Introduction
Atrial fibrillation (AF) is a type of arrhythmia
commonly encountered in clinical practice. AF is associated with a
5-fold increase in the incidence of cardioembolic stroke and a
3-fold increased risk of mortality (1). Accumulating evidence has demonstrated
that left atrial appendage (LAA) is a major site of cardiac
thrombus in patients with AF (1).
Catheter-based LAA closure (LAAC) has recently become an innovative
strategy for preventing cardioembolic events in patients with
nonvalvular AF. The WATCHMAN™ LAAC device and the
AMPLATZER Cardiac Plug are the most widely used closure devices
worldwide, particularly in USA and Europe (2). The method to guide the implantation of
these two types of LAAC devices is well defined (2,3). As
three dimensional (3D) imaging modalities are more reliable and
accurate than two dimensional transesophageal echocardiography (2D
TEE) for the assessment of the LAA orifice size and provide a full
view of the LAA, real time (RT)-3D TEE currently represents the
first-line approach during the procedure of LAAC (2,3).
The LACBES® occluder has been recently
developed by Changhai Hospital and Shanghai PushMed (Shanghai,
China) (4). As a novel LAA device,
there are limited data on the method of optimal sizing for the
LACBES® occluder. Therefore, the present study evaluated
the effectiveness of RT-3D TEE in guiding the LACBES®
device deployment in patients with nonvalvular AF. To the best of
our knowledge, this is the first study to investigate the
feasibility of RT-3D TEE to guide LAAC with the LACBES®
device. The present study has demonstrated that RT-3D TEE is a
feasible, effective and safe method to guide LACBES®
device deployment and has demonstrated that this method is more
accurate than 2D TEE or LAA contrast angiography for device sizing
during LAAC.
Patients and methods
Study population
The present clinical study complied with the
Declaration of Helsinki and was approved by the Ethics Review Board
of Shanghai Ninth People's Hospital, Shanghai Jiaotong University
School of Medicine (Shanghai, China; approval no. 2016-45-C12).
Individual permission was obtained from all the participants using
a standard informed consent procedure.
A total of 22 consecutive patients (9 males and 13
females; age, 74±9.2 years) with nonvalvular AF, CHA2DS2-VASC score
(1) [which assigns points for
congestive heart failure, hypertension, age ≥75 years (doubled),
diabetes, stroke (doubled), vascular disease, age 65–74 years and
sex (female)] ≥2 and contraindication to oral anticoagulants (OACs)
who were admitted to the Department of Cardiology, Shanghai Ninth
People's Hospital Affiliated to Shanghai Jiaotong University School
of Medicine between April and December 2016 were prospectively
enrolled in the present study. The CHA2DS2-VASC score is the most
commonly used method to predict thromboembolic risk in AF (1). Patients with CHA2DS2-VASC score ≥2 are
at high risk of stroke and are recommended to receive OAC treatment
(1). The present study excluded
patients with newly diagnosed AF, hyperthyroidism-related AF,
post-coronary artery bypass grafting AF, significant valvular heart
disease, acute coronary syndrome, myocardial infarction within 3
months, presence of LAA thrombus, New York Heart Association
functional class IV heart failure (5) or left ventricular ejection fraction
(LVEF) <40%, hepatic or renal failure (creatinine clearance,
<30 ml/min), active infectious endocarditis, acute stroke or
transient ischemic attack within 30 days, chronic inflammatory or
neoplastic diseases. Patients undergoing urgent surgery and those
with active peptic ulcers or disorders in blood coagulation were
also excluded. The Hypertension, Abnormal Renal/Liver Function,
Stroke, Bleeding History or Predisposition, Labile INR, Elderly
(>65 years), Drugs/Alcohol Concomitantly score was used to
predict the bleeding risk in AF patients taking anticoagulants
(1).
Transthoracic echocardiography
(TTE)
All patients underwent a routine TTE exam using a
Philips iE Elite ultrasound instrument (Philips Medical Systems,
Inc., Bothell, WA, USA) equipped with an S5-1 probe. Standard
echocardiographic views and measurement were obtained according to
the recommendations of the American Society of Echocardiography
(6).
TEE
All patients underwent TEE examinations 72 h prior
to the procedure, during the procedure, and at 3 months and 1 year
following the procedure. TEE was performed using a 3D matrix-array
transesophageal X7-2t transducer (Philips Medical Systems, Inc.).
The left atrium, interatrial septum and LAA were then evaluated.
The levels of mitral regurgitation, pericardial effusion and left
superior pulmonary venous flow were measured. Using the 2D TEE
mode, measurement of LAA dimensions was obtained at the 0°, 45°,
90° and 135° planes. The largest dimension was considered as the
final result of 2D TEE measurement. Once the LAA was clearly
displayed at 90°, the 3D mode was switched on. 3D TEE imaging was
subsequently performed acquiring the usual pyramidal data set large
enough to include the entire LAA. The zoom mode was used to improve
the visualization of the LAA. The internal area and maximum
diameter of the LAA orifice were measured directly from the
original 3D views, along a plane connecting the origin of the left
circumflex artery to the roof of the LAA, below the ligament of
Marshall. These measurements were assessed online using the iE
Elite ultrasound machine (Philips Medical Systems, Inc.). The LAA
depth (defined as the longest distance from the LAA orifice at the
circumflex artery level to the tip of the LAA) was measured
off-line from the long-axes views, using dedicated software (QLAB
9.1; Philips Medical Systems, Inc.).
Contrast LAA angiography
The diameters of the landing zone were measured by
contrast angiography in the (right anterior oblique 30°/20°)
cranial and caudal views. The largest diameter in the different
views was defined as the final diameter of the landing zone.
Implantation of the LAA closure
device
The LACBES® occluder is made of nitinol
wire mesh and consists of two parts, an anchor cylinder and a
sealing disc, which are filled with a polyester fiber membrane
(4). The anchor cylinder is made of
weaved nitinol wires with 9–12 integrated micro-barbs. It lands at
the landing zone and deeply anchors to the LAA as a supporting
structure for the whole occluder, with the sealing disc sealing the
LAA orifice. The sealing disc is curved inwards and is 6 mm larger
in diameter than the anchor cylinder. The device is available with
anchor cylinder sizes from 16–34 mm (2-mm size increments) and
requires 9–14 French sheaths. The selection of device was based on
the morphological characteristics of the LAA measured by 2D-TEE,
3D-TEE and contrast angiography. The puncture of the atrial septum
was guided by RT-3D TEE. Upon deployment of the occluder, the
device's position and stability were tested by contrast angiography
and TEE.
Follow-up
Upon LAA closure, TTE was performed at months 1, 3,
6 and 12, whereas 3D TEE was performed at months 3 and 12. Device
position, device-related thrombi, left superior pulmonary vein
velocity and pericardial effusion were recorded. Primary endpoints,
defined as the onset of stroke, and serious adverse events such as
hemorrhage, were monitored.
Statistical analysis
Continuous variables were expressed as the mean ±
standard deviation and were compared using unpaired Student's
t-test. For comparisons of >2 groups, one-way analysis of
variance with post-hoc Tukey's test was used. When data were not
normally distributed, they were expressed as the median and range,
and were analyzed with Mann-Whitney U non-parametric tests. More
than two groups were compared using the non-parametric
Kruskal-Wallis test. Categorical variables were expressed as
percentages. Agreement analysis between the device's size and the
landing zone's diameter obtained by RT-3D TEE, 2D TEE and
angiography was evaluated with Bland-Altman plots. Multiple linear
regression analyses were used in a stepwise mode to assess the
correlation among 2D TEE, 3D TEE, LAA angiography and device's
size. A 2-sided P<0.05 was considered to indicate a
statistically significant difference. All analyses were performed
using SPSS version 19.0 software (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
The baseline clinical characteristics of the study
population are presented in Table I.
All patients (n=22) had notably high CHA2DS2-VASC score, and a high
proportion of patients had suffered a previous stroke (45.4%). A
total of 14 patients were contradicted to OACs. A total of 8
patients took OACs at low doses, as 3 patients had bleeding
tendency on OACs at routine doses and 5 patients had a history of
prior bleeding (including prior ulcer bleeding, hematuria due to
kidney stone and prior bronchiectasis hemoptysis). These 8 patients
were not candidates for long-term OACs and thus were enrolled for
the LAAC procedures. All patients underwent successful LAA closure
with the guidance of RT-3D TEE. The duration of the procedure and
the number of occluder devices used were 77.92±21.96 min and
1.14±0.36, respectively. During the procedure, only 1 case of
clinically acceptable residual shunt (2-mm residual shunt) was
noticed, while 2 patients exhibited evidence of minor pericardial
effusion, and no tamponade was observed. No stroke due to the
operation or any other LAAC-related complications were observed.
The flow velocity of left superior pulmonary vein and the intensity
of mitral regurgitation remained unchanged upon LAAC (Table II).
 | Table I.Baseline clinical characteristics. |
Table I.
Baseline clinical characteristics.
| Variable | Value |
|---|
| Age, years | 74±9.2 |
| Male, n (%) | 9 (40.9) |
| BMI,
kg/m2 | 23.52±2.71 |
| Hypertension, n
(%) | 11 (50.0) |
| Diabetes mellitus, n
(%) | 7 (31.8) |
| Coronary heart
disease, n (%) | 16 (72.7) |
| Duration of AF <1
year, n (%) | 4 (18.2) |
| Duration of AF >1
year, n (%) | 18 (81.8) |
| Previous stroke, n
(%) | 10 (45.4) |
| History of
radiofrequency ablation, n (%) | 6 (27.3) |
| Use of OACs, n
(%) | 8 (36.4) |
| CHA2DS2-VASc | 3.7±1.2 |
| HAS-BLED | 3.9±0.9 |
| Prior bleeding, n
(%) | 8 (36.4) |
| Bleeding with
OACs | 4 (18.2) |
 | Table II.Measurements by echo during left
atrial appendage closure procedure and in the follow-up. |
Table II.
Measurements by echo during left
atrial appendage closure procedure and in the follow-up.
| Measurement | Baseline | Immediately after the
procedure | 3 months after the
procedure | 1 year after the
procedure |
|---|
| LA diameter, mm | 41.86±4.69 | 41.45±5.27 | 41.91±4.99 | 42.14±4.75 |
| LVEDD, mm | 47.77±3.60 | 48.36±3.98 | 47.59±3.63 | 47.73±3.28 |
| LVESD, mm | 32.32±3.64 | 32.36±3.78 | 31.91±2.96 | 31.64±2.65 |
| LVEF, (%) | 61.45±4.62 | 61.64±4.23 | 62.14±2.45 | 62.72±2.97 |
| LSPV peak systolic
velocity, cm/sec | 54.1±10.1 | 52.3±8.6 | 56.7±9.3 | 55.5±7.9 |
| Pericardial
effusion | NA | 0 (0) | 0 (0) | 0 (0) |
| Device
displacement | NA | 0 (0) | 0 (0) | 0 (0) |
| Thrombi formed on
device | NA | 0 (0) | 0 (0) | 0 (0) |
| Residual shunt around
device <3 mm | NA | 1 (4.5) | 1 (4.5) | 1 (4.5) |
Follow-up
During the follow-up at 3 months and 1 year, TTE and
TEE were performed. No device displacement, device-related thrombi
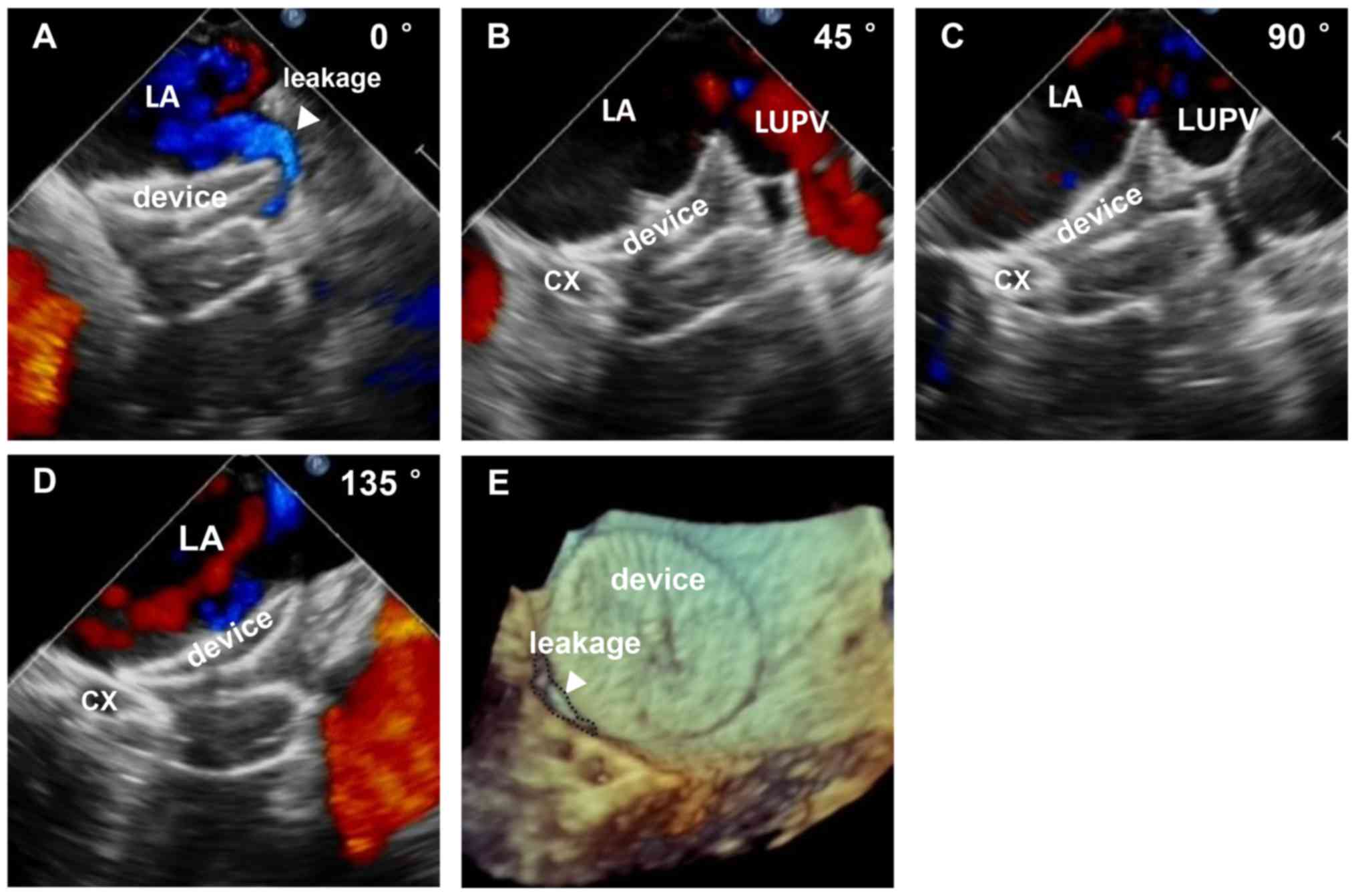
or pericardial effusion were observed. Only 1 patient had
clinically acceptable peri-device leakage (2-mm residual shunt).
All the trans-septum puncture spots were recovered 3 months
following the procedure. LVEF, LA volume and left superior
pulmonary vein velocity at the 3-months and 1-year follow-up were
similar to the baseline levels (Table
II). Additionally, no stroke, major bleeding or adverse
outcomes were recorded (Table
II).
Comparisons among 2D TEE, RT-3D TEE,
LAA contrast angiography and implanted device
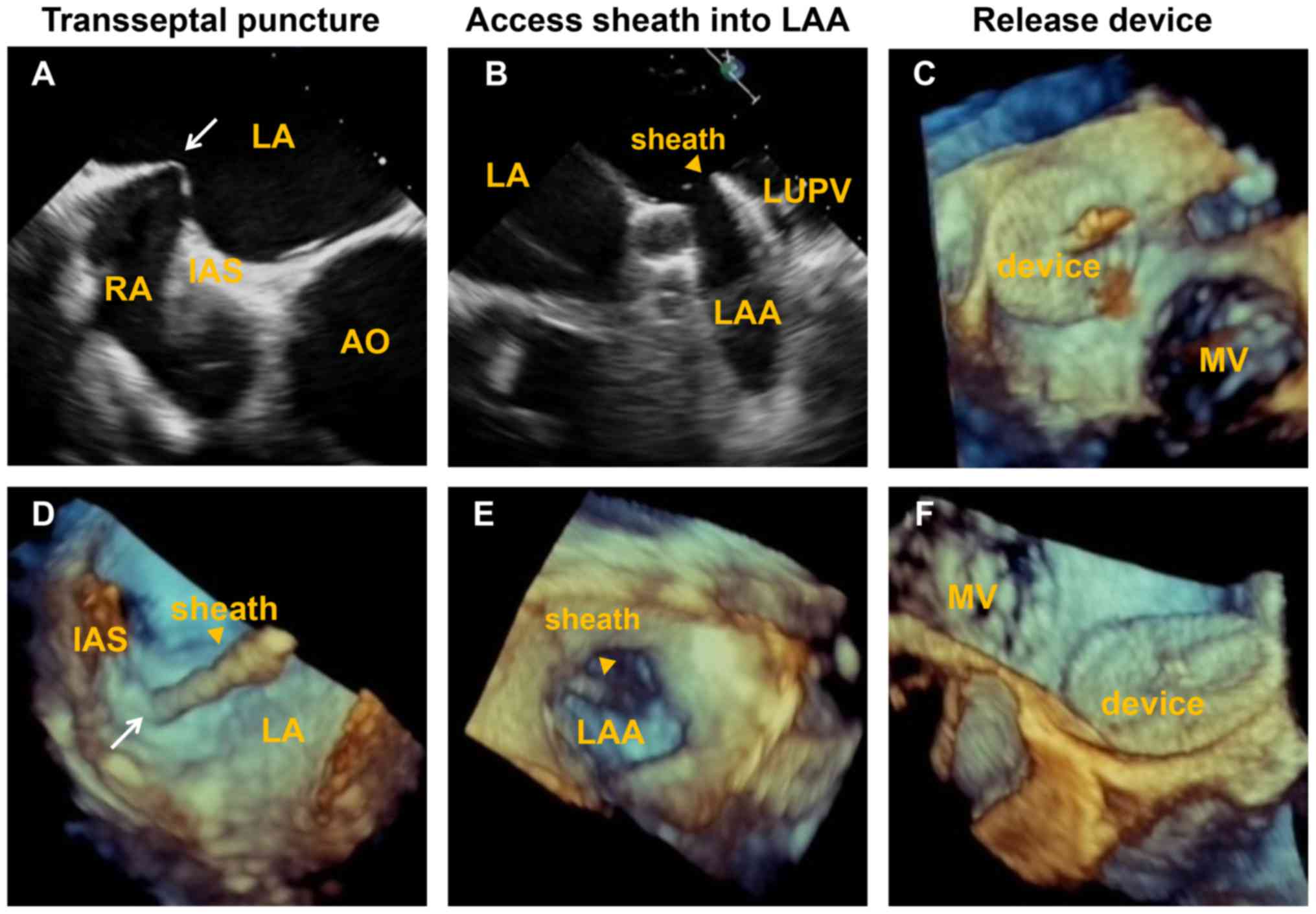
The whole procedure was performed under the guidance
of 2D TEE and RT-3D TEE. The interatrial septal puncture, delivery
of the sheath, release of the occluder device and peri-device
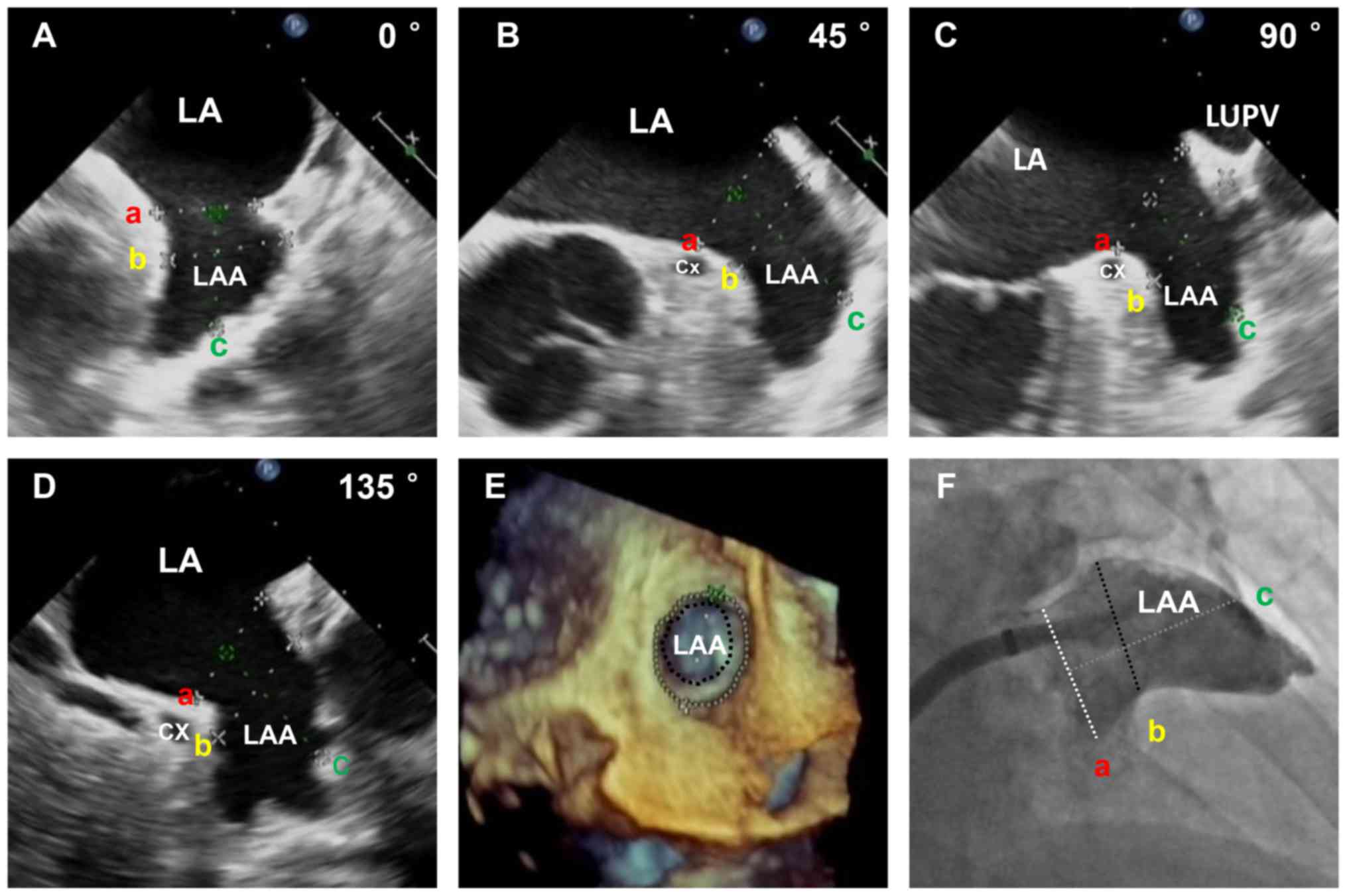
leakage were monitored and evaluated by 2D and 3D TEE (Figs. 1 and 2). The diameter of the landing zone was
measured by 2D TEE, 3D TEE and X-rays (Fig. 3). As presented in Table III, the diameters of the landing
zone obtained by 2D TEE, 3D TEE and contrast angiography were
22.91±4.13, 24.41±3.48 and 23.64±3.79 mm, respectively. The 3D
TEE-derived measurements were slightly larger than those obtained
with 2D TEE and LAA contrast angiography. No statistically
significant difference was observed among the parameters evaluated
by these three methods.
 | Table III.Implanted device size and measurements
by different methods. |
Table III.
Implanted device size and measurements
by different methods.
| Variable | Value |
|---|
| Devices used | 1.14±0.36 |
| Occlusion success
rate | 22 (100) |
| Device size (anchor
cylinder diameter), mm | 25.18±3.74 |
| RT-3D TEE |
|
| Orifice
diameter, mm |
26.36±4.17a |
| Landing
zone diameter, mm | 24.41±3.48 |
| 2D TEE |
|
| Orifice
diameter, mm | 22.64±4.09 |
| Landing
zone diameter, mm | 22.91±4.13 |
| LAA contrast
angiography |
|
| Orifice
diameter, mm | 24.40±4.77 |
| Landing
zone diameter, mm | 23.64±3.79 |
Correlation between different
measurements and device size
Multiple linear regression analysis was utilized to
analyze the correlation between different measurements and device
size. The results revealed that the diameter of the landing zone
obtained by 3D TEE had a better association with the size of the
implanted occlude than that obtained with 2D TEE and LAA
angiography [3D TEE, r=0.60, 95% confidence interval
(CI)=0.226–0.980, P=0.003; 2D TEE, r=0.18, 95% CI=−0.044–0.405,
P=0.11; contrast angiography, r=0.24, 95% CI=−0.130–0.605, P=0.19].
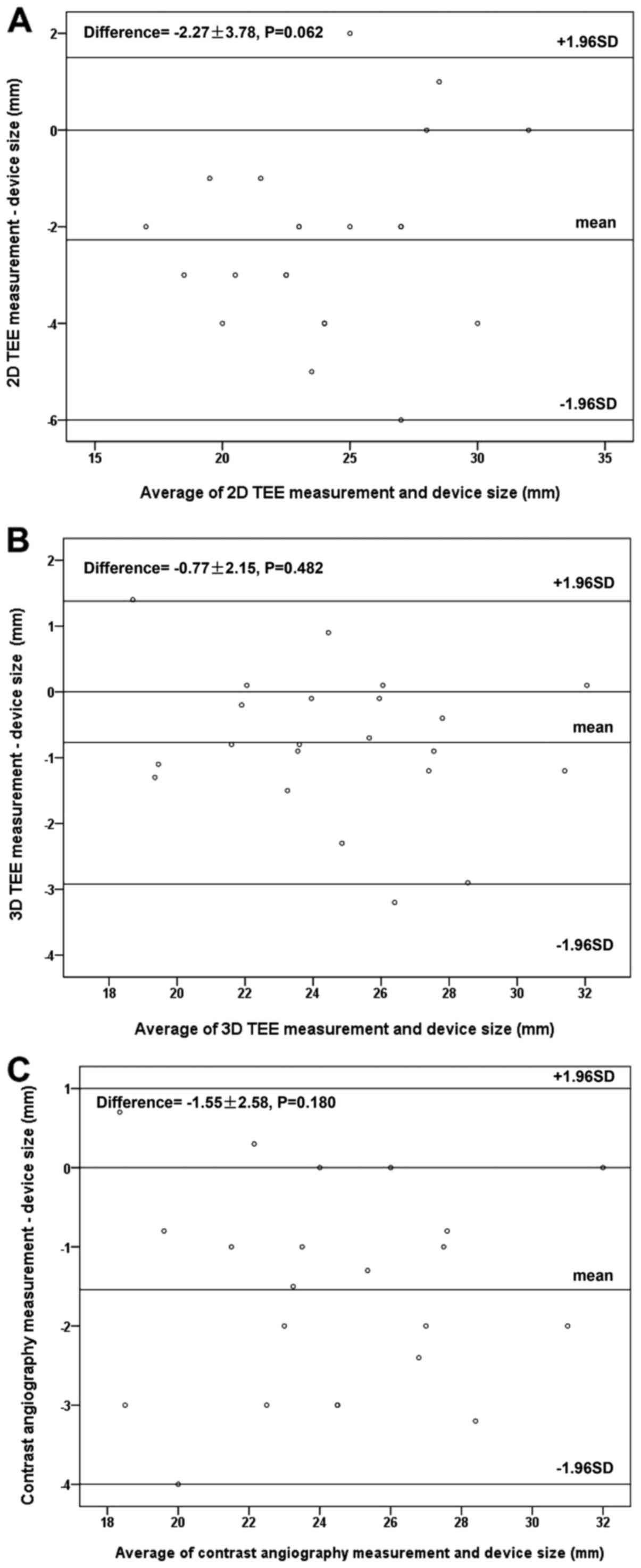
Bland-Altman analysis revealed positive correlation and low
variability between the landing zone diameter measured by 3D TEE
and the device's size (Fig. 4B).
However, 2D TEE underestimated the diameter of the landing zone
with marginal significance (Fig. 4A;
P=0.062).
Discussion
The present study evaluated the feasibility and
safety of RT-3D TEE to guide LAAC with the LACBES®
device. Our main findings can be summarized as follows: i) Compared
with conventional methods such as 2D-TEE and LAA contrast
angiography, RT-3D TEE was more accurate in the decision of
LACBES® device selection and guiding device deployment
during the procedure; and ii) RT-3D TEE was a reliable and safe
imaging modality for LAA occlusion in patients with AF, and was
recommended for routine clinical application in LAAC with
LACBES® devices.
The LACBES® device is a novel member of
the LAA occluder family, which has recently been approved to be
used in clinical trials in certain cardiovascular centers in China
(4). It is composed of two parts, an
anchor cylinder and a sealing disc (4). These two parts are connected by a long,
thin waist. The anchor cylinder integrated with micro-barbs is
harder than the sealing disc. Consequently, the anchor cylinder is
able to land at the landing zone firmly, while the sealing disc is
sufficiently flexible to cover the orifice seamlessly with minimal
damage to the left atrial or LAA wall. As a novel device, there is
little information for determining the optimal size of the device
and guiding the device deployment during the procedure.
In the present study, a total of 22 consecutive
patients underwent LAAC with the LACBES® device between
April and December 2016. As LAAC is an invasive procedure, the
present study excluded patients with severe conditions for safety
and ethical reasons. During the procedure, all patients were
generally anesthetized, mechanically ventilated and guided by 3D-RT
TEE. All the patients tolerated the procedure well and were
resuscitated 2 h following the procedure. The present study
demonstrated that RT-3D TEE was safe and more valuable for LAAC in
clinical practice than the two conventional methods, 2D-TEE and LAA
contrast angiography. This was primarily due to three advantages:
i) The precise determination of the morphology of LAA as well as
the size of the LAA orifice and the landing zone; ii) guiding the
puncture of the atrial septum; and iii) evaluating the position of
the device in the cavity of the atrial appendage.
It is well known that implanting a smaller sized
device may cause peri-device leakage, while using a larger sized
device may increase the risk of LAA perforation and cardiac
tamponade during and following the procedure. Therefore, accurate
measurement of LAA is important for the selection of the optimal
LACBES® device. In 2D TEE, the dimensions of the LAA
orifice and landing zone were measured at the 0°, 45°, 90° and 135°
planes, and the maximum measurement was used for device sizing
(7,8). However, 2D TEE was frequently not
parallel to the ostial plane (9).
Due to the complexity of LAA, 2D TEE is unlikely to provide the
accurate dimension of the LAA. By contrast, RT-3D TEE provides more
information about the anatomy of LAA. The 3D TEE iCrop and iSlice
model is capable of displaying the anatomical structures of LAA
from any angle, including LAA ostial pattern, lobes and internal
anatomy such as the tuberculations in each lobe. Therefore, it
efficiently eradicates the measurement error and provides accurate
information for device selection (10,11). As
a result, the present study revealed that there was a stronger
correlation between the dimension of the orifice and the landing
zone measured by RT-3D TEE and the selection of closure device.
However, device sizing parameters evaluated by 2D-TEE or LAA
contrast angiography were slightly smaller than the size of the
implanted device. Previously, Zhou et al (12) investigated the clinical value of 3D
TEE in the periprocedure of LAA closure using LeFort or LAmbre
devices, both of which are locally developed devices and available
in China. In accordance with the present findings, the landing zone
measured by RT-3D TEE Flexi Slice mode was strongly correlated with
the size of the closure device. Furthermore, all patients in the
present study underwent LAA closure under the guidance of RT-3D
TEE. No cases of adverse events, such as device displacement,
peri-device leakage, pericardial effusion or thrombus, were
recorded immediately following the procedure, or at 3 or 12 months
post-procedure. Only 1 case of clinically acceptable peri-device
leakage (2-mm residual shunt) was observed.
Furthermore, atrial septal puncture is the crucial
step for the LAAC procedure. Appropriate atrial septal puncture
guarantees the sheath and the device to arrive at the LAA through
the puncture point and to remain in a suitable orientation in the
long axis of LAA (11). Co-axiality
is also critical to adjust the device's angle and direction.
Previous studies have demonstrated that a full view of the atrial
septum can be easily obtained by RT-3D TEE, which facilitates the
determination of the optimal puncture site (10,12,13).
With the guidance of RT-3D TEE, no inappropriate puncture,
puncture-related complications or undesirable co-axiality occurred
in the present study. As a result, no deformation of closure
devices or obvious residual leakage was observed during
follow-up.
In addition, RT-3D TEE provided the en face view of
the closure device upon placement as well as the position of the
device in LAA and the location between the adjacent structures. 3D
TEE also displayed the comprehensive position of the device with
respect to the dynamic movement of the mitral valve and the left
superior pulmonary vein.
The present study presented several limitations.
First, this is an observational study with a small number of
patients in a single center. A larger scale multicenter study is
desirable. Secondly, although RT-3D TEE is a more reliable and
effective imaging method to guide LAAC than 2D TEE, 3D TEE is prone
to artifacts from arrythmia and ventilation (11,12).
Particular attention should be paid to acquire 3D images and avoid
motion artifacts during the procedure when patients are generally
anesthetized and ventilated. Thirdly, 3D TEE can only be applied in
experienced centers, as reconstruction of high-quality 3D images
requires proficient skills and is time consuming. Lastly, in the
present study, the closure device selection was predominantly based
on RT-3D TEE, by which the largest size of the landing zone was
obtained. Thus, it could cause a selection bias when evaluating the
potential value of 2D TEE, 3D TEE and LAA angiography in LAAC
device selection. The application of various novel 3D imaging
techniques is likely to minimize the selection bias. Our future
studies will investigate the significance of computed
tomography-based 3D LAA models together with RT-3D TEE in LAAC
procedures.
In conclusion, both 2D TEE and 3D TEE are useful in
LAAC during the peri-procedure and follow-up. However, RT-3D TEE
imaging is a more effective and accurate tool for improving the
quality and safety of LAAC than conventional 2D TEE. It allows
proper evaluation of the LAA's morphology, determination of the
appropriate size of the device, continuous visualization of the
catheter and device implantation, and precise delineation of the
position of the device in LAA. All these aspects contribute to the
intra-operative monitoring and evaluation of the closure in an
accurate manner.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China grant (grant no. 81570037), the
Key Basic Research Program of Shanghai Science and Technology
Committee (grant no. 14JC14044) and the Shanghai Ninth People's
Hospital MDT Program (grant no. 2017-1-015).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ designed the study, analyzed and interpreted the
data, and drafted the manuscript. ZT performed the some of the
transthoracic echocardiography (TTE) and the transesophageal
echocardiography (TEE) examinations, analyzed the TTE/TEE data and
made substantial contributions to the interpretation of the data.
ZH performed the left atrial appendage closure procedures. LZ
performed some of the TTE examinations. CW gave advice on the study
design, interpreted the statistical analysis and critically revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Board of Shanghai Ninth People's Hospital, Shanghai Jiaotong
University School of Medicine (approval no. 2016-45-C12). All
patients provided written, informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
January CT, Wann LS, Alpert JS, Calkins H,
Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD,
Field ME, et al: 2014 AHA/ACC/HRS guideline for the management of
patients with atrial fibrillation: Executive summary: A report of
the American College of Cardiology/American Heart Association Task
Force on practice guidelines and the heart rhythm society.
Circulation. 130:2071–2104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bergmann MW and Landmesser U: Left atrial
appendage closure for stroke prevention in non-valvular atrial
fibrillation: Rationale, devices in clinical development and
insights into implantation techniques. EuroIntervention.
10:497–504. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Möbius-Winkler S, Sandri M, Mangner N,
Lurz P, Dähnert I and Schuler G: The WATCHMAN left atrial appendage
closure device for atrial fibrillation. J Vis Exp. 60:36712012.
|
|
4
|
Tang X, Zhang Z, Wang F, Bai Y, Xu X,
Huang X, Zhao X, Gong S and Qin Y: Percutaneous left atrial
appendage closure with LACBES® occluder-a preclinical
feasibility study. Circ J. 82:87–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lang RM, Badano LP, Mor-Avi V, Afilalo J,
Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA,
Kuznetsova T, et al: Recommendations for cardiac chamber
quantification by echocardiography in adults: An update from the
American Society of echocardiography and the European association
of cardiovascular imaging. J Am Soc Echocardiogr. 28:1–39.e14.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
The criteria committee of the New York
heart association. Nomenclature and criteria for diagnosis of
diseases of the heart and Great Vessels. 9th. Little, Brown &
Co.; Boston: pp. 253–256. 1994
|
|
7
|
Perk G, Lang RM, Garcia-Fernandez MA,
Lodato J, Sugeng L, Lopez J, Knight BP, Messika-Zeitoun D, Shah S,
Slater J, et al: Use of real time three dimensional transesophageal
echocardiography in intracardiac catheter based interventions. J Am
Soc Echocardiogr. 22:865–882. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alli O and Holmes D Jr: Evaluation of the
WATCHMAN left atrialappendage closure device. Expert Rev Med
Devices. 11:541–551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Di Biase L, Horton RP, Nguyen T,
Morhanty P and Natale A: Left atrial appendage studied by computed
tomography to help planning for appendage closure device placement.
J Cardiovasc Electrophysiol. 21:973–982. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nucifora G, Faletra FF, Regoli F, Pasotti
E, Pedrazzini G, Moccetti T and Auricchio A: Evaluation of the left
atrial appendage with real-time 3-dimensional transesophageal
echocardiography: Implications for catheter-based left atrial
appendage closure. Circ Cardiovasc Imaging. 4:514–523. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wunderlich NC, Beigel R, Swaans MJ, Ho SY
and Siegel RJ: Percutaneous interventions for left atrial appendage
exclusion: Options, assessment, and imaging using 2D and 3D
echocardiography. JACC Cardiovasc Imaging. 8:472–488. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou Q, Song H, Zhang L, Deng Q, Chen J,
Hu B, Wang Y and Guo R: Roles of real-time three-dimensional
transesophageal echocardiography in peri-operation of transcatheter
left atrial appendage closure. Medicine (Baltimore). 96:e56372017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Faletra FF, Pedrazzini G, Pasotti E,
Muzzarelli S, Dequarti MC, Murzilli R, Schlossbauer SA, Slater IP
and Moccetti T: 3D TEE during catheter-based interventions. JACC
Cardiovasc Imaging. 7:292–308. 2014. View Article : Google Scholar : PubMed/NCBI
|