Introduction
Shock caused by acute hemorrhage is responsible for
~40% of all trauma-associated fatalities (1,2). It has
long been recognized that multiple organ injuries are associated
with death induced by severe hemorrhagic shock (3–5). The gut
is one of primary organs affected by ischemia following acute
hemorrhage, and ischemic gut injury induces bacteria/endotoxin
translocation that causes further remote organ injury (6). Previous studies have revealed that
unique gut-derived factors carried in the mesenteric lymph, but not
the portal vein, lead to acute organ injury and multiple organ
dysfunction syndrome (MODS) following hemorrhagic shock (7–11).
Previous studies have indicated that blockage of post-hemorrhagic
shock mesenteric lymph (PHSML) return by mesenteric lymph duct
ligation could alleviate the pulmonary injury, cardiac contractile
dysfunction and acute kidney injury (12–15).
Furthermore, PHSML intravenous infusion into naïve rats induced
myocardial contractile dysfunction and decreased RBC deformability
(16,17). These results indicate that the return
of PHSML to blood circulation is involved in the process of
hemorrhagic shock-induced MODS. However, the detailed role of PHSML
return in the pathogenesis of MODS remains unclear; therefore, it
may be useful to investigate the association between PHSML and MODS
following hemorrhagic shock. In addition, previous studies have
demonstrated that the increased trypsin activity and the subsequent
downstream protease cascade serve an important role in hemorrhagic
shock-induced tissue injury and dysfunction besides inflammation
(18,19). Therefore, in the current study, the
effect of intravenous injection of PHSML on trypsin activity,
inflammatory factors, free radical production and multiple organ
injuries in normal rats was investigated. The results revealed that
PHSML has a causal role in mediating multiple organ injuries
following hemorrhagic shock.
Materials and methods
Animals
Adult, male, specific pathogen-free Wistar rats
(n=18; age, 3–4 months; weight, 230–270 g) were purchased from the
Animal Breeding Center of Chinese Academy of Medical Sciences
(Beijing, China). Rats were housed in clear plastic cages at a
temperature of 22–24°C and a humidity of 40–50%, under a 12 h
light/dark cycle with free access to water and food. All rats were
fasted for 12 h, but allowed free access to water, before the
experiments. The animal study was approved by the Institutional
Animal Use and Care Committee of Hebei North University
(Zhangjiakou, China).
Preparation of PHSML
Six rats were used to establish the hemorrhagic
shock model for preparation of PHSML, as previously described
(20,21). Rats were anesthetized with 50 mg/kg
sodium pentobarbital (Beijing Chemical Reagents Institute, Beijing,
China). The femoral artery and mesenteric lymph duct
catheterization were performed for hemorrhage, and mean arterial
pressure (MAP) monitoring and PHSML drainage, respectively.
Following an equilibrium period of 30 min, the hemorrhage was
conducted via the left femoral artery, and the MAP was maintained
at a level of 40 mmHg for 3 h by withdrawing or perfusing shed
blood as needed for the establishment of the hemorrhagic shock
model. Subsequently, the PHSML was drained from 1–3 h of
hypotension. It should be noted that the PHSML collected from 1 rat
with hemorrhagic shock was in the range of 0.15–0.25 ml; then, the
PHSML was centrifuged for 5 min at 315 g at 4°C and stored at −75
to −80°C before further experimentation. Following the collection
of the PHSML, the rats were humanely sacrificed by cervical
dislocation while under deep anesthetic conditions.
Intravenous injection of PHSML
The 12 rats were randomly divided into the control
group and PHSML group (n=6 rats in each group). Following
anesthetization, the femoral artery and vein were catheterized for
MAP monitoring and PHSML (PHSML group) or normal saline (control
group) injection, respectively, as described previously (10,11).
Following a stabilization period of 30 min, cell-free supernatant
fluid from PHSML samples was diluted with an equal amount of saline
and injected into rats intravenously at a dose of 2 ml/kg within 30
min. In the control group rats, the equal volume of saline was
injected intravenously.
Collection and preparation of
samples
At 150 min post-intravenous injection, blood samples
were collected from the abdominal aorta and the plasma was obtained
by centrifugation at 850 × g at 0–4°C for 10 min and stored at −75
to −80°C for assay of biochemical indicators, trypsin, tumor
necrosis factor-α (TNF-α), intercellular adhesion molecule-1
(ICAM-1) and receptor of advanced glycation end-products (RAGE).
Following blood sample collection, the rats were sacrificed as
above. Subsequently, lung, kidney, liver and heart samples were
harvested and fixed. One part of each organ was fixed in 4%
paraformaldehyde at 22–24°C for 1 week to determine morphological
changes, while the remaining part of each organ was homogenized for
the measurement of malondialdehyde (MDA) and TNF-α.
Observation of history
Following 24 h of fixation with paraformaldehyde,
the tissues were dehydrated in an alcohol gradient and embedded in
paraffin. Sections (5 µm) were prepared and stained with
hematoxylin and eosin at room temperature for 3 min and 1 min,
respectively. Morphological changes in the lung, kidney, liver and
myocardium were observed using a light microscope (90i; Nikon
Corporation, Tokyo, Japan) and micrographs were obtained using an
image collection and analysis system (Eclipse; Nikon Corporation)
at a magnification of ×400.
Measurement of biochemical
indicators
To assess the renal, hepatic and myocardial
function, the plasma levels of urea (Shanghai Fahrenheit
Asia-Pacific Biopharmaceutical Co., Ltd., Shanghai, China; cat. no.
20101210), creatinine (Cre; Shanghai Fahrenheit Asia-Pacific
Biopharmaceutical Co., Ltd.; cat. no. 10122101), aspartate
aminotransferase (AST; Wako Pure Chemical Industries, Ltd., Osaka,
Japan; cat. no. EH564), alanine aminotransferase (ALT; cat. no.
EH567; Wako Pure Chemical Industries, Ltd.), total bile acid (TBA;
cat. no. GZD10301; Neusoft Witman Biotechnology Co., Ltd., Nanjing,
China), lactate dehydrogenase 1 (LDH-1; cat. no. 10091902; Zhejiang
Dongsheng Diagnostic Products Co., Ltd., Wenzhou, China) and
creatine kinase MB isoenzyme (CK-MB; cat. no. 20101112; Shanghai
Kehua Bio-engineering Co., Ltd.) were examined using an automatic
biochemical analyzer (7600-110; Hitachi, Ltd., Tokyo, Japan).
Examination of trypsin activity
Using the hydrolysis method, the trypsin activity in
plasma was determined according to the manufacturer's instructions
(Jiancheng Biotechnology Research Institute, Nanjing, China). One
unit of trypsin activity corresponded to the amount of enzyme that
produced an increased absorbance of 0.003 per min at 253 nm, pH 8.0
and 37°C.
ELISA
To determine the changes in ICAM-1, RAGE and TNF-α
in plasma following the PHSML injection, rat-specific ELISA kits
were used according to the manufacturer's recommendations. The
following antibodies were purchased from R&D Systems
(Minneapolis, MN, USA): ICAM-1 (cat. no. MAB5832-100; 1:250), RAGE
(cat. no. AF1616; 1:1,000) and TNF-α (cat. no. MAB510-100; 1:250),
and ELISA kits were prepared by Jiangsu Hope, Inc. (Zhenjiang,
China), while the catalogue numbers were 10632R, 10433R and 10775R,
respectively. The concentration of each examined protein was
calculated by comparing the optical density to that of the standard
curve.
The level of TNF-α in tissue was measured using the
same method. The protein content in homogenates was quantified by
the Coomassie brilliant blue colorimetric method, and values were
normalized to the protein levels.
Examination of MDA
The MDA in tissue homogenates was measured using a
modified thiobarbituric acid (TBA) micro-determination assay
according to the instructions provided by the manufacturer
(Jiancheng Biotechnology Research Institute, Nanjing, China).
Briefly, tissue homogenate (0.1 ml) was mixed with 0.1 ml of
dehydrated alcohol, 0.1 ml TBA and 4.0 ml of developer. Following
incubation in a water bath at 95°C for 40 min, the samples were
cooled in running water and then centrifuged at 1,200 × g at room
temperature for 10 min. The absorbance of the resulting supernatant
at 532 nm was measured and the MDA level was presented as nmol/mg
protein.
Statistical analysis
Data are collected and presented as the mean ±
standard deviation. Statistical analysis was performed using SPSS
16.0 for Windows (SPSS Inc., Chicago, IL, USA). Differences between
the two groups were analyzed using an independent sample Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
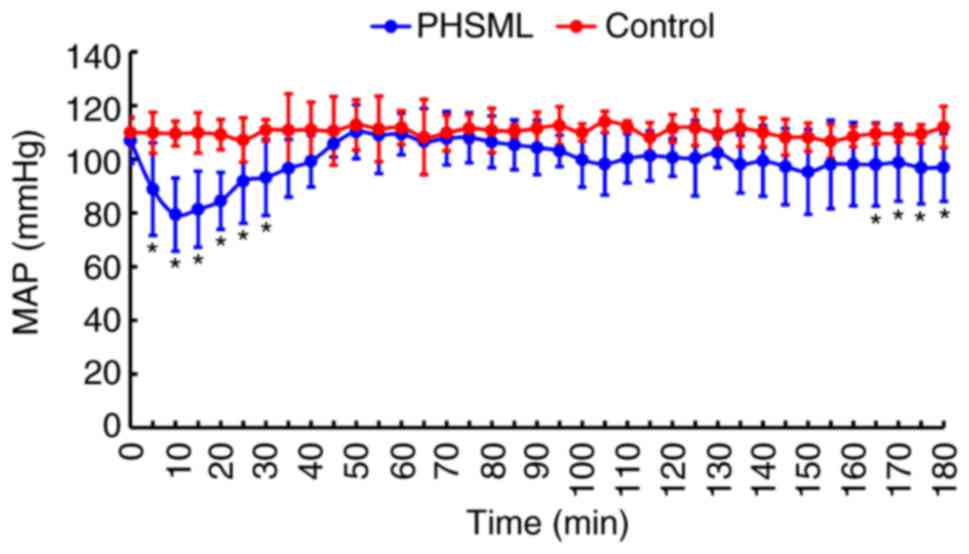
Effect of PHSML on MAP
Intravenous injection of saline did not result in a
significant change in the MAP in rats (105 to 112 mmHg, P>0.05).
By contrast, intravenous injection with PHSML induced a significant
decrease in the MAP during the injection period (P<0.05). This
decreased MAP gradually returned to a normal level. At 90 min, the
MAP started declining again, and reached the minimum at 165–180 min
compared with the control rats (P<0.05; Fig. 1).
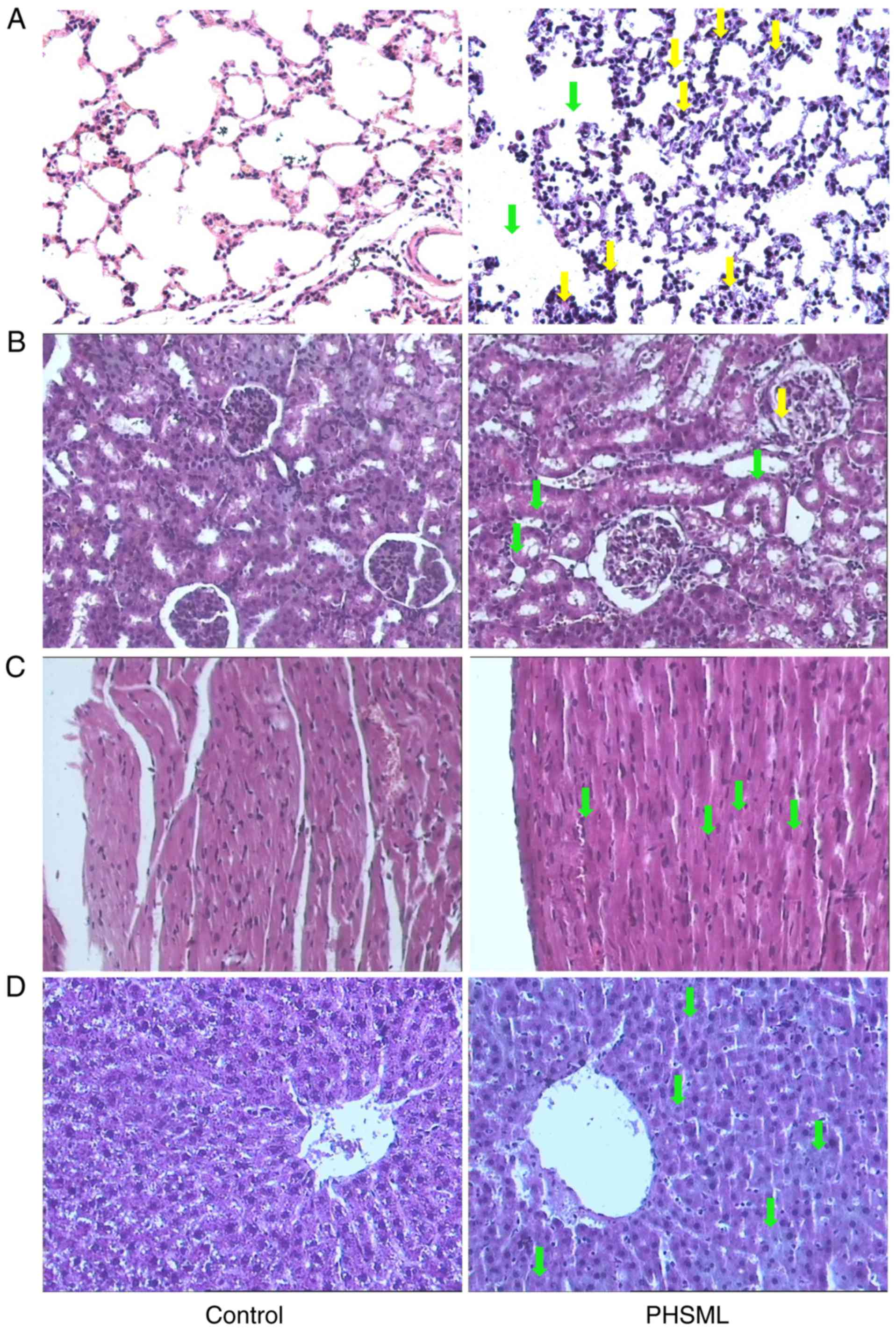
Effect of intravenous injection on the
morphology of the lung, kidney, myocardium and liver
Rats that received saline injection had normal
alveolar architecture, with thin walls covered by alveolar
epithelial cells (Fig. 2A). By
contrast, the rats that received the PHSML injection had broadening
alveolar septum with inflammatory cell infiltration.
In the kidney of the control group rats, there was
normal architecture in the renal glomerulus and tubules, and there
were clear and distinctive proximal and distal convoluted tubules
(Fig. 2B). By contrast, the rats
that received PHSML administration exhibited epithelial cells with
swelling in the tubules, and there was a small amount of protein
deposition in the Bowman's capsule.
Imaging of myocardial sections revealed that the
rats in the control group had normal myocardial fiber bundles and
uniform myocardial cells with central nuclei and clear nuclear
membranes, whereas the PHSML administration induced the
disorganization of myocardial fibers (Fig. 2C).
In the liver sections of control mice, the
hepatocytes were properly organized, with round central nuclei and
clear nuclear membrane (Fig. 2D). In
the rats that received the PHSML injection, the hepatocytes were
slightly disorganized and exhibited edema.
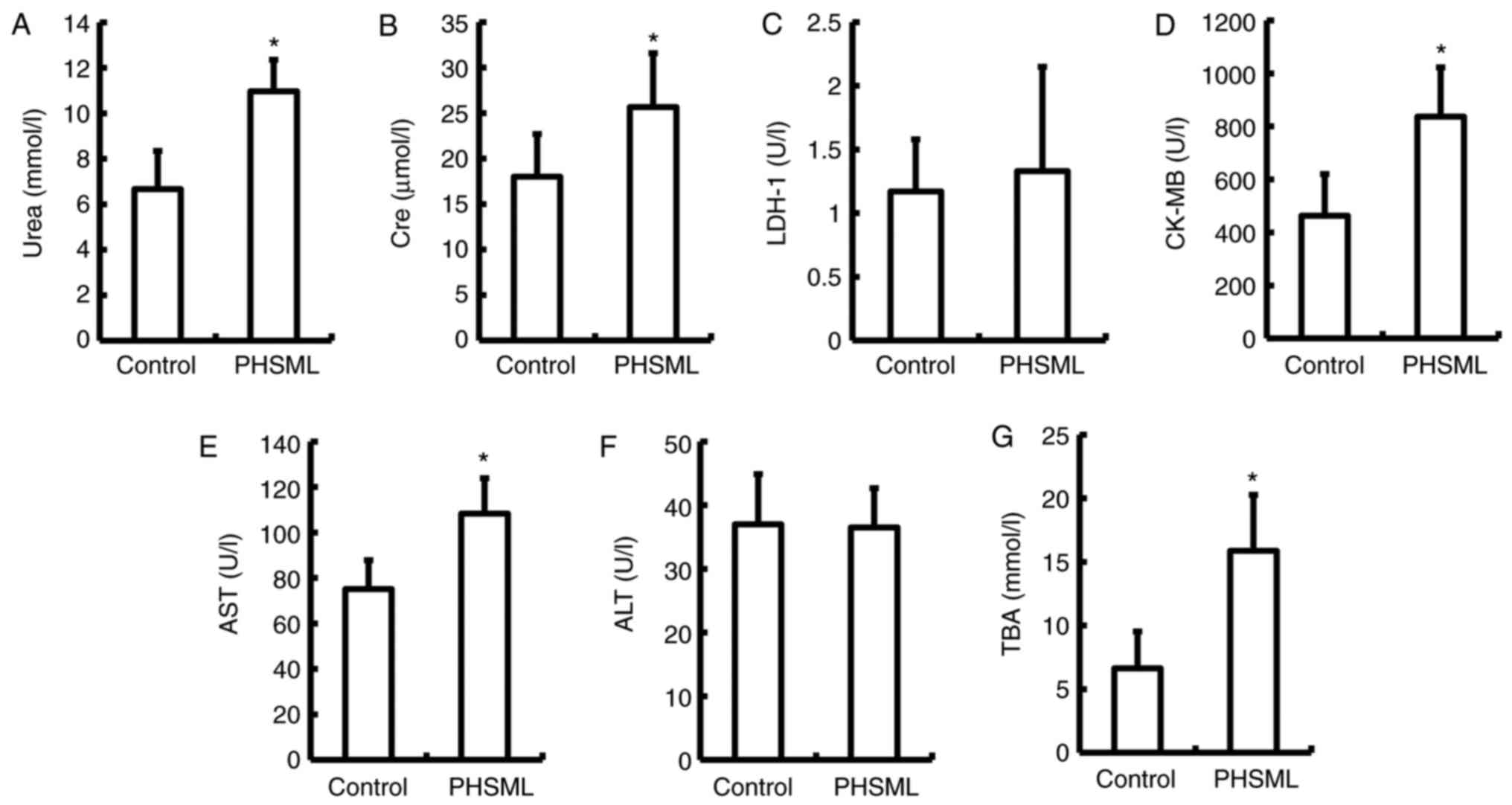
Effect of PHSML on the biochemical
markers in plasma
Urea and Cre, AST and TBA, and CK-MB are markers of
renal function, hepatocyte necrosis and function, and cardiomyocyte
injury, respectively (22–24). In the PHSML group, these indices were
significantly increased compared with the control group
(P<0.05). However, there was no statistical difference in the
level of ALT and LDH-1 between the control and PHSML groups
(P>0.05; Fig. 3).
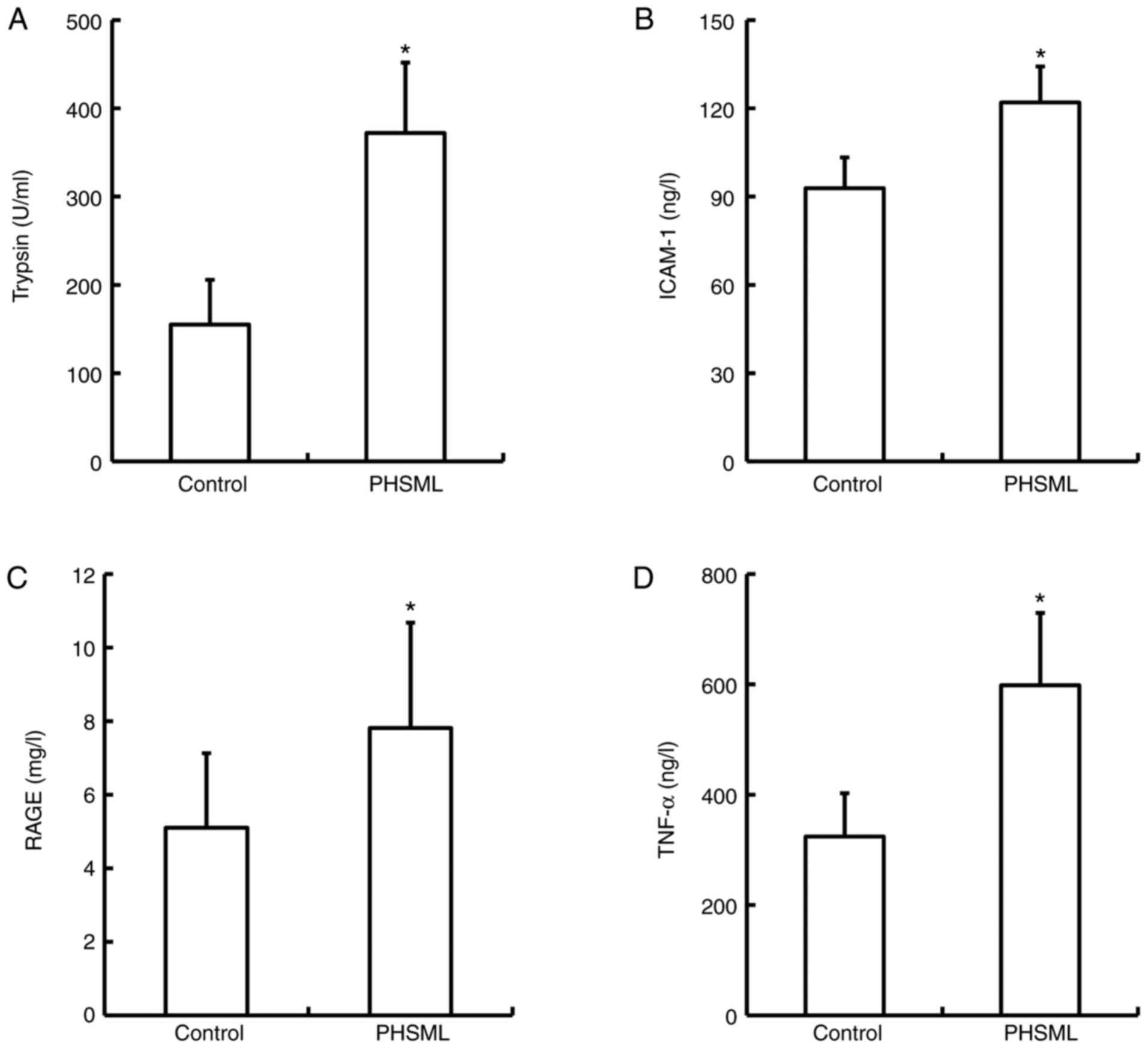
Effect of PHSML on trypsin, ICAM-1,
RAGE and TNF-α in plasma
The activity of trypsin, and ICAM-1, RAGE and TNF-α
levels in plasma samples from the PHSML group (372.00±79.77 U/ml,
24.57±2.43 ng/l, 7.81±2.86 mg/l and 598.16±131.38 ng/l,
respectively) were significantly increased compared with the
control group (155.00±50.62 U/ml, 18.58±2.08 ng/l, 5.10±2.02 mg/l
and 324.28±78.22 ng/l; P<0.05; Fig.
4).
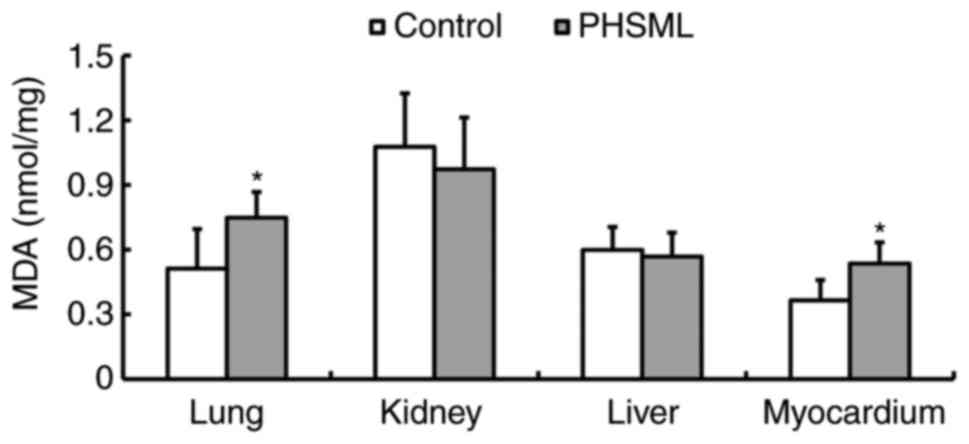
Effect of PHSML injection on the MDA
level in tissues
PHSML administration resulted in a significant
increase in the MDA content in pulmonary and myocardial tissue
homogenates compared with that in the control group (P<0.05;
Fig. 5). However, there was no
statistical difference in the MDA level of hepatic and renal tissue
homogenates between the control and PHSML groups (P>0.05).
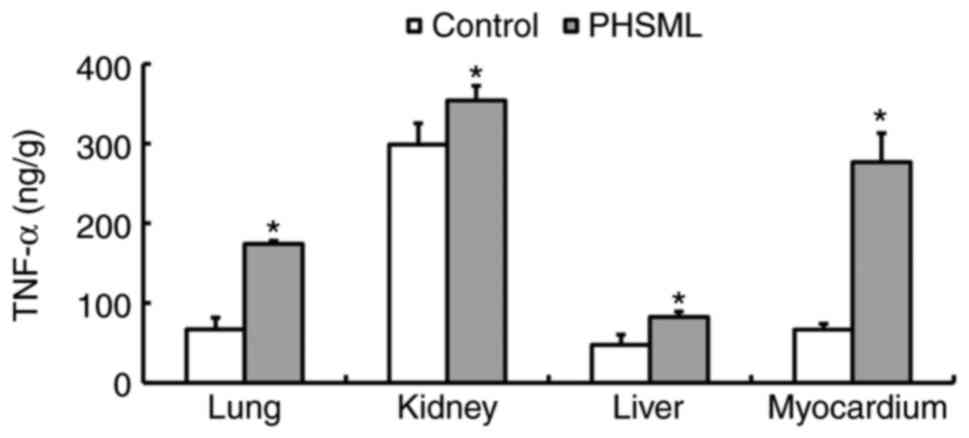
Effect of intravenous PHSML injection
on the TNF-α level in tissues
The results indicated that TNF-α levels were
significantly higher in lung, kidney, myocardium and liver samples
from the PHSML group compared with the control group (P<0.05;
Fig. 6).
Discussion
Based on the previous studies (7–11)
implicating gut-derived factors carried in the mesenteric lymph as
contributing factors to tissue injury and dysfunction caused by
hemorrhagic shock, PHSML was drained from rats with hemorrhagic
shock and injected into normal rats in the current study. The
injected animals exhibited a phased decrease in blood pressure and
damage to the lung, kidney, heart and liver. The results confirm
that the PHSML is an important contributor to organ injuries
following hemorrhagic shock.
In the current study, PHSML injection induced a
significant decrease in MAP at the early and late stage during the
experiment. Thus, the pattern is similar to the effects observed
following LPS administration (25,26).
Similar to LPS administration, PHSML injection has been revealed to
induce damage to red blood cells, which contributes to decreased
microcirculatory blood flow and organ hypoperfusion (27). Intravenous infusion of PHSML was also
reported cause myocardial contractile dysfunction and diminish the
left ventricular developed pressure (LVDP) and the maximal rate of
LVDP rise and fall [±dP/dt(max)] in a previous study (16). This indicated that hypotension at the
early and late stage post-PHSML injection may be caused by the
adverse effects of PHSML on blood flow and myocardial function.
The effects of PHSML injection on tissue morphology,
and biochemical indices that reflect organ function or cell injury
were determined in the current study. Intravenous injection of
PHSML induced structural damage in the lung, kidney, myocardium and
liver, including inflammatory cell infiltration in the alveolar
septum, protein deposition in the Bowman's capsule, disorganization
of myocardial fibers and slight edema in hepatocytes. In addition,
PHSML administration resulted in significant increases in urea,
Cre, AST, TBA and CK-MB in the plasma, which further confirmed the
toxic effect of PHSML at the organ level.
Trypsin, a proteolysis enzyme, is released from
damaged cells, which causes excessive protein catabolism and
exacerbates cell injury (28). In
the current study, the trypsin activity in plasma was significantly
increased by PHSML injection, suggesting that the toxic components
from PHSML caused cell injury resulting in increased trypsin
release. In addition, it is possible that there was a high
concentration of trypsin in the PHSML itself, which exacerbated
organ injury in a positive feedback manner.
RAGE, a member the immunoglobulin super-family, has
a pivotal role in binding advanced glycation end-products (AGEs).
The binding of RAGE with AGEs induces inflammatory responses that
can result in cell injury or tissue damage. Therefore, RAGE can be
used as a marker of inflammation and injury (29). Previous studies have indicated that
the activation of RAGE-dependent signaling is involved in gut
mucosal barrier dysfunction, and the excessive inflammation but
occurs following hemorrhagic shock and resuscitation (30). RAGE signaling also promotes the
mobilization of hematopoietic progenitor cells from bone marrow
(31). In the current study, RAGE
and TNF-α were significantly increased following administration of
PHSML to normal rats. These results indicate the excessive
inflammation derived from increased RAGE is involved in the process
of tissue injury following PHSML injection.
During hemorrhagic shock, increased levels of ICAM-1
induce s adhesion and activation of inflammatory cells, which is a
key indicator of uncontrolled inflammatory responses and organ
dysfunction (32). Interference with
ICAM-1 may be beneficial for regaining and/or maintaining the
balance of inflammation and organ function (32–34). In
the current study, ICAM-1 was increased in plasma, and TNF-α was
increased in plasma, lung, kidney, liver and myocardium samples
following PHSML injection, suggesting that tissue injury was caused
by ICAM-1-induced inflammation.
The excessive production of endogenous free
radicals, such as MDA and reactive oxygen species, can destroy the
structural integrity of cell membranes and organelles, thus
activating a cascade of free radical injury through reactions with
unsaturated fatty acid located in the cell membrane, producing
lipid peroxides (35,36). In the current study, PHSML
administration resulted in an increase in the MDA level in the lung
and myocardium, indicating that the pulmonary and myocardial
injuries were associated with free radical. However, there were no
statistical differences in the MDA contents in the kidney and
hepatic tissue samples, which may be associated with the short
observation time used in the current study.
It should be noted that there are several
limitations in the current study. For example, multiple
intracellular signaling pathways, including the mitogen-activated
protein kinase pathway and IκB kinase-nuclear factor-κB pathway,
coordinate the induction of inflammatory mediators and induce
inflammation during hemorrhagic shock (3,37).
However, in the current study, only the adverse effects of PHSML on
trypsin activity and inflammatory mediator levels were examined;
therefore, whether these signaling pathways are involved in the
mechanism of the PHSML injection-induced inflammatory response
requires further investigation. Additionally, given the role of
white blood cells (WBC) in supporting systemic inflammation,
whether PHSML injection induces an increase in WBC counts in
peripheral blood should be investigated in the future.
In addition, the activity of trypsin and the levels
of ICAM-1, RAGE and TNF-α were not measured in the lymph obtained
from hemorrhagic shock rats. These factors may induce the adverse
effects observed in the current study if there are high
concentrations in the lymph. However, the amount of PHSML injected
to recipient rats was only 0.23–0.27 ml (according the dose of 1
ml/kg), which was much less than the whole rat blood volume of
17.7–20.8 ml (~1/13 body weight); thus, it is likely that the
changes in serum mediators in recipient rats arise mainly from
PHSML stimulation, rather than the factors in PHSML itself from the
donor rats.
In conclusion, the findings of current study
demonstrated that the intravenous injection of PHSML induces organ
injury in normal rats, and that this adverse effect is associated
with increased trypsin activity, inflammatory mediators and free
radicals. Combined with the previous study demonstrating that PHSML
drainage decreases the levels of trypsin activity in the plasma and
ICAM-1, RAGE, TNF-α and MDA in renal tissue (38), these findings further elucidate the
mechanism underlying multiple organ injuries induced by the return
of PHSML following severe hemorrhagic shock.
Acknowledgements
The authors would like to thank Professor Chun-yu
Niu (Institute of Microcirculation, Hebei North University,
Zhangjiakou, Hebei, China) for designing the current study and
obtaining funding.
Funding
The current study was supported by the National
Natural Science Foundation of China (grant no. 30370561) and the
Doctoral Scientific Fund Project of Hebei North University (grant
no. 201710).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ, LZ, RH and YS acquired the data. YZ also wrote
the manuscript. ZZ was involved in the conception and design of the
study, data analysis and interpretation, and critically revised the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Use and Care Committee of Hebei North University
(Zhangjiakou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sauaia A, Moore FA, Moore EE, Moser KS,
Brennan R, Read RA and Pons PT: Epidemiology of trauma deaths: A
reassessment. J Trauma. 38:185–193. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang Y, Ratz PH, Miner AS, Locke VA, Chen
G, Chen Y and Barbee RW: AICAR administration attenuates
hemorrhagic hyperglycemia and lowers oxygen debt in anesthetized
male rabbits. Front Physiol. 8:6922017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Korff S, Loughran P, Cai C, Lee YS, Scott
M and Billiar TR: Eritoran attenuates tissue damage and
inflammation in hemorrhagic shock/trauma. J Surg Res. 184:e17–e25.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Douzinas EE: Hemorrhagic shock
resuscitation: A critical issue on the development of posttraumatic
multiple organ failure. Crit Care Med. 40:1348–1349. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hildebrand F, Andruszkow H, Huber-Lang M,
Pape HC and van Griensven M: Combined hemorrhage/trauma models in
pigs-current state and future perspectives. Shock. 40:247–273.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tarras SL, Diebel LN, Liberati DM and
Ginnebaugh K: Pharmacologic stimulation of the nicotinic
anti-inflammatory pathway modulates gut and lung injury after
hypoxia-reoxygenation injury. Surgery. 154:841–848. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deitch EA, Xu D and Kaise VL: Role of the
gut in the development of injury- and shock induced SIRS and MODS:
The gut-lymph hypothesis, a review. Front Biosci. 11:520–528. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deitch EA: Gut-origin sepsis: Evolution of
a concept. Surgeon. 10:350–356. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deitch EA: Gut lymph and lymphatics: A
source of factors leading to organ injury and dysfunction. Ann N Y
Acad Sci. 1207 Suppl 1:E103–E111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai B, Deitch EA and Ulloa L: Novel
insights for systemic inflammation in sepsis and hemorrhage.
Mediators Inflamm. 2010:6424622010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fanous MY, Phillips AJ and Windsor JA:
Mesenteric lymph: The bridge to future management of critical
illness. JOP. 8:374–399. 2007.PubMed/NCBI
|
|
12
|
Sambol JT, Xu DZ, Adams CA, Magnotti LJ
and Deitch EA: Mesenteric lymph duct ligation provides long term
protection against hemorrhagic shock-induced lung injury. Shock.
14:416–420. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deitch EA, Adams C, Lu Q and Xu DZ: A time
course study of the protective effect of mesenteric lymph duct
ligation on hemorrhagic shock-induced pulmonary injury and the
toxic effects of lymph from shocked rats on endothelial cell
monolayer permeability. Surgery. 129:39–47. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sambol JT, Lee MA, Caputo FJ, Kawai K,
Badami C, Kawai T, Deitch EA and Yatani A: Mesenteric lymph duct
ligation prevents trauma/hemorrhage shock-induced cardiac
contractile dysfunction. J Appl Physiol (1985). 106:57–65. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niu CY, Zhao ZG, Ye YL, Hou YL and Zhang
YP: Mesenteric lymph duct ligation against renal injury in rats
after hemorrhagic shock. Ren Fail. 32:584–591. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sambol JT, Lee MA, Jiang M, Dosi G, Dong
W, Deitch EA and Yatani A: Mesenteric lymph from rats with
trauma-hemorrhagic shock causes abnormal cardiac myocyte function
and induces myocardial contractile dysfunction. J Appl Physiol
(1985). 111:799–807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Condon M, Senthil M, Xu DZ, Mason L, Sheth
SU, Spolarics Z, Feketova E, Machiedo GW and Deitch EA: Intravenous
injection of mesenteric lymph produced during hemorrhagic shock
decreases RBC deformability in the rat. J Trauma. 70:489–495. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rao G, Yadav VR, Awasthi S, Roberts PR and
Awasthi V: Effect of liposome-encapsulated hemoglobin resuscitation
on proteostasis in small intestinal epithelium after hemorrhagic
shock. Am J Physiol Gastrointest Liver Physiol. 311:G180–G191.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Diebel ME, Diebel LN and Liberati DM:
Tranexamic acid and the gut barrier: Protection by inhibition of
trypsin uptake and activation of downstream intestinal proteases.
Am J Surg. 213:489–493. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao ZG, Niu CY, Wei YL, Zhang YP, Si YH
and Zhang J: Mesenteric lymph return is an important contributor to
vascular hyporeactivity and calcium desensitization after
hemorrhagic shock. Shock. 38:186–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao Z, Si Y, Zhang Y, Du S, Zhang L and
Niu C: Postshock mesenteric lymph drainage ameliorates vascular
reactivity and calcium sensitivity through RhoA. J Surg Res.
186:304–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Egli-Spichtig D, Zhang MYH and Perwad F:
Fibroblast growth factor 23 expression is increased in multiple
organs in mice with folic acid-induced acute kidney injury. Front
Physiol. 9:14942018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zinkhan EK, Yu B and Schlegel A: Prenatal
exposure to a maternal high fat diet increases hepatic cholesterol
accumulation in intrauterine growth restricted rats in part through
microRNA-122 inhibition of Cyp7a1. Front Physiol. 9:6452018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen H, Liu S, Liu X, Yang J, Wang F, Cong
X and Chen X: Lysophosphatidic acid pretreatment attenuates
myocardial ischemia/reperfusion injury in the immature hearts of
rats. Front Physiol. 8:1532017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mederle K, Schweda F, Kattler V, Doblinger
E, Miyata K, Höcherl K, Oike Y and Castrop H: The angiotensin II
AT1 receptor-associated protein Arap1 is involved in sepsis-induced
hypotension. Crit Care. 17:R1302013. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Piechota-Polańczyk A and Gorąca A:
Influence of specific endothelin-1 receptor blockers on hemodynamic
parameters and antioxidant status of plasma in LPS-induced
endotoxemia. Pharmacol Rep. 64:1434–1441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Machiedo GW, Zaets SB, Berezina TL, Xu DZ,
Feketova E, Spolarics Z and Deitch EA: Trauma-hemorrhagic
shock-induced red blood cell damage leads to decreased
microcirculatory blood flow. Crit Care Med. 37:1000–1010. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thrower EC, Gorelick FS and Husain SZ:
Molecular and cellular mechanisms of pancreatic injury. Curr Opin
Gastroenterol. 26:484–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Uchida T, Shirasawa M, Ware LB, Kojima K,
Hata Y, Makita K, Mednick G, Matthay ZA and Matthay MA: Receptor
for advanced glycation end-products is a marker of type I cell
injury in acute lung injury. Am J Respir Crit Care Med.
173:1008–1015. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Raman KG, Sappington PL, Yang R, Levy RM,
Prince JM, Liu S, Watkins SK, Schmidt AM, Billiar TR and Fink MP:
The role of RAGE in the pathogenesis of intestinal barrier
dysfunction after hemorrhagic shock. Am J Physiol Gastrointest
Liver Physiol. 291:G556–G565. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiang M, Yuan Y, Fan L, Li Y, Li A, Yin L,
Scott MJ, Xiao G, Billiar TR, Wilson MA and Fan J: Role of
macrophages in mobilization of hematopoietic progenitor cells from
bone marrow after hemorrhagic shock. Shock. 37:518–523. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li R, Zijlstra JG, Kamps JA, van Meurs M
and Molema G: Abrupt reflow enhances cytokine Induced
pro-inflammatory activation of endothelial cells during simulated
shock and resuscitation. Shock. 42:356–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao L, Luo L, Chen J, Xiao J, Jia W and
Xiao Y: Utilization of extracorporeal membrane oxygenation
alleviates intestinal ischemia-reperfusion injury in prolonged
hemorrhagic shock animal model. Cell Biochem Biophys. 70:1733–1740.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu HZ, Liu ZL, Zhao SP, Sun CZ and Yang
MS: Protective mechanism of Panax notoginseng Saponins on rat
hemorrhagic shock model in recovery stage. Cell Biochem Biophys.
70:1719–1724. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Getoff N: Vitamin C: Electron emission,
free radicals and biological versatility. In Vivo. 27:565–570.
2013.PubMed/NCBI
|
|
36
|
Bencini A, Failli P, Valtancoli B and Bani
D: Low molecular weight compounds with transition metals as free
radical scavengers and novel therapeutic agents. Cardiovasc Hematol
Agents Med Chem. 8:128–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kochanek AR, Fukudome EY, Li Y, Smith EJ,
Liu B, Velmahos GC, de Moya M, King D and Alam HB: Histone
deacetylase inhibitor treatment attenuates MAP kinase pathway
activation and pulmonary inflammation following hemorrhagic shock
in a rodent model. J Surg Res. 176:185–194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao ZG, Zhu HX, Zhang LM, Zhang YP and
Niu CY: Mesenteric lymph drainage alleviates acute kidney injury
induced by hemorrhagic shock without resuscitation.
ScientificWorldJournal. 2014:7208362014.PubMed/NCBI
|