Introduction
Amblyopia is a commonly occurring disease of
abnormal visual development characterized by a decrease in
best-corrected visual acuity without any ocular organic disease. It
is typified by deficient spatial vision, such as loss of central
vision, resulting in a reduced spatial contrast sensitivity
function (CSF). Visuotemporal deficits are also associated with
amblyopia (1). To date, there are
four methods of evaluating temporal processing: Time reproduction,
time production, verbal estimation and time comparison. Certain
scholars consider that time comparison, particularly temporal
discrimination, is the purest and best method of measuring time
perception and short time differences compared with time
reproduction and time production (2).
Studies published on temporal discrimination in
amblyopia are rare. Spang and Fahle (3), suggested that the temporal
discrimination of amblyopia deteriorated based on a task of
time-based, figure-ground segregation. However, whether this
deterioration potentially originated from either lower-level
temporal processing deficits or higher-level figure-ground
segregation deficits could not be clarified. In addition, earlier
studies demonstrated that the visual perception of amblyopia was
influenced by attention using tasks of object tracking (4–7), object
enumeration (8), attentional blink
(9), decision making (10), and line bisection (11). Recently, Hou et al (12) identified the degraded attentional
modulation of cortical neural populations in strabismic amblyopia.
Furthermore, attention was involved in the process of temporal
cognition for normal subjects (13,14).
However, how attentional load influences temporal cognition in
amblyopia has yet to be fully elucidated.
The aim of the present study was to determine
whether there is a deficit of temporal frequency discrimination in
amblyopia, and to determine the influences of attentional load on
amblyopic temporal discrimination.
Patients and methods
Subjects
The present study enrolled 20 individuals with
anisometropic amblyopia, 20 individuals with strabismic amblyopia,
and 20 normal subjects who were aged 10–14 years between February
1, 2015 and January 31, 2016. Any subjects who had a history of
neurological dysfunction, such as attention deficit hyperactivity
disorder (ADHD), or had ocular diseases other than amblyopia, or
were not able to cooperate were excluded.
Full optical correction was required during the
present study. All subjects were naive to the purposes of the
experiment. The study was performed in accordance with the
Declaration of Helsinki, and all participants' guardians signed
written informed consent. The experimental protocol was approved by
the institutional review board of West China Hospital of Sichuan
University (Chengdu, China). All information pertaining to the
subjects was anonymized and identified prior to analysis.
Visual stimulus
The stimulus featured a white square-wave flickery
disc against a black background (the contrast was set at 96%). The
disc, which was shown at a central position on the screen,
subtended an arc of ~1° at a viewing distance of ~1 m. There was a
black fixation cross in the middle of the disc that did not flicker
with the disc. The flicker frequency and the flicker duration
exhibited an association of inverse function. The stimulus was
displayed on a 19-inch Samsung CRT computer monitor at a resolution
of 1024×768 pixels, and at an 85-Hz refresh rate.
Procedural details
Prior to performing the temporal discrimination
task, the subjects received a general ocular examination, including
a LogMAR visual acuity test and refraction. Subsequently, the
participants viewed the screen with their tested eyes at a distance
of ~1 m in a darkened room. The temporal discrimination task was
tested at the reference frequency of 5, 10, or 20 Hz. The fellow
eye was tested first, and then the amblyopic eye.
A two-alternative, forced-choice (2AFC) task
procedure, a classical method of measuring frequency discrimination
(15), was performed in the present
study. Each trial consisted of two 800 ms intervals separated by
600 ms. One interval contained the flickery disk of the reference
temporal frequency, whereas the other included the comparison
temporal frequency. The sequence of each was random. Subjects were
required to respond using a keyboard. Pressing the ‘←’ key
suggested that the first interval of the disk flickered faster
compared with the second, whereas pressing the ‘→’ key indicated
the opposite. Regarding the comparison temporal frequency, the
start value of flicker duration was set at 30% of the reference
flicker duration. Pilot experiments indicated that these values
were discriminable. The step size of the staircase was 10% of the
reference flicker duration. The comparison flicker duration varied
initially using an ascending 1-up, 1-down staircase. When an
incorrect response was recorded, staircases changed to an ascending
3-up, 1-down staircase. These staircases ended after seven
reversals; therefore, the numbers of trials per run varied.
Just-noticeable differences (JNDs) were calculated by taking the
geometric mean of the flicker duration differences between the
reference and comparison flicker duration for the last four
reversals of each staircase. JNDs of the amblyopic eye, fellow eye
and normal eye were tested in the present study, and these
represented the thresholds of temporal discrimination.
Attentional load was fulfilled according to the
classical experimental paradigm of double tasks (16). Here, doing mental arithmetic when
testing temporal discrimination was used for double tasks. When the
participant was required to respond by pressing the keyboard, an
arithmetic equation was shown at the top of the screen. The
participant was required to perform the mental arithmetic and read
the result aloud. This action would occupy the working memory
capacity, and divert attention towards the task that was irrelevant
to temporal discrimination. Lower attentional load was performed by
the sum of ones digits, whereas higher attentional load was
performed by the sum of tens digits. JNDs of the amblyopic eye and
normal eye were tested under the condition of different attentional
loads.
Data analysis
Data analysis was performed using SPSS v.16.0
software (IBM Corp., Armonk, NY, USA) and SAS software, v.8.2 (SAS
Institute, Inc., Cary, NC, USA). The age of normal and amblyopic
subjects was compared using Student's t-test. The gender of the two
subjects was compared using chi-square test. If the data met the
normal distribution and homogeneity of variance, JNDs among
amblyopic eyes, fellow eyes and normal eyes (dominant eyes) were
compared using one-way analysis of variance followed by pairwise
comparison using the least significant difference (LSD) method.
Otherwise, those data were analyzed using the Kruskal-Wallis
nonparametric test, and pairwise comparison was performed using the
Nemenyi method featured in the SAS v.8.2 software. The association
between amblyopic temporal discrimination and corrected visual
acuity was analyzed by Pearson correlation analysis. The changes in
JNDs by attentional load between amblyopic and normal eyes were
compared using group t-tests. P<0.05 was considered to indicate
a statistically significant difference.
Results
Comparison of demographic parameters
between normal and amblyopic subjects
Patients in the amblyopic group included 22 males
and 18 females aged 12.1±1.6 years. The normal group included 11
males and 9 females aged 12.2±1.5 years. Age and gender did not
differ significantly between the two groups (P>0.05).
Threshold of temporal
discrimination
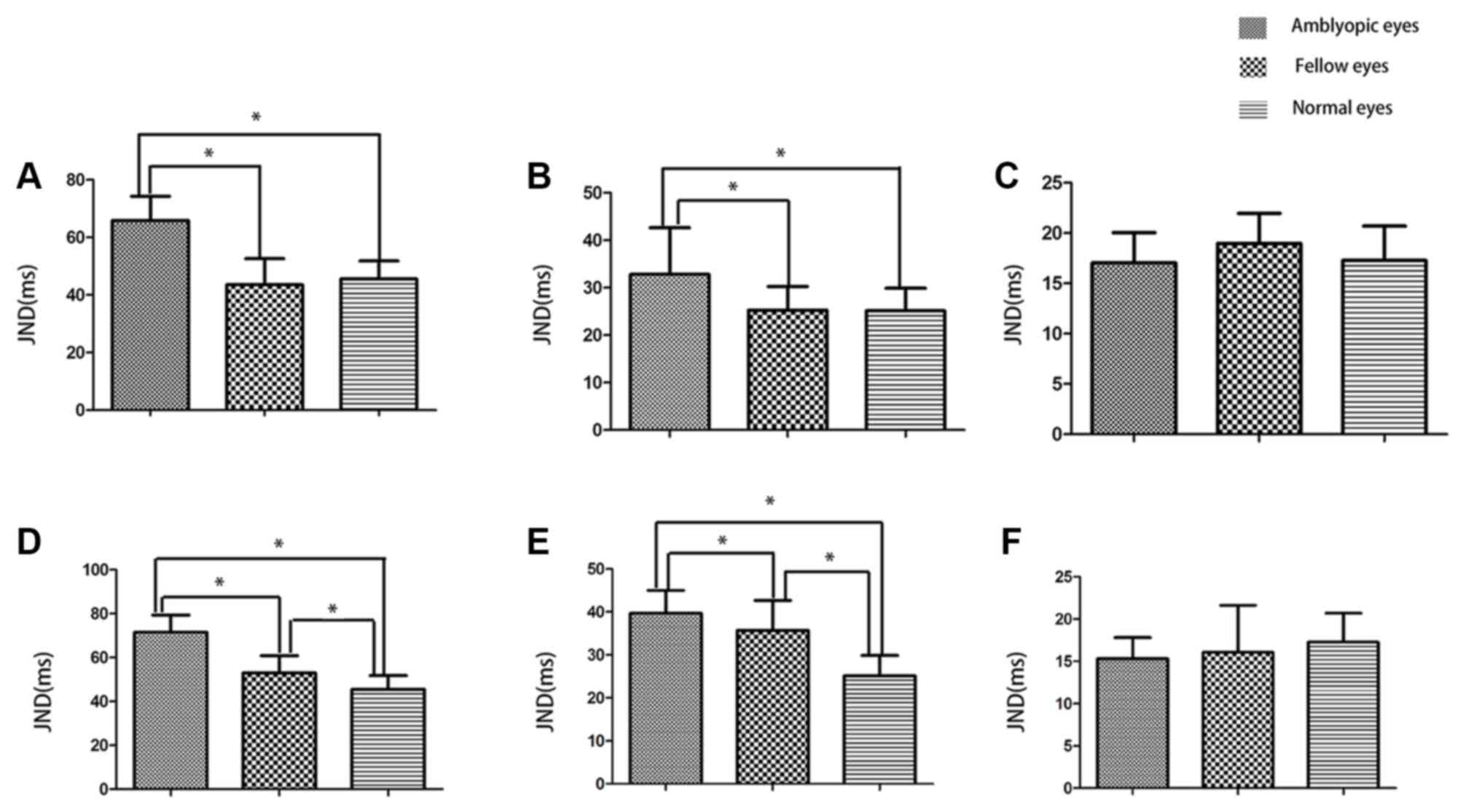
As shown in Fig. 1,
at 5 and 10 Hz, temporal thresholds of amblyopic eyes were
significantly increased compared with fellow eyes, either for
anisometropic amblyopia or for strabismic amblyopia (P<0.05). No
significant differences in the JNDs were identified between fellow
eyes and normal eyes for anisometropic amblyopia (P>0.05).
However, for strabismic amblyopia, temporal thresholds of fellow
eyes were increased compared with normal eyes (P<0.05). At 20
Hz, no significant differences in temporal thresholds among these
eyes were identified.
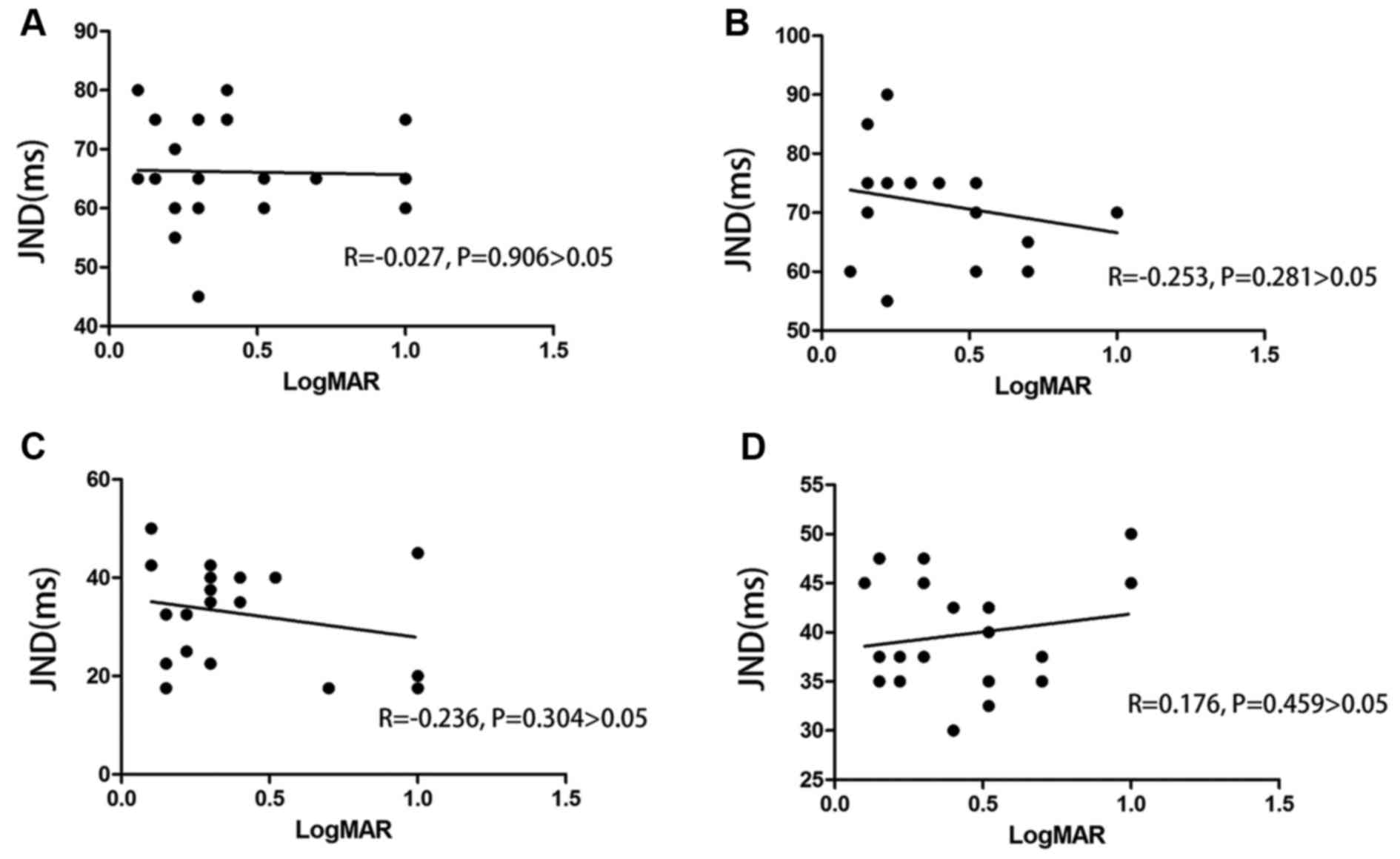
Association between temporal
discrimination deficit and spatial deficit in amblyopia
No significant correlation between the temporal
threshold and LogMAR visual acuity in amblyopia was observed
(P>0.05; Fig. 2).
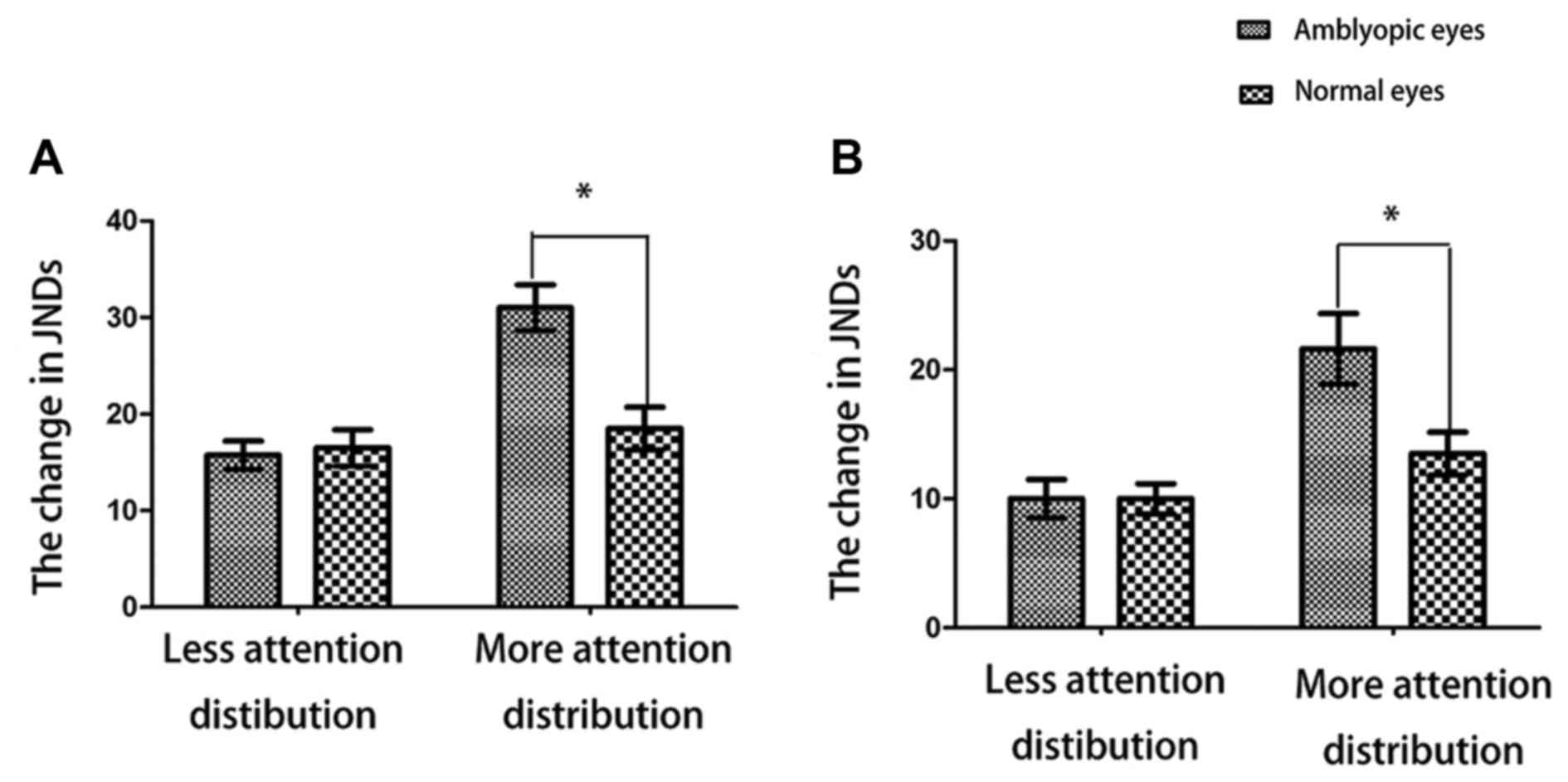
Changes in temporal thresholds by
attentional load in amblyopic and normal eyes
As shown in Fig. 3,
at 5 and 10 Hz, lower attentional load evoked no significantly
different changes in temporal thresholds between amblyopic and
normal eyes. However, higher attentional load caused increased
changes in temporal thresholds in amblyopic eyes compared with
normal eyes.
Discussion
Possible mechanism of temporal
discrimination deficit in amblyopia
The present study revealed that a larger duration
difference for amblyopic eyes compared with normal eyes was
required for differentiation to occur at the lower temporal
frequencies (5 and 10 Hz). This finding was consistent with the
research results obtained for temporal contrast sensitivity
function (TCSF), which was shown to have deteriorated at lower
temporal frequencies and be normal at higher temporal frequencies
(17). Although the present study
focused on the discrimination process and the study investigating
TCSF was focused on the detection process, the two studies
exhibited similar characteristics, indicating that a common
mechanism might exist between temporal detection and temporal
discrimination.
Moulden et al (18) reported two channels for flicker in
the human system. One channel was a low-pass channel with a peak
value of ~5 Hz, whereas the other channel was a band-pass channel
with a peak value of ~10 Hz. The finding reported in the present
study of temporal discrimination declining at 5 and 10 Hz in
amblyopia was similar to the peak values of the two channels.
Therefore, it was possible to hypothesize that this finding was
associated with the channel positions for the flicker.
Temporal discrimination deficit of
fellow eyes in amblyopia and its possible mechanism
Leguire et al (19) proposed that the normal eye of
amblyopia was not normal by testing the CSF. Their results were
subsequently corroborated using visual evoked potential (20). For the temporal deficits of fellow
eyes in amblyopia, numerous studies have evaluated these phenomena,
and these deficits were revealed to be most robust for motion
perception tasks (21–23). To date, and to the best of our
knowledge, the temporal discrimination of the fellow eye in
amblyopia based on assigning the task of flickery disk has rarely
been reported in the literature. The present study revealed a
temporal discrimination deficit in the fellow eye of strabismic
amblyopia, but not for anisometropic amblyopia. It is therefore
possible that different mechanisms might operate between them.
Association between visuotemporal
deficit and visuospatial deficit in amblyopia
Numerous researchers have held different opinions on
the association between temporal deficit and spatial deficit in
amblyopia. Some considered that the temporal deficit in amblyopia
was due to a spatial deficit (24,25).
However, other studies suggested that the temporal deficit in
amblyopia was independent of the spatial deficit (26). In the present study, the contrast of
the flickery stimulus was far beyond the contrast threshold, and
its subtended visual angle was within the scope of amblyopic visual
acuity. Furthermore, the uniform disk contained the spatial
frequency information of the wide band. Thus, our hypothesis was
that the temporal discrimination deficit in amblyopia was not
caused by a spatial deficit.
In addition, our results suggested that no
association existed between the visuotemporal deficit and visual
acuities in amblyopia, which was similar to the results identified
by Huang et al (26).
Related studies about temporal
frequency discrimination in amblyopia
Spang and Fahle (3)
suggested that there was a temporal frequency discrimination
deficit in amblyopia using the figure-ground segregation paradigm
and 4AFC method. However, there were two shortcomings with the
stimulus in that study. First, the stimulus could not always
maintain central fixation, whereas temporal discrimination was
influenced by central fixation and eccentric fixation (26). Secondly, the rotary stimulus included
unnecessary information, such as motion, direction and orientation,
which might have interfered with the results. In the present study,
a flickery disk with a fixation cross was used as the stimulus,
which maintained central fixation and contained pure temporal
information, without any information regarding motion, direction,
and orientation.
Possible mechanism of attentional load
affecting temporal discrimination in amblyopia
In the field of study of diseases involving temporal
discrimination and attention, ADHD has been the most studied. This
attention deficit disease is low in temporal discrimination
(27), which is located in the left
inferior frontal cortex as assessed by functional magnetic
resonance imaging (fMRI) (28). The
cognitive process of attention is exclusively associated with the
frontal lobe (29). Li et al
(30) demonstrated that the white
matter volume of the bilateral inferior frontal lobes was lost in
amblyopes by voxel-based morphometry of MRI. Thus, the discovery of
temporal deficits in amblyopia in the present study may be
associated with attention deficit by frontal inactivation. This
hypothesis will be investigated further in our future studies.
In conclusion, it was shown in the present study
that temporal frequency discrimination is deteriorated in amblyopic
eyes and the fellow eyes of strabismic amblyopes. Furthermore, the
influence of attentional load on temporal frequency discrimination
in amblyopic eyes was increased compared with normal eyes.
Acknowledgements
Not applicable.
Funding
The present study was supported by Sichuan Science
and Technology Program (grant no. 2018SZ0146).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY and JZ designed the investigation and drafted the
manuscript. JW collected and analyzed the patient data. LL
conceptualized the research and revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocol was approved by the
institutional review board of West China Hospital of Sichuan
University (Chengdu, China; no. 201433).
Patient consent for publication
The guardians provided written informed consent for
the publication of any associated data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang X and Liu L: Research progress on
visual defects of amblyopia. Chin J Exp Ophthalmol. 35:1139–1142.
2017.
|
|
2
|
Rammsayer TH: Ageing and temporal
processing of durations within the psychological present. Eur J
Cogn Psychol. 13:549–565. 2001. View Article : Google Scholar
|
|
3
|
Spang K and Fahle M: Impaired temporal,
not just spatial, resolution in amblyopia. Invest Ophthalmol Vis
Sci. 50:5207–5212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ho CS and Giaschi DE: Low- and high-level
motion perception deficits in anisometropic and strabismic
amblyopia: Evidence from fMRI. Vision Res. 49:2891–2901. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ho CS, Paul PS, Asirvatham A, Cavanagh P,
Cline R and Giaschi DE: Abnormal spatial selection and tracking in
children with amblyopia. Vision Res. 46:3274–3283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Secen J, Culham J, Ho C and Giaschi D:
Neural correlates of the multiple-object tracking deficit in
amblyopia. Vision Res. 51:2517–2527. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tripathy SP and Levi DM: On the effective
number of tracked trajectories in amblyopic human vision. J Vis.
8(8): 1–22. 2008.PubMed/NCBI
|
|
8
|
Sharma V, Levi DM and Klein SA:
Undercounting features and missing features: Evidence for a
high-level deficit in strabismic amblyopia. Nat Neurosci.
3:496–501. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Popple AV and Levi DM: The attentional
blink in amblyopia. J Vis. 8(12): 1–9. 2008.PubMed/NCBI
|
|
10
|
Farzin F and Norcia AM: Impaired visual
decision-making in individuals with amblyopia. J Vis. 11:pii: 6.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thiel A and Sireteanu R: Strabismic
amblyopes show a bilateral rightward bias in a line bisection task:
Evidence for a visual attention deficit. Vision Res. 49:287–294.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hou C, Kim YJ, Lai XJ and Verghese P:
Degraded attentional modulation of cortical neural populations in
strabismic amblyopia. J Vis. 16:162016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ziv N and Omer E: Music and time: The
effect of experimental paradigm, musical structure and subjective
evaluations on time estimation. Psychol Music. 39:182–195. 2011.
View Article : Google Scholar
|
|
14
|
Block RA, Hancock PA and Zakay D: How
cognitive load affects duration judgments: A meta-analytic review.
Acta Psychol (Amst). 134:330–343. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arzounian D, de Kerangal M and de
Cheveigné A: A sliding two-alternative forced-choice paradigm for
pitch discrimination. J Acoust Soc Am. 142:1672017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hagmann-von Arx P, Manicolo O,
Perkinson-Gloor N, Weber P, Grob A and Lemola S: Gait in very
preterm school-aged children in dual-task paradigms. PLoS One.
10:e01443632015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spekreijse H, Khoe LH and van der Tweel
LH: A case of amblyopia; electrophysiology and psychophysics of
luminance and contrast. Adv Exp Med Biol. 24:141–156. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moulden B, Renshaw J and Mather G: Two
channels for flicker in the human visual system. Perception.
13:387–400. 1984. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leguire LE, Rogers GL and Bremer DL:
Amblyopia: The normal eye is not normal. J Pediatr Ophthalmol
Strabismus. 27:32–39. 1990.PubMed/NCBI
|
|
20
|
Xiao M, Wei X, Li Y, Xion W and Xu S:
Pattern visual evoked potentials in normal-vision eyes of
post-therapy amblyopia. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
38:704–708. 2013.(In Chinese). PubMed/NCBI
|
|
21
|
Giaschi DE, Regan D, Kraft SP and Hong XH:
Defective processing of motion-defined form in the fellow eye of
patients with unilateral amblyopia. Invest Ophthalmol Vis Sci.
33:2483–2489. 1992.PubMed/NCBI
|
|
22
|
Kiorpes L, Tang C and Movshon JA:
Sensitivity to visual motion in amblyopic macaque monkeys. Vis
Neurosci. 23:247–256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ho CS, Giaschi DE, Boden C, Dougherty R,
Cline R and Lyons C: Deficient motion perception in the fellow eye
of amblyopic children. Vision Res. 45:1615–1627. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hou F, Huang CB, Tao L, Feng L, Zhou Y and
Lu ZL: Training in contrast detection improves motion perception of
sinewave gratings in amblyopia. Invest Ophthalmol Vis Sci.
52:6501–6510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pianta MJ and Kalloniatis M:
Characteristics of anisometropic suppression: Simple reaction time
measurements. Percept Psychophys. 60:491–502. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang PC, Li J, Deng D, Yu M and Hess RF:
Temporal synchrony deficits in amblyopia. Invest Ophthalmol Vis
Sci. 53:8325–8332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Noreika V, Falter CM and Rubia K: Timing
deficits in attention-deficit/hyperactivity disorder (ADHD):
Evidence from neurocognitive and neuroimaging studies.
Neuropsycholia. 51:235–266. 2013. View Article : Google Scholar
|
|
28
|
Hart H, Radua J, Mataix-Cols D and Rubia
K: Meta-analysis of fMRI studies of timing in attention-deficit
hyperactivity disorder (ADHD). Neurosci Biobehav Rev. 36:2248–2256.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mioni G, Stablum F and Cantagallo A: Time
discrimination in traumatic brain injury patients. J Clin Exp
Neuropsychol. 35:90–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Q, Jiang Q, Guo M, Li Q, Cai C and Yin
X: Grey and white matter changes in children with monocular
amblyopia: Voxel-based morphometry and diffusion tensor imaging
study. Br J Ophthalmol. 97:524–529. 2013. View Article : Google Scholar : PubMed/NCBI
|