Introduction
Children undergoing tonsillectomy are at risk of
experiencing complications, including postoperative haemorrhage,
nausea and vomiting, and cognitive dysfunction (1). A previous study has identified the
clinical features of children undergoing tonsillectomy and
presented the adverse outcomes and therapeutic schedule (2). Postoperative vomiting and pain due to
tonsillectomy are two of the most frequent complications in
pediatric patients (3,4). In addition, Eisert et al
(5) indicated that bleeding remains
the most important complication of tonsillectomy in pediatric
patients and coagulation tests are widely applied to assess
bleeding events. Furthermore, the immunological sequela of
tonsillectomy in pediatric patients has also been indicated by
physicians (6). Of note, cognitive
dysfunction following tonsillectomy is a post-operative
complication that occurs most frequently in pediatric patients
(7,8). Therefore, prevention and treatment of
postoperative cognitive dysfunction following tonsillectomy is
essential for pediatric patients (9).
Cognitive impairment following tonsillectomy is a
serious clinical problem as it reduces intelligence and emotional
stability (10,11). Acute coagulatory dysfunction during
adenoidectomy and tonsillectomy has been investigated in a previous
case report on a pediatric patient (12). In addition, postoperative cognitive
dysfunction, characterized by impaired consciousness and disordered
thinking patterns, represents a major complication in pediatric
patients after anesthesia and tonsillectomy (13). Although tonsillectomy provides
numerous advantages, including less bleeding and fewer infections
compared with conventional open procedures, cognitive dysfunction
occurs due to adverse effects on cerebral function (14,15).
Previous evidence indicates that cerebral oxygenation is decreased
following tonsillectomy, as suggested by lightheadedness,
nightmares, nausea, vomiting and constipation, which may be
correlated with neurocognitive changes in pediatric patients
(16). These results suggest that
the cognitive competence of the patients, which is regulated by the
nerve center within the brainstem, may be affected by tonsillectomy
in pediatric patients.
Dexmedetomidine (DEX) is a highly selective
α2-adrenergic receptor agonist and acts as a multifunctional drug
in the treatment of various human diseases (17). A previous study has suggested that
DEX is efficient in the treatment of nerve diseases through the
beneficial effects of acting as an anxiolytic, sedative, analgesic
and blocking the sympathetic nervous system (18). In addition, a clinical study has
indicated that DEX has analgesic, anxiolytic and anti-delirium
effects, while causing little respiratory depression (19). DEX treatment may improve behavioral
disturbances, including aggression, agitation and cognitive
impairment (20). These neurological
function impairments may occur in pediatric patients with
postoperative cognitive dysfunction. Therefore, it was hypothesized
that DEX may be beneficial for restoring cognitive function in
pediatric patients following tonsillectomy.
In the present study, the effects of DEX
administration were assessed in pediatric patients with cognitive
impairment after tonsillectomy. Although a previous study has
suggested that DEX is recommended for decreasing the risk of
postoperative vomiting, as well as to alleviate pain, inflammation
and nausea for patients in intensive care (21), the therapeutic efficacy of DEX on the
restoration of cognitive function has remained elusive. In addition
to the influence of DEX on the recovery of cognitive impairment,
its effects on the levels of interleukin (IL)-6 and −1, tumor
necrosis factor (TNF)-α, C-reactive protein (CRP), neuron-specific
enolase (NSE), superoxide dismutase (SOD), cortisol and melatonin
in pediatric patients following tonsillectomy were also assessed.
The results indicate that DEX improves cognitive impairment in
pediatric patients following tonsillectomy, at least in part,
through the regulation of IL-6, CRP, cortisol and melatonin
levels.
Materials and methods
Patients
Pediatric patients aged 6–12 years who had undergone
tonsillectomy at Qilu Hospital of Shandong University (Jinan,
China) between May 2014 and July 2015 were subjected to a Mini
Mental State Examination (MMSE) and requested to complete a 40-item
quality of life (MONEX-40) questionnaire. The MMSE was used to
screen for cognitive dysfunction. Only patients post tonsillectomy
with post-operative cognitive dysfunction were included in the
study. The patients were randomly divided into two groups, and
double-blinded trails were performed. Further details, including a
description of the inclusion/exclusion criteria and the allocation
method, are specified in a previously published study (22). In a preliminary experiment, the
patients with cognitive dysfunction received DEX (1.0, 5.0, 10.0,
15.0 and 20.0 mg/kg/day) or placebo (PBS) through intravenous
injection for 4 weeks. The dose-limiting toxicities (DLT) and
maximum tolerated dose (MTD) of DEX were 10 and 15 mg/kg,
respectively, determined by the common treatment-emergent adverse
events of DEX as described previously (23).
Study design
The double-blinded study was performed in 3 phases:
Baseline stage, double-blinded treatment phase (4-week
dose-titration treatment, preliminary experiment) and 4-week
post-treatment (maintenance treatment) of those patients who
volunteered to continue to complete the ongoing extension study.
The patients were randomized into two groups which were treated
once a day with DEX or placebo in a double-blinded manner. In the
final investigation, treatment was continued with the 10 mg/kg dose
of DEX or placebo to achieve the ideal effect throughout the
maintenance period.
Outcome measures
The MMSE (24) and
The 40-item Monell Extended Sniffin' Sticks Identification Test
(MONEX-40) (40 items for assessing functional limitation) (25) were used for assessing the pediatric
patients with cognitive dysfunction at prior to treatment and
post-treatment. Clinical cognitive function scores were evaluated
as described in a previous study (26).
Efficacy and safety assessments
Efficacy assessments, including the median percent
reduction scores and response rate, were analyzed with the baseline
values as a reference during the 4-week double-blinded treatment
period with DEX (10 mg/kg) or placebo. In addition, overall safety
and pharmacokinetic analyses were performed according to the
protocols of previous clinical studies (27,28). The
safety assessments regarding the most frequent treatment-emergent
adverse events were performed in all randomized patients. The
dosage of DEX was determined by DLT as described above.
Dose-response analysis was performed when the last dose of the drug
was injected as described previously (29).
ELISA
In the present study, the serum levels of IL-6 (cat.
no. D6050), IL-1 (cat. no. DLB50), TNF-α (cat. no. DTA00C), SOD
(cat. no. DYC3419-2), NSE (cat. no. DY5169-05), CRP (cat. no.
DCRP00), cortisol (cat. no. KGE008B) (all Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and melatonin (cat. no. KA1166; Abnova,
Taipei, Taiwan) were assessed using commercialized ELISA kits. The
ELISAs were performed according to the manufacturer's protocols.
The results were determined by measuring the absorbance at 450 nm
with an ELISA reader and finally converted to the concentrations of
IL-6, IL-1, TNF-α, SOD, NSE, CRP, cortisol and melatonin.
Statistical analysis
All values are expressed as the mean ± standard
error of the mean. All data were analyzed using SPSS software
version 19.0 (IBM Corp., Armonk, NY, USA). Statistical significance
of differences between mean values were assessed by Student's
t-test for paired data. Comparisons of data between multiple groups
were performed by one-way analysis of variance followed by Tukey's
post hoc test. Responder rates and treatment-emergent adverse
events were analyzed with the χ2 test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
A total of 186 pediatric patients with early
postoperative cognitive dysfunction following tonsillectomy were
recruited for the present study. The characteristics of the
patients are summarized in Table I.
The gender distribution within the cohort was equal. The cognitive
dysfunction of the patients was determined by the MMSE at prior to
treatment and post treatment. No other medications or painkillers
were taken during the treatment period.
 | Table I.Characteristics of the study
population post tonsillectomy. |
Table I.
Characteristics of the study
population post tonsillectomy.
| Characteristic | Value |
|---|
| Total patients with
post-operative cognitive dysfunction | 186 (100) |
| Gender |
|
|
Male | 90 (48) |
|
Female | 96 (52) |
| Median age, years
(range) | 8.5
(6–12) |
|
MMSE | 16.5±3.2 |
|
MONEX-40 | 134.4±12.5 |
| Pain
scores |
7.2±2.4 |
| Drug therapy | 186 (100) |
|
DEX | 112 (60) |
|
Placebo | 74 (40) |
Duration of treatment, DLT and MTD of
DEX
The median overall duration of DEX treatment was 4
weeks followed by maintenance treatment. The dosing cohorts of DEX
were 1.0, 5.0, 10.0, 15.0 and 20 mg/kg to evaluate the optimal
dosage. According to the results in Table II, the MTD of DEX was 20 mg/kg once
a day. The DLT was determined as 15 mg/kg of DEX once a day. The
group treated with the lowest dose of DEX presented with the fewest
side effects. Analysis indicated that the common treatment-emergent
adverse events of DEX were hypertension, fatigue, proteinuria,
hypertriglyceridemia, constipation and peripheral edema. Of note,
most of the patients treated with the MTD of DEX required a dose
reduction due to cumulative toxicity. Therefore, most of the
patients that were subsequently enrolled received a dose of 10.0
mg/kg DEX to achieve ideal tolerability and therapeutic efficacy in
the pediatric patients with early postoperative cognitive
dysfunction.
 | Table II.Overall incidence of
treatment-emergent adverse events of DEX. |
Table II.
Overall incidence of
treatment-emergent adverse events of DEX.
|
|
| DEX (mg/kg) |
|---|
|
|
|
|
|---|
| Adverse event | Total (n=32) | 1–10 (n=10) | 15 (n=14) | 20 (n=8) |
|---|
| Hypertension | 5 | 1 | 2 | 2 |
| Fatigue | 3 | 1 | 1 | 1 |
| Proteinuria | 4 | 0 | 1 | 3 |
|
Hypertriglyceridemia | 2 | 0 | 1 | 1 |
| Constipation | 3 | 0 | 1 | 2 |
| Edema
peripheral | 3 | 1 | 1 | 1 |
Treatment-emergent adverse events
associated with DEX
Pediatric patients with early postoperative
cognitive dysfunction after tonsillectomy received DEX therapy with
post-baseline safety evaluation were included in the safety
population. After the administration of the last dose of DEX, it
was determined that the most common treatment-emergent adverse
events of DEX (10 mg/kg) were hypertension and proteinuria (≥10%
each) (Table III). The
administration of DEX at ≥15 mg/kg is not advisable due to more
side effects and therefore, few patients were treated with such
doses. Of the total patient population, 96 completed the overall
maintenance period of the phase III study.
 | Table III.Severity of treatment-emergent
hypertension and proteinuria as adverse events of DEX. |
Table III.
Severity of treatment-emergent
hypertension and proteinuria as adverse events of DEX.
|
|
| DEX (mg/kg) |
|---|
|
|
|
|
|---|
| Adverse
event/grade | Total (n=32) | 1–10 (n=10) | 15 (n=14) | 20 (n=8) |
|---|
| Hypertension | 5 | 1 | 2 | 2 |
| 1 | 1 | 0 | 0 | 1 |
| 2 | 2 | 0 | 1 | 1 |
| 3 | 2 | 1 | 1 | 0 |
| Proteinuria | 4 | 0 | 1 | 3 |
| 1 | 1 | 0 | 0 | 1 |
| 2 | 2 | 0 | 1 | 1 |
| 3 | 1 | 0 | 0 | 1 |
Analysis of the efficacy of DEX in
alleviating cognitive impairment in pediatric patients after
tonsillectomy
In order to investigate the efficacy of DEX in
improving of cognitive function in children affected after
tonsillectomy, the cognitive competence was assessed in 186
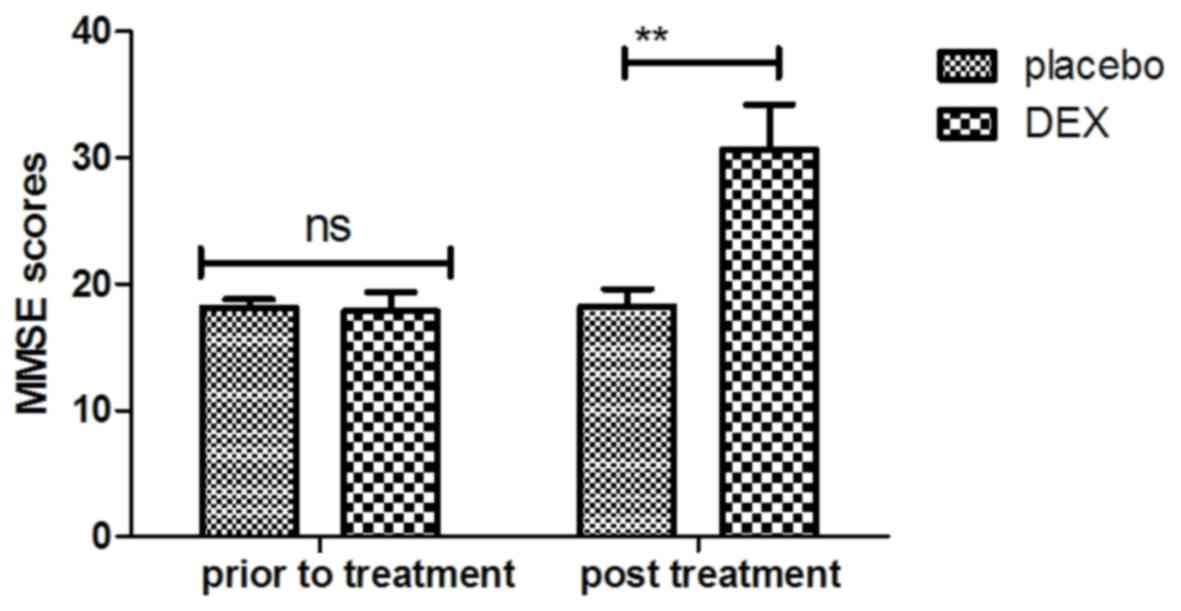
patients using the MMSE and MONEX-40. As presented in Fig. 1, it was demonstrated that DEX
treatment significantly improved the cognitive competence of
children with cognitive dysfunction determined by the MMSE scores.
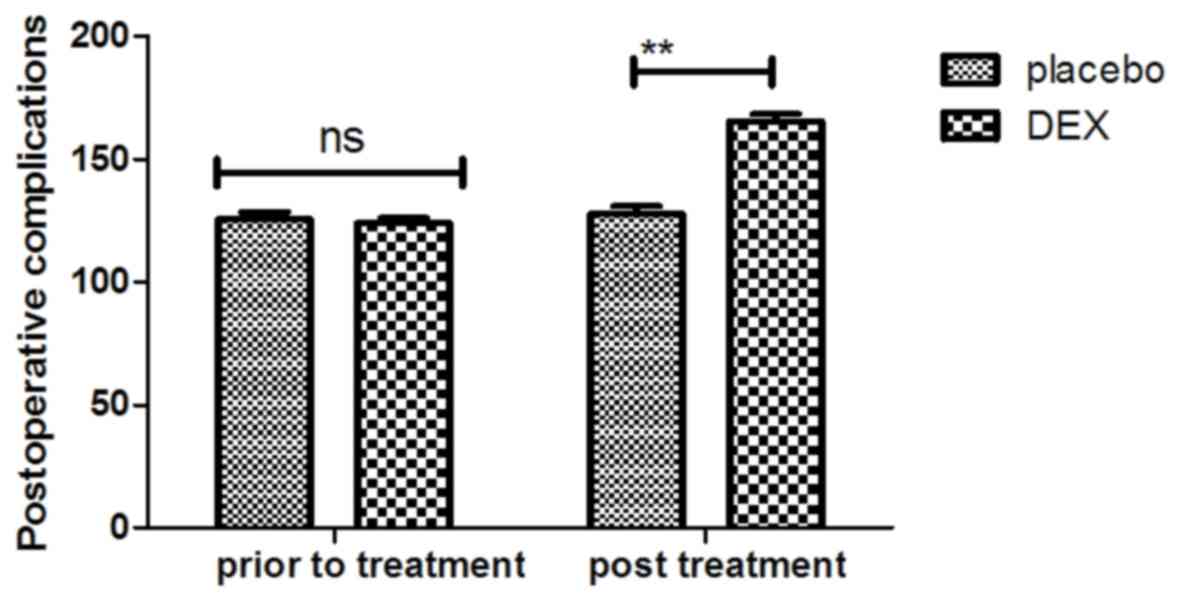
In addition, assessment with the MONEX-40 questionnaire indicated
that DEX treatment reduced postoperative complications (daytime
sedation, lightheadedness, nightmares, nausea, vomiting and
constipation) compared with the placebo group (Fig. 2). It was also observed that the
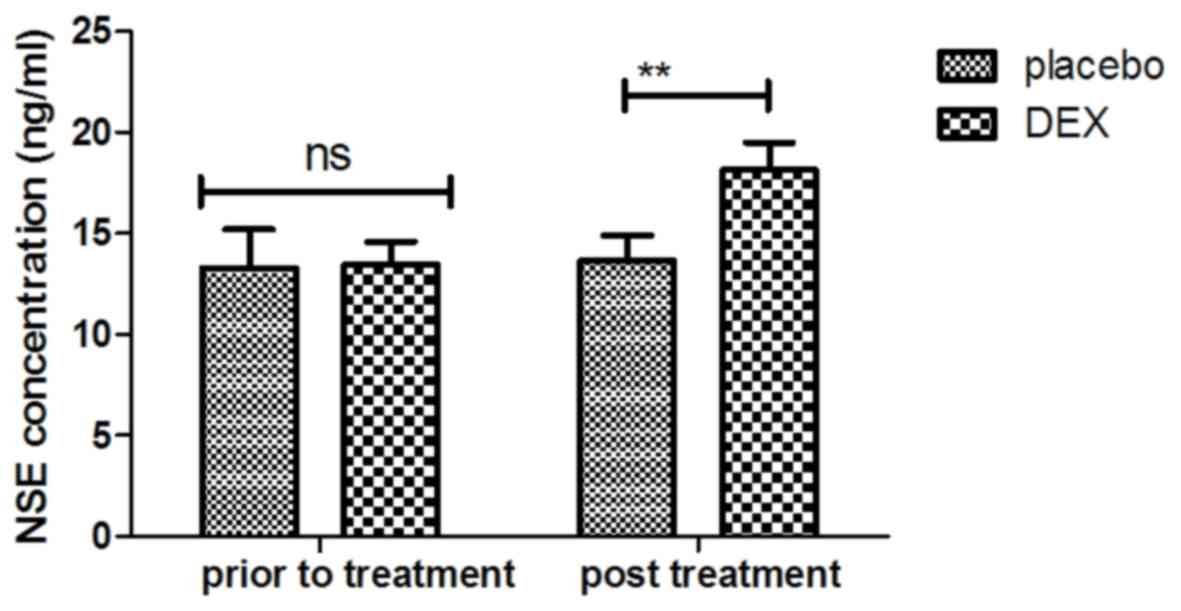
plasma concentration of NSE was increased in patients after DEX
treatment compared with that in the placebo group (Fig. 3). Of note, the plasma concentration
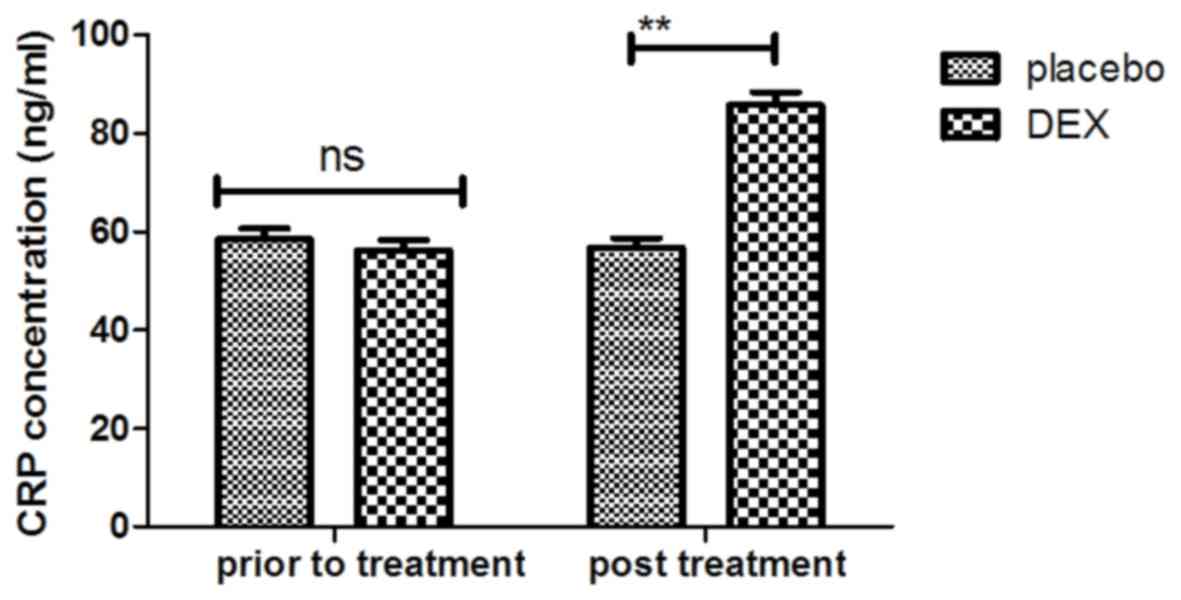
of CRP was decreased in the DEX group (Fig. 4). Furthermore, the serum levels of
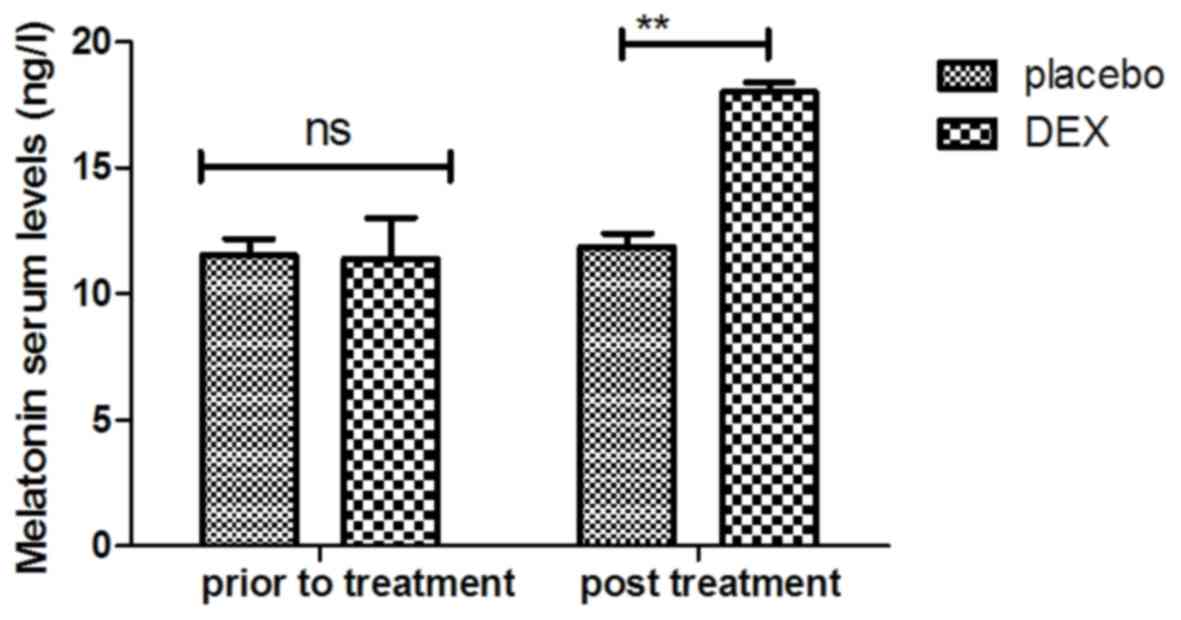
melatonin were increased in patients after DEX treatment compared
with those in the placebo group (Fig.
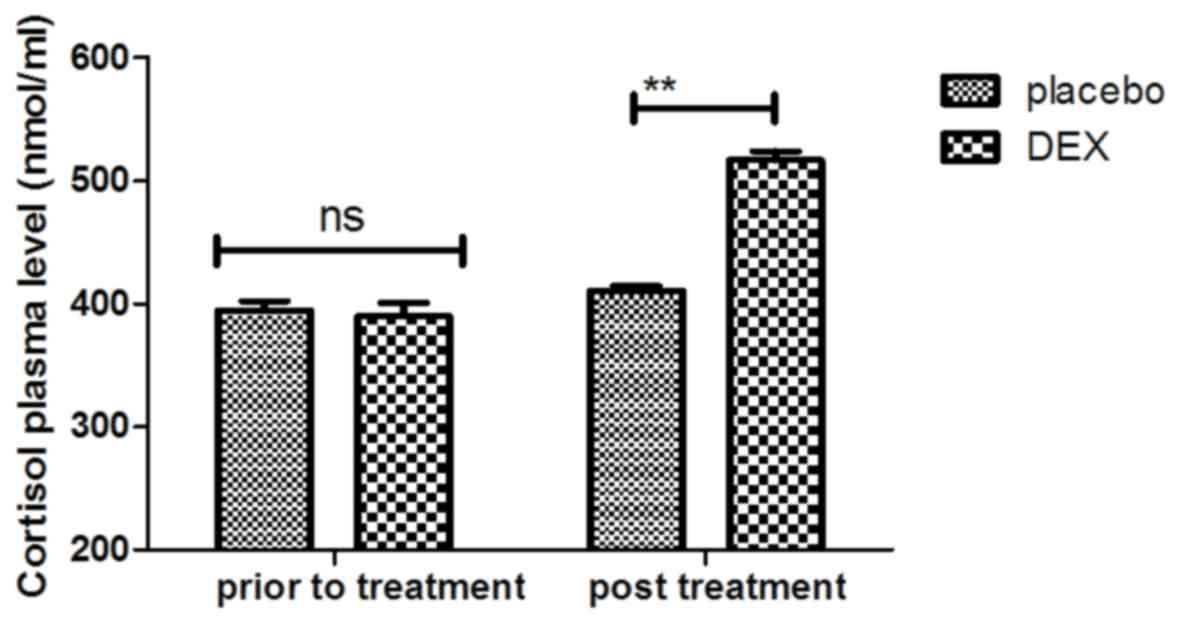
5). Importantly, the plasma concentration of cortisol was
upregulated by DEX compared with that in the placebo group
(Fig. 6). For all these factors no
significant changes were observed in the placebo group between the
prior to treatment and post treatment time points. These results
suggest that DEX improves cognitive competence through the
regulation of nerve growth factors in pediatric patients with
cognitive dysfunction after tonsillectomy.
Analysis of inflammatory factors in
pediatric patients with tonsillectomy after treatment with DEX
The inflammatory factors in pediatric patients with
post-tonsillectomy cognitive dysfunction after treatment with DEX
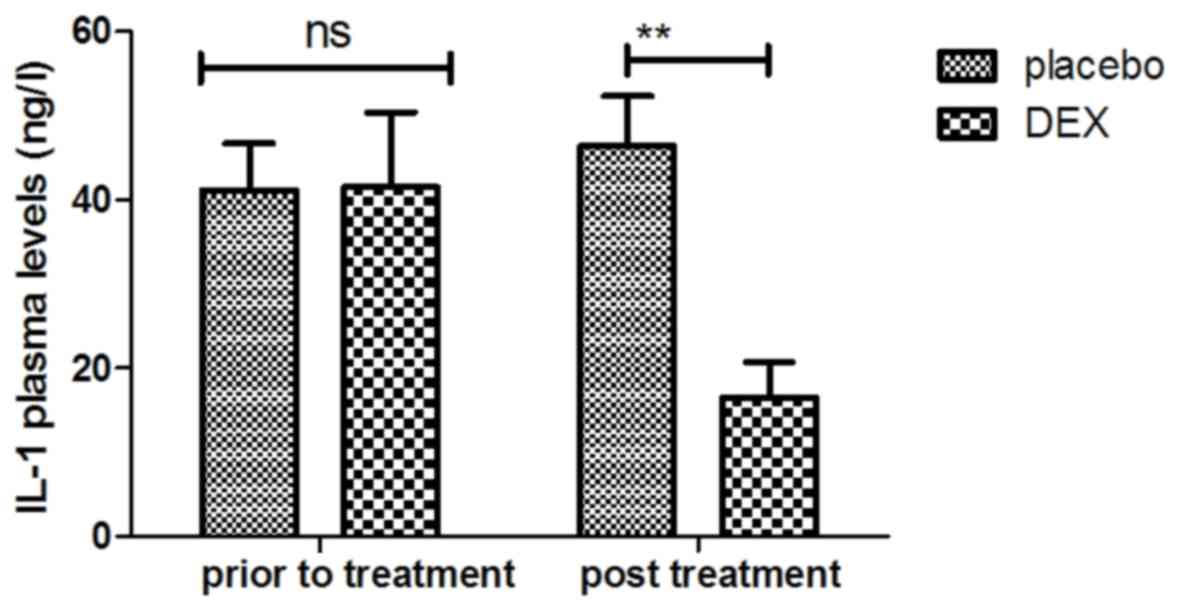
were analyzed. It was indicated that the plasma levels of IL-1 were
increased after DEX treatment (Fig.
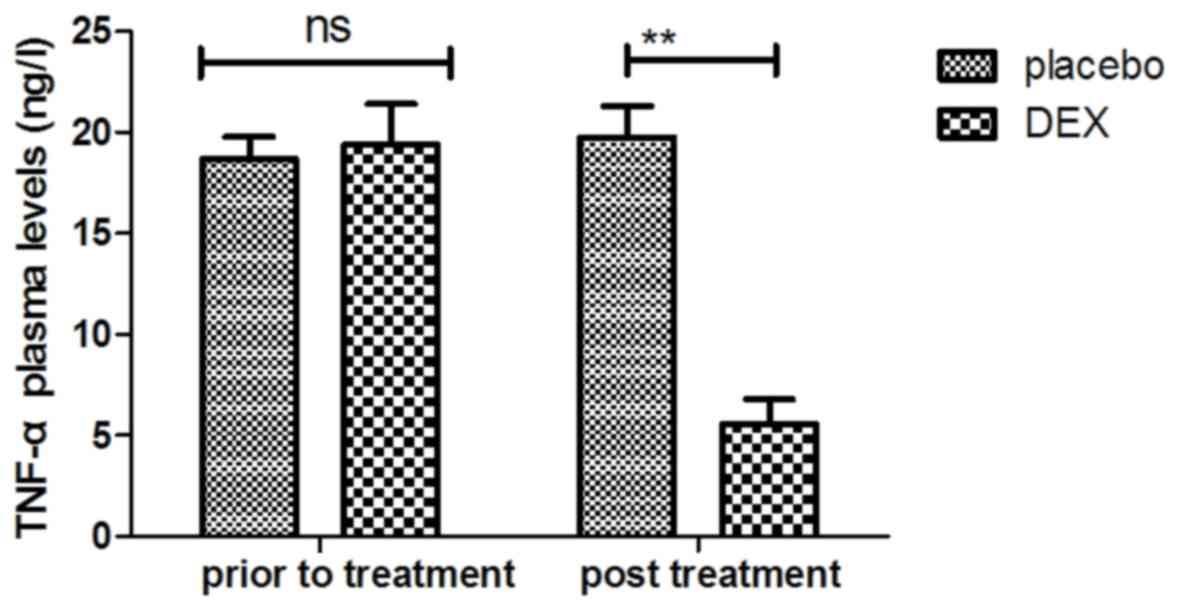
7). In addition, DEX treatment downregulated the plasma levels
of TNF-α to inhibit the inflammatory response (Fig. 8). Furthermore, the plasma
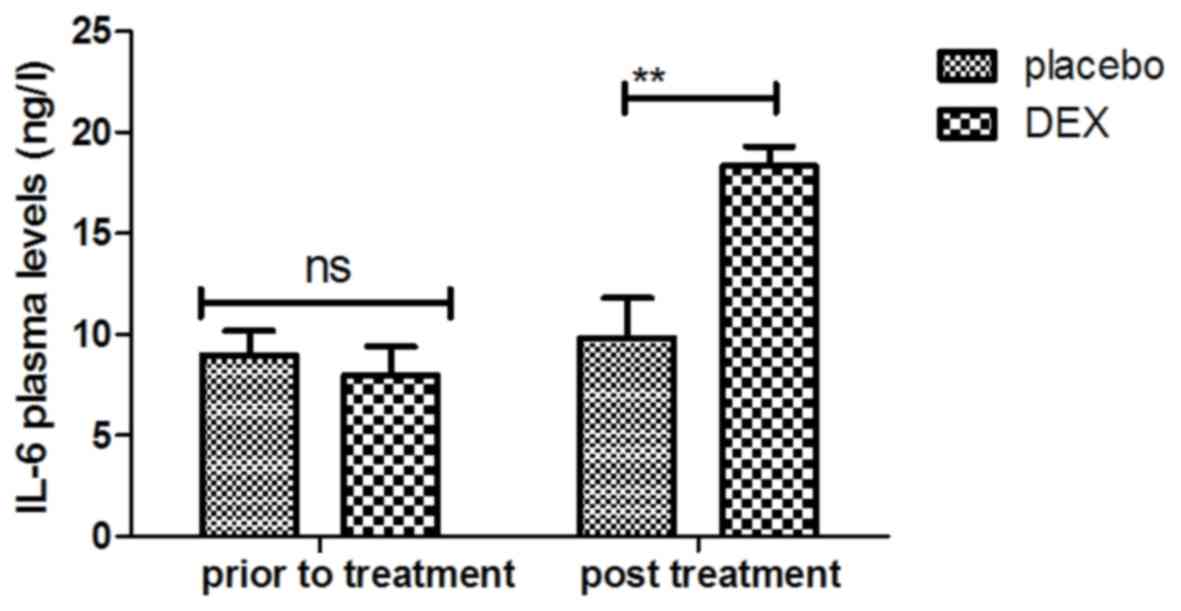
concentration of IL-6 was increased after DEX treatment (Fig. 9). Treatment with DEX also improved
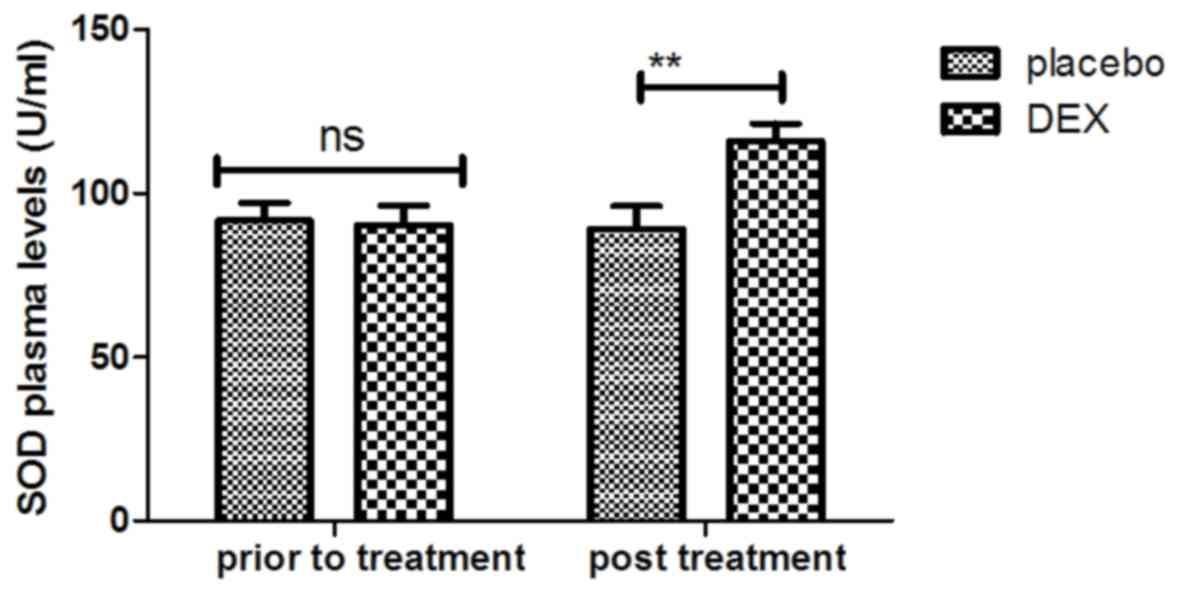
SOD levels in the plasma, which may contribute to the recovery of
cognitive dysfunction after tonsillectomy (Fig. 10). These observations indicate that
inflammatory factors in pediatric patients with tonsillectomy were
improved after treatment with DEX. For the above factors no
significant changes were observed in the placebo group between the
prior to treatment and post treatment time points.
Discussion
Previous studies have indicated that early
postoperative cognitive dysfunction is one of the most common
adverse effects in pediatric patients after tonsillectomy (30,31). DEX
has been approved by the Chinese Food and Drug Administration for
the treatment of inflammation (32).
In addition, it has been suggested that DEX is an efficient drug
for the treatment of postoperative cognitive dysfunction (33,34). In
the present study, the clinical therapeutic effects of DEX on
recovery of cognitive dysfunction in pediatric patients that had
undergone tonsillectomy were investigated. In a preliminary study,
the DLT and MTD of DEX were evaluated in pediatric patients with
cognitive dysfunction after tonsillectomy. The treatment-emergent
adverse events of DEX were analyzed to evaluate the clinical
efficacy and pharmacodynamics. Furthermore, the serum levels of
inflammatory and nerve growth factors were analyzed to determine
the mechanism of DEX-induced recovery of cognitive function. The
results indicate that DEX improves cognitive dysfunction in
children after tonsillectomy through inhibition of the expression
of inflammatory factors and enhancement of neuroprotective protein
expression. These results suggest that DEX may be an efficient
cognitive function-enhancing drug for the treatment of
tonsillectomy-associated cognitive impairment in pediatric
patients.
Postoperative cognitive dysfunction is a
multifactorial adverse event and most frequently occurs in
pediatric and elderly patients after surgery (35). Surgical therapy is the most common
clinical treatment of frequent tonsil inflammation. Tonsillectomy
is also one of the most common surgical procedure performed in
preschool children (36). Although
tonsillectomy provides numerous benefits, various adverse
reactions, including throat pain, haemorrhage, tonsillar fossa
epithelisation and even cognitive dysfunction, may occur in
patients after tonsillectomy (37).
A previous study has reported that cognitive dysfunction may affect
children for up to 6 months after the operation (13). Therefore, the treatment of
postoperative cognitive dysfunction is essential for pediatric
patients in the clinic.
DEX has been increasingly applied during clinical
surgery as a regional anesthetic (38). It is a multifunctional drug and a
highly efficient and specific α2 agonist, which may reduce the
release of norepinephrine, the activity of norepinephrine receptors
exert anxiolytic and calming effects, and ameliorate sleep cycle
disorders (22,39). Numerous clinical trials and studies
have demonstrated that DEX protects cells in the hippocampus
against injury and reduces ischemia-reperfusion injury of human
organs (40,41). The present study further explored the
neuroprotective effects of DEX in pediatric patients with cognitive
dysfunction after tonsillectomy. DEX has sedative, analgesic and
hypnotic effects, and antagonizes sympathetic activity, which
results in improvement of cognitive competence. A previous study
has indicated that recovery from cognitive dysfunction required
>6 weeks for pediatric patients with tonsillectomy (42). The present study only implemented a
4-week observation period and in the placebo group, no improvement
was seen; however, it should be clarified that the post-operative
cognitive dysfunction following tonsillectomy is a non-permanent
condition, and that recovery may take >4 weeks. However, further
study is required to assess the average time of recovery from
cognitive dysfunction in pediatric patients following tonsillectomy
procedures, and the specific effects of DEX and the underlying
molecular mechanisms of its cerebral protection require to be
elucidated.
An integrative review has examined the use of DEX as
an anesthetic for monitored anesthesia care and regional
anesthesia, and has evaluated the effect of DEX on the incidence of
postoperative cognitive dysfunction after non-cardiac and
non-neurologic surgery (43). DEX
acts as an antagonist of transmembrane G protein-coupled receptor
that significantly contributes to distribution of hippocampal focal
adhesion kinase tyrosine phosphorylation in the peripheral nervous
system, autonomic ganglia and central nervous system (44). The major function of DEX is to
selectivity activate α2 adrenergic receptors to regulate the locus
coeruleus of the central nervous system. A phase III
double-blinded, randomized controlled trial has suggested that the
effects of a single low dose of dexamethasone prior to non-cardiac
and non-neurologic surgery and general anesthesia provides
beneficial effects on postoperative cognitive dysfunction (45). Another clinical trial reported on the
preventive effects of low-dose DEX on postoperative cognitive
dysfunction and the quality of recovery in oral cancer patients
through modulating the kinetics of cortisol, expression of
inflammatory cytokines and plasma concentration of melatonin
(46). The present study indicates
that DEX has a beneficial effect on the recovery from cognitive
dysfunction in pediatric patients after tonsillectomy through
inhibition of the expression of inflammatory factors and
enhancement of neuroprotective protein expression. However, as it
was not determined whether the patients had any cognitive
dysfunction prior to surgery, it may not have been
surgery-associated in all cases.
In conclusion, the present study observed that DEX
increased the plasma concentration of cortisol in pediatric
patients with early postoperative cognitive dysfunction after
tonsillectomy, and cortisol is negatively associated with the risk
of cognitive dysfunction. Of note, DEX reduced the serum levels of
IL-1 and TNF-α to decrease nerve injury. Furthermore, DEX treatment
improved the cognitive ability and relieved cognitive dysfunction
caused by tonsillectomy. Taken together, DEX improves cognitive
impairment in children after tonsillectomy through regulation of
the expression of inflammatory factors and neuroprotective
proteins, which may widely apply for impairments of brain function
caused by tonsillectomy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL designed the study. CH and RF performed the
experiments and analyzed the data.
Ethical approval and consent to
participate
This phase-III study (no. QLSDHOS0200810102C) was
performed in strict accordance with the recommendations in the
Guidelines of Qilu Hospital of Shandong University (Jinan, China)
between October 2008 and May 2014. All patients and their guardians
were required to review trial protocols and their amendments, and
provide informed consent.
Consent for publication
All patients provide written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Elgueta MF, Echevarría GC, De la Fuente N,
Cabrera F, Valderrama A, Cabezón R, Muñoz HR and Cortinez LI:
Effect of intravenous fluid therapy on postoperative vomiting in
children undergoing tonsillectomy. Br J Anaesth. 110:607–614. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buckley A and Savage E: Preoperative
information needs of children undergoing tonsillectomy. J Clin
Nurs. 19:2879–2887. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leong AC: A randomised controlled trial to
compare postoperative pain in children undergoing tonsillectomy
using cold steel dissection with bipolar haemostasis versus
coblation technique. Clin Otolaryngol. 34:579–580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parker D, Howe L, Unsworth V and Hilliam
R: A randomised controlled trial to compare postoperative pain in
children undergoing tonsillectomy using cold steel dissection with
bipolar haemostasis versus coblation technique. Clin Otolaryngol.
34:225–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eisert S, Hovermann M, Bier H and Göbel U:
Preoperative screening for coagulation disorders in children
undergoing adenoidectomy (AT) and tonsillectomy (TE): Does it
prevent bleeding complications? Klin Padiatr. 218:334–339. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reichel O, Mayr D, Winterhoff J, de la
Chaux R, Hagedorn H and Berghaus A: Tonsillotomy or
tonsillectomy?-a prospective study comparing histological and
immunological findings in recurrent tonsillitis and tonsillar
hyperplasia. Eur Arch Otorhinolaryngol. 264:277–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prows CA, Zhang X, Huth MM, Zhang K,
Saldaña SN, Daraiseh NM, Esslinger HR, Freeman E, Greinwald JH,
Martin LJ and Sadhasivam S: Codeine-related adverse drug reactions
in children following tonsillectomy: A prospective study.
Laryngoscope. 124:1242–1250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Merry AF, Edwards KE, Ahmad Z, Barber C,
Mahadevan M and Frampton C: Randomized comparison between the
combination of acetaminophen and ibuprofen and each constituent
alone for analgesia following tonsillectomy in children. Can J
Anaesth. 60:1180–1189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liasis A, Nischal KK, Leighton S, Yap S,
Hayward R and Dunaway D: Adenoid-tonsillectomy to treat visual
dysfunction in a child with craniosynostosis. Pediatr Neurosurg.
41:197–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maini S, Osborne JE, Fadl HM, Spyridakou
C, Ogunyemi L and Hill P: Temporomandibular joint dysfunction
following tonsillectomy. Clin Otolaryngol Allied Sci. 27:57–60.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garnett JD and Ramadan HH: Swallowing
dysfunction after tonsillectomy. Otolaryngol Head Neck Surg.
114:813–817. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chu JT, Wu WM and Liu FF: Acute
coagulatory dysfunction during adenoidectomy and tonsillectomy in a
child. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 43:572008.(In
Chinese). PubMed/NCBI
|
|
13
|
Hart JA and Glickman-Simon R:
Phytomedicines for Helicobacter pylori, garlic for hypertension,
intraoperative acupuncture for tonsillectomy, Omega-3 fatty acids
for cognitive decline, qigong for COPD. Explore (NY). 12:141–145.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jimenez N, Anderson GD, Shen DD, Nielsen
SS, Farin FM, Seidel K and Lynn AM: Is ethnicity associated with
morphine's side effects in children? Morphine pharmacokinetics,
analgesic response, and side effects in children having
tonsillectomy. Paediat Anaesth. 22:669–675. 2012. View Article : Google Scholar
|
|
15
|
Bellis JR, Pirmohamed M, Nunn AJ, Loke YK,
De S, Golder S and Kirkham JJ: Dexamethasone and haemorrhage risk
in paediatric tonsillectomy: A systematic review and meta-analysis.
Br J Anaesth. 113:23–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sutters KA, Miaskowski C, Holdridge-Zeuner
D, Waite S, Paul SM, Savedra MC and Lanier B: Time-contingent
dosing of an opioid analgesic after tonsillectomy does not increase
moderate-to-severe side effects in children. Pain Management Nurs.
6:49–57. 2005. View Article : Google Scholar
|
|
17
|
Mason KP and Lerman J: Review article:
Dexmedetomidine in children: Current knowledge and future
applications. Anesth Analg. 113:1129–1142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Zhao B and Li X: Dexmedetomidine
attenuates isoflurane-induced cognitive impairment through
antioxidant, anti-inflammatory and anti-apoptosis in aging rat. Int
J Clin Exp Med. 8:17281–17288. 2015.PubMed/NCBI
|
|
19
|
Ohtsuka M: Dexmedetomidine for
postoperative sedation in elderly patients with cognitive
impairment. Masui. 61:379–383. 2012.(In Japanese). PubMed/NCBI
|
|
20
|
Prommer E: Review article:
Dexmedetomidine: Does it have potential in palliative medicine? Am
J Hosp Palliat Care. 28:276–283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tejani AM and Schwenger E: Comment on
dexmedetomidine systematic review and meta-analysis methodology.
Inten Care Med. 36:1974–1975. 2010. View Article : Google Scholar
|
|
22
|
David MD and De Marchi L: Dexmedetomidine
sedation for awake tracheotomy: Case report and literature review.
J Clin Anesth. 22:360–362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Penel N, Adenis A, Clisant S and
Bonneterre J: Nature and subjectivity of dose-limiting toxicities
in contemporary phase 1 trials: Comparison of cytotoxic versus
non-cytotoxic drugs. Invest New Drugs. 29:1414–1419. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ong HL, Subramaniam M, Abdin E, Wang P,
Vaingankar JA, Lee SP, Shafie S, Seow E and Chong SA: Performance
of Mini-Mental State Examination (MMSE) in long-stay patients with
schizophrenia or schizoaffective disorders in a psychiatric
institute. Psychiat Res. 241:256–262. 2016. View Article : Google Scholar
|
|
25
|
Freiherr J, Gordon AR, Alden EC, Ponting
AL, Hernandez MF, Boesveldt S and Lundström JN: The 40-item Monell
Extended Sniffin' sticks identification test (MONEX-40). J Neurosci
Methods. 205:10–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ansari NN, Naghdi S, Hasson S, Valizadeh L
and Jalaie S: Validation of a Mini-Mental State Examination (MMSE)
for the Persian population: A pilot study. Appl Neuropsychol.
17:190–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gobbi A, Lad D and Karnatzikos G: The
effects of repeated intra-articular PRP injections on clinical
outcomes of early osteoarthritis of the knee. Knee Surg Sports
Traumatol Arthrosc. 23:2170–2177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Filardo G, Kon E, DI Matteo B, DI Marino
A, Sessa A, Merli ML and Marcacci M: Leukocyte-poor PRP application
for the treatment of knee osteoarthritis. Joints. 1:112–120.
2013.PubMed/NCBI
|
|
29
|
Kelly P, Kahlmeier S, Götschi T, Orsini N,
Richards J, Roberts N, Scarborough P and Foster C: Systematic
review and meta-analysis of reduction in all-cause mortality from
walking and cycling and shape of dose response relationship. Int J
Behav Nutri Phys Act. 11:1322014. View Article : Google Scholar
|
|
30
|
Babademez MA, Gul F, Muz E, Muderris T and
Kale H: Impact of partial and total tonsillectomy on adenoid
regrowth. Laryngoscope. 127:753–756. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Spektor Z, Saint-Victor S, Kay DJ and
Mandell DL: Risk factors for pediatric post-tonsillectomy
hemorrhage. Int J Pediat Otorhinolaryngol. 84:151–155. 2016.
View Article : Google Scholar
|
|
32
|
Hui D, Kilgore K, Frisbee-Hume S, Park M,
Tsao A, Guay Delgado M, Lu C, William W Jr, Pisters K, Eapen G, et
al: Dexamethasone for dyspnea in cancer patients: A pilot
double-blind, randomized, controlled trial. J Pain Symptom Manage.
52:8–16.e1. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ding L, Zhang H, Mi W, Wang T, He Y, Zhang
X, Ma X and Li H: Effects of dexmedetomidine on anesthesia recovery
period and postoperative cognitive function of patients after
robot-assisted laparoscopic radical cystectomy. Int J Clin Exp Med.
8:11388–11395. 2015.PubMed/NCBI
|
|
34
|
Ding L, Zhang H, Mi W, He Y, Zhang X, Ma X
and Li H: Effects of dexmedetomidine on recovery period of
anesthesia and postoperative cognitive function after
robot-assisted laparoscopicradical prostatectomy in the elderly
people. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 40:129–135. 2015.(In
Chinese). PubMed/NCBI
|
|
35
|
Zhang Z, Li X, Li F and An L: Berberine
alleviates postoperative cognitive dysfunction by suppressing
neuroinflammation in aged mice. Int Immunopharmacol. 38:426–433.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang JY, Lee H, Zhang Y, Lee JU, Park JH
and Yun EK: The effects of tonsillectomy education using smartphone
text message for mothers and children undergoing tonsillectomy: A
randomized controlled trial. Telemed J E Health. 22:921–928. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kara M, Erdoğan H, Altinişik HB, Aylanç H,
Güçlü O and Dereköy FS: Does topical use of autologous serum help
to reduce post-tonsillectomy morbidity? A prospective, controlled
preliminary study. J Laryngol Otol. 130:662–668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Drown MB: Integrative review utilizing
dexmedetomidine as an anesthetic for monitored anesthesia care and
regional anesthesia. Nurs Forum. 46:186–194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gerlach AT and Murphy CV:
Dexmedetomidine-associated bradycardia progressing to pulseless
electrical activity: Case report and review of the literature.
Pharmacotherapy. 29:14922009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Srinivas NR: Prostaglandin E1 therapy with
alprostadil and risk reduction in early hepatic cellular carcinoma
after liver transplantation. Aliment Pharmacol Ther. 43:172–173.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Barton MH, Darden JE, Clifton S and
Vandenplas M: Effect of firocoxib on cyclooxygenase 2, microsomal
prostaglandin E2 synthase 1, and cytosolic phospholipase A2 gene
expression in equine mononuclear cells. Am J Veter Res.
76:1051–1057. 2015. View Article : Google Scholar
|
|
42
|
Antila H, Manner T, Kuurila K, Salanterä
S, Kujala R and Aantaa R: Ketoprofen and tramadol for analgesia
during early recovery after tonsillectomy in children. Paediatr
Anaesth. 16:548–553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yokoyama M: Impact of preventing the
postoperative cognitive dysfunction in the aging society. Masui.
65:2232016.(In Japanese). PubMed/NCBI
|
|
44
|
Dahmani S, Rouelle D, Gressens P and Mantz
J: Effects of dexmedetomidine on hippocampal focal adhesion kinase
tyrosine phosphorylation in physiologic and ischemic conditions.
Anesthesiology. 103:969–977. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Valentin LS, Pereira VF, Pietrobon RS,
Schmidt AP, Oses JP, Portela LV, Souza DO, Vissoci JR, Luz VF,
Trintoni LM, et al: Effects of single low dose of dexamethasone
before noncardiac and nonneurologic surgery and general anesthesia
on postoperative cognitive dysfunction-a phase III double blind,
randomized clinical trial. PLoS One. 11:e01523082016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Guo Y, Sun L, Zhang J, Li Q, Jiang H and
Jiang W: Preventive effects of low-dose dexmedetomidine on
postoperative cognitive function and recovery quality in elderly
oral cancer patients. Int J Clin Exp Med. 8:16183–16190.
2015.PubMed/NCBI
|