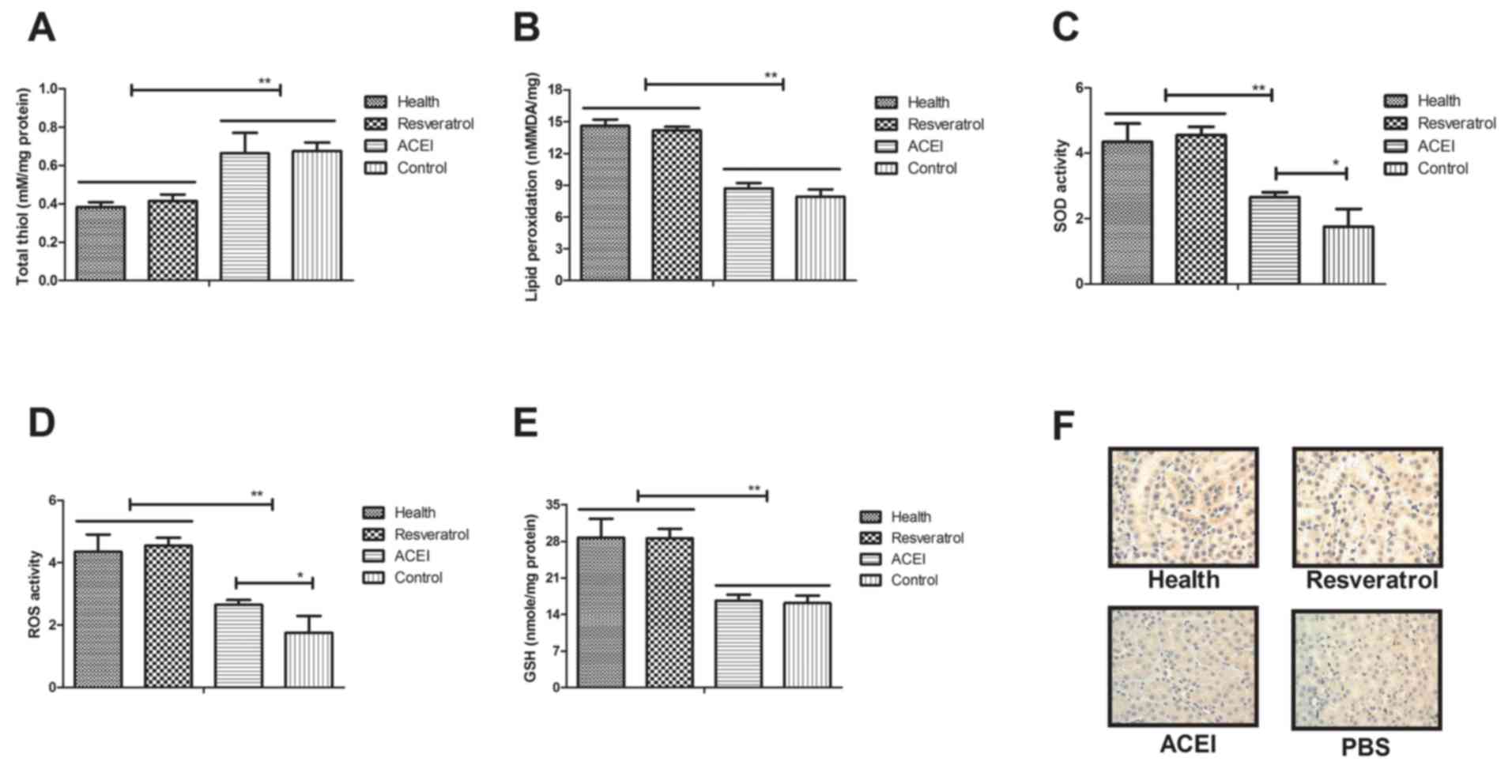 | Figure 5.Regulatory effects of resveratrol on
oxidative stress and anti-oxidant status in mice with chronic renal
failure. (A) Total thiol levels in renal cells. (B) Lipid
peroxidation in the experimental mice. (C) SOD activity, (D) ROS
activity and (E) GSH levels in renal cells of experimental mice.
(F) Immunohistochemical analysis of HNE in renal cells from
experimental mice. Mice with renal failure induced by type II
diabetes were treated with resveratrol (10 mg/kg), the ACEI
aldosterone (10 mg/kg) or the same volume of PBS, and healthy
animals were used as controls (magnification, ×40). *P<0.05,
**P<0.01. ACEI, angiotensin-converting enzyme inhibitor; SOD,
superoxide dismutase; ROS, reactive oxygen species; GSH,
glutathione; HNE, 4-hydroxy-2-nonenal. |
Discussion
Chronic renal failure is a syndrome resulting from
serious metabolic disorders (18).
Chronic renal failure affects nearly 8% of patients with type II
diabetes and impairs the quality of life of affected patients
(34). Studies have indicated that
type II diabetes frequently leads to chronic renal failure due to
long-term insulin injection and disturbance of carbohydrate
metabolism (35,36). In addition, in patients with chronic
renal failure induced by type II diabetes mellitus present
insulinoma treatment with diazoxide is ineffective (37). Furthermore, type II diabetes-induced
chronic renal failure leads to other clinical syndromes and
increases mortality (38,39). From these studies, it is concluded
that type II diabetes may lead to chronic renal failure and result
in other diseases, indicating the importance of the development of
more efficient treatments for chronic renal failure induced by type
II diabetes.
The present study investigated the therapeutic
effects of resveratrol in a mouse model of chronic renal failure
induced by type II diabetes and assessed the molecular mechanisms.
Resveratrol is a naturally occurring polyphenol substance that has
been proven to have multiple functions in the treatment of diabetes
in various animal models (40,41).
However, the effect of resveratrol on chronic renal failure has
remained to be fully elucidated. Resveratrol treatment was reported
to restore peripheral insulin sensitivity in streptozotocin-induced
or insulin receptor substrate 2-deficient diabetic mouse models
(42). The present study found that
insulin resistance, glucose metabolism and body weight in mice with
chronic renal failure induced by type II diabetes were
significantly improved after resveratrol treatment, which confirms
the outcomes reported by previous studies (43,44).
Inflammation is one of the most common
characteristics of patients with chronic renal failure (45). In addition, oxidative stress is
associated with the progression of patients with chronic renal
failure and metabolic syndrome (46). Oxidative stress is also associated
with neutrophil function in cats with chronic renal failure
(47). A previous study found that
resveratrol treatment mitigates hepatic injury, oxidative stress
and apoptosis in a rat model by regulating nuclear factor (NF)-κB
signaling pathway (48). Resveratrol
also protects cells against titanium particle-induced aseptic
loosening through reduction of oxidative stress and inactivation of
NF-κB via inhibition of nitric oxide production, ROS generation and
lipid peroxidation (49). The
present study indicated that resveratrol treatment not only
decreased the apoptosis of renal cells, but also markedly improved
oxidative stress of renal cells isolated from experimental mice.
Furthermore, cytokines, including IL-6, IL-17, IL-1β, TNF-α, IL10
and VEGF, were assessed in the sera of mice with chronic renal
failure induced by type II diabetes. The results demonstrated that
after resveratrol treatment, the decreases of IL-6 and VEGF as well
as the increases of IL-17, IL-1β, TNF-α and IL-10 in the
experimental vs. control mice were inhibited by resveratrol.
Furthermore, the AMPK signaling pathway was found to be involved in
the resveratrol-mediated improvement of the chronic renal failure
induced by type II diabetes.
The AMPK signaling pathway has an important role in
the function of renal cells (50). A
previous study reported that resveratrol attenuated TNF-α-induced
matrix metalloproteinase-3 expression in human nucleus pulposus
cells by activating the AMPK/Sirtuin1 signaling pathway (51). In addition, resveratrol enhances the
anti-tumor effects of temozolomide in glioblastoma via a
ROS-dependent AMPK/tuberous sclerosis 1/mammalian target of
rapamycin signaling pathway (52).
Furthermore, a previous study suggested that resveratrol induces
apoptosis in chemoresistant cancer cells via modulation of the AMPK
signaling pathway (53). These
results suggested that AMPK may be involved in apoptosis and
oxidative stress in renal cells. Importantly, the present results
revealed that resveratrol treatment regulated apoptosis and
oxidative stress via the AMPK signaling pathway. The decreased
activity of AMPK contributes to the recovery of renal cells
isolated from mice with chronic renal failure induced by type II
diabetes. The findings of the present study indicated that
resveratrol treatment improves glucose metabolism and insulin
resistance via activation of glucose-6-phosphate through the AMPK
signaling pathway. Interestingly, resveratrol treatment not only
improved the glucose levels and lipid profile, but also maintained
glucose homeostasis and lipid metabolism in mice with chronic renal
failure. Of note, apoptosis and oxidative stress in renal cells
were inhibited by the treatment with resveratrol, which also
contributed to the increased body weight and insulin
intolerance.
In conclusion, the results of the present study
expanded on the known molecular mechanism of action of resveratrol
in the treatment of mice with chronic renal failure induced by type
II diabetes. Resveratrol treatment markedly improved glucose
metabolism and insulin resistance in mice with chronic renal
failure induced by type II diabetes. The results indicated that
resveratrol treatment targeting AMPK signaling may be a potential
strategy to protect against apoptosis and oxidative stress in renal
cells. Taken together, regulation of the AMPK pathway by
resveratrol may serve as an effective treatment for chronic renal
failure, which also suggests the therapeutic potential of other
compounds modulating the AMPK signaling pathway.
Acknowledgements
The authors would like to thank Dr Zhi Li
(Department of Sports Medicine, North University of China) for
assisting in performing the statistical analysis.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
LZ performed the experiments. HG analyzed and
designed experiments for the present study.
Ethics approval and consent to
participate
This pre-clinical study was approved by the Ethics
Committee of Fenyang College Shanxi Medical University (Fenyang,
China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ibrahim NE, Gaggin HK, Rabideau DJ, Gandhi
PU, Mallick A and Januzzi JL Jr: Worsening renal function during
management for chronic heart failure with reduced ejection
fraction: Results from the Pro-BNP outpatient tailored chronic
heart failure therapy (PROTECT) study. J Card Fail. 23:121–130.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sati A, Jha A, Moulick PS, Shankar S,
Gupta S, Khan MA, Dogra M and Sangwan VS: Corneal endothelial
alterations in chronic renal failure. Cornea. 35:1320–1330. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iwamuro M, Kanzaki H, Tanaka T, Kawano S,
Kawahara Y and Okada H: Lanthanum phosphate deposition in the
gastric mucosa of patients with chronic renal failure. Nihon
Shokakibyo Gakkai Zasshi. 113:1216–1222. 2016.(In Japanese).
PubMed/NCBI
|
|
4
|
Jain P, Kanagal-Shamanna R, Wierda W,
Ferrajoli A, Keating M and Jain N: Membranoproliferative
glomerulonephritis and acute renal failure in a patient with
chronic lymphocytic leukemia: Response to obinutuzumab. Hematol
Oncol Stem Cell Ther. 10:151–154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakano S, Masuda K, Asanuma T and Nakatani
S: The effect of chronic renal failure on cardiac function: An
experimental study with a rat model. J Echocardiogr. 14:156–162.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sathyanarayana V, Patel MT, Raghavan S and
Naresh D: Simultaneous bilateral femur neck fracture in a young
adult with chronic renal failure-a case report and review of
literature. J Orthop Case Reports. 5:24–26. 2015.PubMed/NCBI
|
|
7
|
Malekmakan L, Malekmakan A, Daneshian A,
Pakfetrat M and Roosbeh J: Hypertension and diabetes remain the
main causes of chronic renal failure in Fars Province, Iran 2013.
Saudi J Kidney Dis Transpl. 27:423–424. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mao W, Zhang L, Zou C, Li C, Wu Y, Su G,
Guo X, Wu Y, Lu F, Lin Q, et al: Rationale and design of the
helping ease renal failure with bupi yishen compared with the
angiotensin II antagonist losartan (HERBAAL) trial: A randomized
controlled trial in non-diabetes stage 4 chronic kidney disease.
BMC Complement Altern Med. 15:3162015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takase H, Nakazawa A, Yamashita S,
Toriyama T, Sato K, Ueda R and Dohi Y: Pioglitazone produces rapid
and persistent reduction of vascular inflammation in patients with
hypertension and type 2 diabetes mellitus who are receiving
angiotensin II receptor blockers. Metabolism. 56:559–564. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Varughese GI and Lip GY: Hypertension in
patients with type-II diabetes: Relation to urinary albumin
excretion, endothelial function and inflammation. J Hum Hypertens.
19:421–424. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamamoto S, Okada Y, Mori H, Nishida K,
Uriu K and Tanaka Y: Type 2 diabetes mellitus complicated by
hypertension in Japanese patients: Switching treatment from
high-dose angiotensin II receptor blockers to losartan plus
hydrochlorothiazide. Intern Med. 53:1283–1289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kalaitzidis R and Bakris G: Management of
hypertension in patients with diabetes: The place of angiotensin-II
receptor blockers. Diabetes Obes Metab. 11:757–769. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hasvold LP, Bodegård J, Thuresson M,
Stålhammar J, Hammar N, Sundström J, Russell D and Kjeldsen SE:
Diabetes and CVD risk during angiotensin-converting enzyme
inhibitor or angiotensin II receptor blocker treatment in
hypertension: A study of 15,990 patients. J Hum Hypertens.
28:663–669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Daimon M, Kamba A, Murakami H, Takahashi
K, Otaka H, Makita K, Yanagimachi M, Terui K, Kageyama K, Nigawara
T, et al: Association between pituitary-adrenal axis dominance over
the renin-angiotensin-aldosterone system and hypertension. J Clin
Endocrinol Metab. 101:889–897. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ficek J, Malyszko J and Chudek J: Renalase
and its role in the development of hypertension in patients with
chronic renal failure. Przegl Lek. 72:306–308. 2015.(In Polish).
PubMed/NCBI
|
|
16
|
Finlay E: Review: Most interventions for
preventing bone disease in chronic renal failure improved
biochemical outcomes. Arch Dis Child Edu Pract Ed. 97:402012.
View Article : Google Scholar
|
|
17
|
Kasacka I: Review article-involvement of
gastric APUD cells in chronic renal failure. Acta Histochem.
105:319–327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bos-Touwen I, Schuurmans M, Monninkhof EM,
Korpershoek Y, Spruit-Bentvelzen L, Ertugrul-van der Graaf I, de
Wit N and Trappenburg J: Patient and disease characteristics
associated with activation for self-management in patients with
diabetes, chronic obstructive pulmonary disease, chronic heart
failure and chronic renal disease: A cross-sectional survey study.
PloS One. 10:e01264002015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nair PA, Jivani NB and Diwan NG: Kyrle's
disease in a patient of diabetes mellitus and chronic renal failure
on dialysis. J Family Med Prim Care. 4:284–286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Evans HM, Howe PR and Wong RH: Clinical
evaluation of effects of chronic resveratrol supplementation on
cerebrovascular function, cognition, mood, physical function and
general well-being in postmenopausal women-rationale and study
design. Nutrients. 8:1502016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toth P, Tarantini S, Tucsek Z, Ashpole NM,
Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A
and Ungvari Z: Resveratrol treatment rescues neurovascular coupling
in aged mice: Role of improved cerebromicrovascular endothelial
function and downregulation of NADPH oxidase. Am J Physiol Heart
Circ Physiol. 306:H299–H308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beaudoin MS, Snook LA, Arkell AM, Simpson
JA, Holloway GP and Wright DC: Resveratrol supplementation improves
white adipose tissue function in a depot-specific manner in Zucker
diabetic fatty rats. Am J Physiol Regul Integr Comp Physiol.
305:R542–R551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
González-Rodríguez Á, Santamaría B,
Mas-Gutierrez JA, Rada P, Fernández-Millán E, Pardo V, Álvarez C,
Cuadrado A, Ros M, Serrano M and Valverde ÁM: Resveratrol treatment
restores peripheral insulin sensitivity in diabetic mice in a
sirt1-independent manner. Mol Nutr Food Res. 59:1431–1442. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gencoglu H, Tuzcu M, Hayirli A and Sahin
K: Protective effects of resveratrol against streptozotocin-induced
diabetes in rats by modulation of visfatin/sirtuin-1 pathway and
glucose transporters. Int J Food Sci Nutr. 66:314–320. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carrizzo A, Puca A, Damato A, Marino M,
Franco E, Pompeo F, Traficante A, Civitillo F, Santini L, Trimarco
V and Vecchione C: Resveratrol improves vascular function in
patients with hypertension and dyslipidemia by modulating NO
metabolism. Hypertension. 62:359–366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gulvady AA, Ciolino HP, Cabrera RM and
Jolly CA: Resveratrol inhibits the deleterious effects of
diet-induced obesity on thymic function. J Nutr Biochem.
24:1625–1633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guan Y, Cui ZJ, Sun B, Han LP, Li CJ and
Chen LM: Celastrol attenuates oxidative stress in the skeletal
muscle of diabetic rats by regulating the AMPK-PGC1α-SIRT3
signaling pathway. Int J Mol Med. 37:1229–1238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Madsen A, Bjune JI, Bjorkhaug L, Mellgren
G and Sagen JV: The cAMP-dependent protein kinase downregulates
glucose-6-phosphatase expression through RORα and SRC-2 coactivator
transcriptional activity. Mol Cell Endocrinol. 419:92–101. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu S, Lv Y, Zhao J, Wang J, Zhao X and
Wang S: Inhibitory effects of Shenkang injection and its main
component emodin on the proliferation of high glucoseinduced renal
mesangial cells through cell cycle regulation and induction of
apoptosis. Mol Med Rep. 14:3381–3388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He Y, Chen W, Hu Y, Luo B, Wu L, Qiao Y,
Mo Q, Xu R, Zhou Y, Ren Z, et al: E. adenophorum induces cell cycle
and apoptosis of renal cells through mitochondrial pathway and
caspase activation in saanen goat. PloS One. 10:e01385042015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Santos SS, Carmo AM, Brunialti MK, Machado
FR, Azevedo LC, Assunção M, Trevelin SC, Cunha FQ and Salomao R:
Modulation of monocytes in septic patients: Preserved phagocytic
activity, increased ROS and NO generation, and decreased production
of inflammatory cytokines. Intensive Care Med Exp. 4:52016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hyde GD, Taylor RF, Ashton N, Borland SJ,
Wu HS, Gilmore AP and Canfield AE: Axl tyrosine kinase protects
against tubulo-interstitial apoptosis and progression of renal
failure in a murine model of chronic kidney disease and
hyperphosphataemia. PloS One. 9:e1020962014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alba A, Morales J, Ferrario M, Zehnder C,
Aguiló J, Zavala C, Herzog C, Calabran L, Contreras L, Espinoza R,
et al: Simultaneous kidney and pancreas transplantation (SKPT) in
patients with type 1 diabetes and chronic renal failure: Experience
in 12 patients in Chile. Rev Med Chil. 139:11–18. 2011.(In
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wittmann I, Molnár GA, Wagner L, Köszegi
T, Wagner Z, Laczy B, Tamaskó M, Markó L, Mohás M and Nagy J:
Single dose of acetylsalicylic acid in patients with Type 2
diabetes mellitus and/or chronic renal failure ameliorates anaemia
by decreasing the rate of neocytolysis. Acta Physiol Hung.
94:159–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shamaeva EN, Shestakova MV, Kim IG,
Stoliarevich ES and Tomilina NA: Transplantation of the
kidney-optimal treatment of patients suffering from diabetes
mellitus type 1 with terminal chronic renal failure. Ter Arkh.
79:40–44. 2007.(In Russian). PubMed/NCBI
|
|
37
|
Shimizu M, Suzuki K, Tsuchida K, Kojima M,
Hiraishi H and Aso Y: Insulinoma in a patient with chronic renal
failure due to type 2 diabetes mellitus treated effectively with
diazoxide. Intern Med. 54:621–625. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Miyazato J, Horio T, Takiuchi S, Kamide K,
Sasaki O, Nakamura S, Nakahama H, Inenaga T, Takishita S and Kawano
Y: Left ventricular diastolic dysfunction in patients with chronic
renal failure: Impact of diabetes mellitus. Diabet Med. 22:730–736.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Majdan M, Kurowska M, Orlowska-Kowalik G
and Drop A: Ultrasonographic evaluation of kidneys in type-2
diabetes patients without overt nephropathy and with chronic renal
failure. Wiad Lek. 58:25–28. 2005.(In Polish). PubMed/NCBI
|
|
40
|
Ding DF, You N, Wu XM, Xu JR, Hu AP, Ye
XL, Zhu Q, Jiang XQ, Miao H, Liu C and Lu YB: Resveratrol
attenuates renal hypertrophy in early-stage diabetes by activating
AMPK. Am J Nephrol. 31:363–374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Szkudelska K and Szkudelski T:
Resveratrol, obesity and diabetes. Eur J Pharmacol. 635:1–8. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Thirunavukkarasu M, Penumathsa SV, Koneru
S, Juhasz B, Zhan L, Otani H, Bagchi D, Das DK and Maulik N:
Resveratrol alleviates cardiac dysfunction in
streptozotocin-induced diabetes: Role of nitric oxide, thioredoxin,
and heme oxygenase. Free Radic Biol Med. 43:720–729. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ungvari Z and Csiszar A: Resveratrol
confers endothelial protection in insulin-dependent diabetes
mellitus: Editorial to: ‘Resveratrol shows vasoprotective effect
reducing oxidative stress without affecting metabolic disturbances
in insulin-dependent diabetes of rabbits’ by F. Akar et al.
Cardiovasc Drugs Ther. 25:111–113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huang JP, Huang SS, Deng JY, Chang CC, Day
YJ and Hung LM: Insulin and resveratrol act synergistically,
preventing cardiac dysfunction in diabetes, but the advantage of
resveratrol in diabetics with acute heart attack is antagonized by
insulin. Free Radic Biol Med. 49:1710–1721. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Verheul MK, van Erp SJ, van der Woude D,
Levarht EW, Mallat MJ, Verspaget HW, Stolk J, Toes RE, van der
Meulen-de Jong AE, Hiemstra PS, et al: Anti-carbamylated protein
antibodies: A specific hallmark for rheumatoid arthritis.
Comparison to conditions known for enhanced carbamylation; renal
failure, smoking and chronic inflammation. Ann Rheum Dis.
75:1575–1576. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Medvedeva E, Berezin I, Surkova E, Yaranov
D and Shchukin Y: Galectin-3 in patients with chronic heart
failure: Association with oxidative stress, inflammation, renal
dysfunction and prognosis. Minerva Cardioangiol. 64:595–602.
2016.PubMed/NCBI
|
|
47
|
Keegan RF and Webb CB: Oxidative stress
and neutrophil function in cats with chronic renal failure. J Vet
Intern Med. 24:514–519. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
El-Din Seif SH, El-Lakkany NM, Salem MB,
Hammam OA, Saleh S and Botros SS: Resveratrol mitigates hepatic
injury in rats by regulating oxidative stress, nuclear factor-kappa
B, and apoptosis. J Adv Pharm Technol Res. 7:99–104. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Luo G, Li Z, Wang Y, Wang H, Zhang Z, Chen
W, Zhang Y, Xiao Y, Li C, Guo Y and Sheng P: Resveratrol protects
against titanium particle-induced aseptic loosening through
reduction of oxidative stress and inactivation of NF-κB.
Inflammation. 39:775–785. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lieberthal W, Tang M, Lusco M, Abate M and
Levine JS: Preconditioning mice with activators of AMPK ameliorates
ischemic acute kidney injury in vivo. Am J Physiol Renal Physiol.
311:F731–F739. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang XH, Zhu L, Hong X, Wang YT, Wang F,
Bao JP, Xie XH, Liu L and Wu XT: Resveratrol attenuated
TNF-α-induced MMP-3 expression in human nucleus pulposus cells by
activating autophagy via AMPK/SIRT1 signaling pathway. Exp Biol Med
(Maywood). 241:848–853. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yuan Y, Xue X, Guo RB, Sun XL and Hu G:
Resveratrol enhances the antitumor effects of temozolomide in
glioblastoma via ROS-dependent AMPK-TSC-mTOR signaling pathway. CNS
Neurosci Ther. 18:536–546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hwang JT, Kwak DW, Lin SK, Kim HM, Kim YM
and Park OJ: Resveratrol induces apoptosis in chemoresistant cancer
cells via modulation of AMPK signaling pathway. Ann N Y Acad Sci.
1095:441–448. 2007. View Article : Google Scholar : PubMed/NCBI
|