Introduction
Pernicious placenta previa occurs in patients who
have previously delivered via cesarean section, and in whom the
placenta previa is attached to the scars of the previous uterine
incision (1). The main clinical
symptoms of placenta previa include irregular vaginal bleeding,
hysterorrhexis, postpartum hemorrhage and invasion of the placenta
into pelvic organs. Fatal postpartum hemorrhage is often a danger
to maternal life (2). In this
emergency situation, obstetricians may have to perform obstetric
hysterectomy in order to save the patient's life.
Placenta accreta is the direct invasion of placental
villi into myometrium (3). At
present, the clinical treatment options for placenta previa and
accreta, include internal iliac artery ligation, internal iliac
artery embolization, uterine artery embolization, intrauterine
balloon tamponade and wound suturing (4–6). The
pelvic region is rich in collateral circulation and arterial
anastomoses. Frequently, limited treatment outcomes are achieved
from internal iliac artery ligation, intrauterine balloon tamponade
and wound suturing (7). The methods
of internal iliac artery embolization and uterine artery
embolization were thought to be universally effective (8). However, only a minority of hospitals in
China have hybrid operating rooms and, due to the life-threatening
nature of postpartum hemorrhage or hemorrhagic shock, there may not
be sufficient time for patients to be transferred from the surgical
operating room to another operating room for digital subtraction
angiography (DSA). Therefore, with regard to delayed postpartum
hemorrhage or minor hemorrhage, we consider uterine artery
embolization to be a better treatment choice.
Currently, there are few reports concerning the use
of internal iliac artery balloon occlusion or abdominal artery
balloon occlusion to control postpartum hemorrhage in patients with
pernicious placenta previa (6,9). The
current study reports some successful experience and problems
associated with the use of abdominal artery balloon occlusion in
patients with pernicious placenta previa.
Patients and methods
Patients
The present retrospective study was conducted among
women with pernicious placenta previa accreta/increta who were
treated in The First Affiliated Hospital of Anhui Medical
University (Hefei, China) from June 2016 to November 2017. A total
of 9 patients were diagnosed with placenta previa accreta/increta
by color Doppler ultrasonography and/or magnetic resonance imaging
(10). The age range of the patients
was 23–37 years (mean age, 29.8 years). All patients gave written
informed consent for the use of their data in clinical research,
and the study was approved by the Ethics Committee of Anhui Medical
University.
Prior to cesarean delivery, all patients and their
families were informed of the risks of the procedure, the details
of the abdominal aortic balloon occlusion and cesarean section, and
the possible complications of abdominal aortic balloon occlusion
during cesarean delivery. It was necessary to perform magnetic
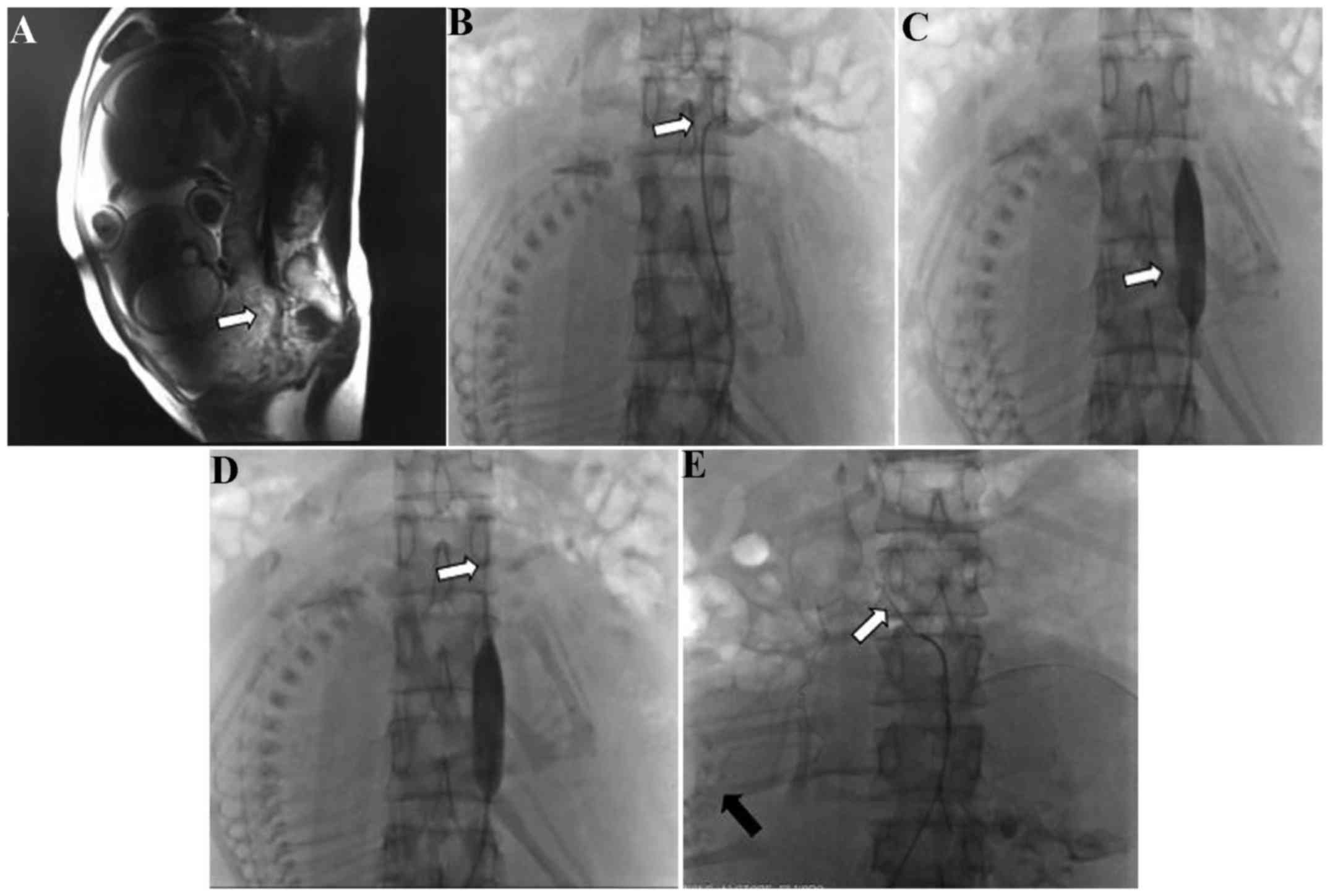
resonance imaging (MRI; Fig. 1A) or
ultrasonography to ascertain the scope and position of the placenta
previa accreta/increta. At the same time, measurements of the
diameter of the abdominal aorta were made according to the results
of the MRI or ultrasonography, in order to select the appropriate
balloon size.
Interventional procedure
On the day of cesarean delivery, in the DSA
operating room, puncture of the right femoral artery and placement
of an 8F arterial sheath (Bard Medical Division, Covington, GA,
USA) was performed in each patient following local anesthesia using
2% lidocaine. Under fluoroscopic guidance, a 5F cobra or pigtail
catheter (Terumo Co., Ltd., Tokyo, Japan) was placed into the
abdominal aorta under angiography using a guide wire to clear the
renal artery; the cobra or pigtail catheter was removed when the
opening level of the renal artery was identified. Similarly, a
balloon catheter was placed into the abdominal artery using a guide
wire, with the balloon positioned below the level of the renal
artery (Fig. 1B-D). Following X-ray
fluoroscopy, the X-ray dose was recorded.
Cesarean procedure
Following the interventional procedure, the patient
was transferred to the surgical operating room to undergo the
cesarean procedure. Once the fetal head had been delivered, the
balloon was inflated with 0.9% saline (15 ml) to block blood flow
immediately. The balloon was inflated for no longer than 15 min,
and was then was deflated for 3 min. Inflation and deflation were
alternated until no evident bleeding was observed and the uterine
wound was then sutured completely. The balloon catheter was
withdrawn following the completion of the whole procedure. The
arterial sheath was removed after 6 h. The estimated blood loss,
the volume of blood products transfused, the duration of the
surgical procedure and the Apgar score at 5 min were recorded
(11). Patients were monitored for
complications for 24 h after surgery.
Results
All observation data are summarized in Table I. The procedure was completely
successful in 7 patients, in whom the maximum estimated volume of
blood loss was ≤2,500 ml. Embolic uterine arterial occlusion was
required in the other 2 cases due to uncontrolled postpartum
hemorrhage. In one of these cases, the estimated blood loss was
4,000 ml. Notably, during the abdominal aortic angiography
procedure, it was observed that the uterine artery originated from
the adrenal artery in this case (Fig.
1E). In the other case, the estimated blood loss was 3,500 ml.
During the cesarean delivery procedure, this patient was observed
to have a high degree of abnormal placental implantation. During
the uterine arterial angiography and embolization, the presence of
double uterine arteries was identified, with one originating from
the internal iliac artery and another from the common iliac artery.
In the study group, the total radiation dose to which the fetus was
exposed was 15.8–24.5 mGy, with a mean of 19.3±2.7 mGy (Table I). The largest transfusion was
received by was patient 2 and the mean number of units of red blood
cells transfused was 3.83. Similarly, fresh frozen plasma transfuse
was positively associated with the transfusion of red blood cells.
The range of duration of surgery was 35–230 min and the mean was
87.6 min. The Apgar score at 5 min range was 6–10 and the mean was
9.1. All patients retained the uterus successfully in the current
study, without the occurrence of severe complications.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Case no. | Age (years) | Gravidity and
parity | Duration of pregnancy
(weeks + days) | Estimated blood loss
(ml) | Red blood cells
transfused (units) | Fresh frozen plasma
transfused (ml) | Duration of surgery
(min) | Uterine artery
origin | Apgar score at 5
min | Fetal radiation
exposure dose (mGy) |
|---|
| 1 | 31 | G4P2 | 32+5 | 500 | 2 | 0 | 75 | Internal iliac
artery | 9 | 16.4 |
| 2 | 30 | G4P1 | 37+2 | 4, 000 | 13 | 1, 200 | 230 | Adrenal artery | 10 | 21.3 |
| 3 | 26 | G2P2 | 34+5 | 2, 500 | 6 | 600 | 72 | Internal iliac
artery | 10 | 15.8 |
| 4 | 29 | G4P1 | 35+5 | 2, 000 | 3.5 | 200 | 80 | Internal iliac
artery | 10 | 19.5 |
| 5 | 23 | G2P1 | 34+4 | 1, 000 | 2 | 0 | 59 | Internal iliac
artery | 6 | 22.3 |
| 6 | 30 | G4P1 | 35+1 | 3, 500 | 6 | 750 | 87 | Internal and common
iliac artery | 9 | 17.9 |
| 7 | 37 | G7P1 | 34+4 | 1, 200 | 2 | 200 | 80 | Internal iliac
artery | 9 | 24.5 |
| 8 | 35 | G3P1 | 36+1 | 1, 000 | 0 | 0 | 70 | Internal iliac
artery | 9 | 17.6 |
| 9 | 27 | G2P0 | 35+6 | 700 | 0 | 0 | 35 | Internal iliac
artery | 10 | 18.7 |
Discussion
Accurate diagnosis of pernicious placenta previa is
very important in the formulation of a detailed plan for subsequent
cesarean delivery. At present, ultrasonography and MRI serve an
important role in the identification of placenta previa
accreta/increta (12).
Three-dimensional color Doppler ultrasound scans have a high
sensitivity and specificity in the diagnosis of pernicious placenta
previa, as they can identify abnormal bleeding from the placental
bed and discern the chorionic villi from the myometrium (10). MRI of the soft tissue has marked
advantages, as it may allow clinicians to detect whether chorionic
villi have invaded into the myometrium and/or peripheral
organs/tissues (10). In the present
study, 2 patients were definitively diagnosed by ultrasonography,
and 7 patients by MRI. During the examination, the diameter of the
abdominal aorta was also measured in order to determine the optimum
balloon size. The selection of an appropriate balloon is essential;
if the balloon is oversized, it may cause injury to the abdominal
arterial endangium during the process of inflation; if it is too
small, it will not be able to effectively control the bleeding
during the cesarean procedure.
Cesarean delivery has been the treatment strategy
for placenta previa for many years (13). However, the control of intractable
bleeding during the surgery is key to the treatment. Traditional
methods of reducing blood loss include changing the uterine suture
method, intrauterine tamponade using a balloon or gauze, uterine
artery or internal iliac artery ligation, and uterine artery or
internal iliac artery embolization (5,14–16). A
number of previous studies have reported that prophylactic
abdominal aortic or internal iliac artery balloon catheter
application reduces bleeding successfully during cesarean delivery
in patients with placenta previa (6,17–19).
However, internal iliac artery balloon occlusion requires the use
of two balloons and bilateral femoral artery puncture; this
increases the economic burden for the patient and is inconvenient
owing to the puncture points requiring pressure homeostasis
following the procedure. Therefore, in the present study, abdominal
aortic balloon occlusion was used as the method of reducing blood
loss during the cesarean delivery, and was demonstrated to be
successful in the majority of the patients with placenta previa.
The minimum estimated blood loss was 500 ml, which is similar to
that reported in studies by Xie et al (18) and Sun et al (19). Although it may be useful in the
majority of patients, the authors of the current study hypothesised
that the abdominal aortic balloon occlusion method might not be
completely effective in cases with an ectopic uterine artery,
large-area placental implantation or poor uterine contraction. In
the present study, the uterine artery of one patient was derived
from the adrenal artery. The estimated blood loss was 4,000 ml
during the cesarean delivery in this case, demonstrating the method
to be almost ineffective; the balloon position was located below
the opening of the renal artery in order to avoid renal injury. The
estimated blood loss for another patient was 3,500 ml due to
large-area placental implantation. Fortunately, uterine artery
embolization was subsequently successfully performed in these
cases. A previous study by Sun et al (19) reported a patient whose estimated
blood loss was 9,000 ml. We suspect this patient may have had an
anomalous uterine artery.
Complications associated with arterial balloon
application have been reported in previous studies (6,18–22),
including ischemic necrosis of the lower limbs, internal iliac
arterial thrombosis, puncture point hematoma, reperfusion injury of
tissues and organs, acute renal failure and initial vessel injury.
In the studies conducted by Xie et al (18) and Sun et al (19), a 12F arterial sheath was used. It is
evident that larger arterial sheaths create a larger femoral
arterial lesion at the puncture site. When pulling out an arterial
sheath, a larger lesion will make compression and hemostasis more
difficult, and a hematoma may easily form at the puncture site. In
the study of Xie et al (18),
a patient presented a hematoma in the right common femoral artery
following arterial sheath removal. In the present study, the
appropriate arterial sheath was chosen according to the size of the
balloon catheter. The maximum arterial sheath was <10F. No
complications occurred in any of the patients. We suggest that the
choice of balloon size and control of the balloon occlusion time
are important factors. If the balloon is too large, it will cause
abdominal aortic injury, and if the balloon occlusion time is too
long, it will cause thrombosis of the lower limb and reperfusion
injury. The optimal occlusion time has been suggested to be in the
range of 25–80 min (18,19,23,24).
However, in the study by Sun et al (19), one patient developed thrombosis in
the internal iliac artery following surgery in which a 20-mm
balloon was selected and ≤40-min periods of occlusion with
intervals of 10 min were used. In the present study, the diameter
of the abdominal aorta was measured by MRI or ultrasonography prior
to surgery to determine the appropriate size of the balloon
catheter. Generally, the diameter of the selected balloon was ≤2 mm
wider than the diameter of the abdominal aorta. Furthermore,
occlusion was maintained for no longer than 15 min at a time with
intervals of 3 min, and no complications occurred in any of the
patients. Therefore, measurement of the abdominal aortic diameter
prior to intervention, clearance of the opening of the renal artery
and control of the duration of balloon occlusion are indicated to
be important for decreasing the risk of complications.
Additionally, minimizing the fetal radiation
exposure dose is an important factor when considering these
methods. The latest report about mean fetal radiation exposure dose
recommended a dose of 4.2±1.9 mGy (19). However, we consider it difficult to
achieve this target, particularly when using DSA. The X-ray dose of
DSA far exceeds that of fluoroscopy at the same exposure time
(25). In the present study, the
mean fetal radiation exposure dose was 19.3±2.7 mGy, which is far
less than the standard dose of ≤150 mGy recommended by the National
Committee on Radiological Protection (26). Insertion of the balloon into the
aorta was performed rapidly in all patients by experienced
interventional radiologists to minimize radiation exposure.
In conclusion, the prophylactic use of abdominal
aortic balloon catheters is a relatively safe and effective method
of treating patients with placenta previa, which may control
hemorrhaging during cesarean delivery and reduce the risk of
hysterectomy. However, the control of balloon occlusion-associated
complications requires further consideration. The present study had
certain limitations, such as a small sample size and the absence of
a control group. A control group was not included in the current
study because the method used was demonstrated to be very effective
in previous studies (18,19). However, since uncontrolled postpartum
hemorrhage occurred in cases with abnormal uterine arteries in the
present study, it was considered necessary to report these findings
in a timely manner in order to avoid ineffective balloon
occlusion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZHP, ZX, BSZ, GBZ, WS, LXT and XZZ performed the
surgery, analyzed the data and prepared the manuscript. ZHP and ZX
were responsible for study conception and design.
Ethics approval and consent to
participate
All patients gave written informed consent for the
use of their data in clinical research. The current study was
approved by the Ethics Committee of Anhui Medical University.
Patient consent for publication
Written informed consent was provided for
publication of the patients' data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
He Y and Chen D: New understanding of the
diagnosis and management of pernicious placenta previa. Chin J
Perinat Med. 18:494–496. 2015.(In Chinese).
|
|
2
|
Sumigama S, Itakura A, Ota T, Okada M,
Kotani T, Hayakawa H, Yoshida K, Ishikawa K, Hayashi K, Kurauchi O,
et al: Placenta previa increta/percreta in Japan: A retrospective
study of ultrasound findings, management and clinical course. J
Obstet Gynecol Res. 33:606–611. 2007. View Article : Google Scholar
|
|
3
|
Silver RM and Barbour KD: Placenta accreta
spectrum: Accreta, Increta, and percreta. Obstet Gynecol Clin North
Am. 42:381–402. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen ZY, Li J, Shen J, Jin J, Zhang W and
Zhong W: Direct puncture embolization of the internal iliac artery
during cesarean delivery for pernicious placenta previa coexisting
with placenta accreta. Int J Gynecol Obstet. 135:264–267. 2016.
View Article : Google Scholar
|
|
5
|
Xu JQ: Effectiveness of embolization of
the internal iliac or uterine arteries in the treatment of massive
obstetrical and gynecological hemorrhages. Eur Rev Med Pharmacol
Sci. 19:372–374. 2015.PubMed/NCBI
|
|
6
|
Cui SH, Zhi YX, Cheng GM, Zhang K, Zhang L
and Shen L: Retrospective analysis of placenta previa with abnormal
placentation with and without prophylactic use of abdominal aorta
balloon occlusion. Int J Gynecol Obstet. 137:265–270. 2017.
View Article : Google Scholar
|
|
7
|
Arduini M, Epicoco G, Clerici G,
Bottaccioli E, Arena S and Affronti G: B-Lynch suture, intrauterine
balloon, and endouterine hemostatic suture for the management of
postpartum hemorrhage due to placenta praevia accrete. Int J
Gynaecol Obstet. 108:191–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ganguli S, Stecker MS, Pyne D, Baum RA and
Fan CM: Uterine artery embolization in the treatment of postpartum
uterine hemorrhage. J Vasc Interv Radiol. 22:169–176. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carnevale FC, Kondo MM, de Oliveira Sousa
W Jr, Santos AB, da Motta Leal Filho JM, Moreira AM, Baroni RH,
Francisco RP and Zugaib M: Perioperative temporary occlusion of the
internal iliac arteries as prophylaxis in cesarean section at risk
of hemorrhage in placenta accreta. Cardiovasc Intervent Radiol.
34:758–764. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ayati S, Leila L, Pezeshkirad M, Seilanian
Toosi F, Nekooei S, Shakeri MT and Golmohammadi MS: Accuracy of
color Doppler ultrasonography and magnetic resonance imaging in
diagnosis of placenta accreta: A survey of 82 cases. Int J Reprod
Biomed (Yazd). 15:225–230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Crawford JS: The Apgar scoring system. Dev
Med Child Neurol. 4:441–444. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Balcacer P, Pahade J, Spektor M, Staib L,
Copel JA and McCarthy S: Magnetic resonance imaging and sonography
in the diagnosis of placental invasion. J Ultrasound Med.
35:1445–1456. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deng L, Chang Q, Wang Y, Wang L, Li Y and
Hu Q: Tourniquet device for hemorrhage control during cesarean
section of complete placenta previa pregnancies. J Obstet Gynaecol
Res. 40:399–404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Danisman N, Kahyaoglu S, Celen S, Akselim
B, Tuncer EG, Timur H, Kaymak O and Kahyaoglu I: The outcomes of
surgical treatment modalities to decrease ‘near miss’ maternal
morbidity caused by peripartum hemorrhage. Eur Rev Med Pharmacol
Sci. 18:1092–1097. 2014.PubMed/NCBI
|
|
15
|
Kaya B, Tuten A, Daglar K, Onkum M, Sucu
S, Dogan A, Unal O and Guralp O: B-Lynch uterine compression
sutures in the conservative surgical management of uterine atony.
Arch Gynecol Obstet. 291:1005–1014. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ko HK, Shin JH, Ko GY, Gwon DI, Kim JH,
Han K and Lee SW: Efficacy of prophylactic uterine artery
embolization before obstetrical procedures with high risk for
massive bleeding. Korean J Radiol. 18:355–360. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Angileri SA, Mailli L, Raspanti C, Ierardi
AM, Carrafiello G and Belli AM: Prophylactic occlusion balloon
placement in internal iliac arteries for the prevention of
postpartum haemorrhage due to morbidly adherent placenta: Short
term outcomes. Radiol Med. 122:798–806. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie L, Wang Y, Luo FY, Man YC and Zhao XL:
Prophylactic use of an infrarenal abdominal aorta balloon catheter
in pregnancies complicated by placenta accreta. J Obstet Gynaecol.
37:557–561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun WJ, Duan SH, Xin G, Xiao J, Hong F,
Hong H, Wu Y and Xu Y: Safety and efficacy of preoperative
abdominal aortic balloon occlusion in placenta increta and/or
percreta. J Surg Res. 222:75–84. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bishop S, Butler K, Monaghan S, Chan K,
Murphy G and Edozien L: Multiple complications following the use of
prophylactic internal iliac artery balloon catheterisation in a
patient with placenta. Int J Obstet Anesth. 20:70–73. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sewell MF, Rosenblum D and Ehrenberg H:
Arterial embolus during commom iliac balloon catheterization at
cesarean hysterectomy. Obstet Gynecol. 108:746–748. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei X, Zhang J, Chu Q, Du Y, Xing N, Xu X,
Zhou Y and Zhang W: Prophylactic abdominal aorta balloon occlusion
during caesarean section: A retrospective case series. Int J Obstet
Anesth. 27:3–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Masamoto H, Uehara H, Gibo M, Okubo E,
Sakumoto K and Aoki Y: Elective use of aortic balloon occlusion in
caesarean hysterectomy for placenta previa percreta. Gynecol Obstet
Invest. 67:92–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andoh S, Mitani S, Nonaka A, Suzuki S,
Tamaki F, Ohmori K and Kimura E: Use of temporary aortic balloon
occlusion of the abdominal aorta was useful during cesarean
hysterectomy for placenta accrete. Masui. 60:217–219. 2011.(In
Japanese). PubMed/NCBI
|
|
25
|
Harrington DP, Boxt LM and Murray PD:
Digital subtraction angiography: Overview of technical principles.
AJR Am J Roentgenol. 139:781–786. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
The 2007 recommendations of the
international commission on radiological protection. ICRP
publication 103. Ann ICRP. 37:1–332. 2007.
|