Introduction
Zoledronic acid (ZA) is a nitrogen-containing
bisphosphonate, administered intravenously to treat several bone
diseases, including osteoporosis, hypercalcemia, Paget's disease,
bone metastasis of a tumor and myeloma (1–3). ZA is
selectively accumulated in bone tissue due to its high affinity to
bone (4). ZA induces osteoclast
apoptosis and consequently inhibits bone resorption as well as bone
remodeling (5,6). One of the side effects of
bisphosphonates is bisphosphonate-related osteonecrosis of the jaw
(BRONJ), first noted in 2003 and now recognized as a well-known
complication (7,8). It is occasionally necessary to treat
BRONJ, which is associated with the consequent suffering of
patients, by performing resective surgery (9). Although the mechanism of action of
bisphosphonates in BRONJ remains to be fully understood, the
inflammatory process from a bacterial infection and trauma are
suspected to cause and intensify the severity of the affliction
(10,11).
Lipopolysaccharide (LPS), a component from the cell
walls of Gram-negative bacteria, induces immune responses of
macrophages, thereby increasing the production of nitric oxide (NO)
as well as various cytokines, including tumor necrosis factor
(TNF)-α and interleukin (IL)-6 (12,13). NO
regulates various pathophysiological processes and its
overproduction damages the surrounding tissue resulting in
increased inflammation (14). A
previous study revealed that the induction of proinflammatory
cytokines by LPS is enhanced by pretreatment of ZA and functions
due to the reduction of suppressor of cytokine signaling-1
(15).
Polydeoxyribonucleotide (PDRN), consisting of a
mixture of deoxyribonucleotide polymers, is easily extracted from
the sperm of trout and highly purified by a classified extractive
process (16). Following the
cleavage of PDRN by active cell membrane enzymes, it provides a
source for purine and pyrimidine deoxynucleosides and
deoxyribonucleotides (17).
Adenosine, a purine nucleoside, activates adenosine A2A
receptor and the stimulation of the receptor is suspected to
decrease inflammatory cytokine secretion (16,18,19).
Previous studies have demonstrated that PDRN significantly reduces
the clinical signs of arthritis in mice, by decreasing the
circulating levels of several inflammatory cytokines (20) and increasing vascular endothelial
growth factor (VEGF) production, indicating decreased ischemic
tissue damage and an enhanced oxygen supply (21). Ramanathan et al (22) also reported that the adenosine
A2A receptor and LPS increased VEGF expression through
transcriptional regulation of the VEGF promoter. VEGF acts as a
main regulatory factor for endothelial cell proliferation and
increases neovascularization (23).
Therefore, VEGF may contribute to enhanced vascularization in
BRONJ, which was compromised by increased endothelial cell
apoptosis (24). Subsequently, the
pathological condition in BRONJ can be improved through increased
vascularization with VEGF. Thus, the present study aimed to
evaluate whether PDRN regulates the expression of inflammatory
cytokines in macrophages pretreated with ZA and LPS.
Materials and methods
Cell culture
The macrophage cell line, RAW 264.7, was purchased
from the Korea Cell Line Bank (Korean Cell Line Research
Foundation, Seoul, Korea). The cells were maintained in high
glucose Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 100 µg/ml
streptomycin, 100 U/ml penicillin (Lonza Group, Ltd., Basel,
Switzerland) and 10% heat-inactivated fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) in a 37°C humidified incubator with
5% CO2 for all experiments. At the occurrence of BRONJ,
which may be caused by dental procedures or intraoral trauma,
bisphosphonates accumulate in bone tissue, subsequently, a
bacterial infection occurs and causes an increase in LPS. In the
present study, in order to mimic the aforementioned situation, ZA
was incubated with RAW 264.7 cells, which were then exposed to LPS.
Control cells were maintained without ZA and LPS.
Cell viability analysis
Cytotoxicity was assessed by the MTT assay. RAW
264.7 cells were seeded in 96-well plates at a density of
1×105 cells/well containing 200 µl high glucose DMEM
supplemented by 100 µg/ml streptomycin, 100 U/ml penicillin and 10%
heat-inactivated fetal bovine serum, and incubated with 1, 10 or
100 µM ZA (Novartis International AG, Basel, Switzerland) for 24 h.
The cells were then exposed to 0.01, 0.1 or 1 µg/ml LPS
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and various
concentrations of PDRN (1, 10 or 100 µg/ml; PharmaResearch Products
Co., Ltd., Seongnam, Republic of Korea) in serum free medium
at 37°C for 24 h. MTT solution (Sigma-Aldrich; Merck KGaA) was
added to a final concentration of 0.5 mg/ml and the plates were
incubated at 37°C for 1 h in a humidified incubator. The medium was
then removed, the formazan precipitate was solubilized in
dimethylsulphoxide and the solution was measured at 570 nm by a
microplate reader.
Measurement of NO production
NO production was measured using Nitric oxide (NO)
Detection kit (cat. no. 21021; Intron Biotechnology, Sungnam,
Republic of Korea). RAW 264.7 cells were plated at a density of
1×105 cells/well in 96-well culture plates and incubated
with 10 µM ZA for 24 h at 37°C, followed by exposure to 0.1 µg/ml
LPS and various concentrations of PDRN (1, 10 or 100 µg/ml) in
serum free medium at 37°C for 24 h. Nitrite levels in the culture
medium were measured for NO estimation. The supernatant collected
from the culture plate (100 µl) and N1 buffer (50 µl) were added to
a new 96-well plate in triplicate. The plate was maintained at room
temperature for 10 min, and N2 buffer (50 µl) was subsequently
added. The absorbance of the content of each well was measured at a
wavelength of 540 nm with a microplate reader. The nitrite
concentration was calculated using a nitrite standard curve,
according to the manufacturer's protocol.
Western blot analysis of inflammatory
factors in whole cell lysate
Cell proteins of RAW 264.7 cells were collected.
Briefly, cells were plated at a density of 2×105
cells/dish in a 100 mm culture dish and incubated with 10 µM ZA for
24 h, followed by incubation with 0.1 µg/ml LPS and various
concentrations of PDRN (1, 10 or 100 µg/ml) in serum free medium at
37°C for 24 h. Cells were harvested and gently homogenized in
radioimmunoprecipitation assay buffer consisting of 20 mM Tris-HCl
(pH 7.5), 150 mM NaCl, 1 mM Na2 EDTA, 1 mM EGTA, 1%
Nonidet P-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate,
1 mM β-glycerophosphate, 1 mM Na3VO4, 1 µg/ml
leupeptin (Cell Signaling Technology, Inc., Danvers, MA, USA) and 1
mM phenylmethylsulfonyl fluoride (Sigma-Aldrich; Merck KGaA).
Subsequently, the lysates were incubated for 20 min at 4°C,
centrifuged at 16,000 × g for 20 min at 4°C, following which they
were rapidly frozen. The lysates were measured for protein
quantification using Bio-Rad Protein Assay kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). A total of 30 µg/lane
protein was subjected to western blot analysis using 12% SDS-PAGE
gel and transferred to nitrocellulose membrane (GE Healthcare Life
Sciences, Little Chalfont, UK). Membranes were incubated in a
blocking buffer containing 5% skim milk for 1 h at room
temperature. They were subsequently incubated overnight at 4°C with
the following primary antibodies: Mouse β-actin, inducible NO
synthase (iNOS), TNF-α and VEGF (cat. nos. SC-4778, SC-7271,
SC-52746 and SC-7269, respectively), rabbit A2A (cat.
no. 13937; all 1:1,000; Santa Cruz Biotechnology Inc., Dallas, TX,
USA), IL-1β and IL-6 (cat. nos. bs-6319r and bs-0782r,
respectively; both 1:1,000; Bioss Antibodies, Inc., Woburn, MA,
USA). The membranes were then incubated at room temperature for 1 h
with anti-mouse and anti-rabbit secondary horseradish
peroxidase-conjugated antibodies (cat. no. PI-2000 and PI-1000,
respectively; both 1:1,000; Vector Laboratories, Inc., Burlingame,
CA, USA). Bands were detected using an enhanced chemiluminescence
detection kit (Bio-Rad Laboratories, Inc.). Densitrometric analysis
of the resultant bands was performed using Molecular
Analyst™ software, version 1.4.1 (Bio-Rad Laboratories,
Inc.).
Statistical analysis
Statistical analysis was performed using one-way
analysis of variance followed by Duncan's post hoc test using SPSS
software (version 23.0; IBM Corp., Armonk, NY, USA). Data are
expressed as the mean + standard error of the mean. P<0.05
indicated that the difference between groups was statistically
significant.
Results
ZA+LPS decreases and PDRN increases
cell viability
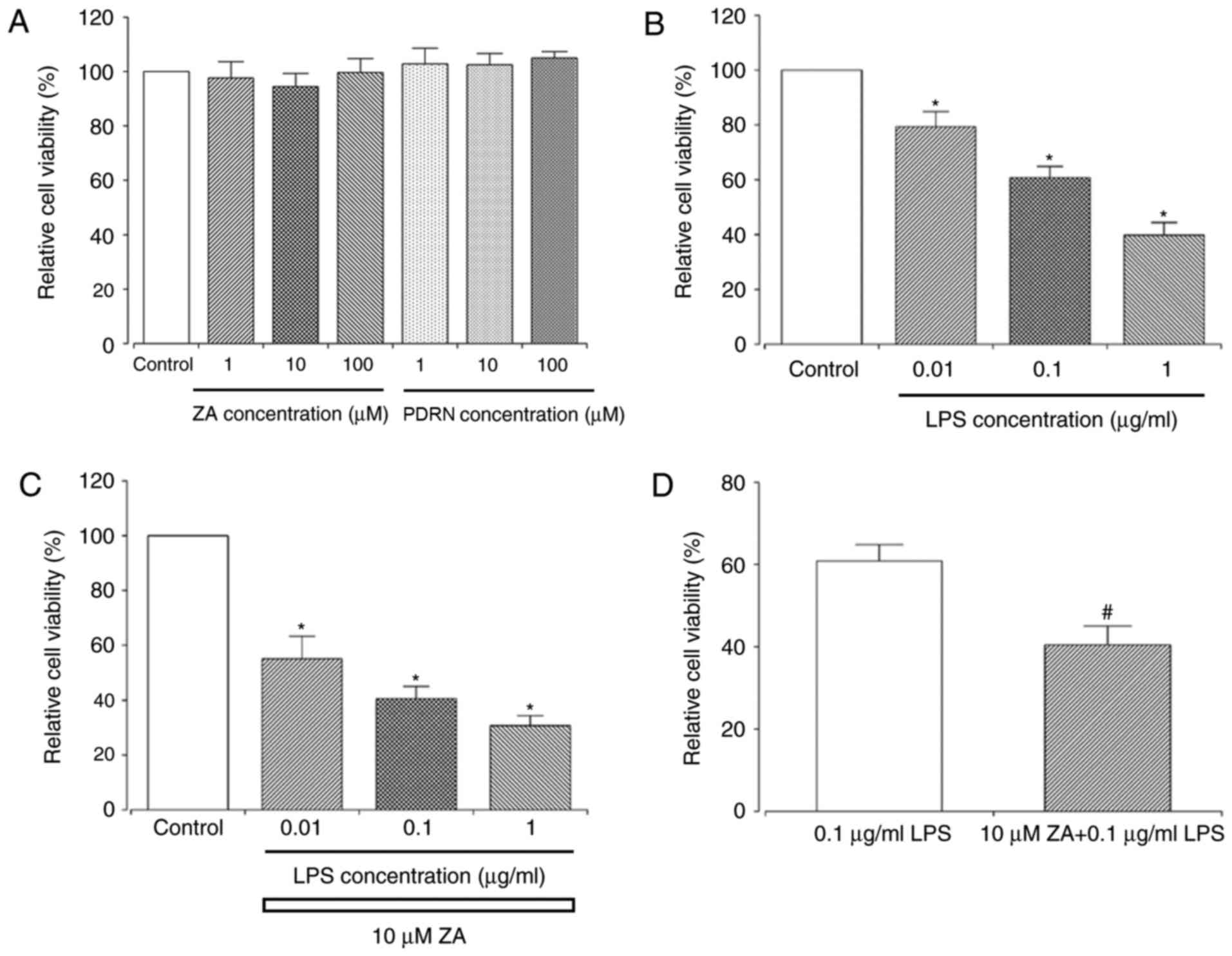
Single treatment with either ZA or PDRN had no
significant effect on the viability of RAW 264.7 cells at any
concentration (1, 10 or 100 µM ZA; 1, 10 or 100 µg/ml PDRN;
Fig. 1A). However, there was a
significant decrease in cell viability when cells were exposed to
LPS alone (0.01, 0.1 or 1 µg/ml; Fig.
1B) or ZA+LPS (10 µM ZA + 0.01, 0.1 or 1 µg/ml LPS; Fig. 1C) compared with control cells.
Treatment with ZA+LPS (10 µM + 0.1 µg/ml) significantly decreased
the cell viability by more than 20% compared with the cells treated
with LPS alone (0.1 µg/ml; Fig. 1D).
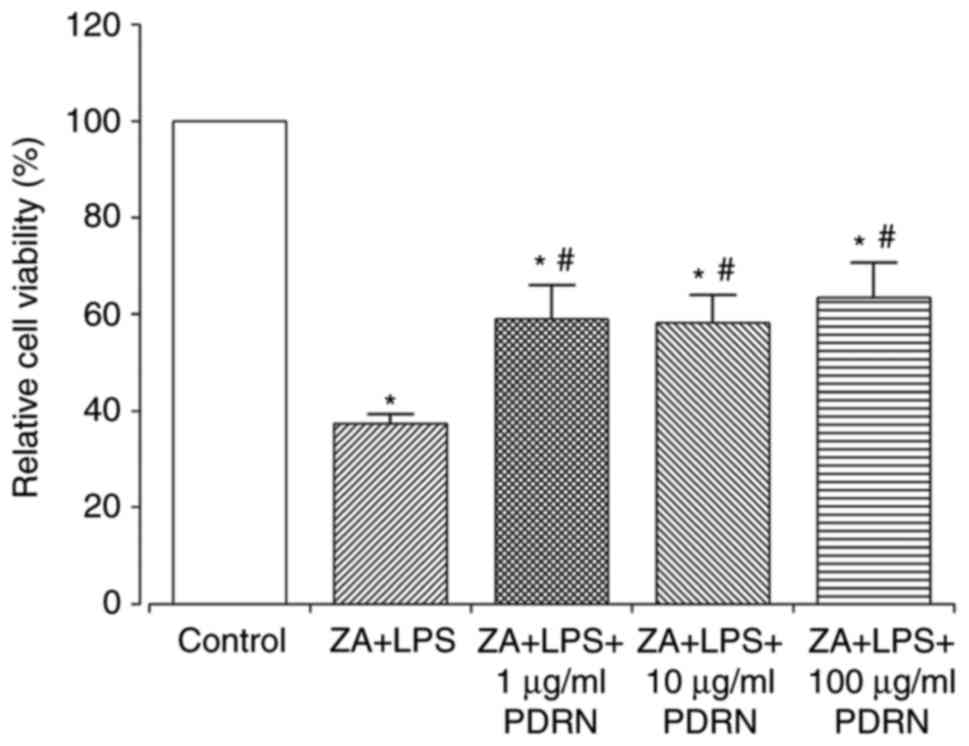
Treatment with 1, 10 or 100 µg/ml PDRN significantly increased cell
viability of the ZA+LPS-stimulated RAW 264.7 cells compared with
ZA+LPS-stimulated cells (Fig. 2).
However, the cell viabilities in ZA+LPS-stimulated cells with or
without PDRN treatment were significantly low compared with the
control group.
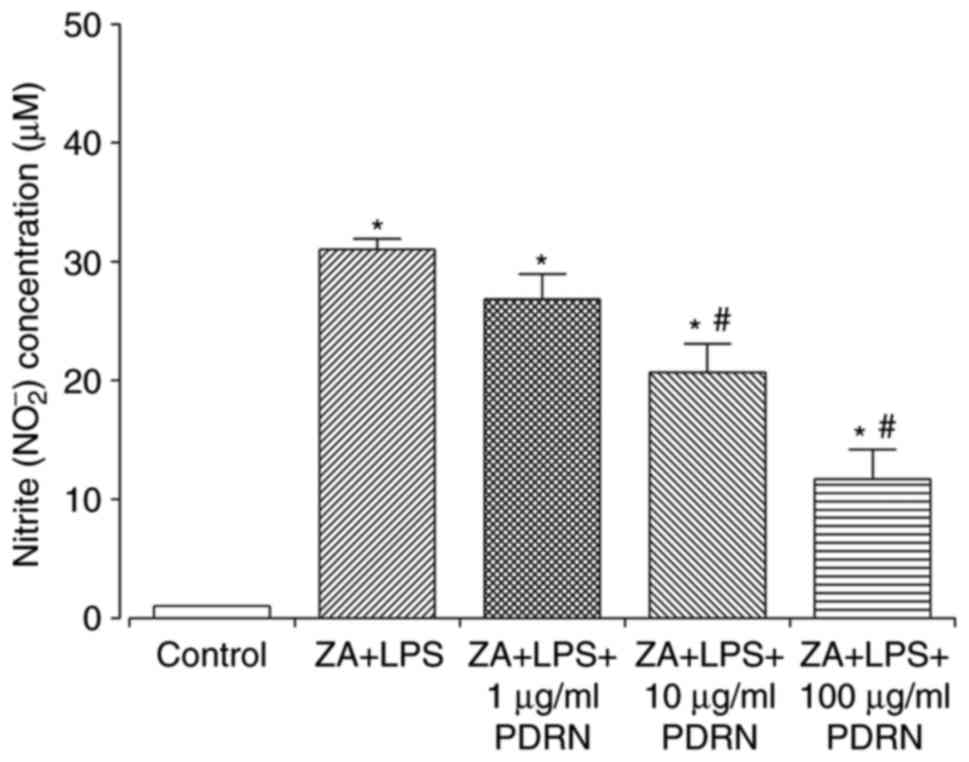
PDRN decreases NO production
ZA+LPS (10 µM + 0.1 µg/ml, respectively)-stimulated
RAW 264.7 cells demonstrated significantly enhanced NO production
compared with the control group (Fig.
3). NO production significantly decreased in what appeared to
be in a dose-dependent manner following 10 and 100 µg/ml of PDRN
treatment compared with ZA+LPS-stimulated RAW 264.7 cells.
PDRN decreases the expression of
inflammatory cytokines
iNOS, IL-1β, IL-6, and TNF-α inflammatory cytokine
expression levels were significantly increased following
ZA+LPS-stimulation of RAW 264.7 cells compared with cells in the
control group (Fig. 4). However, as
PDRN concentration (1, 10 or 100 µg/ml) increased, iNOS expression
decreased compared with the ZA+LPS-stimulation of RAW 264.7 cells.
Similarly, IL-1β and IL-6 expression significantly decreased
following PDRN treatment (1, 10 or 100 µg/ml) compared with
ZA+LPS-stimulation of RAW 264.7 cells. Although 1 µg/ml PDRN
demonstrated no significant differences, 10 or 100 µg/ml PDRN
significantly suppressed TNF-α expression compared with
ZA+LPS-stimulated RAW 264.7 cells.
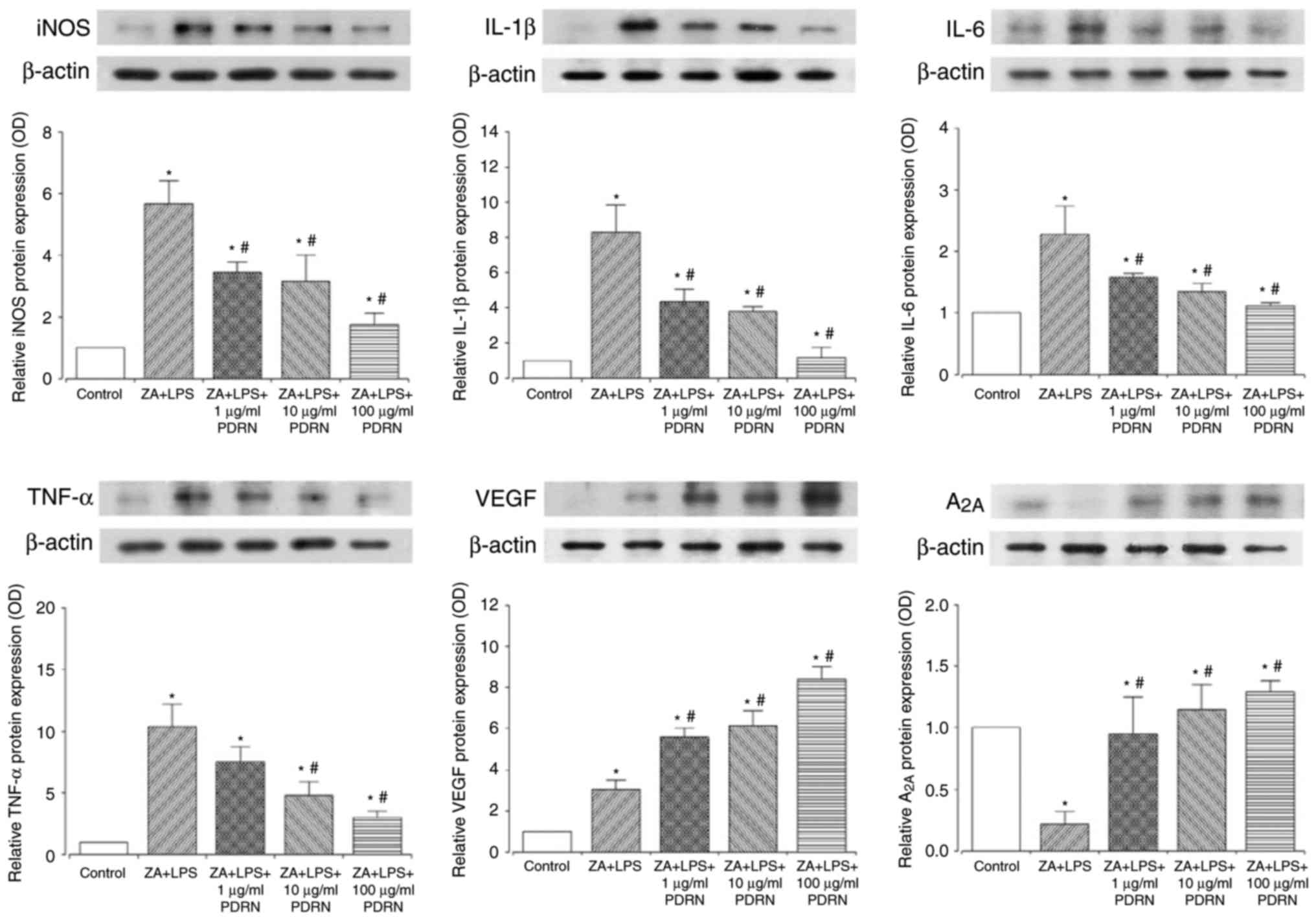 | Figure 4.PDRN decreased inflammatory cytokine
expression and increased A2A and VEGF expression. The
expression levels of iNOS, IL-1β, IL-6, TNF-α, A2A and
VEGF were measured in RAW 264.7 cells treated with 10 µM ZA for 24
h, and then with 0.1 µg/ml LPS and PDRN (1, 10, and 100 µg/ml) for
24 h. Data are presented as mean ± standard error of the mean.
*P<0.05 compared with the control group. #P<0.05
compared with the ZA+LPS group. ZA, zoledronic acid; LPS,
lipopolysaccharide; PDRN, polydeoxynucleotide; iNOS, inducible
nitric oxide synthase; IL, interleukin; TNF, tumor necrosis factor;
VEGF, vascular endothelial growth factor. |
PDRN increases A2A and VEGF
expression
VEGF expression was significantly increased by
ZA+LPS-stimulation compared with control cells, and the expression
was further and significantly increased by PDRN treatment in a
marked dose-dependent manner compared with the ZA+LPS-stimulation
of RAW 264.7 cells (Fig. 4). PDRN
(100 µg/ml) treatment demonstrated the most potent increase in VEGF
expression. ZA+LPS-stimulation significantly lowered the expression
of A2A compared with the control group. However, the
expression of A2A was enhanced by PDRN treatment in what
appeared to be a dose-dependent manner compared with control and
ZA+LPS-stimulated cells.
Discussion
Several factors are associated with BRONJ, including
dental procedures, trauma and inflammation. Previous studies have
elucidated the development of BRONJ along with bacterial infection
(25–28). LPS, which is found in the cell wall
of Gram negative bacteria, induces inflammatory cytokines and NO
via nuclear factor-κB activation and NOS. Furthermore, Muratsu
et al (15) previously
suggested that ZA enhances LPS-stimulated proinflammatory reactions
in macrophages, with a consequent increase in LPS-induced
apoptosis. PDRN has demonstrated effectiveness in decreasing the
circulating levels of several inflammatory cytokines and increasing
VEGF production in previous studies (20,21). The
present study was therefore undertaken to analyze the effect of
PDRN on RAW 264.7 cells pretreated with ZA and stimulated with LPS,
by measuring inflammatory mediators.
In the present study, the cell viability of RAW
264.7 was increased by ZA pre-treatment and LPS stimulation. This
is due to nitrogen-containing bisphosphonates causing cell
apoptosis by inhibiting the mevalonate pathway (4) and LPS stimulation increasing
inflammatory cytokine expression levels (15). Notably, the viability of RAW 264.7
cells stimulated by ZA and LPS were significantly enhanced by PDRN
treatment.
Western blotting revealed that PDRN treatment
significantly decreased the NO production and iNOS expression of
RAW 264.7 cells. iNOS catalyzes the synthesis of NO from the amino
acid L-arginine as an immune defense mechanism in macrophages;
overproduction of NO causes oxidative damage to the surrounding
tissue, with a consequent increase of the inflammatory response
(29). Furthermore, proinflammatory
cytokines, including IL-1β, IL-6 and TNF-α were decreased by PDRN
treatment in what appeared to be a dose-dependent manner.
Overexpressed TNF-α stimulated the release of free radicals such as
NO, inducing cell injury (30,31).
Administration of nitrogen-containing bisphosphonate induced the
release of IL-1β and IL-6 by macrophages or monocytes, resulting in
an acute phase response; one of the known adverse effects of
bisphosphonates (32). The results
of the current study therefore indicate that PDRN downregulates
inflammatory cytokines, and may help in prevention and management
of BRONJ.
The present study also demonstrated that PDRN
increased A2A receptors and VEGF expression on
ZA-pretreated and LPS-stimulated RAW 264.7 cells in what appeared
to be a dose-dependent manner. As ZA and LPS induce cell apoptosis,
it was hypothesized that adenosine A2A receptor
expression decreased in the ZA and LPS-treated cells, and that the
expression of the receptor recovered following PDRN treatment.
However, the mechanism of the recovered adenosine A2A
receptor expression should be further evaluated in a future study.
ZA and LPS treatment (10 µM and 0.1 µg/ml, respectively) increase
VEGF expression without PDRN. Koide et al (33) suggested that LPS induces VEGF
overproduction in macrophages, and Ben-Av et al (34) explained that inflammatory mediators,
including IL-1β, prostaglandin E2 and TNF-α, increase VEGF
expression.
The activation of adenosine A2A receptors
also indicated an increase of VEGF release through the signaling
pathways activated by LPS treatment (35). This suggested that PDRN, an
A2A receptor agonist, is capable of restoring blood flow
and tissue architecture by modulating VEGF expression (36). Additionally, the PDRN-stimulated
increase of A2A receptors caused VEGF production during
pathological conditions characterized by low tissue perfusion
(16,21). Thus, it was hypothesized that PDRN
can be used to counteract the inflammatory process inducing
osteonecrosis of the jaw. Although other mechanisms may also be
involved, these results demonstrate that PDRN increased cell
viability and suppressed the inflammatory process.
In conclusion, the present study demonstrated that
LPS potently accelerated inflammation in RAW 264.7 cells, following
pretreatment with ZA, and that PDRN acted as a suppresser of
inflammatory cytokines. Additionally, increased VEGF expression,
either directly by PDRN or indirectly by other cytokines and
A2A receptors, may have contributed to increased
vascularization and subsequently improved the pathological
condition of BRONJ. As inflammation and LPS may stimulate the
occurrence of BRONJ, the authors of the present study postulated
that PDRN is possibly a candidate for the therapeutic management of
BRONJ.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the
National Research Foundation of Korea (grant nos.
NRF-2014R1A1A1002630 and NRF-2016R1A2B4014600).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
J-HH, JJ and LH wrote the manuscript. JJ, LH and
I-GK performed the experiments. OHN and MSK analyzed the data.
J-HH, JJ, J-WL and B-JC interpreted the data and revised the
manuscript. D-WL conceived and managed the study design, and gave
final approval of the version to be published.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BRONJ
|
bisphosphonate-related osteonecrosis
of the jaw
|
|
PDRN
|
polydeoxynucleotide
|
References
|
1
|
Pennanen N, Lapinjoki S, Urtti A and
Mönkkönen J: Effect of liposomal and free bisphosphonates on the
IL-1 beta, IL-6 and TNF alpha secretion from RAW 264 cells in
vitro. Pharm Res. 12:916–922. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berenson JR and Lipton A: Bisphosphonates
in the treatment of malignant bone disease. Annu Rev Med.
50:237–248. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lipton A: Emerging role of bisphosphonates
in the clinic-antitumor activity and prevention of metastasis to
bone. Cancer Treat Rev. 34 Suppl 1:S25–S30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Beek ER, Cohen LH, Leroy IM, Ebetino
FH, Löwik CW and Papapoulos SE: Differentiating the mechanisms of
antiresorptive action of nitrogen containing bisphosphonates. Bone.
33:805–811. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pataki A, Muller K, Green JR, Ma YF, Li QN
and Jee WS: Effects of short-term treatment with the
bisphosphonates zoledronate and pamidronate on rat bone: A
comparative histomorphometric study on the cancellous bone formed
before, during, and after treatment. Anat Rec. 249:458–468. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rogers MJ: New insights into the molecular
mechanisms of action of bisphosphonates. Curr Pharm Des.
9:2643–2658. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marx RE: Pamidronate (Aredia) and
zoledronate (Zometa) induced avascular necrosis of the jaws: A
growing epidemic. J Oral Maxillofac Surg. 61:1115–1117. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ruggiero SL, Dodson TB, Fantasia J,
Goodday R, Aghaloo T, Mehrotra B and O'Ryan F: American Association
of Oral and Maxillofacial Surgeons: American Association of oral
and maxillofacial surgeons position paper on medication-related
osteonecrosis of the jaw-2014 update. J Oral Maxillofac Surg.
72:1938–1956. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spinelli G, Torresetti M, Lazzeri D, Zhang
YX, Arcuri F, Agostini T and Grassetti L: Microsurgical
reconstruction after bisphosphonate-related osteonecrosis of the
jaw: Our experience with fibula free flap. J Craniofac Surg.
25:788–792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsurushima H, Kokuryo S, Sakaguchi O,
Tanaka J and Tominaga K: Bacterial promotion of
bisphosphonate-induced osteonecrosis in Wistar rats. Int J Oral
Maxillofac Surg. 42:1481–1487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoff AO, Toth BB, Altundag K, Johnson MM,
Warneke CL, Hu M, Nooka A, Sayegh G, Guarneri V, Desrouleaux K, et
al: Frequency and risk factors associated with osteonecrosis of the
jaw in cancer patients treated with intravenous bisphosphonates. J
Bone Miner Res. 23:826–836. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie C, Kang J, Li Z, Schauss AG, Badger
TM, Nagarajan S, Wu T and Wu X: The açaí flavonoid velutin is a
potent anti-inflammatory agent: Blockade of LPS-mediated TNF-α and
IL-6 production through inhibiting NF-κB activation and MAPK
pathway. J Nutr Biochem. 23:1184–1191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nicholas C, Batra S, Vargo MA, Voss OH,
Gavrilin MA, Wewers MD, Guttridge DC, Grotewold E and Doseff AI:
Apigenin blocks lipopolysaccharide-induced lethality in vivo and
proinflammatory cytokines expression by inactivating NF-kappaB
through the suppression of p65 phosphorylation. J Immunol.
179:7121–7127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taira J, Nanbu H and Ueda K: Nitric
oxide-scavenging compounds in Agrimonia pilosa Ledeb on LPS-induced
RAW264.7 macrophages. Food Chem. 115:1221–1227. 2009. View Article : Google Scholar
|
|
15
|
Muratsu D, Yoshiga D, Taketomi T, Onimura
T, Seki Y, Matsumoto A and Nakamura S: Zoledronic acid enhances
lipopolysaccharide-stimulated proinflammatory reactions through
controlled expression of SOCS1 in macrophages. PLoS One.
8:e679062013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Altavilla D, Bitto A, Polito F, Marini H,
Minutoli L, Di Stefano V, Irrera N, Cattarini G and Squadrito F:
Polydeoxyribonucleotide (PDRN): A safe approach to induce
therapeutic angiogenesis in peripheral artery occlusive disease and
in diabetic foot ulcers. Cardiovasc Hematol Agents Med Chem.
7:313–321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Squadrito F, Bitto A, Altavilla D,
Arcoraci V, De Caridi G, De Feo ME, Corrao S, Pallio G, Sterrantino
C, Minutoli L, et al: The effect of PDRN, an adenosine receptor A2A
agonist, on the healing of chronic diabetic foot ulcers: Results of
a clinical trial. J Clin Endocrinol Metab. 99:E746–E753. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gomez G and Sitkovsky MV: Targeting G
protein-coupled A2a adenosine receptors to engineer inflammation in
vivo. Int J Biochem Cell Biol. 35:410–414. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cronstein BN: Adenosine, an endogenous
anti-inflammatory agent. J Appl Physiol (1985). 76:5–13. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bitto A, Polito F, Irrera N, D'Ascola A,
Avenoso A, Nastasi G, Campo GM, Micali A, Bagnato G, Minutoli L, et
al: Polydeoxyribonucleotide reduces cytokine production and the
severity of collagen-induced arthritis by stimulation of adenosine
A(2A) receptor. Arthritis Rheum. 63:3364–3371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeong EK, Jang HJ, Kim SS, Lee SY, Oh MY,
Kim HJ, Eom DW, Ham JY and Han DJ: Protective effect of
polydeoxyribonucleotide against renal ischemia-reperfusion injury
in mice. Transplant Proc. 48:1251–1257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ramanathan M, Pinhal-Enfield G, Hao I and
Leibovich SJ: Synergistic up-regulation of vascular endothelial
growth factor (VEGF) expression in macrophages by adenosine A2A
receptor agonists and endotoxin involves transcriptional regulation
via the hypoxia response element in the VEGF promoter. Mol Biol
Cell. 18:14–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ziebart T, Pabst A, Klein MO, Kämmerer P,
Gauss L, Brüllmann D, Al-Nawas B and Walter C: Bisphosphonates:
Restrictions for vasculogenesis and angiogenesis: Inhibition of
cell function of endothelial progenitor cells and mature
endothelial cells in vitro. Clin Oral Investig. 15:105–111. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Naik NH and Russo TA:
Bisphosphonate-related osteonecrosis of the jaw: The role of
actinomyces. Clin Infect Dis. 49:1729–1732. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ikeda T, Kuraguchi J, Kogashiwa Y, Yokoi
H, Satomi T and Kohno N: Successful treatment of
bisphosphonate-related osteonecrosis of the jaw (BRONJ) patients
with sitafloxacin: New strategies for the treatment of BRONJ. Bone.
73:217–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boff RC, Salum FG, Figueiredo MA and
Cherubini K: Important aspects regarding the role of microorganisms
in bisphosphonate-related osteonecrosis of the jaws. Arch Oral
Biol. 59:790–799. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakaguchi O, Kokuryo S, Tsurushima H,
Tanaka J, Habu M, Uehara M, Nishihara T and Tominaga K:
Lipopolysaccharide aggravates bisphosphonate-induced osteonecrosis
in rats. Int J Oral Maxillofac Surg. 44:528–534. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Burney S, Caulfield JL, Niles JC, Wishnok
JS and Tannenbaum SR: The chemistry of DNA damage from nitric oxide
and peroxynitrite. Mutat Res. 424:37–49. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Makkonen N, Hirvonen MR, Teräväinen T,
Savolainen K and Mönkkönen J: Different effects of three
bisphosphonates on nitric oxide production by RAW 264
macrophage-like cells in vitro. J Pharmacol Exp Ther.
277:1097–1102. 1996.PubMed/NCBI
|
|
31
|
Kobayashi Y: The regulatory role of nitric
oxide in proinflammatory cytokine expression during the induction
and resolution of inflammation. J Leukoc Biol. 88:1157–1162. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adami S, Bhalla AK, Dorizzi R, Montesanti
F, Rosini S, Salvagno G and Lo Cascio V: The acute-phase response
after bisphosphonate administration. Calcif Tissue Int. 41:326–331.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koide N, Odkhuu E, Naiki Y, Tsolmongyn B,
Ito K, Komatsu T, Yoshida T and Yokochi T: Augmentation of
LPS-induced vascular endothelial cell growth factor production in
macrophages by transforming growth factor-β1. Innate Immun.
20:816–825. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ben-Av P, Crofford LJ, Wilder RL and Hla
T: Induction of vascular endothelial growth factor expression in
synovial fibroblasts by prostaglandin E and interleukin-1: A
potential mechanism for inflammatory angiogenesis. FEBS Lett.
372:83–87. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
De Ponti C, Carini R, Alchera E, Nitti MP,
Locati M, Albano E, Cairo G and Tacchini L: Adenosine A2a
receptor-mediated, normoxic induction of HIF-1 through PKC and
PI-3K-dependent pathways in macrophages. J Leukoc Biol. 82:392–402.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Polito F, Bitto A, Galeano M, Irrera N,
Marini H, Calò M, Squadrito F and Altavilla D:
Polydeoxyribonucleotide restores blood flow in an experimental
model of ischemic skin flaps. J Vasc Surg. 55:479–488. 2012.
View Article : Google Scholar : PubMed/NCBI
|