Introduction
Ovarian cancer is one of the most common female
malignant tumor types, which has a high mortality rate and a 5 year
survival rate of <50% (1–3). The poor prognosis of patients with
ovarian cancer is largely attributed to the intrinsic molecular
changes that occur, and certain oncogenes and tumor suppressors
have been determined to serve key functions in ovarian cancer
growth (4,5). Therefore, a better understanding of the
molecular mechanism that underlies ovarian cancer development and
progression is urgently required for the development of novel
therapeutic strategies.
The human genome encodes a large number of
non-coding RNAs, including microRNAs (miRs) and long non-coding
RNAs (lncRNAs) (6–10). miRs directly bind to the
3′-untranslated region (3′-UTR) of their target mRNAs, which leads
to translation repression or mRNA degradation (4,11,12). In
addition to targeting numerous protein-coding genes, miRs can also
target lncRNAs (13).
The lncRNA growth arrest-specific transcript 5
(GAS5), which is located at 1q25 and contains ~630 nucleotides
(6), has been reported to serve a
tumor suppressive function in several common cancer types,
including liver (13), breast
(14), lung (15), renal (16) and colorectal cancer (17). In ovarian cancer, the downregulation
of GAS5 promotes tumor cell proliferation, migration and invasion
and indicates poor patient prognosis (18). Furthermore, Gao et al
(19) reported that GAS5 could
induce ovarian cancer cell apoptosis via the disruption of
mitochondrial membrane potential and the promotion of pro-apoptotic
protein expression, including Bcl-2-associated X, Bcl-2 homologous
antagonist killer, cleaved-caspase 3 and cleaved-caspase 9.
However, to the best of our knowledge, the underlying molecular
mechanism through which GAS5 participates in ovarian cancer growth
has not been previously studied.
miR-21 has been demonstrated to be upregulated in
numerous cancer types, including ovarian cancer, and has been
demonstrated to act as an oncogene (20–22).
Targeting miR-21-3p could inhibit the proliferation and
invasiveness of ovarian cancer cells (20). Recently, the targeting relationship
between miR-21 and GAS5 has been reported in multiple common cancer
types, including liver (13), lung
(15), cervical (23) and breast cancer (14). However, to the best of our knowledge,
the underlying mechanism by which GAS5 regulates miR-21 expression
in ovarian cancer has not been previously reported. In addition,
Sprouty homolog 2 (SPRY2), a member of the Sprouty family, has been
demonstrated to serve a suppressive function in ovarian cancer.
Patients with ovarian cancer whose tumors express SPRY2 at low
levels have a significantly poorer prognosis compared with those
who have tumors with high SPRY2 expression (24). However, the regulatory mechanism
underlying SPRY2 expression in ovarian cancer remains unclear.
The aim of the current study was to investigate the
underlying mechanism by which GAS5 regulates ovarian cancer cell
proliferation, and the involvement of miR-21 and SPRY2 in this
process.
Materials and methods
Clinical samples
Ovarian cancer tissues as well as adjacent normal
tissues were collected from 53 patients with ovarian cancer at The
First Affiliated Hospital of Xinxiang Medical University (Weihui,
China) between June 2013 and April 2016. These 53 female patients
were between 37 and 69 years old, with a mean age of 58.3 years
old. These patients were assessed using FIGO staging (25). The clinical characteristics of these
patients are summarized in Table I.
None of the patients received radiation therapy or chemotherapy
prior to surgical resection. Inclusion criteria were as follows: i)
Primary surgical patient, ii) complete records regarding
pre-operative chemotherapy and past medical history. Exclusion
criteria were as follows: i) Non-primary surgical patient, ii)
missing or incomplete records regarding pre-operative chemotherapy
or past medical history, iii) previous history of any malignancy.
Following resection, tissues were immediately stored in liquid
nitrogen until use. The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Xinxiang Medical
University (Weihui, China). Written informed consent was obtained
from all patients.
 | Table I.Association between growth
arrest-specific transcript 5 expression and clinicopathological
characteristics of patients with ovarian cancer. |
Table I.
Association between growth
arrest-specific transcript 5 expression and clinicopathological
characteristics of patients with ovarian cancer.
| Variable | Cases (n=53) | Low expression
(n=28) | High expression
(n=25) | P-value |
|---|
| Age, years |
|
|
| 0.578 |
|
<55 | 22 | 13 | 9 |
|
|
≥55 | 31 | 15 | 16 |
|
|
Differentiation |
|
|
| 0.074 |
|
Well/moderate | 38 | 17 | 21 |
|
|
Poor | 15 | 11 | 4 |
|
| FIGO stage |
|
|
| 0.011a |
|
I–II | 32 | 12 | 20 |
|
|
III–IV | 21 | 16 | 5 |
|
Cell culture and transfection
Human ovarian cancer cell lines, OVCAR-3, SKOV3,
3AO, ES-2, HO-8910, A2780 and COC1, as well as the normal human
ovarian epithelial cell line HOSEpiC, were purchased from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). All cells were cultured in Dulbecco's modified Eagle's
medium (DMEM, Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Thermo Fisher Scientific,
Inc.) at 37°C with 5% CO2 and were passaged every other
day using 0.25% trypsin (Thermo Fisher Scientific, Inc.).
A2780 cells were transfected with 50 nM GAS5
expression plasmid (Yearthbio, Changsha, China), blank vector
(Yearthbio), miR-21 inhibitor (anti-miR-21, cat. no. YB00238;
Yearthbio) or negative control (NC) inhibitor (anti-NC, cat. no.
YB00102; Yearthbio), or were co-transfected with either 50 nM GAS5
expression plasmid and 50 nM miR-21 mimic (cat. no. YB00237;
Yearthbio), 50 nM GAS5 expression plasmid and 50 nM scrambled miR
mimic (miR-NC, Yearthbio), 50 nM GAS5 expression plasmid and 50 nM
SPRY2 small interfering (si)RNA (cat. no. YB00588; Yearthbio) or 50
nM GAS5 expression plasmid and 50 nM NC siRNA (cat. no. YB00501;
Yearthbio) using Lipofectamine 2000 (Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Subsequent
experiments were conducted at 48 h following transfection.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cell lines
using TRIzol reagent (Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. A total of 1 µg RNA was converted into
cDNA using a SuperScript® One-Step RT-PCR kit (Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
qPCR was performed to examine the expression levels of GAS5, miR-21
and SPRY2 mRNA using the Applied Biosystems™ PowerUp™ SYBR Green
Master Mix kit (Thermo Fisher Scientific, Inc.) in an ABI 7500
fluorescence qPCR machine (Applied Biosystems; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. U6 was
used as the internal reference for GAS5 and miR-21, while GAPDH was
used as the internal reference for SPRY2. The PCR reaction
conditions were as follows: 95°C for 3 min, followed by 40 cycles
at 95°C for 15 sec and 60°C for 30 sec. The relative expression
levels were analyzed using the 2−ΔΔCq method (26). The primer sequences used are shown in
Table II.
 | Table II.Primers used in quantitative
polymerase chain reaction. |
Table II.
Primers used in quantitative
polymerase chain reaction.
| Primer | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| miR-21 |
CAGTGCGTGTCGTGGAGT |
ACGGGTAGCTTATCAGACTGA |
| U6 |
CAAATTCGTGAAGCGTTCCATA |
AGTGCAGGGTCCGAGGTATTC |
| GAS5 |
CGACTCCTGTGAGGTATGGTG |
ATCCTTCCTTGGGGACACAAC |
| SPRY2 |
CCTACTGTCGTCCCAAGACCT |
GGGGCTCGTGCAGAAGAAT |
| GAPDH |
GGAGCGAGATCCCTCCAAAAT |
GGCTGTTGTCATACTTCTCATGG |
MTT assay
An MTT assay was performed to assess cell
proliferation. A2780 cells (5,000 cells per well) were seeded in
96-well plates, and 100 µl DMEM containing 0.5 g/l MTT (Thermo
Fisher Scientific, Inc.) was added into each well. Following
incubation at 37°C for 12, 24, 48 or 72 h, the medium was removed
and 50 µl dimethyl sulfoxide (Thermo Fisher Scientific, Inc.) was
added into each well. The cells were then incubated at 37°C for 10
min. Cell proliferation was measured at a wavelength of 570 nm
using the Varioskan LUX Multimode Microplate Reader (Thermo Fisher
Scientific, Inc.).
Bioinformatics analysis
Bioinformatics analysis was performed to explore the
targeting relationship among GAS5, miR-21 and SPRY2 using the
miRecords resource and PicTar algorithms (c1.accurascience.com/miRecords/).
Luciferase reporter gene assay
Firefly luciferase reporter plasmids containing
either wild-type (WT) miR-21 with GAS5 binding sequences,
mutant-type (MT) miR-21 with no GAS5 binding sequences, WT SPRY2
3′-UTR with miR-21 binding sequences or MT SPRY2 3′-UTR without
miR-21 binding sequences were purchased from Shanghai Genechem Co.,
Ltd. (Shanghai, China). To study the targeting relationship between
GAS5 and miR-21, A2780 cells were co-transfected with a GAS5
plasmid or its control vector, and a WT or MT miR-21 luciferase
reporter plasmid using Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. To
clarify the targeting relationship between miR-21 and SPRY2, A2780
cells were co-transfected with miR-21 mimic or miR-NC, and a WT or
MT SPRY2 3′-UTR luciferase reporter plasmid using Lipofectamine
2000 (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Following transfection for 48 h,
luciferase activity was determined using the Dual-Luciferase
Reporter Assay system (Promega Corporation, Madison, WI, USA). The
firefly luciferase activities were normalized to Renilla luciferase
activity.
Western blot analysis
A2780 cells were lysed in cold
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.). Then, the protein concentration was determined using the BCA
Protein Assay kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Protein (50 µg/well) was separated using
12% SDS-PAGE and was then transferred to a polyvinylidene fluoride
(PVDF) membrane (Thermo Fisher Scientific, Inc.). The PVDF membrane
was blocked in 5% non-fat dried milk in phosphate-buffered saline
(PBS; Thermo Fisher Scientific, Inc.) at 4°C overnight. Following
washing in PBS with Tween-20 (PBST) for 10 min at room temperature,
the PVDF membrane was incubated with a rabbit anti-human SPRY2
antibody (1:50; cat. no. ab50317; Abcam, Cambridge, MA, USA) or a
rabbit anti-human GAPDH antibody (1:50; cat. no. ab9485; Abcam) for
3 h at room temperature. Following washing in PBST for 10 min at
room temperature, the PVDF membrane was incubated with a
horseradish peroxidase conjugated goat anti-rabbit secondary
antibody (1:5,000; cat. no. ab6721, Abcam) for 1 h at room
temperature. Following another wash in PBST for 10 min at room
temperature, the immune complex on the PVDF membrane was detected
using the Enhanced Chemiluminescence Western Blotting kit (Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Protein expression was determined using Image-Pro Plus software 6.0
(Media Cybernetics, Inc., Rockville, MD, USA), and GAPDH was used
as the internal reference.
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS 19.0 software (IBM Corp., Armonk, NY, USA) was used for
statistical analysis. Data were analyzed using Student's t-test for
two-group comparisons or ANOVA for comparisons of multiple groups
followed by Turkey's post hoc test. A chi-square test was used to
evaluate associations between GAS5 expression and
clinicopathological characteristics. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of GAS5, miR-21 and SPRY2
in ovarian cancer
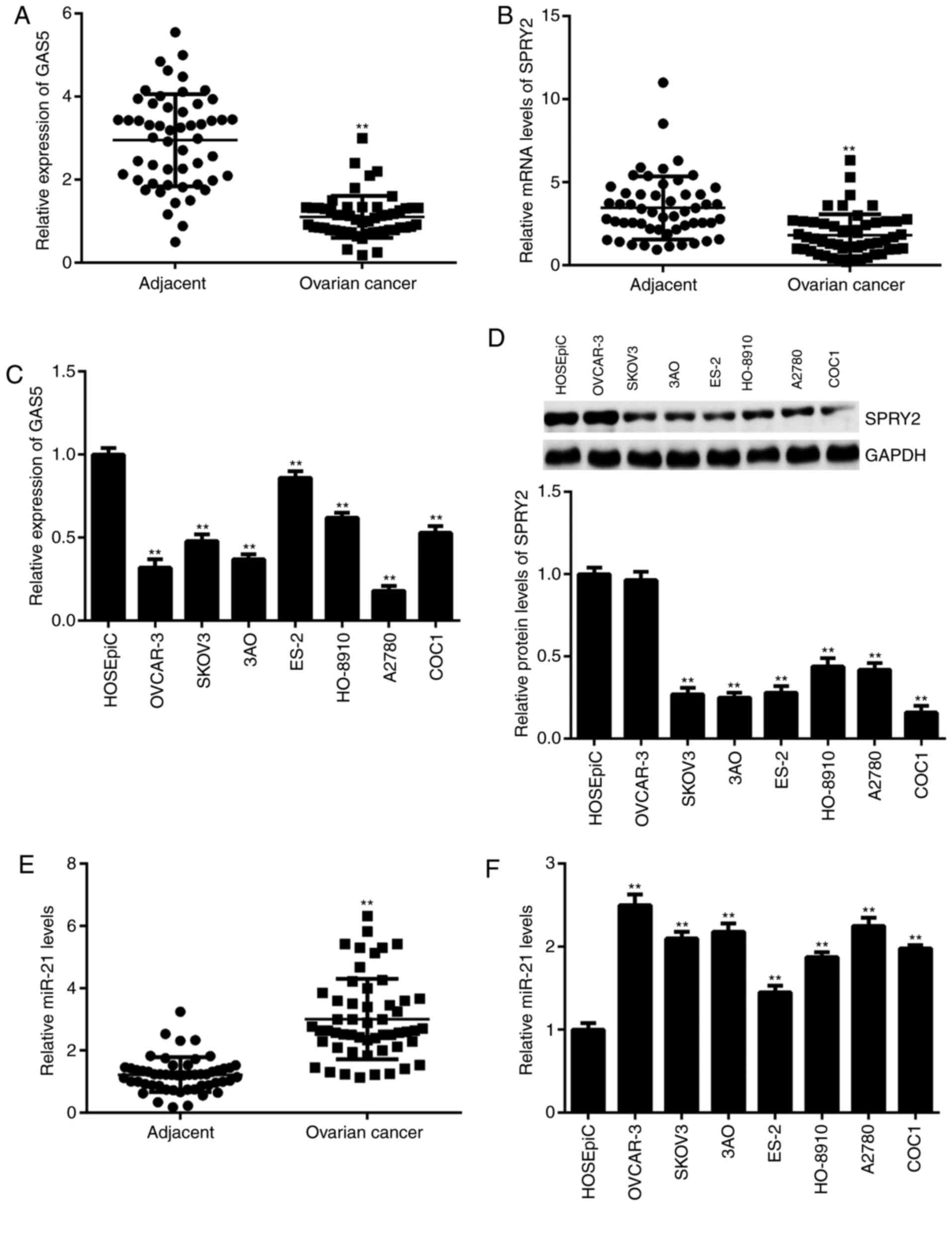
The expression of GAS5, miR-21 and SPRY2 was
examined in ovarian cancer tissues and cell lines. As shown in
Fig. 1A and B, the expression of
GAS5 and SPRY2 was significantly downregulated in ovarian cancer
tissues compared with adjacent non-tumor tissues. Similar findings
were also obtained in ovarian cancer cell lines except for OVCAR-3,
when compared with normal ovarian epithelial cells (Fig. 1C and D). In addition, downregulation
of GAS5 was significantly associated with advanced clinical stage
in patients with ovarian cancer (Table
I). By contrast, the expression of miR-21 was significantly
higher in ovarian cancer tissues and cell lines compared with
adjacent non-tumor tissues and normal ovarian epithelial cells,
respectively (Fig. 1E and F). In
summary, these findings demonstrate that GAS5 and SPRY2 are
downregulated, while miR-21 is upregulated, in ovarian cancer. As
A2780 cells showed the lowest levels of GAS5, they were selected
for use in subsequent experiments.
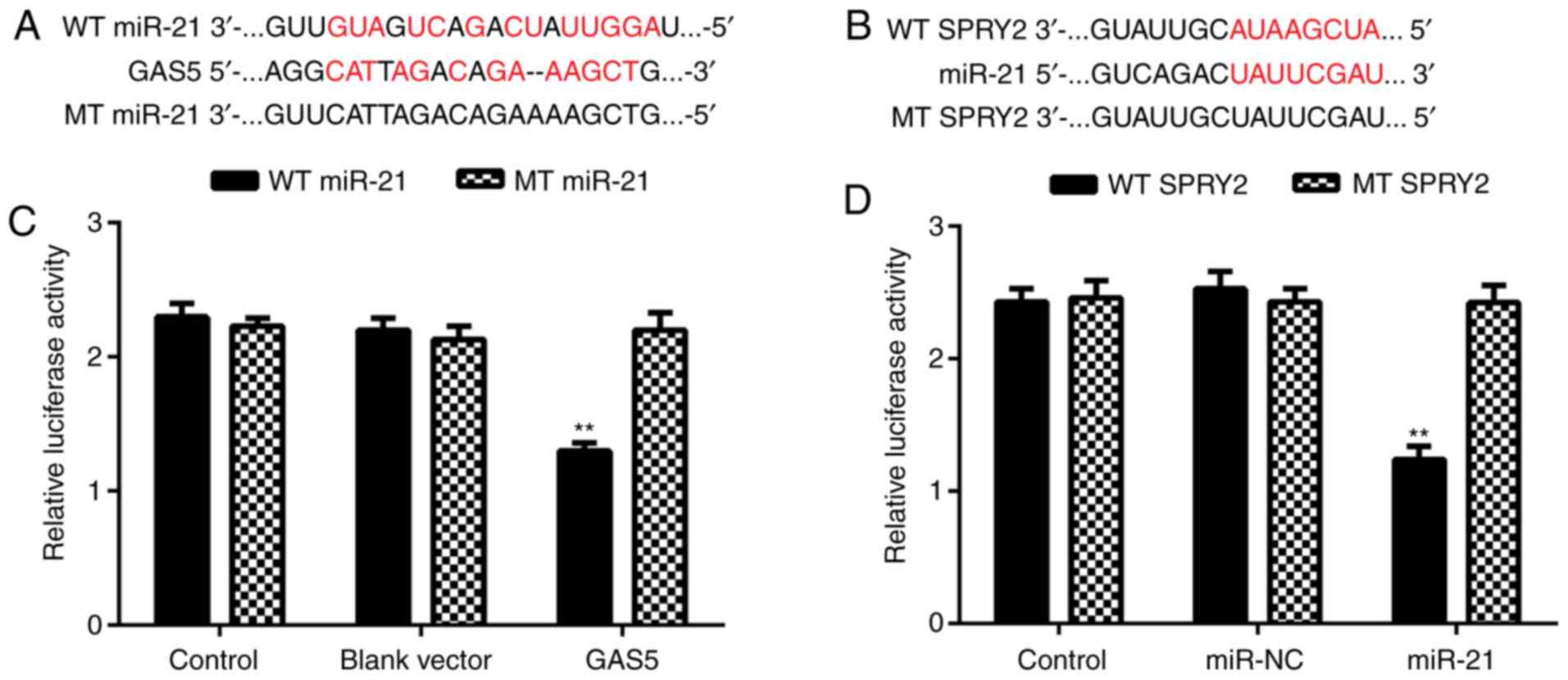
miR-21 is a target of GAS5 and SPRY2
is a target gene of miR-21 in A2780 cells
Bioinformatics analysis was performed to explore the
targeting relationship among GAS5, miR-21 and SPRY2 using the
miRecords resource and PicTar algorithms (c1.accurascience.com/miRecords/). The data indicated
that miR-21 contained GAS5 binding sites and SPRY2 3′-UTR contained
miR-21 binding sites (Fig. 2A and
B). Therefore, miR-21 may be a target of GAS5, and SPRY2 may be
a target gene of miR-21 in ovarian cancer cells. To clarify these
predictions, a luciferase reporter assay was performed. The results
indicated that ovarian cancer cells that were co-transfected with a
GAS5 expression plasmid and a WT miR-21 reporter plasmid exhibited
significantly reduced luciferase activity compared with the control
group, but cells that were co-transfected with a GAS5 expression
plasmid and an MT miR-21 reporter plasmid exhibited no effect on
reporter luciferase activity (Fig.
2C). Therefore, miR-21 was demonstrated to be a target of GAS5
in ovarian cancer cells. Similarly, ovarian cancer cells that were
co-transfected with an miR-21 mimic and a WT SPRY2 3′-UTR reporter
plasmid exhibited a significant decrease in luciferase activity
compared with the control group, but cells that were co-transfected
with an miR-21 mimic and an MT SPRY2 reporter plasmid demonstrated
no difference with respect to luciferase activity (Fig. 2D). Thus, SPRY2 was identified to be a
target gene of miR-21 in ovarian cancer A2780 cells.
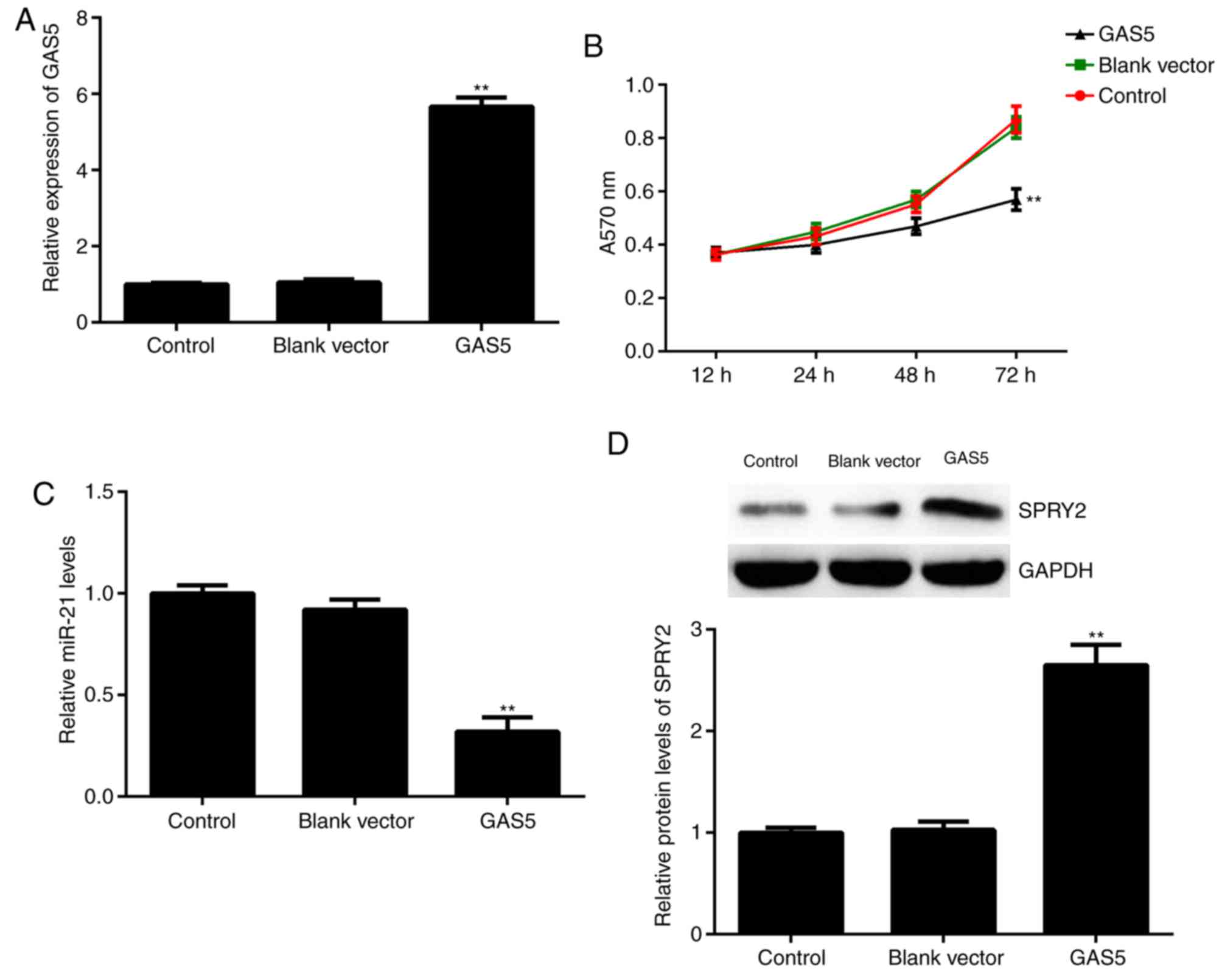
Overexpression of GAS5 inhibits A2780
cell proliferation and affects the expression of miR-21 and
SPRY2
Based on the finding that GAS5 was significantly
downregulated in ovarian cancer, A2780 cells were transfected with
a GAS5 plasmid. Following transfection, the expression of GAS5 was
significantly increased in the GAS5 group compared with the control
group (Fig. 3A). The results from
the MTT assay further indicated that, compared with the control
group, GAS5 overexpression significantly decreased A2780 cell
proliferation at 72 h (Fig. 3B).
Furthermore, the effects of GAS5 on the expression of miR-21 and
SPRY2 were studied. The data indicated that overexpression of GAS5
resulted in a significant decrease in miR-21 expression and a
significant increase in SPRY2 expression in A2780 cells (Fig. 3C and D). Therefore, GAS5 could
inhibit ovarian cancer cell proliferation and affect the expression
of miR-21 and SPRY2.
Overexpression of miR-21 reduces SPRY2
expression and rescues GAS5-mediated A2780 cell proliferation
The effects of miR-21 downregulation on ovarian
cancer cell proliferation and SPRY2 expression were studied. Since
the expression of miR-21 was significantly upregulated in ovarian
cancer tissues and cell lines, A2780 cells were transfected with an
miR-21 inhibitor to knock down its expression. qPCR data indicated
that miR-21 levels were significantly reduced in the anti-miR-21
group compared with the control group, but transfection with an NC
inhibitor did not affect miR-21 levels (Fig. 4A). Furthermore, it was identified
that the knockdown of miR-21 significantly inhibited A2780 cell
proliferation at 72 h, which was accompanied by significantly
increased expression of SPRY2 (Fig. 4B
and C).
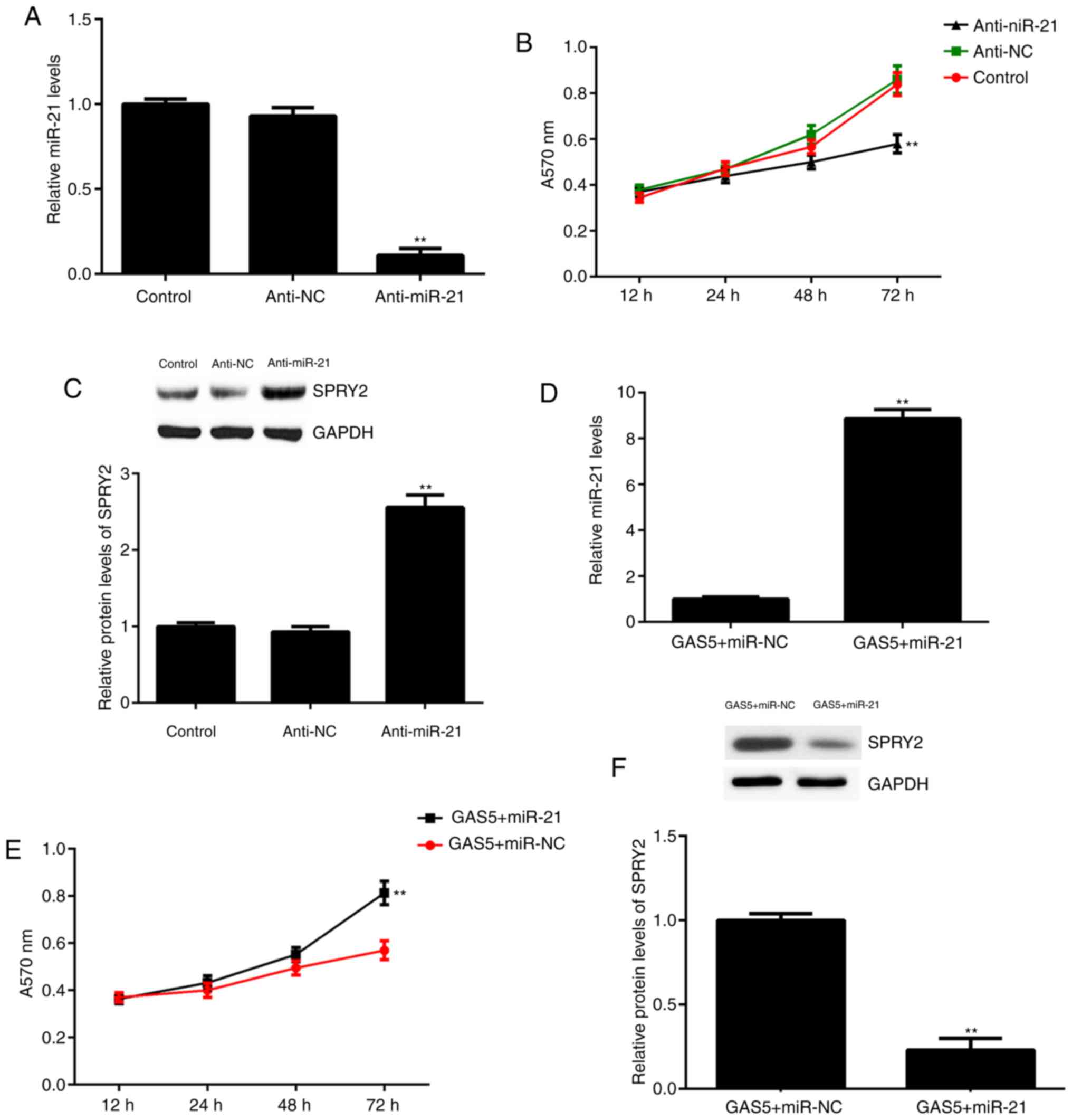 | Figure 4.Effects of miR-21 inhibition and
overexpression. A2780 cells were transfected with miR-21 inhibitor
or NC inhibitor. Non-transfected cells were used as the control
group. Following transfection, (A) qPCR was used to examine miR-21
levels, (B) an MTT assay was used to determine cell proliferation
and (C) western blot analysis was used to examine the protein
levels of SPRY2. **P<0.01 vs. Control. Then, A2780 cells were
co-transfected with GAS5 expression plasmid and miR-21 mimic or
scramble miR (miR-NC). Following transfection, (D) qPCR was used to
examine miR-21 levels, (E) an MTT assay was used to determine cell
proliferation and (F) western blot analysis was used to examine the
protein levels of SPRY2. **P<0.01 vs. GAS5+miR-NC. GAS5, growth
arrest-specific transcript 5; SPRY2, Sprouty homolog 2; qPCR,
quantitative polymerase chain reaction; NC, negative control; miR,
microRNA; anti-miR-21, miR-21 inhibitor. |
To further confirm that miR-21 acts as the
downstream effector of GAS5, A2780 cells were co-transfected with a
GAS5 expression plasmid and a miR-21 mimic. Following transfection,
the expression of miR-21 was significantly higher in the
GAS5+miR-21 group compared with the GAS5+miR-NC group (Fig. 4D). Further investigation revealed
that the proliferation of A2780 cells was significantly increased
in the GAS5+miR-21 group at 72 h compared with the GAS5+miR-NC
group, which suggests that miR-21 overexpression attenuated the
suppressive effects of GAS5 on A2780 cell proliferation (Fig. 4E). In addition, the overexpression of
miR-21 also attenuated the promoting effects of GAS5 on the
expression of SPRY2 in A2780 cells (Fig.
4F).
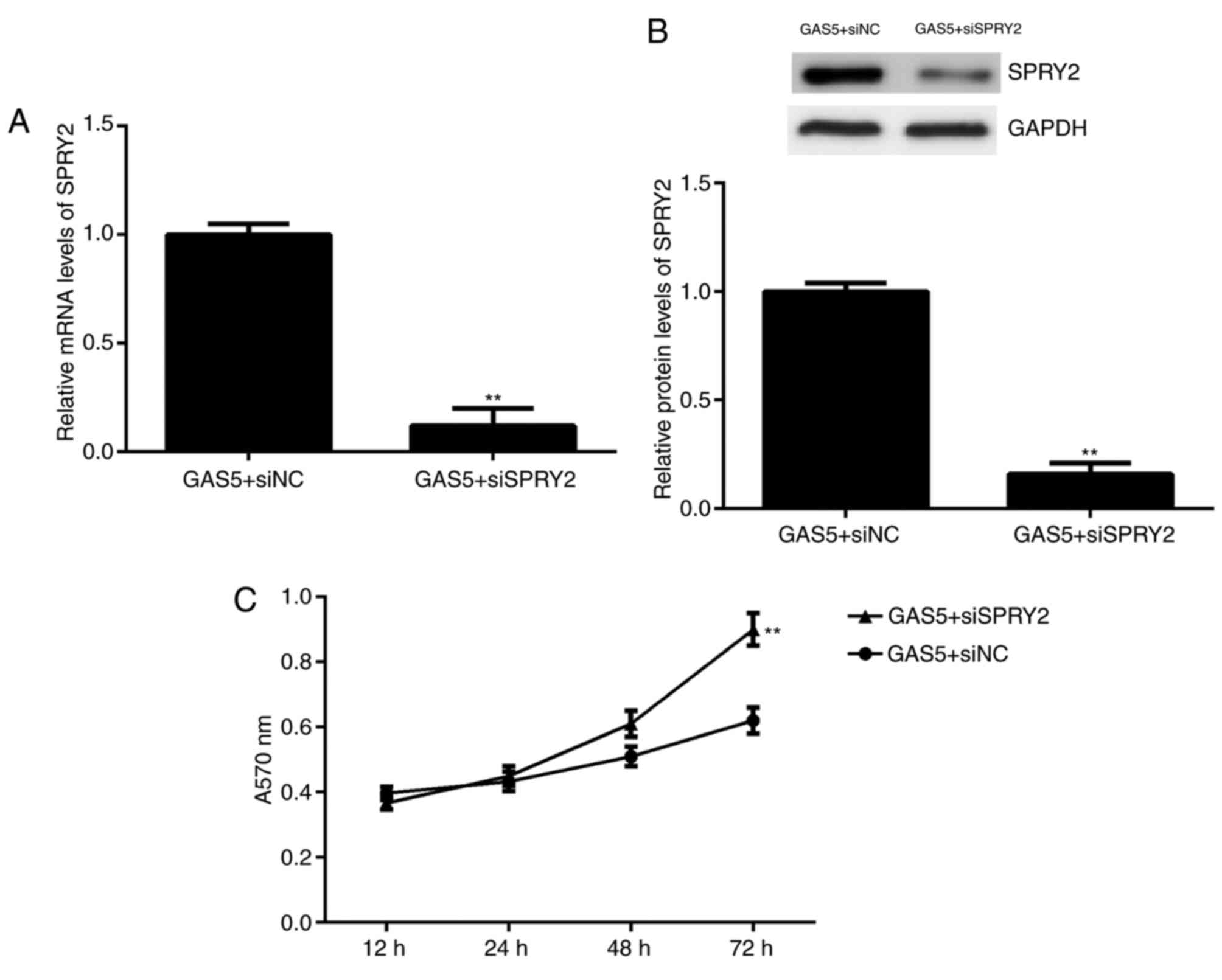
Knockdown of SPRY2 rescues the
inhibitory effects of GAS5 on A2780 cell proliferation
Finally, it was investigated whether SPRY2 was
involved in GAS5-mediated ovarian cancer cell proliferation. A2780
cells were co-transfected with a GAS5 plasmid and SPRY2 siRNA.
Following transfection, the mRNA and protein levels of SPRY2 were
significantly downregulated in the GAS5+siSPRY2 group compared with
the GAS5+siNC group (Fig. 5A and B).
The results from the MTT assay indicated that the proliferation of
A2780 cells was significantly increased in the GAS5+siSPRY2 group
compared with the GAS5+siNC group at 72 h (Fig. 5C). These findings demonstrate that
inhibition of SPRY2 rescues, at least partially, the suppressive
effects of GAS5 overexpression on ovarian cancer cell
proliferation.
Discussion
To the best of our knowledge, the underlying
molecular mechanism through which lncRNA GAS5 regulates ovarian
cancer growth has not been previously studied. In the present
study, it was reported that GAS5 and SPRY2 were downregulated and
that miR-21 was upregulated in ovarian cancer. Downregulation of
GAS5 was significantly associated with advanced clinical stage of
patients with ovarian cancer. miR-21 was identified to be a target
gene of GAS5 and SPRY2 was identified to be a target gene of miR-21
in ovarian cancer A2780 cells. GAS5 overexpression significantly
inhibited the proliferation of ovarian cancer cells, which was
accompanied by the downregulation of miR-21 and the upregulation of
SPRY2. Overexpression of miR-21 resulted in a significant decrease
in A2780 cell proliferation, which was accompanied by reduced SPRY2
expression. Furthermore, miR-21 overexpression attenuated the
suppressive effects of GAS5 on A2780 cell proliferation and rescued
the promoting effects of GAS5 on SPRY2 expression. Furthermore, the
knockdown of SPRY2 also rescued the suppressive effects of GAS5 on
the proliferation of A2780 cells.
Recently, the association between GAS5 and miR-21
has been reported in multiple physiological and pathological
processes (13–15). For instance, GAS5 inhibits cardiac
fibroblast activation and fibrosis by targeting miR-21 through the
phosphatase and tensin homolog (PTEN)/matrix metalloproteinase-2
signaling pathway (27).
Furthermore, GAS5 contributes to the pathogenesis of osteoarthritis
via the inhibition of miR-21 expression (28). Additionally, the tumor suppressive
role of GAS5 has been reported in numerous cancer types (13). For instance, GAS5 suppresses cancer
cell proliferation by acting as a molecular sponge for miR-21 and
directly interacting with miR-21, which reverses the repression of
PTEN, the endogenous target of miR-21 (14). Hu et al (13) reported that GAS5 suppresses the
migration and invasion of hepatocellular carcinoma cells via the
inhibition of miR-21. In addition, GAS5 and miR-21 are also
involved in chemoresistance (15).
Cao et al (15) demonstrated
that inhibition of GAS5 reduces the chemosensitivity of non-small
cell lung cancer cells to cisplatin through the upregulation of
miR-21 and thus the downregulation of PTEN. In the present study,
for the first time, the targeting relationship between miR-21 and
GAS5 in ovarian cancer was reported. The present data indicated
that GAS5 was significantly downregulated, while miR-21 was
significantly upregulated, in ovarian cancer tissues. Luciferase
reporter gene assay data confirmed that miR-21 was a direct target
of GAS5 in A2780 cells and that the overexpression of GAS5
significantly reduced ovarian cancer cell proliferation by directly
targeting miR-21.
Furthermore, it was identified in the current study
that SPRY2 was also downregulated in ovarian cancer tissues, and
luciferase reporter gene assay data confirmed that SPRY2 was a
target gene of miR-21 in A2780 cells. SPRY2 is a member of the
Sprouty family and inhibits the activity of receptor tyrosine
kinase signaling; this protein is also required for its growth
factor-stimulated translocation to membrane ruffles (29,30). It
has been widely demonstrated that SPRY2 acts as a tumor suppressor
in some common human cancer types (31–33). For
instance, SPRY2 is downregulated in renal cell carcinoma, and its
low expression is associated with poor prognosis (34). Furthermore, SPRY2 may suppress the
proliferation and invasiveness of renal cell carcinoma cells
(34). Recently, Masoumi-Moghaddam
et al (24) reported that
SPRY2 is significantly downregulated in ovarian cancer, which is
consistent with the present findings, and that patients whose
tumors express SPRY2 at low levels have a significantly poorer
prognosis compared with those who have tumors with high SPRY2
expression. In the present study, it was identified that SPRY2 is a
direct target gene of miR-21 in A2780 cells. A targeting
relationship between miR-21 and SPRY2 has also been identified in
other cancer types (35,36). For instance, Kwak et al
(35) reported that the
downregulation of SPRY2 by miR-21 triggers malignancy in human
gliomas. Huo et al (37)
reported that disruption of pre-miR-21 sequences inhibits the
proliferation, migration and invasiveness of ovarian cancer cells,
which is accompanied by increased expression of SPRY2. In the
present study, it was identified that GAS5 overexpression reduced
miR-21 expression and increased the expression of its target gene,
SPRY2. It was also demonstrated that the knockdown of SPRY2
expression rescued the inhibitory effects of GAS5 on A2780 cell
proliferation. These findings suggest that SPRY2 is a downstream
effector of GAS5/miR-21 signaling in A2780 cells.
To the best of our knowledge, this is the first
study to report that GAS5 exerts inhibitory effects on ovarian
cancer cell proliferation, at least in part through the
downregulation of miR-21 expression and therefore the upregulation
of SPRY2 expression. These findings suggest that the
GAS5/miR-21/SPRY2 signaling pathway may be a potential therapeutic
target in ovarian cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in the published article.
Authors' contributions
SL collected the clinical tissues. NM, SL, QZ, HW
and HQ performed the in vitro experiments. NM wrote the
manuscript. SW designed the study and revised the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Xinxiang Medical
University (Weihui, China). Written informed consent was obtained
from all patients.
Consent for publication
Written informed consent for the publication of
their data was obtained from all patients involved in the
study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29;. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y, Li J, Xu C and Zhang X:
MicroRNA-139-5p inhibit cell proliferation and invasion by
targeting RHO-associated coiled-coil containing protein kinase 2 in
ovarian cancer. Oncol Res. Jun 14–2017. View Article : Google Scholar
|
|
5
|
Hua F, Li CH, Chen XG and Liu XP: Long
noncoding RNA CCAT2 knockdown suppresses tumorous progression by
sponging miR-424 in epithelial ovarian vancer. Oncol Res.
26:241–247. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ebert MS and Sharp PA: Emerging roles for
natural microRNA sponges. Curr Biol. 20:R858–R861. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Zi Y, Wang W and Li Y: Long
noncoding RNA MEG3 inhibits cell proliferation and metastasis in
chronic myeloid leukemia via targeting miR-184. Oncol Res.
26:297–305. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang S, Hui Y, Li X and Jia Q: Silencing
of lncRNA-CCDC26 restrains the growth and migration of glioma cells
in vitro and in vivo via targeting miR-203. Oncol Res. Jun 9–2017.
View Article : Google Scholar
|
|
9
|
Zhang Y, Dai Q, Zeng F and Liu H:
WITHDRAWN: MALAT1 promotes the proliferation and metastasis of
osteosarcoma cells by activating the Rac1/JNK pathway via targeting
miR-509. Oncol Res. May 26–2017.
|
|
10
|
Li Z, Guo J, Ma Y, Zhang L and Lin Z:
Oncogenic role of MicroRNA-30b-5p in glioblastoma through targeting
proline-rich transmembrane protein 2. Oncol Res. 26:219–230. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang M, Zhai X, Ge T, Yang C and Lou G:
miR-181a-5p promotes proliferation and invasion, and inhibits
apoptosis of cervical cancer cells via regulating inositol
polyphosphate-5-phosphatase A (INPP5A). Oncol Res. Jun
23–2017.(Epub ahead of print). View Article : Google Scholar
|
|
12
|
Zhou Y, Yang C, Wang K, Liu X and Liu Q:
MicroRNA-33b inhibits the proliferation and migration of
osteosarcoma cells via targeting hypoxia-inducible factor-1α. Oncol
Res. 25:397–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu L, Ye H, Huang G, Luo F, Liu Y, Yang X,
Shen J, Liu Q and Zhang J: Long noncoding RNA GAS5 suppresses the
migration and invasion of hepatocellular carcinoma cells via
miR-21. Tumour Biol. 37:2691–2702. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li W, Zhai L, Wang H, Liu C, Zhang J, Chen
W and Wei Q: Downregulation of LncRNA GAS5 causes trastuzumab
resistance in breast cancer. Oncotarget. 7:27778–27786.
2016.PubMed/NCBI
|
|
15
|
Cao L, Chen J, Ou B, Liu C, Zou Y and Chen
Q: GAS5 knockdown reduces the chemo-sensitivity of non-small cell
lung cancer (NSCLC) cell to cisplatin (DDP) through regulating
miR-21/PTEN axis. Biomed Pharmacother. 93:570–579. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiao HP, Gao WS, Huo JX and Yang ZS: Long
non-coding RNA GAS5 functions as a tumor suppressor in renal cell
carcinoma. Asian Pac J Cancer Prev. 14:1077–1082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Y, Shen Z, Yan Y, Wang B, Zhang J,
Shen C, Li T, Ye C, Gao Z, Peng G, et al: Long non-coding RNA GAS5
inhibits cell proliferation, induces G0/G1 arrest and apoptosis,
and functions as a prognostic marker in colorectal cancer. Oncol
Lett. 13:3151–3158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Huang H, Li Y, Li L, Hou W and You
Z: Decreased expression of long non-coding RNA GAS5 promotes cell
proliferation, migration and invasion, and indicates a poor
prognosis in ovarian cancer. Oncol Rep. 36:3241–3250. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao J, Liu M, Zou Y, Mao M, Shen T, Zhang
C, Song S, Sun M, Zhang S, Wang B, et al: Long non-coding RNA
growth arrest-specific transcript 5 is involved in ovarian cancer
cell apoptosis through the mitochondria-mediated apoptosis pathway.
Oncol Rep. 34:3212–3221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Báez-Vega PM, Vargas Echevarría IM,
Valiyeva F, Encarnación-Rosado J, Roman A, Flores J,
Marcos-Martínez MJ and Vivas-Mejía PE: Targeting miR-21-3p inhibits
proliferation and invasion of ovarian cancer cells. Oncotarget.
7:36321–36337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou L, Yang ZX, Song WJ, Li QJ, Yang F,
Wang DS, Zhang N and Dou KF: MicroRNA-21 regulates the migration
and invasion of a stem-like population in hepatocellular carcinoma.
Int J Oncol. 43:661–669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang X, Cheng Y, Li P, Tao J, Deng X,
Zhang X, Gu M, Lu Q and Yin C: A lentiviral sponge for miRNA-21
diminishes aerobic glycolysis in bladder cancer T24 cells via the
PTEN/PI3K/AKT/mTOR axis. Tumour Biol. 36:383–391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wen Q, Liu Y, Lyu H, Xu X, Wu Q, Liu N,
Yin Q, Li J and Sheng X: Long noncoding RNA GAS5, which acts as a
tumor suppressor via microRNA 21, regulates cisplatin resistance
expression in cervical cancer. Int J Gynecol Cancer. 27:1096–1108.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Masoumi-Moghaddam S, Amini A, Wei AQ,
Robertson G and Morris DL: Sprouty 2 protein, but not Sprouty 4, is
an independent prognostic biomarker for human epithelial ovarian
cancer. Int J Cancer. 137:560–570. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Javadi S, Ganeshan DM, Qayyum A, Iyer RB
and Bhosale P: Ovarian cancer, the revised FIGO staging system, and
the role of imaging. AJR Am J Roentgenol. 206:1351–1360. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tao H, Zhang JG, Qin RH, Dai C, Shi P,
Yang JJ, Deng ZY and Shi KH: LncRNA GAS5 controls cardiac
fibroblast activation and fibrosis by targeting miR-21 via
PTEN/MMP-2 signaling pathway. Toxicology. 386:11–18. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song J, Ahn C, Chun CH and Jin EJ: A long
non-coding RNA, GAS5, plays a critical role in the regulation of
miR-21 during osteoarthritis. J Orthop Res. 32:1628–1635. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lim J, Wong ES, Ong SH, Yusoff P, Low BC
and Guy GR: Sprouty proteins are targeted to membrane ruffles upon
growth factor receptor tyrosine kinase activation. Identification
of a novel translocation domain. J Biol Chem. 275:32837–32845.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lim J, Yusoff P, Wong ES, Chandramouli S,
Lao DH, Fong CW and Guy GR: The cysteine-rich sprouty translocation
domain targets mitogen-activated protein kinase inhibitory proteins
to phosphatidylinositol 4,5-bisphosphate in plasma membranes. Mol
Cell Biol. 22:7953–7966. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu C, Liang S, Xiao S, Lin Q, Chen X, Wu
Y and Fu J: MicroRNA-27b inhibits Spry2 expression and promotes
cell invasion in glioma U251 cells. Oncol Lett. 9:1393–1397. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chandramouli S, Yu CY, Yusoff P, Lao DH,
Leong HF, Mizuno K and Guy GR: Tesk1 interacts with Spry2 to
abrogate its inhibition of ERK phosphorylation downstream of
receptor tyrosine kinase signaling. J Biol Chem. 283:1679–1691.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu G, Qin XQ, Guo JJ, Li TY and Chen JH:
AKT/ERK activation is associated with gastric cancer cell
resistance to paclitaxel. Int J Clin Exp Pathol. 7:1449–1458.
2014.PubMed/NCBI
|
|
34
|
Li P, Tao L, Yang J, Cai H, Ju X, Li J,
Shao P, Cao Q, Qin C, Meng X and Yin C: Sprouty2 is associated with
prognosis and suppresses cell proliferation and invasion in renal
cell carcinoma. Urology. 82(253): e1–e7. 2013.PubMed/NCBI
|
|
35
|
Kwak HJ, Kim YJ, Chun KR, Woo YM, Park SJ,
Jeong JA, Jo SH, Kim TH, Min HS, Chae JS, et al: Downregulation of
Spry2 by miR-21 triggers malignancy in human gliomas. Oncogene.
30:2433–2442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Feng YH, Wu CL, Shiau AL, Lee JC, Chang
JG, Lu PJ, Tung CL, Feng LY, Huang WT and Tsao CJ:
MicroRNA-21-mediated regulation of Sprouty2 protein expression
enhances the cytotoxic effect of 5-fluorouracil and metformin in
colon cancer cells. Int J Mol Med. 29:920–926. 2012.PubMed/NCBI
|
|
37
|
Huo W, Zhao G, Yin J, Ouyang X, Wang Y,
Yang C, Wang B, Dong P, Wang Z, Watari H, et al: Lentiviral
CRISPR/Cas9 vector mediated miR-21 gene editing inhibits the
epithelial to mesenchymal transition in ovarian cancer cells. J
Cancer. 8:57–64. 2017. View Article : Google Scholar : PubMed/NCBI
|